Introduction
Toll-like receptors (TLRs), a class of
toll-homologous protein molecules expressed in mammals, are
pattern-recognition receptors that are present in macrophages,
mononuclear cells, dendritic cells, B cells and other immune cells
(1). At present, 13 types of TLRs
have been identified in mammals, namely TLR1 to TLR13, including
TLR1-TLR11 in humans (2).
Interactions between TLRs and their specific pathogen-associated
molecular patterns mediate intracellular signaling pathways,
increasing the levels of various chemical regulatory factors and
cell surface molecules, thereby triggering innate, and adaptive
immune responses. TLR9 specifically recognizes the unmethylated
cytosine-phosphate-guanosine (CpG) motifs in bacteria, viruses and
plasmids or synthetic double-, or single-stranded
oligodeoxynucleotides (ODNs). CpG ODNs are synthetic DNA
nucleotides containing unmethylated CpG sequences. As powerful
immune modulators and immune adjuvants, the effectiveness of CpG
ODNs has attracted increasing attention, particularly as potential
therapies for malignant tumors that are difficult to treat
(3). Wang et al (4) demonstrated that the immunomodulatory
oligonucleotide had potent antitumor effects when used as
monotherapy and in combination with conventional chemotherapeutic
agents on non-small cell lung cancer (NSCLC) cells via TLR9.
Previous studies have demonstrated that CpG ODNs may
contribute to the induction of apoptosis, inhibition of cancer cell
growth and enhancement of radiotherapeutic, and chemotherapeutic
sensitivity in various types of cancer (3,5–7). Several studies have also confirmed that
TLR9 is highly expressed in numerous types of cancer cells,
including lung, ovarian, pancreatic and breast cancer (8–11).
However, there are disagreements regarding the associations between
TLR9 expression, tumor development, and sensitivity to radiation
and chemotherapy. According to a previous study, subsequent to
recognizing CpG ODNs, TLR9 may activate the mitogen-activated
protein kinase 14 (p38)/mitogen-activated protein kinase (MAPK)
signal pathway (12); however, the
downstream signal transduction pathway and subsequently target gene
expressions have been inconclusive (13,14).
Our previous studies demonstrated that the
combination of TLR9 and its agonist CpG ODNs increased the
radiosensitivity of A549 lung cancer cells through reducing cell
survival and colony formation ability, increasing the G2/M phase
block and inducing cell apoptosis. CpG ODNs increased the
radiosensitivity of radioresistant A549 cells, which may be
mediated through the upregulation of TLR9 expression (15,16).
Furthermore, our subsequent study demonstrated that the combination
of TLR9 and its agonist CpG ODNs markedly increased the levels of
cellular tumor antigen p53 (p53) protein phosphorylation induced by
X-ray, which may be involved in the G2/M phase arrest and apoptosis
induced by X-ray in human lung cancer A549 cells (17). Additional studies have indicated that
p53 phosphorylation serves an important, and even a crucial,
regulatory role in its own activation (18,19).
Activation of p53 may result in cell growth retardation, induction
of apoptosis or adaptation to DNA damage and survival, via
regulating the expression of downstream target genes (20,21).
Wild-type p53 has also been demonstrated to enhance the sensitivity
of tumor cells to radiotherapy by inhibiting oncogene expression,
arresting tumor cells in the G2/M phase, inhibiting tumor cell
repair of radiation damage, and promoting apoptosis (14,15). Based
on the aforementioned studies, we hypothesized that TLR9 may
strengthen the effect of X-ray irradiation (IR) on NSCLC and
consequently improve the radiosensitivity of lung cancer cells.
Additionally, we also hypothesize that activation of the p53
pathway may be involved in this process; TLR9 expression may affect
the proliferation and apoptosis of tumor cells through altering the
expression of p53, and the associated downstream pathway via
activating p38/MAPK signaling pathways. However, previous studies
examining whether the TLR9-p53-p38/MAPK signaling pathways are
involved in the process whereby TLR9 combines with CpG ODN to
improve the radiosensitivity of lung cancer cells have not yet been
sufficient.
In the present study, TLR9 gene expression was
silenced using small interfering (si)RNA interference technology.
Cells were then stimulated with CpG ODN 7909 and subjected to X-ray
IR, and the radiation sensitivity and expression levels of p38,
wild-type p53 and downstream target genes, including B-cell
lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and genome
polyprotein (p21) were investigated. Therefore, the present study
provides a preliminary investigation into the role of TLR9 in NSCLC
radiotherapy, including the potential associated downstream pathway
involved, which may assist in developing novel methods for
predicting and improving the outcome of radiotherapy in NSCLC.
Materials and methods
Materials
Rabbit monoclonal antibodies against TLR9 (cat. no.
5845), p38 (cat. no. 8690), p53 (cat. no. 2527), Bax (cat. no.
2772), Bcl-2 (cat. no. 4223), p21 (cat. no. 2947) and β-actin (cat.
no. 4970), were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). CpG ODN 7909, comprising a nucleotide sequence
of 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′, was synthesized, purified and
analyzed as previously described (11).
Cell culture and small interfering
(si)RNA transfection
The human NSCLC A549 cell line was obtained from
Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences (Shanghai, China) and grown in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). The
cell lines were cultured at 37°C in a humidified air containing 5%
CO2. When the cells reached 70% confluence, they were
transfected with 100 nM siRNA targeting: Human TLR9 mRNA forward,
5′-CUAGACCUGUCCCACAAUATT-3′ and reverse,
5′-UAUUGUGGGACAGGUCUAGTT-3′; wild-type p53 mRNA forward,
5′-CCACUGGAUGGAGAAUAUUTT-3′ and reverse,
5′-AAUAUUCUCCAUCCAGUGGTT-3′; or scramble (control) siRNA 1 forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′; scramble (control) siRNA 2 forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGAGAATT-3′), using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. All siRNAs were fluorescein amidite
(FAM)-labeled, and were obtained from Shanghai GenePharma Co., Ltd.
(Shanghai, China). Subsequent to incubation for 6 h at 37°C in a
humidified incubator containing 5% CO2, the medium was
replaced with RPMI-1640 medium supplemented with 10% fetal bovine
serum. Following another incubation for 18 h at 37°C, the cells
were used for subsequent experimentation. Then, 24 h after
transfection, the expression of green fluorescent protein was
observed under an Olympus IX73 fluorescence microscope (Olympus
Corporation, Tokyo, Japan) to evaluate the transfection efficiency.
A total of 500 cells were selected randomly, and the percentage of
cells expressing green fluorescent protein was calculated.
Radiation treatment
A549 cells and other transfected cells were exposed
to various doses of 6 MV X-ray IR, which was performed with the use
of a linear accelerator (Varian Medical Systems Inc., Palo Alto,
CA, USA), and the dose rate at an IR distance of 100 cm was 2
Gy/min, as determined by Fricke's chemical dosimeter (15). Various doses of radiation (2, 4, 6, 8
and 10 Gy) were applied in the colony formation assay, while 10 Gy
radiation was used for the remaining experiments.
Western blot analysis
Proteins were extracted from the cells with 10% SDS
cell lysis solution (including 1:100 phenylmethylsulfonyl fluoride;
Beyotime Institute of Biotechnology, Haimen, China) and protein
concentration was measured using a BCA assay kit (Beyotime
Institute of Biotechnology). Then, 6% SDS-PAGE (Beyotime Institute
of Biotechnology) was used to separate the protein extracts (~50
µg/lane), which was then transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
then blocked with tris-buffered saline with 1% Tween-20 (TBST)
containing 5% skimmed milk for 2 h at room temperature, and
subsequently incubated with the primary antibodies (TLR9, p38, p53,
Bax, Bcl-2, p21 or β-actin; dilution, 1:800) at room temperature.
The membranes were then washed with TBST three times, followed by
incubation with the horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (cat no. 4970; 1:5,000; Cell
Signaling Technology, Inc.) for 1 h at room temperature. The
membranes were finally washed with TBST and protein expression was
determined with enhanced chemiluminescence kit (ECL; EMD
Millipore). β-actin was applied as an endogenous reference for
quantification. The images were analyzed using Adobe Photoshop CS3
software (Adobe Systems, Inc., San Jose, CA, USA).
