Introduction
Congenital deafness is one of the most common birth
defects and its incidence rate is ~1-3‰ worldwide in 2003 (1). Ministry of Health figures from China
identify 115,000 children under the age of 7 years with
severe-to-profound deafness and 30,000 babies born each year with
hearing impairment (2). Overall,
hearing loss is usually categorized into 3 subgroups;
sensorineural, conductive or mixed. The primary cause of hearing
loss is attributed to genetic or environmental factors (3). Genetic transmission accounts for 50% of
cases of congenital deafness, and of these, ~30% are syndromic and
70% are non-syndromic (4). Syndromic
hearing loss (SHL) or non-(N) SHL refers to hearing loss with or
without clinical symptoms, respectively (5). In total, ~77% of NSHL cases are due to
autosomal recessive inheritance (6),
10–20% are autosomal dominant, 1% are X-linked and <1% are due
to mitochondrial inheritance (7). In
the Chinese population, extensive studies of deafness molecular
epidemiology have demonstrated that a number of NSHL cases are
caused by multiple mutated genes, including the gap junction
protein β-2 gene (GJB2 gene), solute carrier family 26 member 4
(SLC26A4) gene (PDS gene) and mitochondrial gene, mitochondrially
encoded 12S RNA (6).
There is frequent co-morbidity following cochlear
implantation and the child/person remains handicapped (7). Cochlear implants are costly and
therefore burden the affected families and patients (8). Given the increased incidence of hearing
loss among newborns (1) and the huge
cost of treatments and care, preventing this birth defect is
strongly recommended. Pregnancy termination may be considered an
extreme action taken for a deaf fetus, and therefore
preimplantation genetic diagnosis (PGD) has become an important
option for avoiding the birth of affected children without risking
abortion following prenatal diagnosis (9,10). PGD is
not only used for single gene disorders (SGDs) (8) but is also used for human leukocyte
antigen matching (10) and inherited
types of cancer (11). The primary
goal of PGD is to aid parents who are attempting to conceive
healthy offspring.
Current methods of PGD for SGDs are direct mutation
detection using polymerase chain reaction (PCR) or Sanger
sequencing; however, PGD accuracy and diagnostic efficiency have
been limited due to amplification failures, allelic drop-out (ADO),
mosaicism and contamination (12).
Linkage analysis, including mutation detection combined with short
tandem repeat (STR) identifier analysis, increases the efficiency
of PGD diagnosis and confirms the genetic diagnosis (13). Linkage analysis based on STR is
usually associated with choosing 3–8 genetic markers within 2 Mb of
the mutation. Compared with STR analysis, karyomapping provides the
analysis of more single nucleotide polymorphism (SNP) markers and
has been used to diagnose SGDs in the clinic with high efficiency,
accuracy and reliability (14–17).
However, the dependence on DNA from family members may limit its
application (18). If detailed
information on affected relatives is not available, karyomapping
may be performed in parallel with conventional PCR methods for
direct detection of the mutation to increase the accuracy of the
genetic diagnosis (19). With certain
genes that have decreased SNP coverage, performing PCR testing
(including STR analysis) or direct mutation detection in parallel
is necessary (20).
Next-generation sequencing (NGS) is a rapidly
developing technology that produces enormous amounts of data with a
wide range of applications (21). PGD
with NGS provides more informative genetic markers in high
throughput (22), and whole genome
amplification (WGA) provides novel possibilities for diagnosis and
parameters for evaluation of SGDs. NGS also allows for analysis of
aneuploidy or translocation of all chromosomes and mutations
responsible for any single-gene disease, using one biopsy and one
process (23). Furthermore, in the
absence of suitable affected family members in SGD cases, NGS-based
linkage analysis may still correctly diagnose the embryos by using
the affected embryo as the proband.
The development of different NGS platforms and
decreased costs enable their introduction into PGD (21). In the present study, Ion Torrent
technology was used, which incorporates the use of non-optical,
single-nucleotide, semiconductor-based sequencing on an Ion Torrent
Personal Genome Machine (PGM). This technology combines aspects of
parallel sequencing, including bead-based emulsion PCR but employs
a complementary metal oxide semiconductor chip with microwells that
serve as pH-sensitive pixels to detect the release of a hydrogen
ion (registered as an electrical signal) when a nucleotide is
incorporated during sequencing by synthesis (24). This approach suggests the requirement
for chemiluminescent dyes, serial optical image acquisition, a
motorized camera stage and extensive storage of preanalytic files
for subsequent processing (25).
To the best of our knowledge, this is the first
report of an NGS-based PGD case on an Ion Torrent PGM platform for
non-syndromic sensorineural hearing loss caused by the SLC26A4
mutation.
