Introduction
Tumorigenesis is initiated by the activation of
oncogenes and the inactivation of tumor suppressor genes, leading
to an increase in cell proliferation and a decrease in apoptosis.
The proto-oncogene Pim-2 was originally identified as a result of a
proviral insertion discovered in a murine T cell lymphoma (1). Overexpression of Pim-2 has been reported
to occur in lymphoma (2), leukemia
(3) and multiple myeloma (MM)
(4). Other previous studies have also
suggested that Pim-2 promoting the growth of solid tumors,
including prostate cancer (5),
gastric liver carcinomas (6) and
colorectal carcinoma (7). These
observations highlight that Pim-2 serves roles in the tumorigenesis
of a number of hematological neoplasms in addition to solid
tumors.
Pim kinases are a family of serine/threonine kinases
that includes three highly homologous members (Pim1, Pim2 and
Pim3). Pim kinases are important regulators of normal cell cycle
progression. Pim-1 and Pim-2 have a similar function, highlighted
by a study that demonstrated that Pim-1 and Pim-2 genes induced
lymphomas alone or in synergy with c-myc (8). Pim kinases inhibit cell growth via the
regulation of cell cycle progression (9). Phosphorylation of M-phase inducer
phosphatase 1 by Pim-1 amplifies the effects of this critical
G1/S-phase phosphatase (10). In addition, the stability of
cyclin-dependent kinase (CDK) inhibitor p21, which inhibits
G1/S-phase progression, was enhanced by Pim-2
phosphorylation and inhibited cell proliferation in HCT116 cells
(7). However, Pim-2 can function as a
potent survival factor; Pim-2 has been revealed to be upregulated
and associated with the progression of chronic lymphatic leukemia,
diffuse large B-cell lymphoma, mantle cell lymphoma and MM
(11–13). However, the molecular mechanisms
underlying the association between Pim-2 and cell cycle regulators
remain unclear in lung cancer and these neoplasms.
p21 Cip1/WAF1 (p21) is a negative modulator of cell
cycle progression and inhibits the activity of cyclin/CDK2
complexes, which phosphorylate retinoblastoma protein (Rb) and
promote E2F transcription factor 1 (E2F1)-induced proliferation by
inducing phosphorylation of its transactivation domain, thus
promoting the induction of genes required for S-phase progression
(14,15). DNA damage results in p53-dependent
induction of p21 during p53-induced apoptosis (16). However, the regulation of p21
expression is primarily regulated at the transcriptional level and
may occur via a p53-dependent or p53-independent mechanism
(17). Whether the mutation of the
p53 gene affects the link between Pim-2 and the p21 signaling
pathway is investigated in the present study.
The present study demonstrated that Pim2 was
expressed in solid tumors (lung cancer) and hematological neoplasms
(leukemia and MM). Downregulation of Pim-2 decreased cell
proliferation and cell cycle arrest in the
G0/G1 phase via the p21 signaling pathway.
Furthermore, the process in the H1299 (p53−) cell line
was not p53-dependent.
Materials and methods
Cell culture and transfection
K562 chronic myelogenous leukemia cell line,
RPMI-8226MM cell line and H1299 and A549 non-small cell lung
carcinoma cell lines were obtained from the American Type Culture
Collection (Manassas, VA, USA), and grown in RPMI-1640 medium
(Boehringer, Ingelheim, Germany) supplemented with 10%
heat-inactivated fetal calf serum (Boehringer), 100 µg/ml
penicillin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 100 U/ml streptomycin (Gibco; Thermo Fisher Scientific,
Inc.), in a humidified atmosphere (37.5°C; 5% CO2).
Transfections were performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Silencer validated short interfering
(si)RNA for Pim-2 (sense, 5′-GUGCCAAACUCAUUGAUUUTT-3′ and
antisense, 5′-AAAUCAAUGAGUUUGGCACTT-3′) and scrambled siRNA (sense,
5′-AUCCGCGCGAUAGUACGUATT-3′ and antisense,
5′-UACGUACUAUCGCGCGGAUTT-3′) were used. siRNA was diluted to 20 µM
with DEPC water and placed in a 6-well plate. A total of 5 µl siRNA
(20 µM), 5 µl Lipofectamine® 2000 and 100 µl culture
media was added per siRNA mastermix tube and agitated gently. This
was incubated for 15 min at room temperature to allow complex
formation between siRNA and lipids. Media was removed from the
cells and 1,900 µl fresh media was added to each 6-well plate.
siRNA mixture (110 µl per well) was added drop-wise while gently
swirling the plate. Cells were cultured for 48 h at 37.5°C prior to
harvesting for analysis.
Proliferation assay
Cell viability was evaluated using the tetrazolium
salt-based cell counting kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). Cells were seeded into
96-well plates at 1.5×105 cell/ml in 200 µl complete
medium (RPMI medium + serum). Plates were incubated for siRNA
transfection for 48 h at 37°C in 5% CO2, then 20 µl
CCK-8 reagent was added to the wells followed by incubation for 1.5
h at 37.5°C. The optical density (OD) was evaluated at 450 nm
within 15 min. The experiment was repeated 3 times with each sample
in triplicate. Cell viability was determined using the following
equation: Proliferation (%)=(OD450 of isogarcinol group/OD450 of
control group) ×100%.
Cell cycle analysis by flow
cytometry
Cell cycle analysis was performed using a
FACSCalibur (BD Bioscience, Franklin Lakes, NJ, USA). Cells
(5×105 cells) were fixed in 70% ethanol for ≥4 h at 4°C
and stained with 20 µg/ml propidium iodide supplemented with 10
µg/ml RNaseA for 30 min at room temperature. Resulting DNA
distributions were analyzed by Modifit (version 4.0; Verify
Software House, Inc., Topsham, ME, USA) for the proportions of
cells in the phases of the cell cycle.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from A549, H1299, RPMI8226
and K562 cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) at 4°C for 24 h. A total of 1 µg purified
total RNA was reverse transcribed to complementary DNA using the
SuperScript First-Strand Synthesis System (Invitrogen; Thermo
Fisher Scientific, Inc.). RT-qPCR was performed using SYBR Premix
Ex Taq (Takara Biotechnology Co., Ltd., Dalian, China) and the
Thermal Cycler Dice Real Time system (Takara Biotechnology Co.,
Ltd.) in a 96-well plate, according to the manufacturer's protocol.
The optimized parameters for PCR were: 95°C for 2 min, 94°C for 10
sec, 61.5°C for 30 sec and 72°C for 40 sec (40 cycles). The primers
used for RT-qPCR were as follows: Human Pim-2, sense
5′-TTGGGAAGGAATGGAAGATG-3′ and anti-sense,
5′-CAGGAGAACAAACAGCAAGC-3′; human GAPDH sense
5′-AATCCCATCACCATCTTCCA-3′ and antisense,
5′-TGGACTCCACGACGTACTCA-3′. The Pim-2 expression levels were
evaluated using the 2−ΔΔCq method, using GAPDH as an
internal control (18).