Colony formation assay
Following X-ray IR, cells were collected and used to
prepare a single-cell suspension. According to the radiation dose
(0, 2, 4, 6, 8 and 10 Gy), cells were seeded in triplicate in 60-mm
petri dishes at densities of 500, 500, 1,000, 500, 1,000 and 8,000
cells/dish, respectively. Following incubation at 37°C in an
incubator for 2 weeks, the cells were washed twice with PBS, fixed
with 4% paraformaldehyde for 30 min and stained with 0.5% crystal
violet for 15 min at room temperature. Subsequently, cell colonies
containing >50 cells were counted. The formed colonies were
observed using light microscope (×100). The colony formation rate
was calculated as the number of colonies divided by the number of
seeded cells. According to single-hit, multi-target model, D0, Dq,
N and SF2 were obtained (22). D0
represents the slope rate of survival curve, which indicates the
radiosensitivity of cells; Dq represents the initial shoulder of
survival curve, which is associated with the efficacy of the DNA
repair system of the cell; and N is an extrapolation value of D0,
which is an associated parameter reflecting primary radiation
sensitivity of cells. SF2 was the survival fraction following 2 Gy
irradiation and used as an index of intrinsic radiosensitivity. The
sensitivity enhancement ratio (SER) was calculated as the D0
(lethal dose in one-hit multi-target model) value of cells
receiving IR treatment alone in the control group divided by the D0
value of other cells receiving IR treatment alone, or CpG ODN 7909
plus IR treatment.
Apoptosis assays
Apoptosis was evaluated with the Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (BD
Biosciences, San Jose, CA, USA) according to the manufacturer's
protocol. All cells, including adherent and non-adherent cells,
were collected at 48 h subsequent to IR, and washed twice with cold
PBS. Following centrifugation at 800 × g for 5 min at 4°C, the
deposits were resuspended in binding buffer, and incubated with
FITC-conjugated Annexin-V and propidium iodide for 15 min at room
temperature in the dark. Immediately, the cells were analyzed using
a Cytomics™ FC500 flow cytometer (Beckman Coulter, Inc., Fullerton,
CA, USA). Data were analyzed using Summit version 5.2 software
(Beckman Coulter, Inc.).
Statistical analysis
All experiments were repeated three times.
Statistical analysis was performed using SPSS 20.0 (IBM Corp.,
Armonk, NY, USA) and GraphPad Prism 5.0 (fitting the survival curve
in a single-hit, multi-target model) (GraphPad Software, Inc., La
Jolla, CA, USA). Values are presented as the mean ± standard
deviation of triplicate experiments. One-way analysis of variance
followed by Bonferroni post-hoc test was used for data with ≥3
groups or Student's t-test for data with two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Silencing of TLR9 expression in A549
cell lines by siRNA
To knock down the expression of TLR9, siRNA
targeting TLR9 were applied to the cells. A total of 24 h after
transfection, the transfection efficiency was observed under an
Olympus IX73 fluorescence microscope (Olympus Corporation, Tokyo,
Japan). Scramble siRNA (control siRNA) was applied to exclude the
effect of the liposome transfection on the measured effects. All
siRNAs were FAM-labeled. Following transfection, >70% of cells
in each transfected group were FAM-positive, indicating high
transfection efficiency. Western blot analyses revealed decreased
TLR9 expression levels in TLR9 siRNA-transfected cells compared
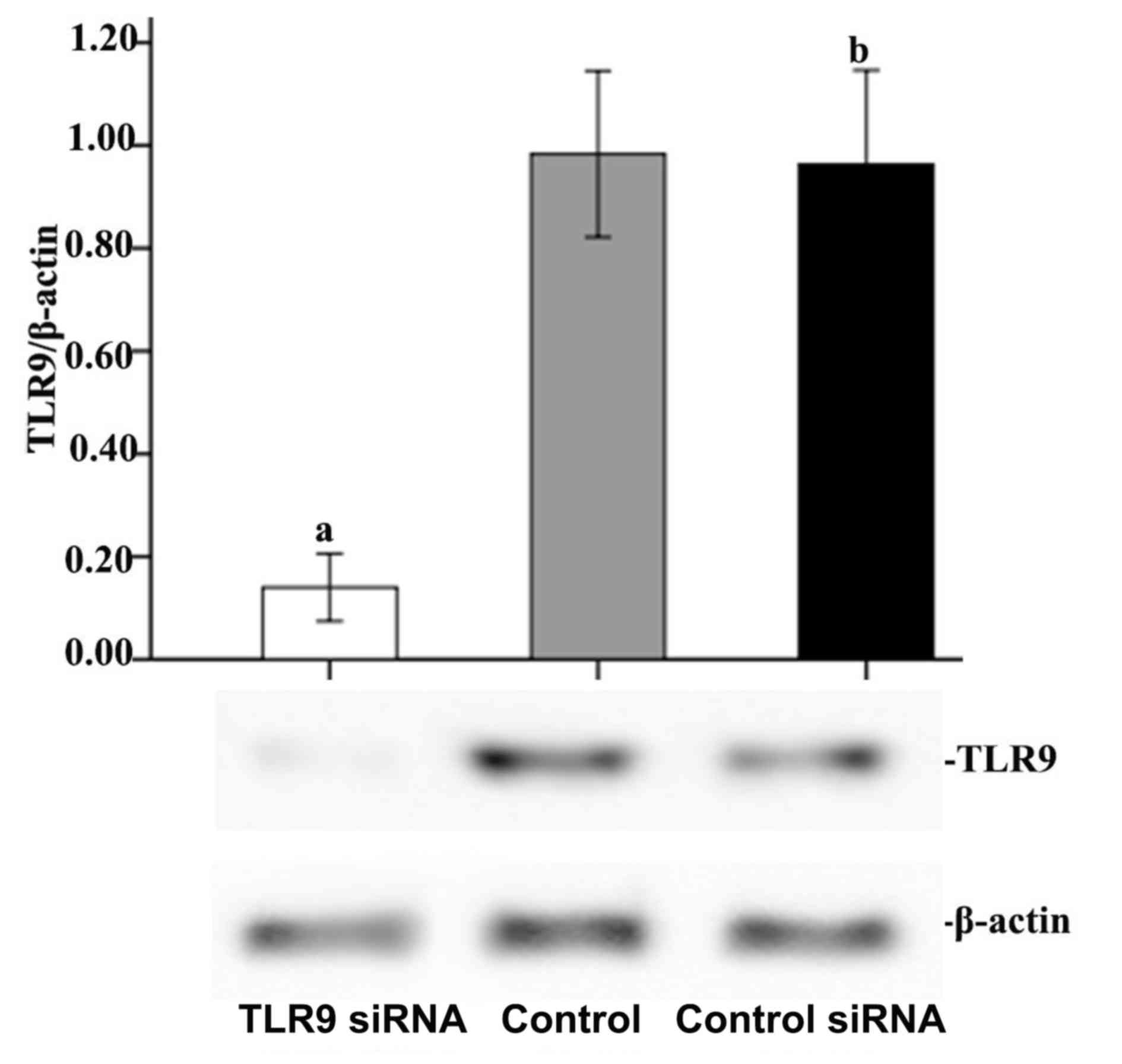
with untransfected cells. As presented in Fig. 1, 24 h subsequent to transfection, TLR9
protein expression was significantly reduced in A549 cells
transfected with TLR9 siRNA compared with that in control cells,
demonstrating 85.76% inhibition based on the densitometric analysis
(P<0.05). However, there was no significant difference between
the control siRNA and control groups with regard to TLR9 protein
expression, which suggested that transfection using Lipofectamine
had very little or no effect on A549 cells.
Clonogenic assays for determination of
radiosensitivity of A549 cells in response to CpG ODN 7909 combined
with TLR9
Colony formation assays were performed to evaluate
the radiation-enhancing effects of CpG ODN 7909 combined with TLR9.
As demonstrated in Fig. 2 and
Table I, the cell survival curve,
fitted using a single-hit, multi-target model, indicated that A549
cells treated with CpG ODN 7909 plus IR exhibited decreased colony
formation ability and lower values of D0, Dq, N and SF2 compared
with cells treated with IR alone; the sensitivity enhancement
ratios (SER) was 1.28. However, following the knockdown of TLR9
expression by siRNA, CpG ODN 7909 was unable to decrease the colony
forming capacity of lung cancer cells and the SER was 1.01.