Materials and methods
Patient information and ethics
A couple (maternal age, 30; paternal age, 31) who
had a 7-year-old daughter affected by non-syndromic sensorineural
hearing loss attended the Reproductive Medicine Center, Department
of Obstetrics and Gynecology in the First Affiliated Hospital of
Anhui Medical University (Anhui, China) for PGD. The result of
target NGS revealed that the daughter carried a causative compound
heterozygous mutation c.919-2 A>G (IVS7-2 A>G) and c.1707+5
G>A (IVS15+5 G>A) in intron 7 and 15 of the SLC26A4 gene. DNA
testing of the couple using Sanger sequencing of SLC26A4 confirmed
the presence of a maternal splicing mutation c.919-2 A>G and a
paternal splicing mutation c.1707+5 G>A that were inherited by
the child.
The couple was counseled about PGD and signed
informed consent forms for intracytoplasmic sperm injection (ICSI)
treatment and PGD. The Ethics Committee of Anhui Medical University
approved the present study.
Pre-clinical test: NGS-based
haplotyping of the family
Haplotyping for the pre-analytic testing of the
family was performed using genomic DNA extracted from peripheral
blood of the mother, father and child in order to establish the
mutation-associated haplotype based on informative SNPs. This was
achieved by using a magnetic genomic DNA kit (Tiangen Biotech Co.,
Ltd., Beijing, China). A total of 160 high-frequency SNPs located 3
Mb upstream, 3 Mb downstream of SLC26A4 and 11 high-frequency SNP
markers, including mutation sites in the SLC26A4 gene, were
selected for NGS-based SNP haplotyping. These SNPs were then
submitted to a primer design website (www.ampliseq.com). Following DNA purification
(Agencourt AMPure XP; Beckman Coulter, Inc., Brea, CA, USA) the
target region was amplified using multiplex PCR. The reaction
contained 10 µl 2X Ion AmpliSeq™ Primer Pool, 4 µl 5X Ion AmpliSeq™
HiFi Master mix, 6 µl 10 ng DNA and nuclease-free water, and an Ion
AmpliSeq™ Library kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was used. The target area was amplified according to the
following procedure: 99°C for 2 min for 1 cycle, 99°C for 15 sec
and 60°C for 4 min for 21 cycles. Samples were then incubated at
4°C for the next step. The Amplicon Library Preparation protocol
(using Ion AmpliSeq™ Library kits) was used as recommended by the
supplier (Thermo Fisher Scientific, Inc.). Template preparation was
performed using an Ion One Touch 2 system and an Ion One Touch
Enrichment System in accordance to the manufacturers protocol
(version 2.0; Ion Onetouch 200 Template kit; Thermo Fisher
Scientific, Inc.). The template positive Ion Sphere Particles were
sequenced on an Ion Torrent PGM (Thermo Fisher Scientific, Inc.)
using the 318 chip following the protocol provided by the Ion
Sequencing kit (version 2.0; Thermo Fisher Scientific, Inc.)
(26). Informative SNPs located 3 Mb
upstream and 2.5 Mb downstream of the SLC26A4 gene [Minor Allele
Frequency, (MAF)>0.2] in the genomes of Han Chinese in Beijing
and the Southern Han Chinese from the 1,000 Genomes Project were
selected for NGS-based haplotyping.
ICSI procedure, embryo biopsy and
vitrification
A standard pituitary downregulation protocol
(26) was used for ovarian
stimulation This was performed with Gonal-F (Merck Serono,
Darmstadt, Germany) in a long-protocol cycle. When ≥2 follicles
were >18 mm in diameter, 250 µg recombinant human chorionic
gonadotropin (Ovitrelle; Merck Serono) was administered via
hypodermic injection (27) and the
oocytes were retrieved 36 h later, guided by transvaginal
ultrasound. ICSI was performed for insemination of mature oocytes
to avoid contamination by extraneous sperm. Fertilization was
assessed 16–18 h after ICSI. A ~14 µm hole was made in the zona
pellucida of the embryos using a ZILOS-tk laser (Hamilton Thorne,
Inc., Beverly, MA, USA) on the morning of day 3 after ICSI.