Western blot analysis
Western blot analysis evaluated the content of Pim-2
P53, P21, CDK2 Rb and phosphorylated (p) Rb in cell extracts
following siRNA transfection for 48 h. Cells were cultured with
nuclear factor-κB (NF-κB) inhibitor (Ro 106-9920; Tocris
Bioscience, Bristol, UK) at 37.5°C for 48 h prior to harvesting for
analysis of NF-κB and Pim-2 expression levels. Cells were lysed
with a lysis buffer (20 mM Tris-HCl pH 8.0, 150 mM NaCl, 2 mM EDTA,
100 mM NaF,1% NP40, 1 µg/ml leupeptin, 1 µg/ml anti-pain and 1 mM
phenylmethylsulfonyl fluoride),and the protein concentrations were
determined using a BCA protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Proteins (30 µg) were separated using 8%
SDS-PAGE. Following electrophoresis, the SDS-PAGE gels were
transferred electronically to polyvinylidene difluoride (PVDF)
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). PVDF
membranes were blocked using a solution containing 5% skimmed milk
and incubated overnight at 4°C with the following antibodies:
Anti-p21 (cat. no. 2947), anti-CDK2 (cat. no. 2546), anti-pRb (cat.
no. 9308), anti-Rb (cat. no. 9303), anti-NF-κB (cat. no. 8242),
anti-GAPDH (cat. no. 5174; all Cell Signaling Technology, Inc.,
Danvers, MA, USA), anti-Pim-2 (cat. no. ab97475; Abcam, Cambridge,
UK) and anti-p53 (cat. no. sc-126; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) were diluted using PBS (1:1,000). Following
washing with Tris-buffered saline with Tween-20, the membranes were
incubated for 1 h at room temperature with horseradish
peroxidase-conjugated anti-rabbit IgG sheep antibody diluted using
PBS (1:2,000; cat no. ab6721; Abcam) or horseradish
peroxidase-conjugated anti-mouse IgG sheep antibody diluted using
PBS (1:2,000; cat no. ab6785; Abcam). Reactive proteins were
visualized using an Immobilon Western horseradish peroxidase
chemiluminescence kit (EMD Millipore, Billerica, MA, USA).
Immunocytochemistry
Cells were prepared as monolayer on 6-well plates.
Monolayers were washed with PBS twice, fixed in 4% ice-cold
paraformaldehyde solution for 10 min and subsequently blocked in
PBS supplemented with 2% rabbit serum for 1 h at room temperature.
Samples were incubated with rabbit anti-Pim-2 (dilution, 1:500)
overnight at 4°C followed by a secondary fluorescein
isothiocyanate-conjugated anti-rabbit antibody (1:200; ab150077;
Abcam) for 1 h at room temperature. Following three washes,
monolayers were mounted on glass slides with ProLong antifade
mounting medium with DAPI (Molecular Probes; Thermo Fisher
Scientific, Inc.). Images were observed under a fluorescence
microscope (magnification, ×200).
Statistical analysis
All results are expressed as the mean and standard
deviation of numerous independent experiments. Multiple comparisons
of the data were performed by Student's t-test to determine
statistical significance of detected differences. P<0.05 was
considered to indicate a statistically significant difference.
Results
Pim-2 expression and localization
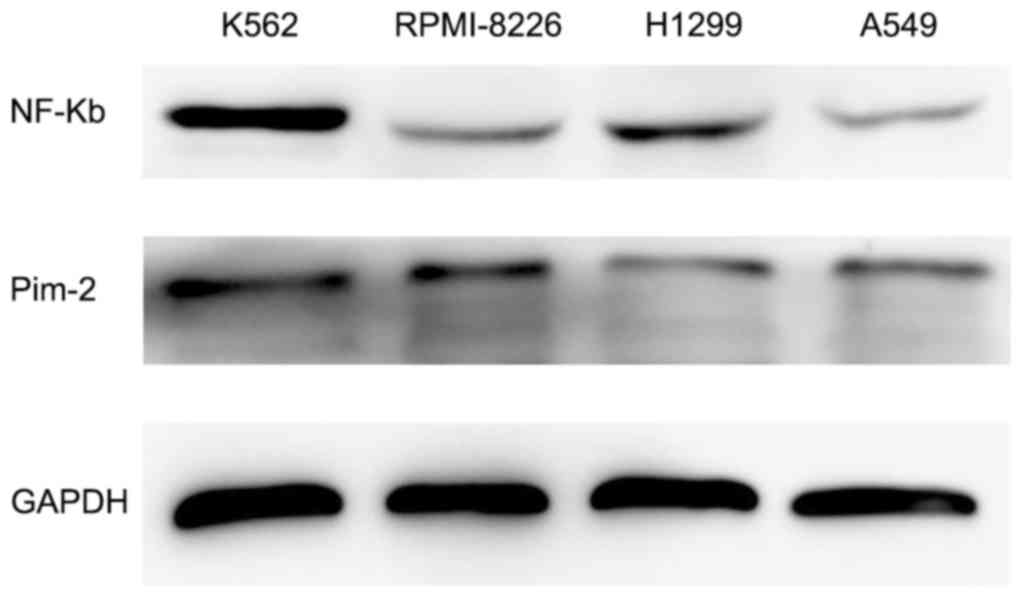
Western blotting was performed to evaluate the
expression levels of Pim-2 in K562, RPMI-8226, H1299 and A549 cell
lines. Western blotting demonstrated clear expression of Pim-2 and
NF-κB in K562 cells, but lower expression levels in RPMI-8226,
H1299 and A549 cell lines (Fig. 1).
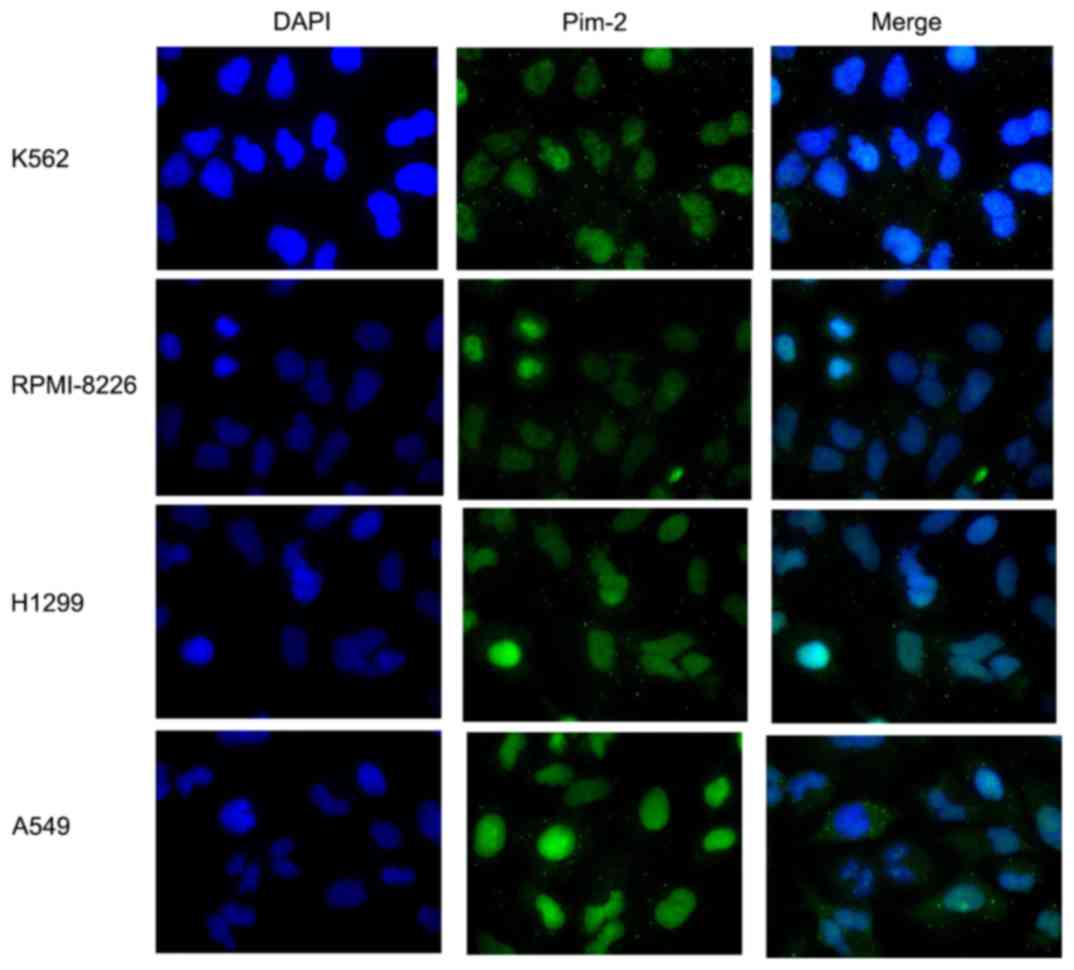
Immunocytochemistry analysis of all four cell lines revealed that
Pim-2 was predominantly located in the cytoplasm (Fig. 2).