Control-siRNA transfection had no significant effect on the colony
formation ability of A549 cells.
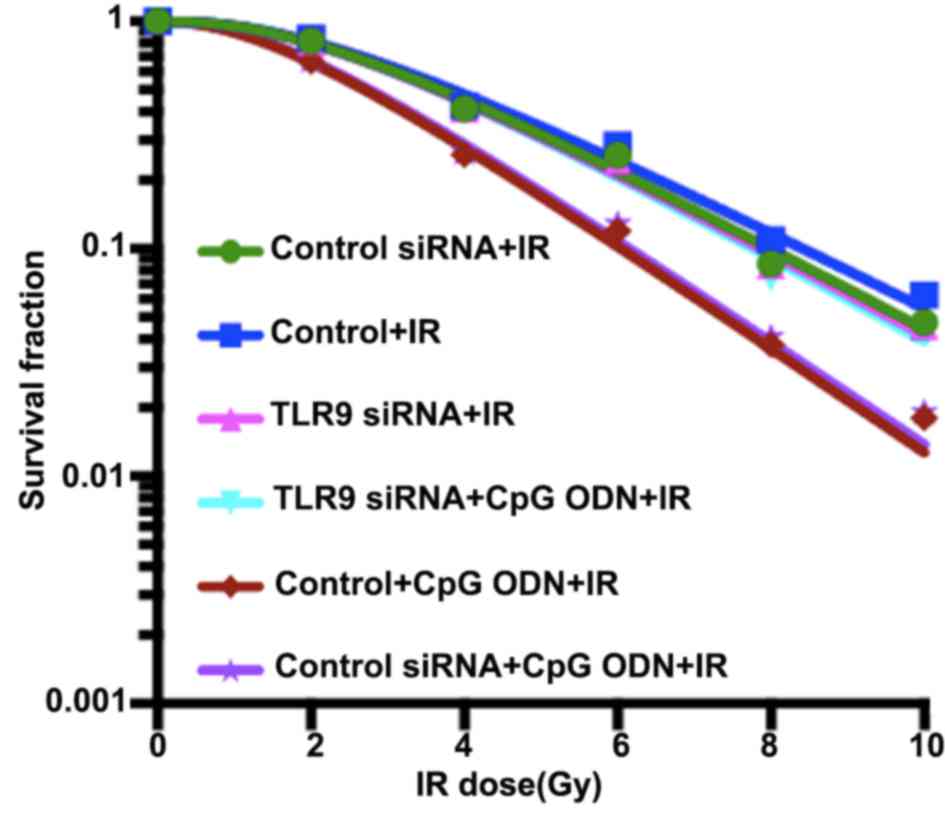 | Figure 2.Activation of TLR9 by CpG ODN 7909
enhances the radiosensitivity of A549 cells. Compared with that of
untransfected control cells, the dose-survival curve of TLR9
siRNA-transfected cells exhibited a broader initial shoulder
(indicating increase in Dq) and a smaller slope rate (indicating
increase in D0). TLR9 siRNA-transfected cells were more resistant
to cell death post-IR. These results implied that CpG ODN 7909 plus
IR resulted in decreased colony formation ability in untransfected
control cells compared with TLR9 siRNA-transfected cells, with
sensitivity enhancement ratios of 1.28 and 1.01, respectively.
TLR9, toll-like receptor 9; CpG ODNs,
cytosine-phosphorothioate-guanine-containing oligodeoxynucleotides;
IR, irradiation; TLR9, toll-like receptor 9; si, small interfering;
D0, slope rate of survival curve; Dq, initial shoulder of survival
curve. |
 | Table I.Comparison of radiosensitivity of
untransfected, control siRNA-transfected and TLR9 siRNA-transfected
A549 cells treated with IR or CpG ODN 7909. |
Table I.
Comparison of radiosensitivity of
untransfected, control siRNA-transfected and TLR9 siRNA-transfected
A549 cells treated with IR or CpG ODN 7909.
| Group | D0 | Dq | N | SF2 | SER |
|---|
| TLR9 siRNA +
IR | 2.38 | 4.39 | 2.85 | 0.82 | 1.03 |
| Control + IR | 2.46 | 4.56 | 2.86 | 0.84 | – |
| Control siRNA +
IR | 2.42 | 4.40 | 2.82 | 0.82 | 1.02 |
| TLR9 siRNA + CpG
ODN + IR | 2.41 | 4.40 | 2.82 | 0.81 | 1.01 |
| Control + CpG ODN +
IR | 1.92 | 2.90 | 2.52 | 0.67 | 1.28 |
| Control siRNA + CpG
ODN + IR | 1.91 | 3.00 | 2.57 | 0.68 | 1.29 |
Effect of TLR9 expression on apoptosis
in A549 cells treated with CpG ODN 7909 plus IR
Flow cytometry was applied to analyze apoptosis. As
indicated in Fig. 3, in the untreated
control, TLR9 siRNA-transfected and control siRNA-transfected
groups, there was no significant difference in apoptosis rates
between untreated cells and cells treated with CpG ODN 7909 alone.
Furthermore, in all three groups, compared with the untreated
cells, there was a significantly increased apoptosis rate in cells
treated with IR alone and those treated with CpG ODN 7909 plus IR
(P<0.05). In the untransfected control and control siRNA groups,
increased apoptosis was observed in cells treated with CpG ODN 7909
plus IR compared with that in cells treated with IR alone; however,
this was not observed in the TLR9 siRNA group. Compared with the
corresponding cells in the control group, cells in the TLR9 siRNA
group that were untreated or treated with CpG ODN 7909 or IR alone
did not exhibit any significant difference in the rate of
apoptosis; however, cells in the TLR9 siRNA group treated with CpG
ODN 7909 combined with IR exhibited a significantly decreased
apoptosis rate compared with those in the control group. When
receiving the same treatment, there was no significant difference
between the untransfected control and control siRNA groups with
regard to the apoptosis rate.