Blastocyst biopsy was performed on day 5 or 6 using the laser when
the trophectoderm cells herniated out of the zona pellucida. The
quality of blastocysts were scored between 1 and 6 according to
their expansive degree and development condition of inner cell mass
and trophectoderm outlined by Gardner's grading system (28). Categorization was as follows; 1, an
early blastocyst with a blastocoels cavity <50% of the embryo
volume; 2, a blastocyst with a blastocoels that is ≥50% of the
embryo volume; 3, a blastocyst with a blastocoel that is 100% of
the whole embryo; 4, an expanded blastocyst with a blastocoel
filling the embryo and a thinning zona pellucida; 5, a hatching
blastocyst with the trophectoderm beginning to extrude from the
zona pellucida; and 6, a hatched blastocyst which has completely
escaped from the zona pellucida. Additionally, according to the
development condition of the inner cell mass and trophectoderm, the
blastocyst inner cell mass and trophectoderm were graded (A-C). The
inner cell mass was graded as follows: A, numerous cells packed
tightly; B, several cells loosely grouped; and C, only very few
cells. The trophectoderm was graded as follows: A, large numbers of
cells forming a cohesive epithelium; B, few cells forming a loose
epithelium; and C, very few large cells. Biopsied blastocysts were
then vitrified using a Kitazato vitrification kit (Kitazato, Tokyo,
Japan). The biopsied cells and fragments were each placed in 2 µl
PBS (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in 0.2 ml PCR
tubes and multiple displacement amplification (MDA) was
performed.
Multiple displacement amplification
and NGS
MDA was performed using a REPLI-g single cell kit
(Qiagen GmbH, Hilden, Germany). A total of 4 µl trophectoderm cells
(supplied with PBS) were placed into a microcentrifuge tube and 3
µl prepared Buffer D2 (provided by the kit) was added. Following
incubation at 65°C for 10 min, 3 µl stop solution was added. For
each reaction, 40 µl master mix (9 µl H2Osc, 29 µl REPLI-g Reaction
Buffer and 2 µl REPLI-g DNA Polymerase, from the REPLI-g single
cell kit) was added to 10 µl obtained solution and incubated at
30°C for 8 h. The reaction was stopped by incubation at 65°C for 3
min to inactivate the Phi29 polymerase (REPLI-g sc DNA polymerase)