Inhibition of Pim-2 mRNA and protein
expression levels by Pim-2 specific siRNA
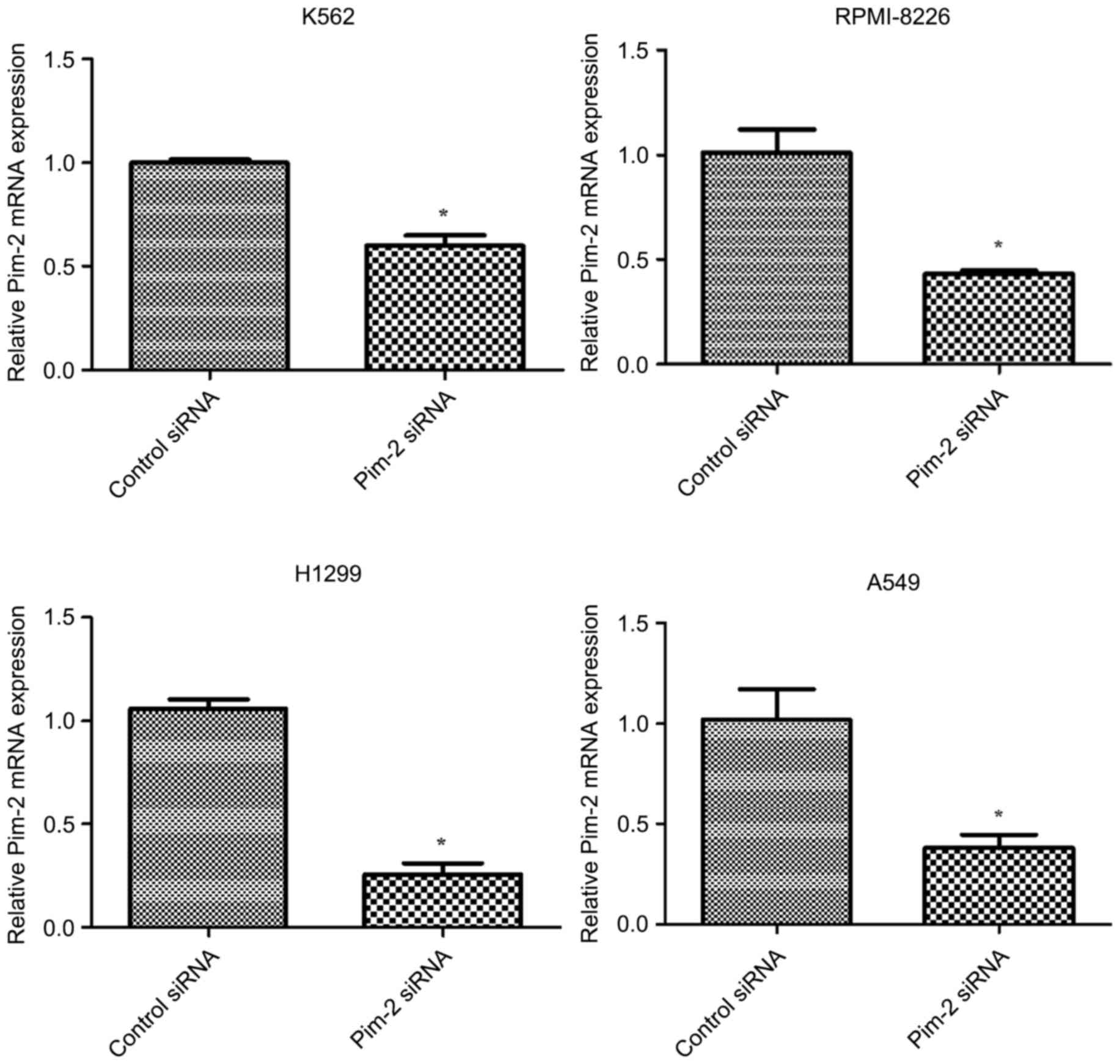
In order to investigate the role of Pim-2 in the
cancer cell lines tested, Pim-2 was knocked down using Pim-2 siRNA
in K562, RPMI-8226, H1299 and A549 cell lines. The degree of Pim-2
expression knockdown by specific siRNA was determined by RT-qPCR
analysis and western blotting. Pim-2-specific siRNAs significantly
decreased Pim-2 mRNA levels (P<0.05; Fig. 3) and markedly decreased protein
expression levels in all four cell lines (Fig. 4); however, siRNA knockdown exhibited
the highest efficiency in H1299 and A549 cells (70 and 62%
inhibition at the mRNA level, respectively). In addition, Pim-2
specific siRNAs markedly decreased the protein expression level of
NF-κB (Fig. 4).
Pim-2 silencing suppresses cell
proliferation
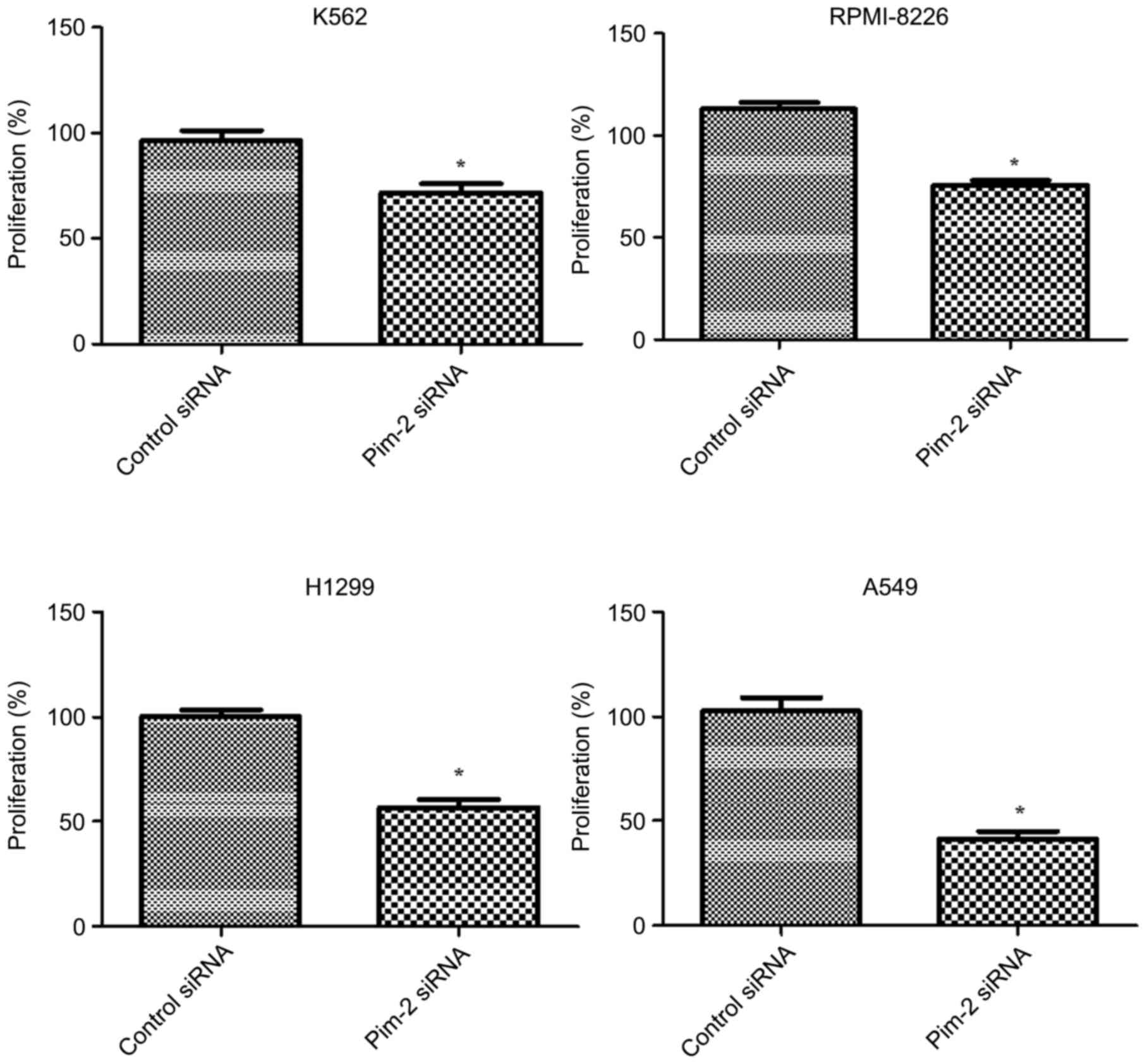
In order to determine whether knockdown of Pim-2
expression by siRNA had an inhibitory effect on cancer cell growth,
cell proliferation was determined using CCK-8. Proliferation was
significantly reduced by 29 (K562), 24 (RPMI-8226), 44 (H1299) and
59% (A549) in Pim-2 siRNA knockdown cells when compared with the
control cells (P<0.05) at 48 h after incubation (Fig. 5). These results suggested that Pim-2
may serve a pivotal role in cell proliferation.