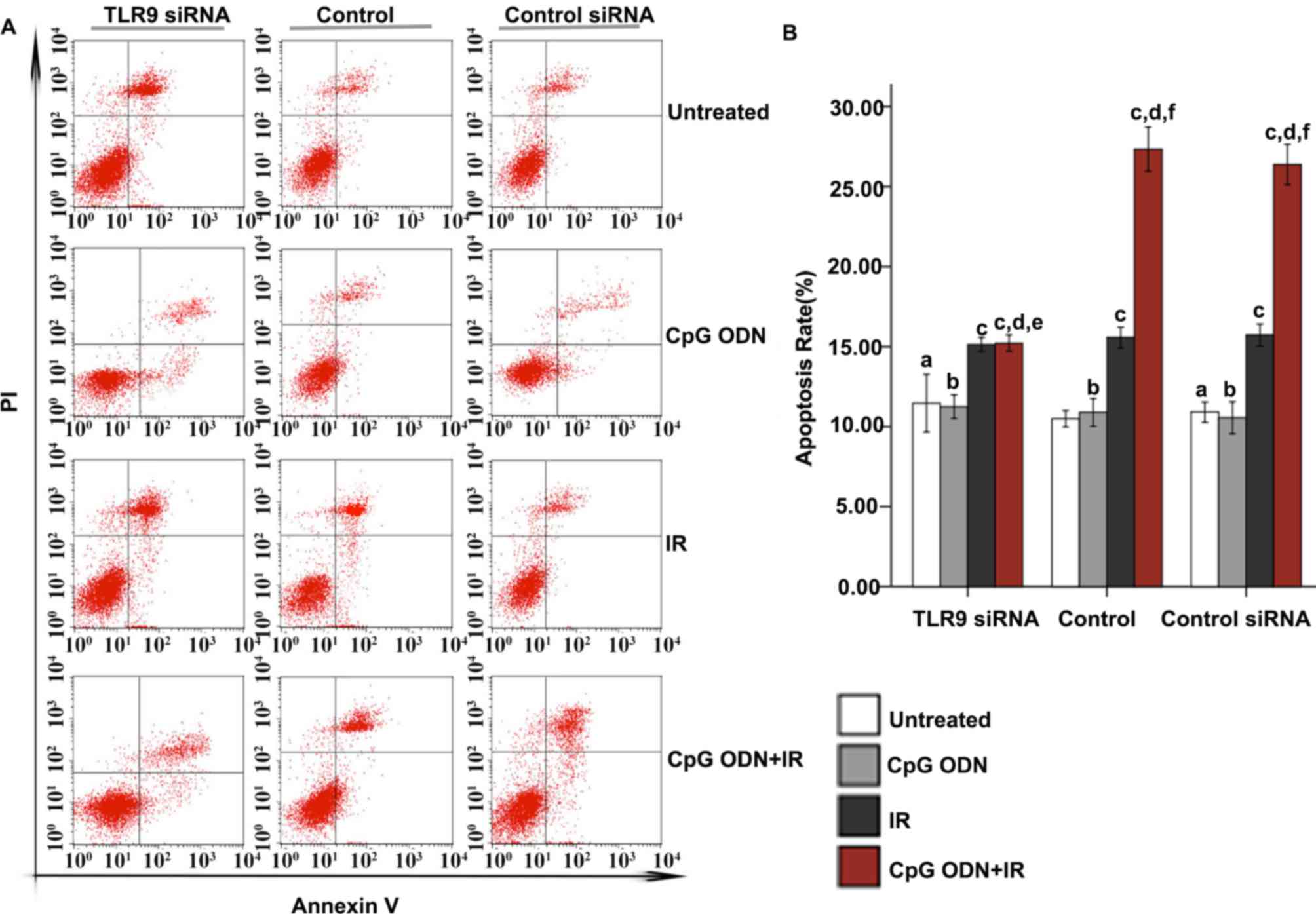 | Figure 3.Effect of TLR9 siRNA on apoptosis in
A549 cells treated with CpG ODN plus IR. At 48 h following IR,
apoptosis was evaluated using Annexin V-fluorescein
isothiocyanate/PI staining and flow cytometric analysis. (A)
Representative flow cytometry results: Bottom right quadrant, cells
stained primarily by Annexin V (early apoptotic cells); top right
quadrant, cells stained by PI and Annexin V (late apoptotic cells);
top left quadrant, cells stained primarily by PI (necrotic cells);
bottom left quadrant, cells negative for Annexin V and PI. (B) The
rates of apoptosis (cells in bottom right and top right quadrants)
in each transfection and treatment group were quantified and
presented as the mean ± standard deviation. aNot
significantly different (P>0.05) compared with untreated cells
in the control group. bNot significantly different
(P>0.05) compared with untreated cells in the same group.
cP<0.05 compared with untreated cells in the same
group. dP<0.05 compared with CpG ODN-treated cells in
the same group. eNot significantly different (P>0.05)
compared with IR-treated cells in the same group.
fP<0.05 compared with IR-treated cells in the same
group. TLR9, toll-like receptor 9; CpG ODNs,
cytosine-phosphorothioate-guanine-containing oligodeoxynucleotides;
IR, irradiation; PI, propidium iodide; si, small interfering; CITC,
fluorescein isothiocyanate. |
Effects of TLR9 activation by CpG ODN 7909 on the
p53-mediated pathway in A549 lung cancer cells
Effect of TLR9 activation by CpG ODN
7909 on the expression of p38
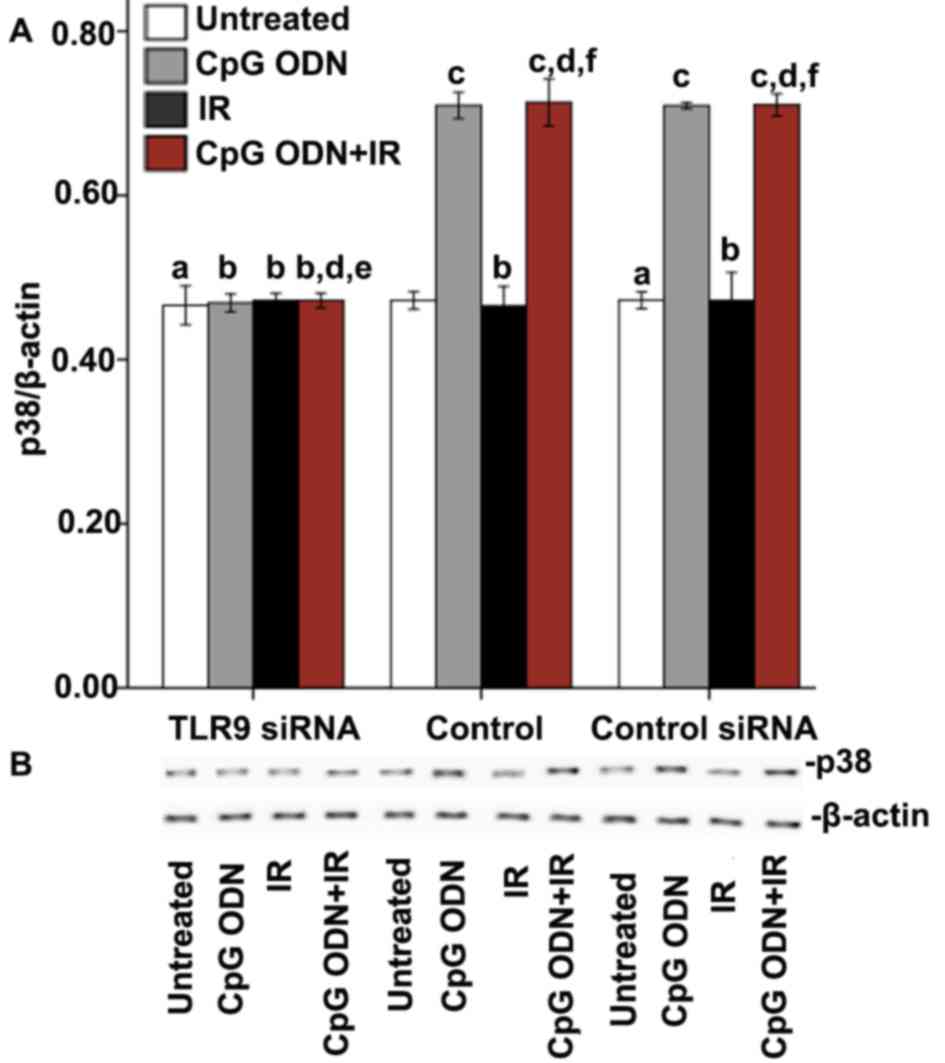
The expression of p38 was detected by western blot
analysis to explore whether the interaction between CpG ODN and
TLR9 activated the p38/MAPK signaling pathway. As presented in
Fig. 4, in the untransfected control
and control siRNA groups, the cells treated with CpG ODN 7909 alone
and those treated with CpG ODN 7909 plus IR exhibited increased p38
expression levels compared with untreated cells. By contrast, the
treatments had no significant effect on p38 levels in the TLR9
siRNA group. In all the three groups, compared with untreated
cells, there was no significant difference in p38 levels in cells
treated with IR alone.
Effect of CpG ODN 7909-mediated TLR9
activation on the expression of p53 pathway-associated
proteins
X-ray IR may lead to apoptosis via activation of the
p53 signal pathway (16). In order to
investigate whether TLR9 activation by CpG ODN 7909 enhanced the
radiosensitivity of A549 lung cancer cells of via the p53 pathway,
various p53 pathway-associated proteins, including p53, Bax, Bcl-2
and p21, were detected by western blotting. As indicated in
Fig. 5, in the control and control
siRNA groups, significantly increased expression levels of p53, Bax
and p21, and unchanged expression levels of Bcl-2 were observed in
cells treated with CpG ODN 7909 alone compared with those in the
untreated cells. By contrast, no significant difference was
observed in the TLR9 siRNA group. In all the three groups, compared
with the untreated cells, increased expression levels of p53, Bax
and p21, and decreased Bcl-2 expression were observed in cells
treated with IR alone and in cells treated with CpG ODN 7909 plus
IR. In the untransfected control and control siRNA groups, the
levels of p53, Bax and p21 expression were increased, and the Bcl-2
levels were decreased significantly in the cells treated with CpG
ODN7909 plus IR compared with the cells treated with IR alone;
however, the combined treatment did not lead to the same effects in
the TLR9 siRNA group. When receiving the same treatment, no
significant difference between control and control siRNA groups for
all detected p53 pathway associated proteins was observed.