and amplified DNA was stored at −20°C until required.
The following steps were similar to the construction
of the haplotype map for the family. Library preparation was then
performed and followed by chip loading for PGM (Thermo Fisher
Scientific, Inc.) sequencing. The procedure was performed according
to the manufacturer's protocol (Thermo Fisher Scientific, Inc.).
The data from the PGM sequencing were analyzed by Beijing Jia Renhe
Medical Technology Co., Ltd. (Beijing, China). Amniocentesis was
performed at 19 weeks of gestation.
Results
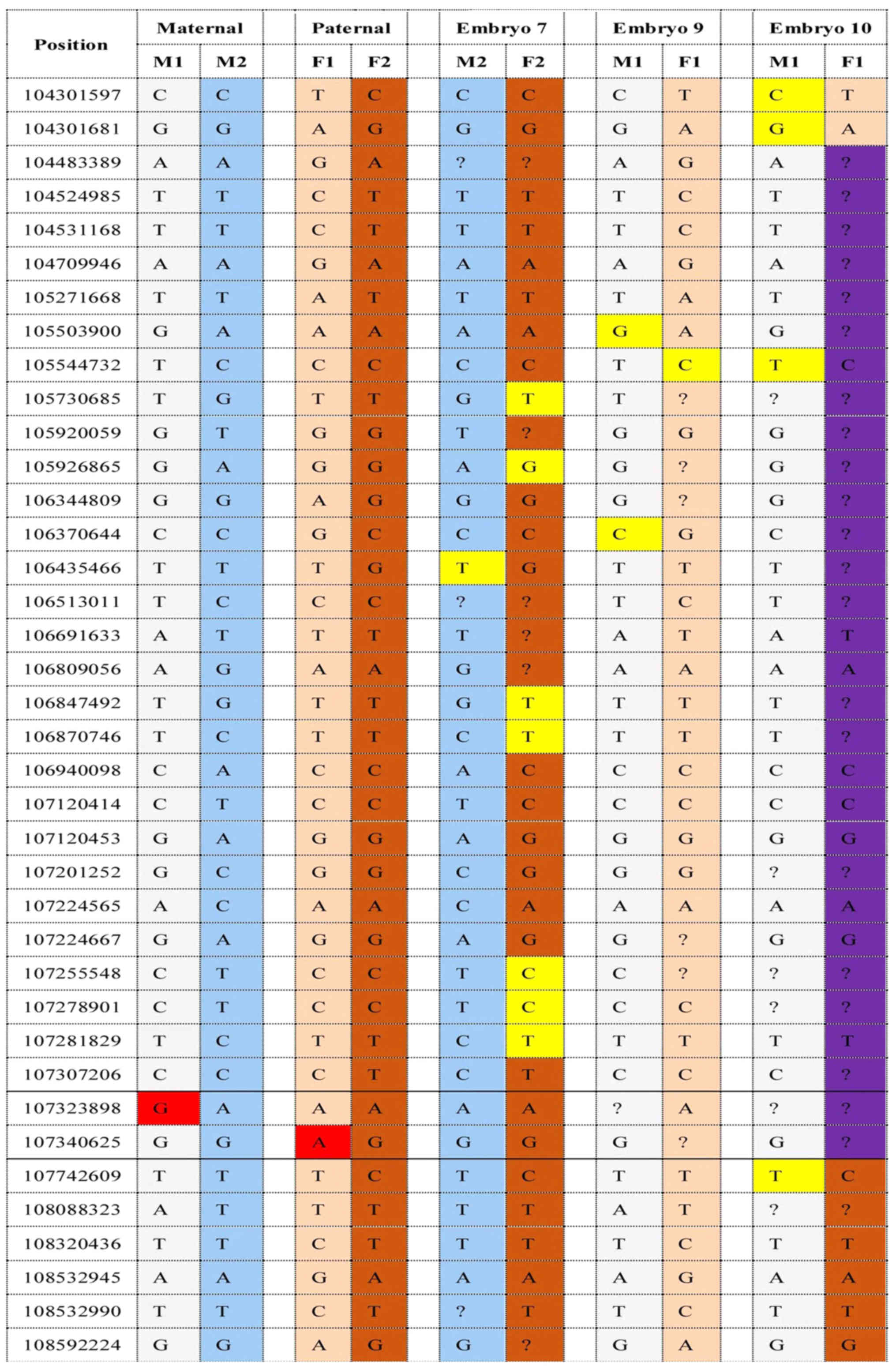
Haplotyping for the pre-analytic testing of the
family was successfully performed. A total of 60 informative SNPs
including mutation sites were identified in the family of NSHL.
Following controlled ovarian stimulation, 14 oocytes were retrieved
and 11 of them were suitable for ICSI; however, only 7 fertilized
oocytes developed into hatched blastocysts, the remaining 4 were
arrested. The 7 hatched blastocysts were biopsied successfully on
day 5 or 6 of culture. Biopsied trophectoderm cells (5–10 cells)
from embryo 1, 3, 4, 7, 9 and 10 were successfully amplified using
MDA, whereas the biopsies from embryo 6 failed to amplify. All
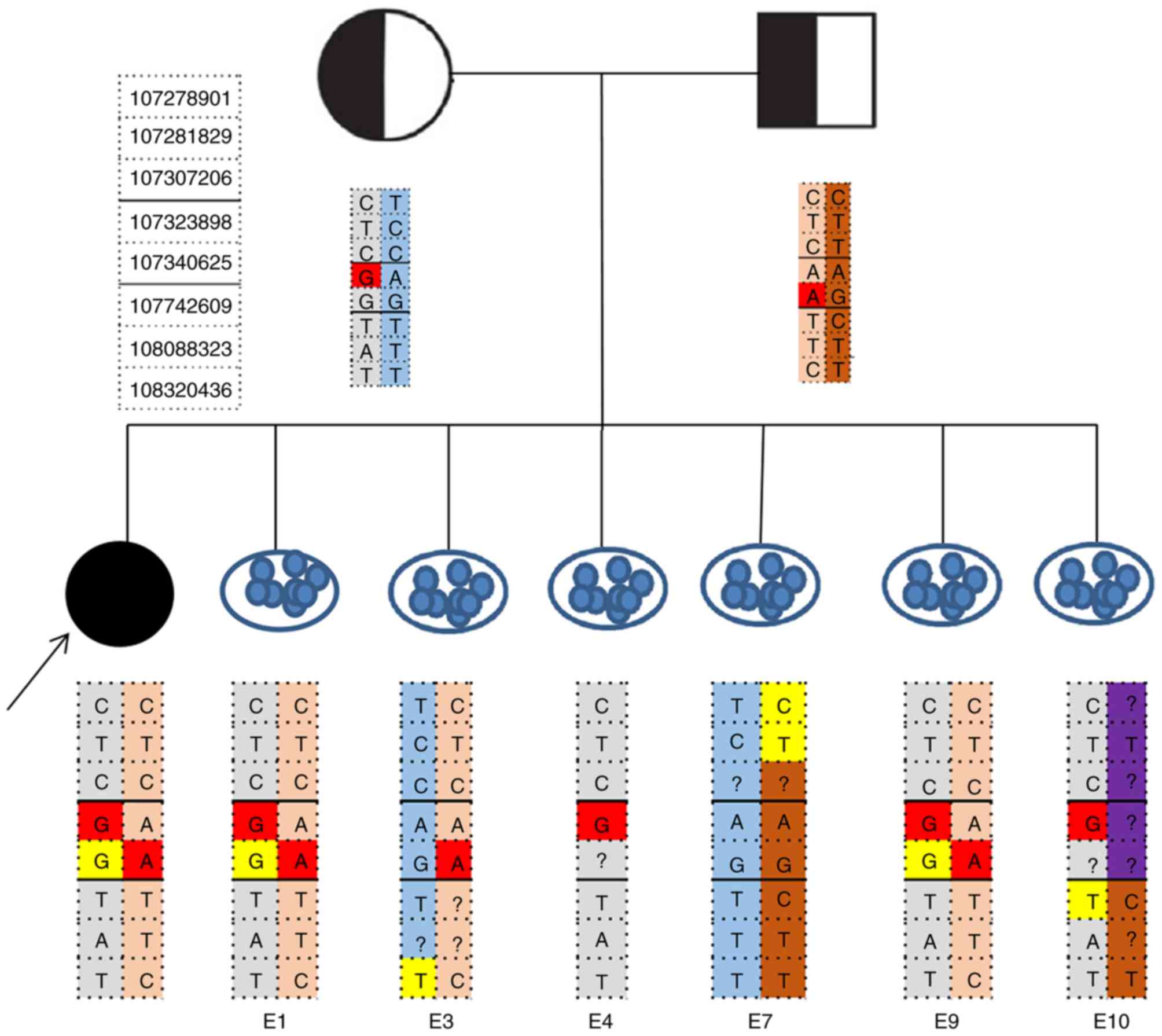
embryos were then analyzed by NGS. On the basis of the embryonic
SLC26A4 genotypes results (Figs. 1
and 2), embryo 7 was genotypically
normal, embryo 3 revealed a carrier pattern with the normal
maternally inherited allele. Two embryos (embryo 1 and 9) were
affected, inheriting affected parental haplotypes. Embryo 4, which
inherited only the maternally affected haplotype, was diagnosed as
monosomic. Paternal recombination occurred in embryo 10 and the
diagnosis was ambiguous. The quality of the blastocyst was assessed
based on Gardner's grading system (Table
I) (28). No external DNA
contamination events occurred in any tested embryos. Three months
after oocyte retrieval, embryo 7, which was diagnosed as being
normal, was transferred back to the mother in frozen-thawed embryo
transfer cycle, resulting in a single ongoing pregnancy. Prenatal
diagnosis by amniocentesis at 19 weeks demonstrated concordance of
the embryo. Remaining embryos were cryopreserved.
 | Table I.The quality of biopsied blastocysts