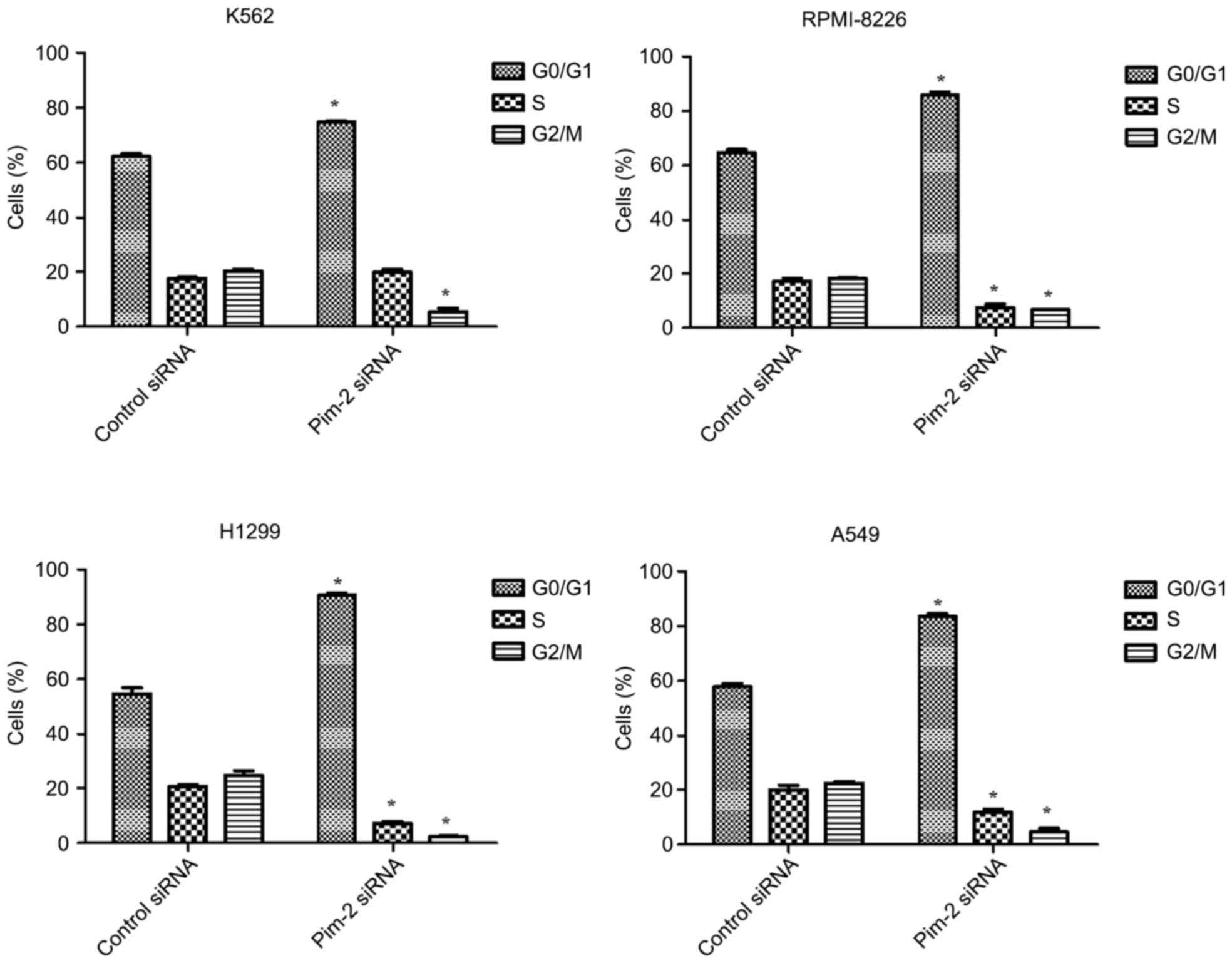
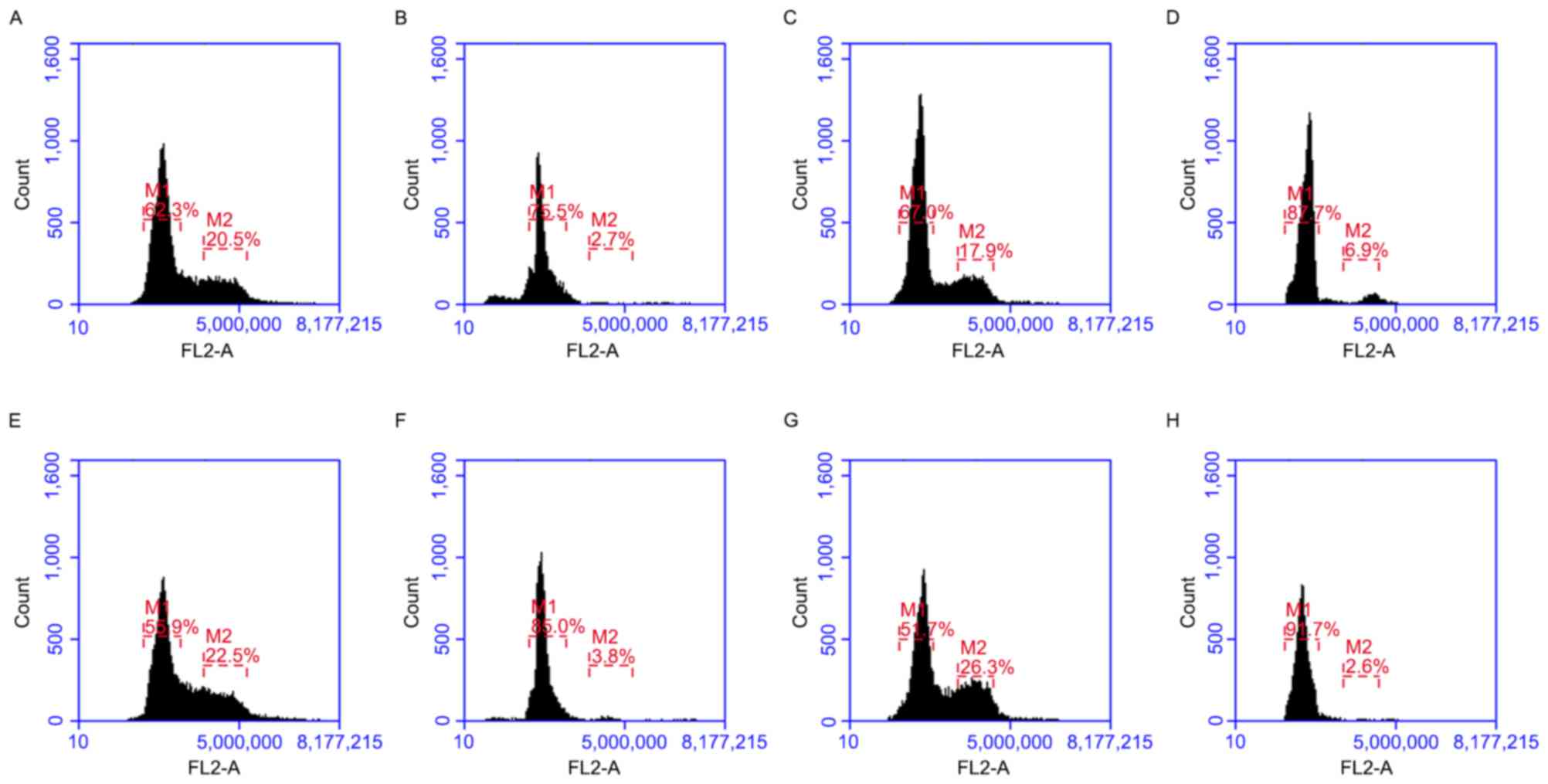
Pim-2 silencing arrests cells in the
G0/G1 phase of the cell cycle
Cell cycle changes following inhibition of Pim-2
were analyzed by flow cytometry. Separation of cells in the
G0/G1, S and G2/M phases were
based on linear fluorescence intensity following staining with
propidium iodide. Cell cycle analysis demonstrated a significant
increase in the percentage of cells in the
G0/G1 cell cycle phase following transfection
with Pim-2 siRNA compared with the control for all cell lines
tested (P<0.05; Figs. 6 and
7). A concomitant significant
decrease in the percentage of cells in the G2/M cell
cycle phase was observed in all cell lines compared with the
control (P<0.05) and a significant decrease in the percentage of
cells in the S cell cycle phase was observed in RPMI-8226, H1299
and A549 cells compared with the control (P<0.05). Therefore,
downregulation of Pim-2 induces accumulation of cells in the
G0/G1 phase of the cycle.
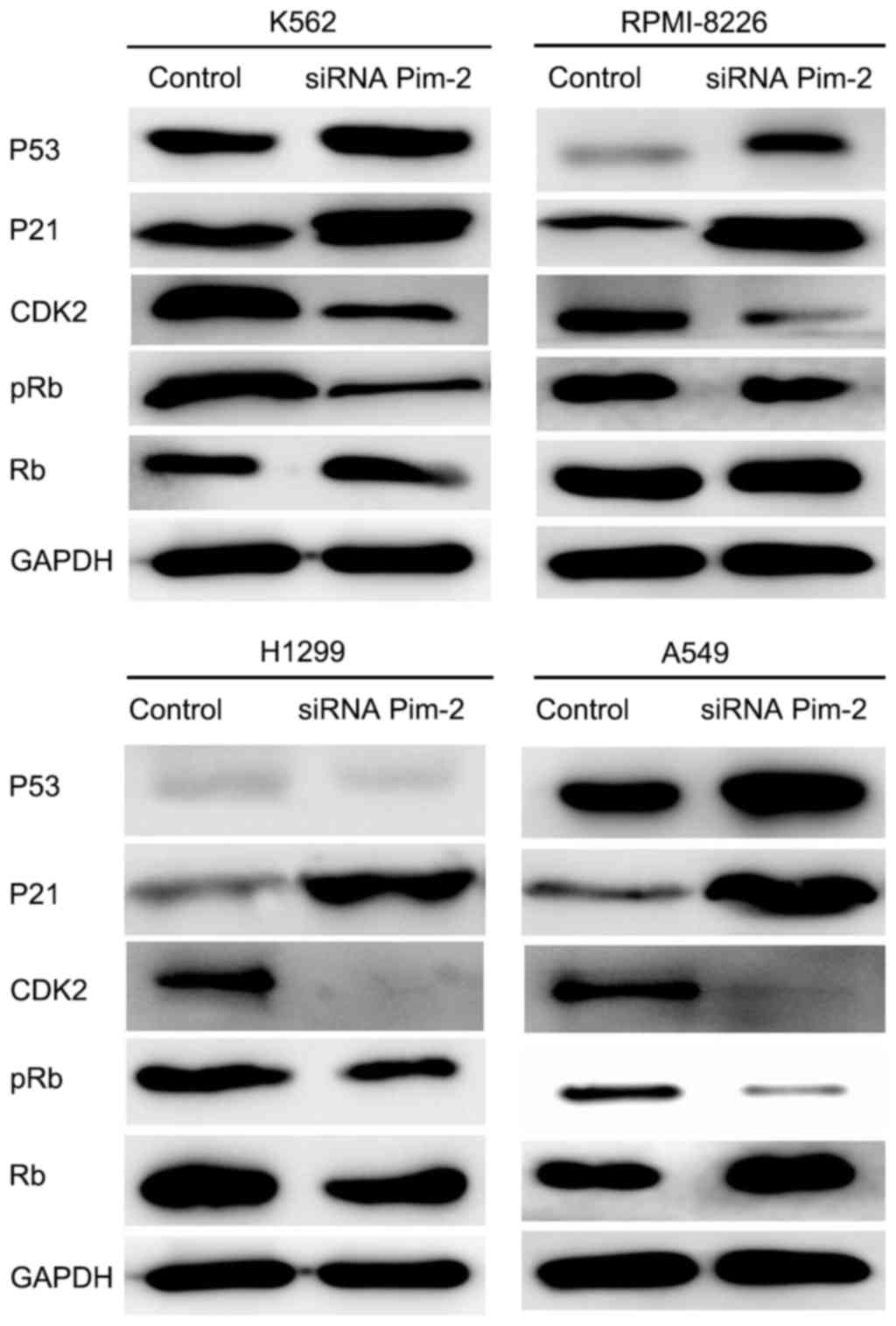
Downregulation of Pim-2 kinase induces
cell cycle arrest at the G0/G1 cell cycle
phase and is associated with changes in expression of cell