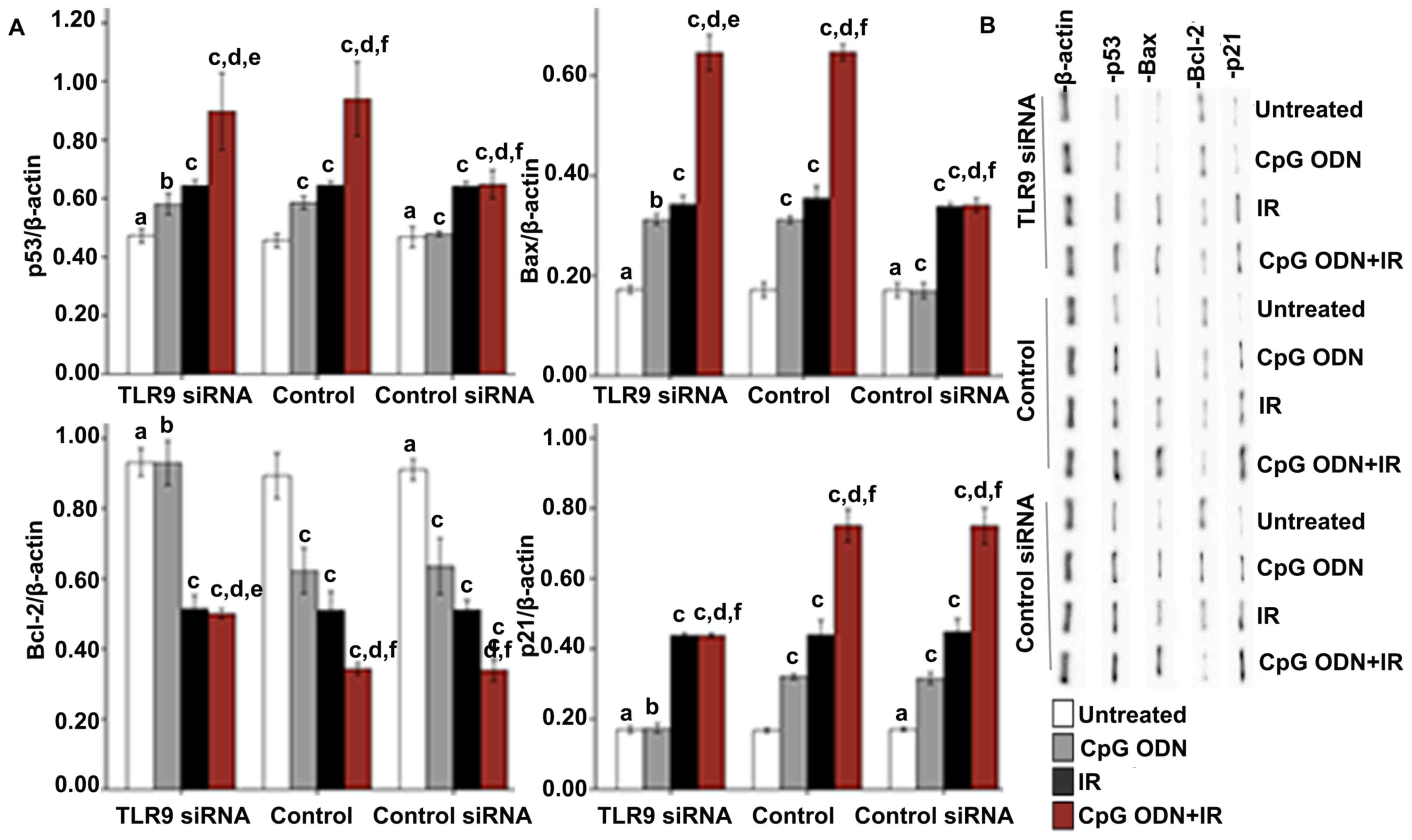 | Figure 5.Effect of TLR9 activation by CpG ODN
7909 on p53 pathway-associated proteins in A549 cells. (A)
Quantification and (B) representative image of western blot
analyses applied to detect the expression of p53 pathway-associated
proteins, including p53, Bax, Bcl-2 and p21. The graph presents
data as the mean ± standard deviation from three independent
determinations of optical density of the protein western blot
bands. aNot significantly different (P>0.05) compared
with untreated cells in the control group. bNot
significantly different (P>0.05) compared with untreated cells
in the same group. cP<0.05 compared with untreated
cells in the same group. dP<0.05 compared with CpG
ODN-treated cells in the same group. eNot significantly
different (P>0.05) compared with IR-treated cells in the same
group. fP<0.05 compared with IR-treated cells in the
same group. TLR9, toll-like receptor 9; CpG ODNs,
cytosine-phosphorothioate-guanine-containing oligodeoxynucleotides;
IR, irradiation; p53, cellular tumor antigen p53; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein; p21, genome
polyprotein. |
Effect of blocking the p53 signal
transduction pathway on apoptosis in A549 cells treated with CpG
ODN 7909 plus IR
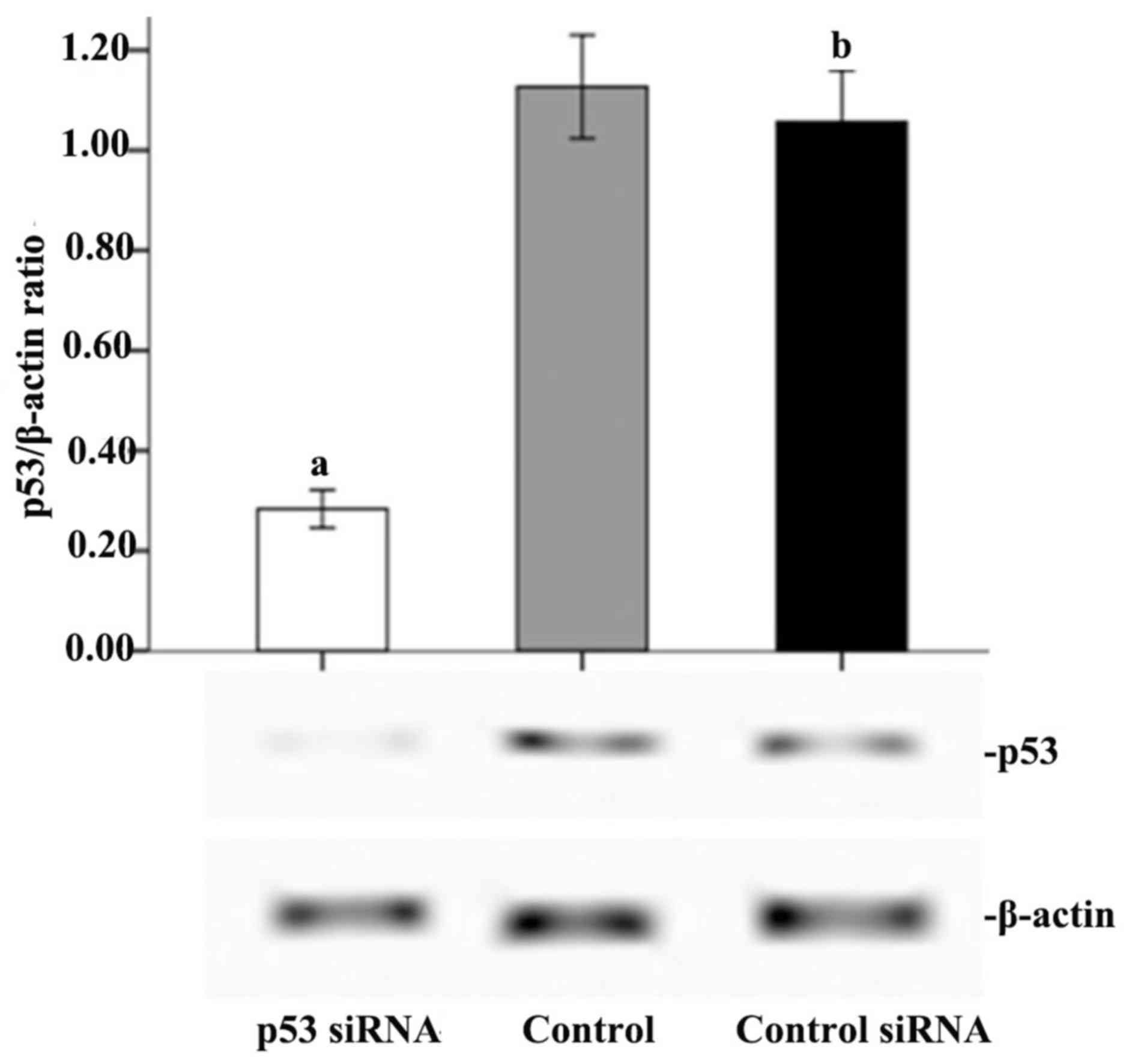
As demonstrated in Fig.