and result of haplotype analysis. |
Table I.
The quality of biopsied blastocysts
and result of haplotype analysis.
| Embryo | Quality of
blastocystsa | Result |
|---|
| 1 | 5BB | Pathogenic |
| 3 | 5BB | Carrier |
| 4 | 6BB | Pathogenic,
monosomic |
| 6 | 5BB | Amplification
failure |
| 7 | 5BA | Normal |
| 9 | 5BB | Pathogenic |
| 10 | 5CC | Recombination |
Discussion
The present study may provide the first successful
application of NGS-based PGD using a semiconductor technology, an
Ion Torrent platform, for nonsyndromic sensorineural hearing loss
caused by compound heterozygous mutation in SLC26A4.
Although hearing loss is not life-threatening,
children with hearing loss may encounter multiple issues, given
that spoken language is the predominant form of communication and
social interaction (29). Adequate
auditory stimulation and sufficient language exposure in early
childhood are critical in their subsequent linguistic acquisition,
cognitive development and psychosocial functioning (29). Hearing loss causes hearing disability,
affects mental health and places a heavy burden on the patient's
family and society. The 2006 National Sample Survey in China
(30) demonstrated that hearing
impairment affected ~20 million people, accounting for 24.16% of
all people with disabilities. In 2011, ~30,000 babies are born with
congenital hearing impairment annually in China and nearly 1/2 of
these cases of congenital deafness are estimated to be associated
with genetic factors (31). Children
affected with nonsyndromic deafness using cochlear implants require
more hospitalization for postoperative complications, including
device extrusion requiring further surgery and wound infection
(32).
Given the high incidence of hearing loss among
newborns and huge cost of treatments and care (8), the medical genetic services in China
strongly recommend to prevent this birth defect (1,30,31). PGD is now clinically established
globally for preventing the birth defect. It may detect birth
defects at the embryo stage, avoiding the trauma of prenatal
diagnosis and the possibility of terminating affected pregnancies,
which may be appropriate for deafness since abortion for deafness
is viewed as a nonacceptable option for the majority of the
population (9).