cycle-associated proteins
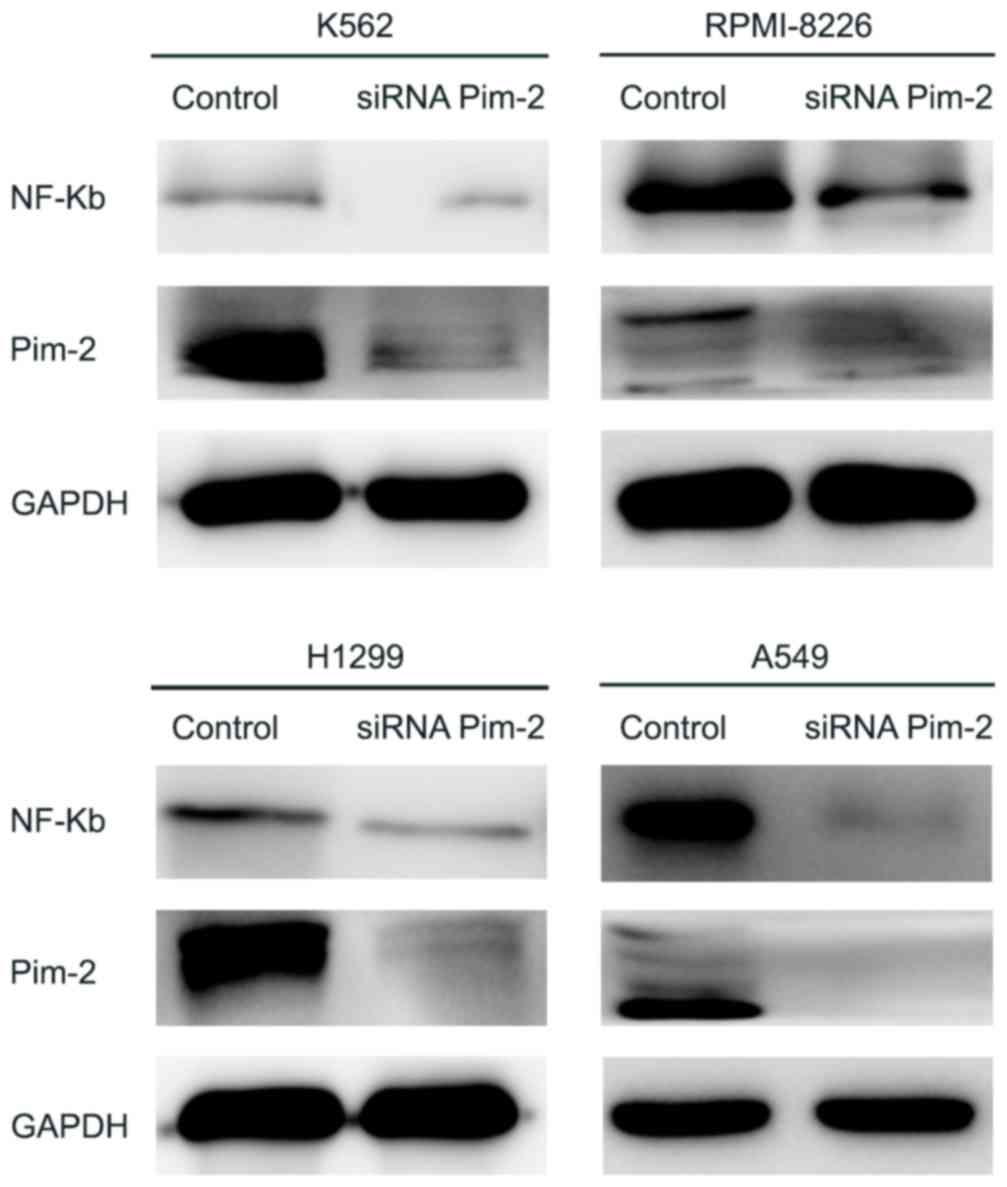
Western blotting was performed to investigate the
effect of Pim-2 knockdown on the expression level of cell
cycle-associated proteins, including CDK inhibitors, p21Cip1/WAF1,
CDK2, Rb, pRb and tumor suppressor protein p53. Following the
inhibition of Pim-2 in K562, RPMI-8226, H1299 and A549 cells by
siRNA, p21 expression was markedly increased and CDK2 expression
was markedly decreased in all four cell lines compared with the
control (Fig. 8). The p21 protein, as
a member of the Cip/Kip family of CDK2 inhibitors, binds to and
inhibits CDK2/cyclin complexes during the G1 phase
(14,19), which is in accordance with the results
from the present study. Rb is a ‘master controller’ of the cell
cycle, attributed to its intricate involvement in the regulation of
the G1 to S phase transition (20). Mitogenic stimulation during
G1 cell cycle phase induces sequential activation of
CDK2-cyclin E complexes, which hyperphosphorylate Rb and thereby
induce the release of active E2F1 to drive G1 to S-phase
progression (21). Therefore, Rb may
be affected by the downregulation of Pim-2. To confirm this