6, p53 was successfully knocked down by transfection of the
cells with p53 siRNA. Therefore, the p53-mediated signal
transduction pathway was blocked to verify its effect on the
apoptosis of cells A549 cells treated with CpG ODN 7909 plus IR. As
indicated in Fig. 7, the rate of
apoptosis of the untreated cells, cells treated with CpG ODN7909 or
IR respectively, and CpG ODN 7909 plus IR in the p53 siRNA group
all decreased notably compared with those in the control group. In
p53 siRNA, control or control siRNA groups, the apoptosis rate of
cells treated with CpG ODN7909 alone did not vary significantly
compared with those of cells without any treatment; but there was a
significantly increased apoptosis rate in cells treated with IR
alone or CpG ODN7909 combined with IR compared with that in cells
without any treatment (P<0.05); in addition, compared with cells
treated with IR or CpG ODN7909 alone, increased apoptosis was
observed in the cells treated with CpG ODN7909 plus IR. No
significant difference between control and control siRNA groups for
the apoptosis rate was observed in any treatment group.
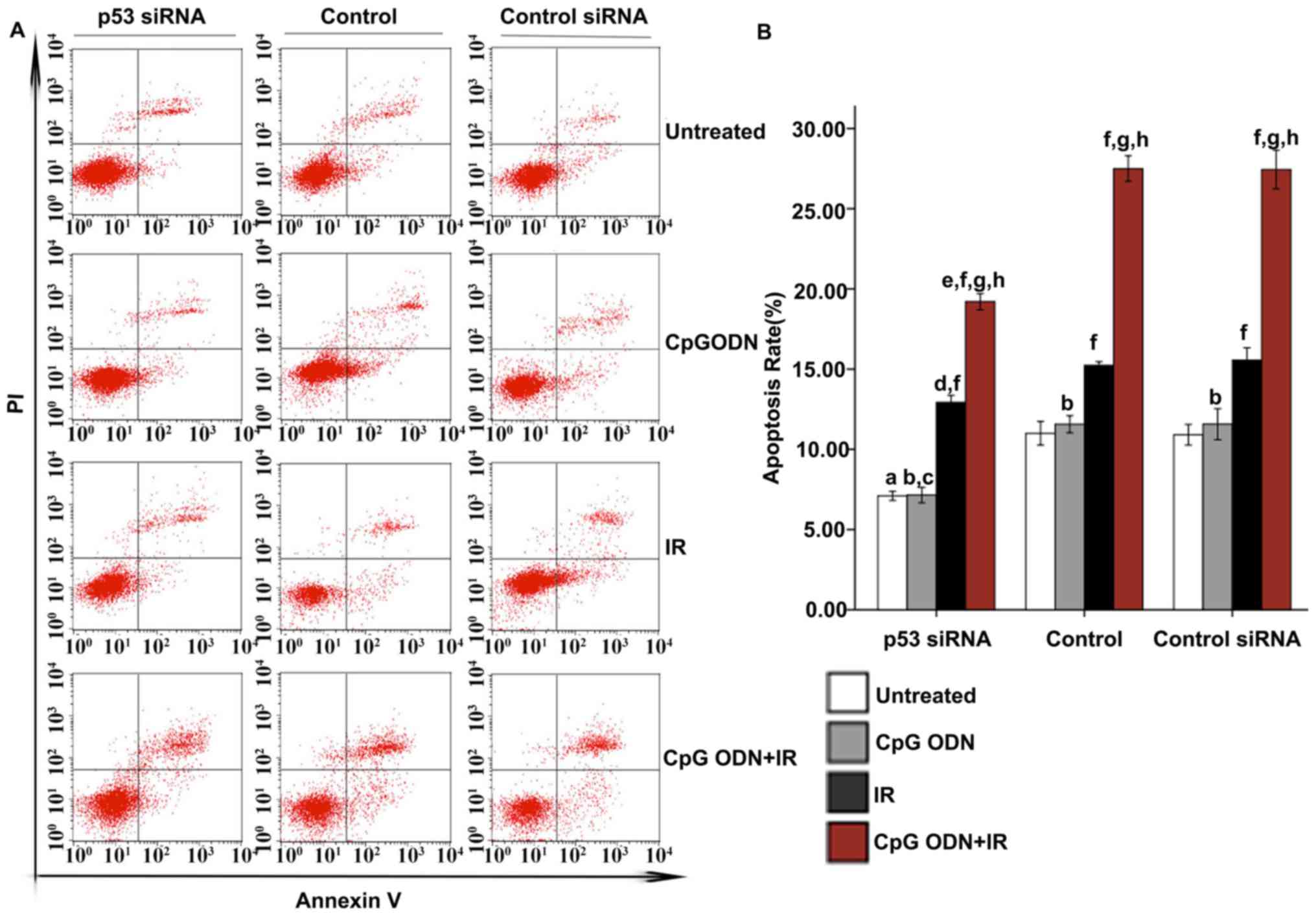 | Figure 7.Effect of blocking the p53
transduction pathway on apoptosis. At 48 h following IR, the
apoptosis rates of all cells were evaluated using Annexin V-FITC/PI
staining and flow cytometric analysis. (A) Representative flow
cytometry results: Bottom right quadrant, cells stained primarily
by Annexin V (early apoptotic cells); top right quadrant, cells
stained by both PI and Annexin V (late apoptotic cells); top left
quadrant, cells stained primarily by PI (necrotic cells); bottom
left quadrant, cells negative for both Annexin V and PI. (B) The
rates of apoptosis (cells in both bottom right and top right
quadrants) in each transfection and treatment group were quantified
and presented as the mean ± standard deviation.
aP<0.05 compared with untreated cells of the control
group. bNot significantly different (P>0.05) compared
with untreated cells in the same group. cP<0.05
compared with CpG ODN-treated cells of the control group.
dP<0.05 compared with IR-treated cells of the control
group. eP<0.05 compared with CpG ODN plus IR-treated
cells of the control group. fP<0.05 compared with
untreated cells in the same group. gP<0.05 compared
with CpG ODN-treated cells in the same group. hP<0.05
compared with IR-treated cells in the same group. CpG ODNs,
cytosine-phosphorothioate-guanine-containing oligodeoxynucleotides;
IR, irradiation; FITC, fluorescein isothiocyanate; PI, propidium
iodide; p53, cellular tumor antigen p53; si, small interfering. |
Discussion
As a monotherapy and combined with other treatment
methods, CpG ODNs have exhibited vast potential for the treatment
of cancer (5,23). Using in vitro experiments and
in vivo animal models, previous studies have identified that
CpG ODNs combined with chemotherapy or radiation may increase the
therapeutic effects of these conventional therapies in treating
cancer (16,24,25). CpG
ODNs may have broad application prospects for the prevention and
treatment of malignant tumors (26).
It has been demonstrated previously that CpG ODNs exert their
effects by interacting with TLR9 and activating the subsequent
pathways (27).
In the present study, the effect of CpG ODNs
treatment and TLR9 expression on the radiosensitivity of A549 lung
cancer cells was investigated, and whether the p53 signaling
pathway was the downstream pathway involved in exerting these
effects was explored.