PGD for nonsyndromic deafness based on a single cell
protocol (33,34) has been reported. However, the single
cell protocol for PGD failed to avoid the misdiagnosis caused by
ADO. PGD for nonsyndromic deafness based on Sanger sequencing and
STR linkage analysis has been applied (9,35);
however, the number of STR markers used for linkage analysis are
limited and when a crossover event occurred, low density STR
markers may still result in misdiagnosis.
To overcome increased ADO rates due to small amounts
of genetic material and improve the accuracy of diagnosis, newly
developed methods involving WGA and high-resolution methods,
including karyomapping may be used in SGD-PGD.
Karyomapping, which uses genome-wide linkage to
reveal the inheritance of genetic disease loci present in one or
both parents, is efficient, accurate and reliable (17). However, it also has certain
limitations. For example, the dependence on DNA samples from family
members may limit its application (18). It is not appropriate for cases when
detailed information of affected relatives is not available. In
addition, when certain genes have decreased SNP coverage, PCR
testing including STR analysis or direct mutation detection is
necessary and need to be performed in parallel (18). Although karyomapping has the potential
for providing a simultaneous identification of aneuploidy, it has
not been validated for microdeletions (19) and cannot detect sequence-identical
chromosome duplication that may result from malsegregation of
chromosomes during the early cleavage divisions of the embryo
(12). In addition, the cost of
consumables required for karyomapping is significantly increased
compared with that of the reagents required for conventional PGD
methods.
Previously, NGS provided unprecedented
high-throughput, highly parallel and base-pair resolution data for
genetic analysis (36). It provides
powerful application not only to molecular diagnostics but may also
help advance research, due to an interest in long-term solutions,
including prevention of disease/disabilities in future family
members and advanced rehabilitation and therapeutics based on
research outcome (37). Treff et
al (36) applied NGS-based SNP
haplotyping in PGD for single-gene disorders and provided
blastocyst PGD results with consistency using established
methodologies. Chen et al (22) reported PGD for the patient affected by
congenital contractual arachnodactyly and spinal and bulbar
muscular atrophy, illustrating the reliability of NGS-based SNP
haplotyping. In the present study, mutations and high-frequency SNP
markers were selected for haplotyping using NGS. The unaffected
blastocyst was transferred to the patient who became pregnant.
Compared with karyomapping, NGS-based haplotying may
still correctly diagnose the embryos using the affected embryo as a
proband under the condition of the absence of affected family
members (20). Comprehensive
aneuploidy screening of blastocysts may also be performed
simultaneously and NGS has the advantage of detecting chromosome
microdeletion and microduplication (38,39).
To conclude, to the best of our knowledge, this is
the first study in which NGS-based haplotying was combined with WGA
and applied to PGD for nonsyndromic sensorineural hearing loss
caused by compound heterozygous mutation of the SLC26A4 gene using
an Ion Torrent platform. NGS combined with WGA applied in PGD may
potentially offer a powerful means to prevent genetic transmission
and therefore may benefit the family and society's health care
system.
Acknowledgements
The authors would like to thank laboratory
technicians, Lixian Xing and Yaohua Zhu, at Peking Jabrehoo Med
Tech., Ltd. (Beijing, China) for their assistance with sequencing
and data analysis. The present study was supported by the Key
Science and Technology Project of Anhui Province (grant no.
1604a0802077) and the College Natural Science Project of Anhui
Province (grant no. KJ2015A057).
References
|
1
|
White KR: Early hearing detection and
intervention programs: Opportunities for genetic services. Am J Med
Genet A. 130A:1–36. 2004. View Article : Google Scholar
|
|
2
|
Liang Q and Mason B: Enter the
dragon-China's journey to the hearing world. Cochlear Implants Int.