hypothesis, p-Rb and Rb expression levels were evaluated by western
blotting. A marked reduction of p-Rb following the knockdown of
Pim-2 was observed compared with the control (Fig. 8).
p53, which is known as a ‘guardian’ of the genome,
regulates responses to genotoxic stress through the modulation of
the transcription of a number of genes encoding proteins involved
in cell cycle control, including p21 Cip1/WAF1 (22). Following downregulation of Pim-2
kinase in K562 (p53+), RPMI-8226 (p53+),
H1299 (p53−) and A549 (p53+) cells (Fig. 8), + and -notation refers to p53
expression, p53 expression was markedly increased compared with the
control in K562 (p53+), RPMI-8226 (p53+) and
A549 (p53+) cell lines, but not in H1299 cells
(p53−; Fig. 8).
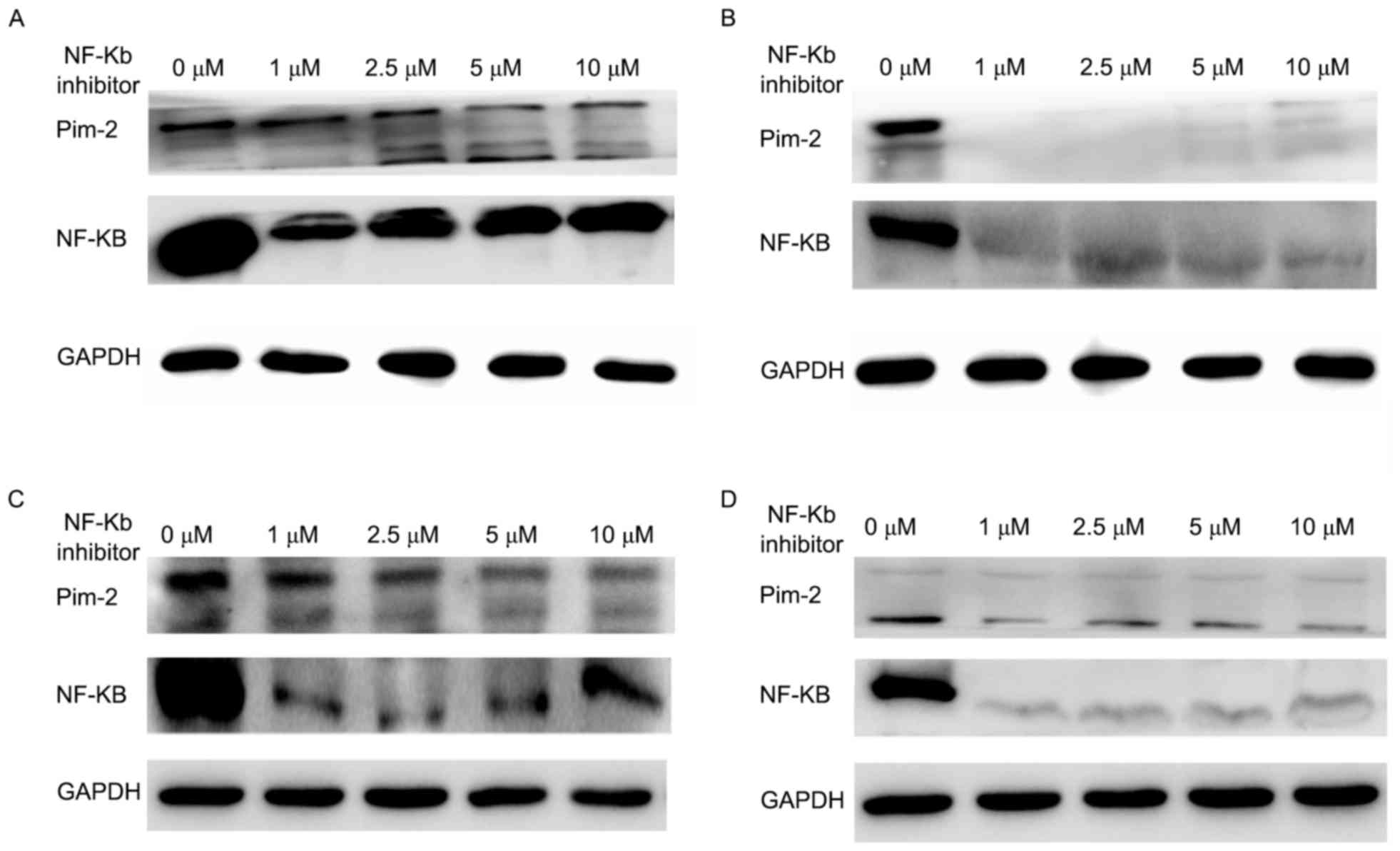
NF-κB inhibition decreases the
expression of Pim-2
Since treatment with Pim-2 siRNA also markedly
decreased the expression of NF-κB, cells were treated with an NF-κB
inhibitor (Ro 106-9920) at an increasing concentration for 48 h to
analyze the effect on Pim-2 expression. Pim-2 expression was
suppressed following the inhibition of NF-κB, but not in a
concentration-dependent manner at the concentrations tested
(Fig. 9).