The results of colony forming assays revealed that
the activation of TLR9 by CpG ODN 7909 significantly increased the
radiosensitivity of A549 lung cancer cells, whereas CpG ODNs used
alone did not significantly affect radiosensitivity when the
expression of TLR9 was knocked down. This indicated that the
interaction of CpG ODNs with TLR9 was responsible for increasing
sensitivity of the cancer cells to X-ray IR.
Cell apoptosis is an important factor affecting
tumor development; the tumorigenesis and progression of malignant
tumors depends on the inhibition of the cell death processes, and
unlimited malignant hyperplasia of tumor cells. Therefore,
interventions that may cause tumor cell apoptosis represent
potential tumor treatment strategies. In the present study, X-ray
IR alone induced marked increases in apoptosis in the three groups
of cells (untransfected, TLR9 siRNA-transfected and control
siRNA-transfected A549 cells) compared with the respective
untreated cells, whereas CpG ODN 7909 alone had no significant
effect on apoptosis in any group. Furthermore, the combined
apoptosis-inducing effect of TLR9 and CpG ODNs following IR
treatment was significantly increased compared with that of X-ray
IR alone, which was indicated by the lack of increase in apoptosis
following the application of the combined treatment in cells with
TLR9 gene silencing. This evidence suggests that the interaction of
CpG ODNs with TLR9 is associated with the significant enhancement
of radiation-induced apoptosis in NSCLC cells to a certain
extent.
The interaction between CpG ODNs and TLR9 activates
the p38/MAPK signaling pathway, and subsequently the p53 signal
pathway (28,29). The p38/MAPK pathway is involved in
various biological effects, including the stress response, cell
proliferation and apoptosis (30). A
previous study identified that the overexpression of p38 MAPK
proteins by the transfection of exogenous p38 MAPK into cells
improved the curative effect of various pro-apoptotic genes
dependent on the p38/MAPK signal pathway, and p38 MAPK may regulate
apoptosis by activating p53 (13,31). This
suggests that TLR9 may affect the proliferation and apoptosis of
tumor cells through altering the expression of p53 via activation
of the p38/MAPK pathway. It is well-known that the p53-mediated
apoptotic pathway is important in the apoptotic cell death of
tumors; the tumor suppressor p53 regulates cell cycle arrest,
apoptosis and DNA repair processes by controlling various target
genes that contain p53 sequence-specific DNA binding sites
(32–34).
X-ray IR may result in apoptosis in various types of
human cancer and consequently kill cancer cells; increased
apoptosis rates usually indicate increased radiosensitivity
(35). A previous study has
demonstrated that X-ray IR may increase wild-type p53 protein
levels and subsequently induce p53-dependent apoptotic cell death
(36).
In order to explore whether the p53-mediated
apoptotic pathway was involved in signal transduction downstream of
TLR9, the levels of the p53 pathway-associated proteins p53, Bax,
Bcl-2 and p21 were examined. Bax and Bcl-2 are pro-apoptotic and
anti-apoptotic genes, respectively, in the Bcl-2 family. Bax is the
primary regulator of Bcl-2 and serves an important role in
modulating tumor cells (37,38). X-ray IR may increase the expression of
Bax in cancer cells and result in apoptosis (39). A previous study indicated that p21, a
member of the cyclin-dependent kinase inhibitor family, may cause
G2/M phase arrest by inhibiting the function of cyclin-dependent
kinase 1 (40). As a target gene of
the p53 signal transduction pathway and upstream gene of certain
pro-apoptotic genes, p21 may be involved in the IR-induced
apoptosis of cancer cells (41).
The results of the present study indicated that CpG
ODN 7909 treatment increased the expression of p38, p53, Bax and
p21, and decreased the expression of Bcl-2, whereas TLR9 knockdown
attenuated the effect of CpG ODN 7909 treatment alone. This
suggested that CpG ODN 7909 may activate the p38 MAPK-p53 pathway,
but only through TLR9. X-ray IR alone significantly increased the
expression of p53, Bax and p21, and decreased the expression of
Bcl-2, but did not significantly alter the expression of p38.
Furthermore, the effect of X-ray IR on the p53 pathway was not
affected by the expression of TLR9. The combined treatment of CpG
ODN 7909 and X-ray IR did not result in an increased expression of
p38 compared with that in cells treated with CpG ODN 7909 alone,
but did induce increased expression of p53, Bax and p21 and
decreased expression of Bcl-2 in cells treated with either CpG ODN
7909 or X-ray IR single treatment. The results regarding the p38
MAPK-p53 pathway-associated expression combined with effect of TLR9
expression on apoptosis indicate that TLR9 activation by CpG ODN
7909 may activate the p38 MAPK-p53 pathway, but not induce
apoptosis. A potential reason may be that TLR9 activation by CpG
ODN 7909 may activate other pathways that counteract the effect of
the p53 pathway on inducing apoptosis directly. However, the
activation of the p38 MAPK-p53-mediated pathway may significantly
enhance the IR-induced apoptosis of A549 cells, thus increasing the
radiosensitivity of A549 cells. Additional studies are required to
establish the underlying reason and mechanism for this.
In order to verify whether the p53 pathway affects
the radiosensitivity of A549 lung cancer cells, the expression of
p53 was knocked down. The results indicated that, although the
apoptosis rate was decreased compared with that in control cells,
X-ray IR may induce apoptosis in p53 siRNA-transfected cells. In
addition, combined treatment of CpG ODN 7909 and IR also produced
this effect, which indicated that the activation of the p53 pathway
was partially, but not fully, responsible for IR-induced apoptosis,
and therefore contributed to enhancing the radiosensitivity of A549
lung cancer cells.
In conclusion, the results of the present study
demonstrated that TLR9 activation by CpG ODN 7909 may produce a
therapeutic effect by enhancing the radiotherapeutic sensitivity of
A549 cells. This mechanism is at least partially associated with
the activation of the TLR9-p53-mediated pathway. The results of the
present study may be useful in improving the effectiveness of
radiotherapy for lung cancer and in developing a TLR9-targeted
treatment with CpG ODNs as a radiosensitizer. However, a number of
factors remain unclear, including the following: The specific and
exact mechanisms of the interaction of CpG ODNs with TLR9; the
process by which identification of CpG ODNs by immune or tumor
cells leads to the eventual effects; whether CpG ODNs may be
developed as a radiosensitizer, and applied to patients safely and
effectively in a clinical setting; and what the optimal dose and
administration route would be. Therefore, additional studies are
required to address to these issues.
Acknowledgements
Not applicable
Funding
The present study was supported by a grant from the
Shanghai Municipal Health Bureau Commission of Health and Family
Planning (grant no. 20134y156).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
TKQ was the guarantor of integrity of entire study,
study concepts/study design and definition of intellectual content.
SJY, XL and XBZ performed data analysis/data acquisition, SJY, WC,
XC and QZ performed the literature research and SJY and TKQ
performed manuscript editing. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Mutwiri G: TLR9 agonists: Immune
mechanisms and therapeutic potential in domestic animals. Vet
Immunol Immunopathol. 148:85–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoneda K, Sugimoto K, Shiraki K, Tanaka J,
Beppu T, Fuke H, Yamamoto N, Masuya M, Horie R, Uchida K and Takei
Y: Dual topology of functional Toll-like receptor 3 expression in
human hepatocellular carcinoma: Differential signaling mechanisms
of TLR3-induced NF-kappaB activation and apoptosis. Int J Oncol.
33:929–936. 2008.PubMed/NCBI
|
|
3
|
He H, Genovese KJ, Swaggerty CL, Nisbet DJ
and Kogut MH: Differential induction of nitric oxide,
degranulation, and oxidative burst activities in response to
microbial agonist stimulations in monocytes and heterophils from
young commercial turkeys. Vet Immunol Immunopathol. 123:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Rayburn ER, Wang W, Kandimalla ER,
Agrawal S and Zhang R: Chemotherapy and chemosensitization of
non-small cell lung cancer with a novel immunomodulatory
oligonucleotide targeting Toll-like receptor 9. Mol Cancer Ther.