14 Suppl 1:S26–S31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith RJ, Bale JF Jr and White KR:
Sensorineural hearing loss in children. Lancet. 365:879–890. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taneja MK: Preimplantation genetic
diagnosis: Its role in prevention of deafness. Indian J Otolaryngol
Head Neck Surg. 66:1–3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mahboubi H, Dwabe S, Fradkin M, Kimonis V
and Djalilian HR: Genetics of hearing loss: Where are we standing
now? Eur Arch Otorhinolaryngol. 269:1733–1745. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu RM, Liu HJ, Cong JL, Sun AL, Du JD and
Sun CM: Genetic characteristics of the couple with non-syndromic
sensorineural hearing loss and fertility guidance. Int J Clin Exp
Med. 8:21746–21754. 2015.PubMed/NCBI
|
|
7
|
Park MK, Sagong B, Lee JD, Bae SH, Lee B,
Choi KS, Choo YS, Lee KY and Kim UK: A1555G homoplasmic mutation
from A1555G heteroplasmic mother with Pendred syndrome. Int J
Pediatr Otorhinolaryngol. 78:1996–1999. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nadège C, Valérie G, Laura F, Hélène DB,
Vanina B, Olivier D, Bernard F and Laurent M: The cost of cochlear
implantation: A review of methodological considerations. Int J
Otolaryngol. 2011:2108382011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Altarescu G, Eldar-Geva T, Brooks B,
Zylber-Haran E, Varshaver I, Margalioth EJ, Levy-Lahad E and
Renbaum P: Preimplantation genetic diagnosis (PGD) for nonsyndromic
deafness by polar body and blastomere biopsy. J Assist Reprod
Genet. 26:391–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuliev A, Rechitsky S, Tur-Kaspa I and
Verlinsky Y: Preimplantation genetics: Improving access to stem
cell therapy. Ann N Y Acad Sci. 1054:223–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drüsedau M, Dreesen JC, Derks-Smeets I,
Coonen E, van Golde R, van Echten-Arends J, Kastrop PM, Blok MJ,
Gómez-García E, Geraedts JP, et al: PGD for hereditary breast and
ovarian cancer: The route to universal tests for BRCA1 and BRCA2
mutation carriers. Eur J Hum Genet. 21:1361–1368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kou S: Preimplantation genetic diagnosis:
An update on current technologies and ethical considerations.
Reprod Med Biol. 15:69–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qubbaj W, Al-Swaid A, Al-Hassan S,
Awartani K, Deek H and Coskun S: First successful application of
preimplantation genetic diagnosis and haplotyping for congenital
hyperinsulinism. Reprod Biomed Online. 22:72–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Natesan SA, Bladon AJ, Coskun S, Qubbaj W,
Prates R, Munne S, Coonen E, Dreesen JC, Stevens SJ, Paulussen AD,
et al: Genome-wide karyomapping accurately identifies the
inheritance of single-gene defects in human preimplantation embryos
in vitro. Genet Med. 16:838–845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giménez C, Sarasa J, Arjona C, Vilamajó E,
Martínez-Pasarell O, Wheeler K, Valls G, Garcia-Guixé E and Wells
D: Karyomapping allows preimplantation genetic diagnosis of a
de-novo deletion undetectable using conventional PGD technology.
Reprod Biomed Online. 31:770–775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Handyside AH, Harton GL, Mariani B,
Thornhill AR, Affara N, Shaw MA and Griffin DK: Karyomapping: A
universal method for genome wide analysis of genetic disease based
on mapping crossovers between parental haplotypes. J Med Genet.
47:651–658. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thornhill AR, Handyside AH, Ottolini C,
Natesan SA, Taylor J, Sage K, Harton G, Cliffe K, Affara N,
Konstantinidis M, et al: Karyomapping - a comprehensive means of
simultaneous monogenic and cytogenetic PGD: Comparison with
standard approaches in real time for Marfan syndrom. J Assist
Reprod Genet. 32:347–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Natesan SA, Handyside AH, Thornhill AR,
Ottolini CS, Sage K, Summers MC, Konstantinidis M, Wells D and
Griffin DK: Live birth after PGD with confirmation by a
comprehensive approach (karyomapping) for simultaneous detection of
monogenic and chromosomal disorders. Reprod Biomed Online.
29:600–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Konstantinidis M, Prates R, Goodall NN,
Fischer J, Tecson V, Lemma T, Chu B, Jordan A, Armenti E, Wells D
and Munné S: Live births following Karyomapping of human
blastocysts: Experience from clinical application of the method.
Reprod Biomed Online. 31:394–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren Y, Zhi X, Zhu X, Huang J, Lian Y, Li
R, Jin H, Zhang Y, Zhang W, Nie Y, et al: Clinical applications of
MARSALA for preimplantation genetic diagnosis of spinal muscular
atrophy. J Genet Genomics. 43:541–547. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lukaszuk K, Pukszta S, Ochman K, Cybulska
C, Liss J, Pastuszek E, Zabielska J and Woclawek-Potocka I: Healthy
baby born to a robertsonian translocation carrier following
next-generation sequencing-based preimplantation genetic diagnosis:
A case report. AJP Rep. 5:e172–e175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Diao Z, Xu Z, Zhou J, Wang W, Li
J, Yan G and Sun H: The clinical application of preimplantation
genetic diagnosis for the patient affected by congenital
contractural arachnodactyly and spinal and bulbar muscular atrophy.