Discussion
Overexpression of Pim in cancer, particularly
hematopoietic malignancies, is thought to serve a role in promoting
survival and proliferation and inhibition of the expression of
Pim-2 in tumor cells may be an effective strategy for treating
tumors that overexpress Pim (9,23). The
present study investigated the expression level of Pim-2 in solid
tumors (lung cancer) and hematopoietic malignancies (leukemia and
multiple myeloma), in addition to the effect of Pim-2-targeted
siRNA on cell proliferation and the cell cycle in the K562 leukemia
(p53+), RPMI-8266 (p53+) MM, and H1299
(p53−) and A549 (p53+) lung cancer cell
lines. The present study demonstrated the expression levels of
Pim-2 in hematopoietic malignancies (K562, MM) and solid tumors
(H1299, A549). Pim-2 was primarily expressed in the cytoplasm.
These results were consistent with previous studies that
demonstrated that Pim-2 was widely expressed in various types of
cancer (11–13). Therefore Pim-2 may be a potential
therapy target for novel cancer treatments.
The present study aimed to elucidate the role of
Pim-2 in proliferation by inhibiting its expression using siRNA.
Treatment of K562, RPMI-8226, H1299 and A549 cells with Pim-2
targeted siRNA resulted in a significant decrease in Pim-2
expression. The efficiency and effects of Pim-2 knockdown were more
apparent in H1299 and A549 cells compared with the other cell lines
tested. In addition, downregulation of Pim-2 led to the
downregulation of NF-κB, a nuclear factor activated by various
upstream factors that regulates a number of downstream signaling
pathways, and thus serves various roles in the inflammatory
response, cell proliferation and tumorigenesis (24).
A CCK-8 assay revealed a significant decrease in the
proliferation rate of cells following treatment with Pim-2 siRNA
compared with control siRNA (P<0.05) in the experimental cell
lines used. In order to investigate the molecular mechanisms
underlying the effects of Pim-2 expression on cell proliferation,
cell cycles were characterized using flow cytometry analysis. The
results demonstrated a significant increase in the proportion of
cells in the G0/G1 phase following treatment
with Pim-2 siRNA compared with cells treated with control siRNA.
There was a concomitant significant decrease in the percentage of S
and G2/M phase cells following treatment with Pim-2
siRNA compared with cells treated with control siRNA. These results
suggest that Pim-2 may serve a role in cell cycle progression; the
delay in progression from G0/G1 to S or
arrest in the G0/G1 cell cycle phase may be
the reason for the anti-proliferative effect of Pim-2 suppression
in cells.
Since downregulation of Pim-2 resulted in the
accumulation of cells in the G0/G1 phase, the
expression levels of cell cycle regulators were investigated in the
present study. The results revealed that CDK2 and pRb were markedly
downregulated, whereas p21 was markedly upregulated following
treatment with Pim-2 siRNA. These results suggest that the
inhibition of CDK2 and pRb expression levels via upregulated p21 is
involved in mediating the effects of Pim-2 downregulation on
G0/G1 arrest in lung cancer and hematopoietic
malignancies. Pim-2 phosphorylation of p21Cip1/WAF1 inhibits cell
proliferation in human colon carcinoma (7). Further studies are required in order to
verify the results of the present study and to elucidate the
molecular mechanisms underlying Pim-2 regulation of p21.
Expression of the p21 gene is tightly controlled by
the tumor suppressor p53 (7,25). The present study revealed that p21 was
significantly highly expressed in the p53(+) cell lines
K562, RPMI-8226 and A549 compared with the p53(−) cell
line H1299. Downregulation of Pim-2 decreased cell proliferation
and arrested cells in the G0/G1 phase of the
cell cycle in p53(+) and p53(−) cells,
indicating that p21 was upregulated by a p53-independent signaling
pathway following downregulation of Pim-2. Further studies are
required to verify the existence of a p53-independent signaling
pathway in this context. Regardless of the type of damage and the
temporal pattern of p53, induction of p21 occurs only in the
presence of DNA damage, and not following spontaneous expression of
p53 that occurs without damage (26).
Thus, Pim-2 may regulate the cell proliferation via p21 without
p53. In addition, downregulation of Pim-2 increased the expression
level of p53 in p53(+) cell lines but not H1299 cells
[p53(−)]. Therefore the association between Pim-2 and
p53 in lung cancer, MM and leukemia requires further investigation.
The elevated expression of Pim oncogenes has been suggested to
suppress p53 by regulating E3 ubiquitin-protein ligase Mdm2 in
mantle cell lymphoma (27).
In addition to the resulting downregulation of NF-κB
following Pim-2 downregulation, Pim-2 expression was markedly
decreased following treatment with an NF-κB inhibitor. This
suggests that there is an association between NF-κB and Pim-2 in
cancer cells. Pim-2 has previously been demonstrated to activate
API-5 to in order to inhibit the apoptosis of hepatocellular
carcinoma cells via the NF-κB signaling pathway (28). The ability of Pim-2 to serve as an
oncogene in vivo depends on sustained NF-κB activity in
lymphoma (29). NF-κB may be a
downstream factor of the Pim-2 signaling pathway. However, bone
marrow stromal cells (BMSCs) and osteoclasts have been demonstrated
to upregulate Pim-2 expression level in MM cells via the
interleukin-6/signal transducer and activator of transcription 3
and NF-κB signaling pathways, respectively (4). Pim-2 and NF-κB promote cell survival in
response to a wide variety of proliferative signals (30,31).
Numerous previous studies revealed that downstream factors of Pim-2
include the translational repressor 4E-binding protein 1, the BH3
protein BCL2 associated agonist of cell death and tuberous
sclerosis 2 (TSC2) (13,32). NF-kB has been demonstrated to regulate
TSC2-dependent cell survival (33).
Thus, there are certain signals between Pim-2 and NF-κB that have
not been described previously. Previous studies have suggested that
Pim-2 may be an important survival factor in cancer proliferation
and requires further attention (11–13).
In conclusion, the present study demonstrated that
Pim-2 was highly expressed in cell lines derived from solid tumors
(A549 and H1299 lung cancer cell lines) and hematopoietic
malignancies (K562 leukemia cell line and RPMI-8226MM cell line).