J Assist Reprod Genet. 33:1459–1466. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liss J, Chromik I, Szczyglińska J,
Jagiełło M, Łukaszuk A and Łukaszuk K: Current methods for
preimplantation genetic diagnosis. Ginekol Pol. 87:522–526. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roy S, LaFramboise WA, Nikiforov YE,
Nikiforova MN, Routbort MJ, Pfeifer J, Nagarajan R, Carter AB and
Pantanowitz L: Next-generation sequencing informatics: Challenges
and strategies for implementation in a clinical environment. Arch
Pathol Lab Med. 140:958–975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mardis ER: The impact of next-generation
sequencing technology on genetics. Trends Genet. 24:133–141. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tenedini E, Artuso L, Bernardis I, Artusi
V, Percesepe A, De Rosa L, Contin R, Manfredini R, Pellacani G,
Giannetti A, et al: Amplicon-based next-generation sequencing: An
effective approach for the molecular diagnosis of epidermolysis
bullosa. Br J Dermatol. 173:731–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Drakopoulos P, Blockeel C, Stoop D, Camus
M, de Vos M, Tournaye H and Polyzos NP: Conventional ovarian
stimulation and single embryo transfer for IVF/ICSI. How many
oocytes do we need to maximize cumulative live birth rates after
utilization of all fresh and frozen embryos? Hum Reprod.
31:370–376. 2016.PubMed/NCBI
|
|
28
|
Gardner DK, Lane M, Stevens J, Schlenker T
and Schoolcraft WB: Blastocyst score affects implantation and
pregnancy outcome: Towards a single blastocyst transfer. Fertil
Steril. 73:1155–1158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin A, Liu C, Zhang Y, Wu J, Mai M, Ding
H, Yang J and Zhang X: The carrier rate and mutation spectrum of
genes associated with hearing loss in South China hearing female
population of childbearing age. BMC Med Genet. 14:572013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang QJ, Zhao YL, Rao SQ, Guo YF, He Y,
Lan L, Yang WY, Zheng QY, Ruben RJ, Han DY and Shen Y: Newborn
hearing concurrent gene screening can improve care for hearing
loss: A study on 14,913 Chinese newborns. Int J Pediatr
Otorhinolaryngol. 75:535–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morton NE: Genetic epidemiology of hearing
impairment. Ann N Y Acad Sci. 630:16–31. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Postelmans JT, Cleffken B and Stokroos RJ:
Post-operative complications of cochlear implantation in adults and
children: Five years' experience in Maastricht. J Laryngol Otol.
121:318–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liss J, Mirecka A, Kitowska K and Lukaszuk
K: Preimplantaion genetic diagnosis of hearing loss with 35delG
mutation in GJB2 gene-preliminary report. Otolaryngol Pol.
65:443–446. 2011.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu CC, Lin SY, Su YN, Fang MY, Chen SU and
Hsu CJ: Preimplantation genetic diagnosis (embryo screening) for
enlarged vestibular aqueduct due to SLC26A4 mutation. Audiol
Neurootol. 15:311–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiong W, Wang D, Gao Y, Gao Y, Wang H,
Guan J, Lan L, Yan J, Zong L, Yuan Y, et al: Reproductive
management through integration of PGD and MPS-based noninvasive
prenatal screening/diagnosis for a family with GJB2-associated
hearing impairment. Sci China Life Sci. 58:829–838. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Treff NR, Fedick A, Tao X, Devkota B,
Taylor D and Scott RT Jr: Evaluation of targeted next-generation
sequencing-based preimplantation genetic diagnosis of monogenic
disease. Fertil Steril. 99:1377–1384.e6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Idan N, Brownstein Z, Shivatzki S and
Avraham KB: Advances in genetic diagnostics for hereditary hearing
loss. J Basic Clin Physiol Pharmacol. 24:165–170. 2013.PubMed/NCBI
|
|
38
|
Watson CT, Marques-Bonet T, Sharp AJ and
Mefford HC: The genetics of microdeletion and microduplication
syndromes: An update. Annu Rev Genomics Hum Genet. 15:215–244.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Russo CD, Di Giacomo G, Cignini P, Padula
F, Mangiafico L, Mesoraca A, D'Emidio L, McCluskey MR, Paganelli A
and Giorlandino C: Comparative study of aCGH and Next Generation
Sequencing (NGS) for chromosomal microdeletion and microduplication
screening. J Prenat Med. 8:57–69. 2014.PubMed/NCBI
|