Further knockdown of Pim-2 by using siRNA potently inhibited
proliferation and promoted cell cycle arrest at the
G0/G1 phase. Pim-2 overexpression may be
associated with cell cycle progression via downregulation of p21,
without p53-dependence. Further investigation of the functional
role of Pim-2 may lead to an improved understanding of the
molecular mechanisms underlying lung cancer and hematopoietic
malignancies. Combinations of drugs that induce suppression of
Pim-2 may be an effective strategy for treatment of lung cancer and
hematopoietic malignancies, and therefore require further
evaluation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81570106, 81400088
and 81400085), the anticancer major special project of Tianjin
(grant no. 12ZCDZSY18000) and the Tianjin Municipal Natural Science
Foundation (grant nos. 14JCYBJC25400 and 15JCYBJC24300). The
authors wish to thank the Tianjin Institute of Lung Cancer for
their support in the lab.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Breuer ML, Cuypers HT and Berns A:
Evidence for the involvement of pim-2, a new common proviral
insertion site, in progression of lymphomas. EMBO J. 8:743–748.
1989.PubMed/NCBI
|
|
2
|
Yoshida S, Kaneita Y, Aoki Y, Seto M, Mori
S and Moriyama M: Identification of heterologous translocation
partner genes fused to the BCL6 gene in diffuse large B-cell
lymphomas: 5′-RACE and LA-PCR analyses of biopsy samples. Oncogene.
18:7994–7999. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amson R, Sigaux F, Przedborski S, Flandrin
G, Givol D and Telerman A: The human protooncogene product p33pim
is expressed during fetal hematopoiesis and in diverse leukemias.
Proc Natl Acad Sci USA. 86:pp. 8857–8861. 1989; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asano J, Nakano A, Oda A, Amou H, Hiasa M,
Takeuchi K, Miki H, Nakamura S, Harada T, Fujii S, et al: The
serine/threonine kinase Pim-2 is a novel anti-apoptotic mediator in
myeloma cells. Leukemia. 25:1182–1188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dai H, Li R, Wheeler T, Diaz de Vivar A,
Frolov A, Tahir S, Agoulnik I, Thompson T, Rowley D and Ayala G:
Pim-2 upregulation: Biological implications associated with disease
progression and perinueral invasion in prostate cancer. Prostate.
65:276–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gong J, Wang J, Ren K, Liu C, Li B and Shi
Y: Serine/threonine kinase Pim-2 promotes liver tumorigenesis
induction through mediating survival and preventing apoptosis of
liver cell. J Surg Res. 153:17–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Zhang Y, Gu JJ, Davitt C, Reeves R
and Magnuson NS: Pim-2 phosphorylation of p21(Cip1/WAF1) enhances
its stability and inhibits cell proliferation in HCT116 cells. Int
J Biochem Cell Biol. 42:1030–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Lugt NM, Domen J, Verhoeven E,
Linders K, van der Gulden H, Allen J and Berns A: Proviral tagging
in E mu-myc transgenic mice lacking the Pim-1 proto-oncogene leads
to compensatory activation of Pim-2. EMBO J. 14:2536–2544.
1995.PubMed/NCBI
|
|
9
|
Morishita D, Katayama R, Sekimizu K,
Tsuruo T and Fujita N: Pim kinases promote cell cycle progression
by phosphorylating and down-regulating p27Kip1 at the
transcriptional and posttranscriptional levels. Cancer Res.
68:5076–5085. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Losman J, Chen XP, Jiang H, Pan PY,
Kashiwada M, Giallourakis C, Cowan S, Foltenyi K and Rothman P:
IL-4 signaling is regulated through the recruitment of
phosphatases, kinases, and SOCS proteins to the receptor complex.
Cold Spring Harb Symp Quant Biol. 64:405–416. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen AM, Grinblat B, Bessler H, Kristt D,
Kremer A, Schwartz A, Halperin M, Shalom S, Merkel D and Don J:
Increased expression of the hPim-2 gene in human chronic
lymphocytic leukemia and non-Hodgkin lymphoma. Leuk Lymphoma.
45:951–955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gomez-Abad C, Pisonero H, Blanco-Aparicio
C, Roncador G, González-Menchén A, Martinez-Climent JA, Mata E,
Rodríguez ME, Muñoz-González G, Sánchez-Beato M, et al: PIM2
inhibition as a rational therapeutic approach in B-cell lymphoma.
Blood. 118:5517–5527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J, Zavorotinskaya T, Dai Y, Niu XH,
Castillo J, Sim J, Yu J, Wang Y, Langowski JL and Holash J: Pim2 is
required for maintaining multiple myeloma cell growth through
modulating TSC2 phosphorylation. Blood. 122:1610–1620. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harper JW, Elledge SJ, Keyomarsi K,
Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley
L, Swindell E, et al: Inhibition of cyclin-dependent kinases by
p21. Mol Biol Cell. 6:387–400. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harbour JW, Luo RX, Dei Santi A, Postigo
AA and Dean DC: Cdk phosphorylation triggers sequential
intramolecular interactions that progressively block Rb functions
as cells move through G1. Cell. 98:859–869. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mlynarczyk C and Fåhraeus R: Endoplasmic
reticulum stress sensitizes cells to DNA damage-induced apoptosis
through p53-dependent suppression of p21(CDKN1A). Nat Commun.
5:50672014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gartel AL and Tyner AL: Transcriptional
regulation of the p21((WAF1/CIP1)) gene. Exp Cell Res. 246:280–289.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gartel AL, Serfas MS and Tyner AL:
p21-negative regulator of the cell cycle. Proc Soc Exp Biol Med.
213:pp. 138–149. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bertoli C, Skotheim JM and de Bruin RA:
Control of cell cycle transcription during G1 and S phases. Nat Rev
Mol Cell Biol. 14:518–528. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amaravadi R and Thompson CB: The survival
kinases Akt and Pim as potential pharmacological targets. J Clin
Invest. 115:2618–2624. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang JX, Mikami K, Venugopal S, Li Y and
Török NJ: Apoptotic body engulfment by hepatic stellate cells
promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent
pathways. J Hepatol. 51:139–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gartel AL and Radhakrishnan SK: Lost in
transcription: p21 repression, mechanisms, and consequences. Cancer
Res. 65:3980–3985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loewer A, Batchelor E, Gaglia G and Lahav
G: Basal dynamics of p53 reveal transcriptionally attenuated pulses
in cycling cells. Cell. 142:89–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hogan C, Hutchison C, Marcar L, Milne D,
Saville M, Goodlad J, Kernohan N and Meek D: Elevated levels of
oncogenic protein kinase Pim-1 induce the p53 pathway in cultured
cells and correlate with increased Mdm2 in mantle cell lymphoma. J
Biol Chem. 283:18012–18023. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren K, Zhang W, Shi Y and Gong J: Pim-2
activates API-5 to inhibit the apoptosis of hepatocellular
carcinoma cells through NF-kappaB pathway. Pathol Oncol Res.
16:229–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hammerman PS, Fox CJ, Cinalli RM, Xu A,
Wagner JD, Lindsten T and Thompson CB: Lymphocyte transformation by
Pim-2 is dependent on nuclear factor-kappaB activation. Cancer Res.
64:8341–8348. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
White E: The pims and outs of survival
signaling: Role for the Pim-2 protein kinase in the suppression of
apoptosis by cytokines. Genes Dev. 17:1813–1816. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fox CJ, Hammerman PS, Cinalli RM, Master
SR, Chodosh LA and Thompson CB: The serine/threonine kinase Pim-2
is a transcriptionally regulated apoptotic inhibitor. Genes Dev.
17:1841–1854. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghosh S, Tergaonkar V, Rothlin CV, Correa
RG, Bottero V, Bist P, Verma IM and Hunter T: Essential role of
tuberous sclerosis genes TSC1 and TSC2 in NF-kappaB activation and
cell survival. Cancer Cell. 10:215–226. 2006. View Article : Google Scholar : PubMed/NCBI
|