5:1585–1592. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holtick U, Scheulen ME, von
Bergwelt-Baildon MS and Weihrauch MR: Toll-like receptor 9 agonists
as cancer therapeutics. Expert Opin Investig Drugs. 20:361–372.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrangolini G, Tortoreto M, Perego P,
Carenini N, De Cesare M, Balsari A, Zunino F and Pratesi G:
Combination of metronomic gimatecan and CpG oligodeoxynucleotides
against an orthotopic pancreatic cancer xenograft. Cancer Biol
Ther. 7:596–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brignole C, Marimpietri D, Di Paolo D,
Perri P, Morandi F, Pastorino F, Zorzoli A, Pagnan G, Loi M, Caffa
I, et al: Therapeutic targeting of TLR9 inhibits cell growth and
induces apoptosis in neuroblastoma. Cancer Res. 70:9816–9826. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Droemann D, Albrecht D, Gerdes J, Ulmer
AJ, Branscheid D, Vollmer E, Dalhoff K, Zabel P and Goldmann T:
Human lung cancer cells express functionally active Toll-like
receptor 9. Respir Res. 6:12005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu HQ, Wang B, Zhu SK, Tian Y, Zhang JH
and Wu HS: Effects of CPG ODN on biological behavior of PANC-1 and
expression of TLR9 in pancreatic cancer. World J Gastroenterol.
17:996–1003. 2011.PubMed/NCBI
|
|
10
|
Tuomela J, Sandholm J, Karihtala P,
Ilvesaro J, Vuopala KS, Kauppila JH, Kauppila S, Chen D, Pressey C,
Härkönen P, et al: Low TLR9 expression defines an aggressive
subtype of triple-negative breast cancer. Breast Cancer Res Treat.
135:481–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kundu SD, Lee C, Billips BK, Habermacher
GM, Zhang Q, Liu V, Wong LY, Klumpp DJ and Thumbikat P: The
toll-like receptor pathway: A novel mechanism of infection induced
carcinogenesis of prostate epithelial cells. Prostate. 68:223–229.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang JH, Park JY and Kim SK: Dependence
on p38 MAPK signalling in the up-regulation of TLR2, TLR4 and TLR9
gene expression in Trichomonas vaginalis-treated HeLa cells.
Immunology. 118:164–170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Amicis F, Giordano F, Vivacqua A,
Pellegrino M, Panno ML, Tramontano D, Fuqua SA and Andò S:
Resveratrol, through NF-Y/p53/Sin3/HDAC1 complex phosphorylation,
inhibits estrogen receptor gene expression via p38MAPK/CK2
signaling in human breast cancer cells. FASEB J. 25:3695–3707.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riazantseva NV, Novitskiĭ VV, Kaĭgorodova
EV, Chasovskikh NIu and Starikova EG: Mitogenactivated protein
kinases JNK and p38 as redox-dependent molecular targets correction
of programmed cell death disturbances in oxidative stress
condition. Usp Fiziol Nauk. 40:3–11. 2009.(In Russian). PubMed/NCBI
|
|
15
|
Zha L, Qiao T, Yuan S and Lei L:
Enhancement of radiosensitivity by
CpG-oligodeoxyribonucleotide-7909 in human non-small cell lung
cancer A549 cells. Cancer Biother Radiopharm. 25:165–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan L, Xu G, Qiao T, Chen W, Yuan S and Li
X: CpG-ODN 7909 increases radiation sensitivity of
radiation-resistant human lung adenocarcinoma cell line by
overexpression of Toll-like receptor 9. Cancer Biother Radiopharm.
28:559–564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu XQ, Qiao TK, Chen W and Yuan SJ: Role
of ATM kinase in the effect of CpG-oligodeoxynucleotide-7909 on
X-ray-induced G2/M phase arrest and apoptosis in A549 cells. Chin J
Radiol Med Prot. 3:270–273. 2012.(In Chinese).
|
|
18
|
Viadiu H, Fronza G and Inga A: Structural
studies on mechanisms to activate mutant p53. Subcell Biochem.
85:119–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Merino D and Malkin D: p53 and hereditary
cancer. Subcell Biochem. 85:1–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan I, Garikapati KR, Shaik AB, Makani
VKK, Rahim A, Shareef MA, Reddy VG, Pal-Bhadra M, Kamal A and Kumar
CG: Design, synthesis and biological evaluation of 1, 4-dihydro
indeno[1,2-c] pyrazole linked oxindole analogues as potential
anticancer agents targeting tubulin and inducing p53 dependent
apoptosis. Eur J Med Chem. 144:104–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Parvathaneni S, Lu X, Chaudhary R, Lal A,
Madhusudan S and Sharma S: RECQ1 expression is upregulated in
response to DNA damage and in a p53-dependent manner. Oncotarget.
8:75924–75942. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spring E and Holmberg P: Evaluation of
experimental irradiation fractionation with the single-hit,
multi-target model. Acta Radiol Ther Phys Biol. 7:297–306. 1968.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang L, Wu X, Wan M, Yu Y, Yu Y and Wang
L: CpG oligodeoxynucleotides with double stem-loops show strong
immunostimulatory activity. Int Immunopharmacol. 15:89–96. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sommariva M, de Cesare M, Meini A, Cataldo
A, Zaffaroni N, Tagliabue E and Balsari A: High efficacy of
CpG-ODN, cetuximab and cisplatin combination for very advanced
ovarian xenograft tumors. J Transl Med. 11:252013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Liu X, Qiao T and Zhang Q:
Radiosensitization by CpG ODN7909 in an epidermoid laryngeal
carcinoma Hep-2 cell line. J Int Med Res. 45:2009–2022. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sfondrini L, Sommariva M, Tortoreto M,
Meini A, Piconese S, Calvaruso M, Van Rooijen N, Bonecchi R,
Zaffaroni N, Colombo MP, et al: Anti-tumor activity of CpG-ODN
aerosol in mouse lung metastases. Int J Cancer. 133:383–393. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xing N, Qiao T, Zhuang X, Yuan S, Zhang Q
and Xu G: CpG oligodeoxyribonucleotide 7909 enhances
radiosensitivity via downregulating Oct-4 expression in
radioresistant lung cancer cells. Onco Targets Ther. 8:1443–1449.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Honda K, Yanai H, Mizutani T, Negishi H,
Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh WC and Taniguchi T:
Role of a transductional-transcriptional processor complex
involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc
Natl Acad Sci USA. 101:pp. 15416–15421. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong MY, Gao JL, Cui JZ, Wang KJ, Tian YX,
Li R, Wang HT and Wang H: Effect of c-Jun NH2-terminal
kinase-mediated p53 expression on neuron autophagy following
traumatic brain injury in rats. Chin Med J (Engl). 125:2019–2024.
2012.PubMed/NCBI
|
|
30
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Strittmatter F, Gratzke C, Walther S,
Göttinger J, Beckmann C, Roosen A, Schlenker B, Reich O, Stief CG
and Hennenberg M: Alpha1-adrenoceptor signaling in the human
prostate involves regulation of p38 mitogen-activated protein
kinase. Urology. 78:969.e7–e13. 2011. View Article : Google Scholar
|
|
32
|
Aloni-Grinstein R, Schwartz D and Rotter
V: Accumulation of wild-type p53 protein upon gamma-irradiation
induces a G2 arrest-dependent immunoglobulin kappa light chain gene
expression. EMBO J. 14:1392–1401. 1995.PubMed/NCBI
|
|
33
|
Liu R, Ji P, Liu B, Qiao H, Wang X, Zhou
L, Deng T and Ba Y: Apigenin enhances the cisplatin cytotoxic
effect through p53-modulated apoptosis. Oncol Lett. 13:1024–1030.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang P, Cui J, Wen J, Guo Y, Zhang L and
Chen X: Cisplatin induces HepG2 cell cycle arrest through targeting
specific long noncoding RNAs and the p53 signaling pathway. Oncol
Lett. 12:4605–4612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vellanki SH, Grabrucker A, Liebau S,
Proepper C, Eramo A, Braun V, Boeckers T, Debatin KM and Fulda S:
Small-molecule XIAP inhibitors enhance gamma-irradiation-induced
apoptosis in glioblastoma. Neoplasia. 11:743–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Borges HL, Chao C, Xu Y, Linden R and Wang
JY: Radiation-induced apoptosis in developing mouse retina exhibits
dose-dependent requirement for ATM phosphorylation of p53. Cell
Death Differ. 11:494–502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rossé T, Olivier R, Monney L, Rager M,
Conus S, Fellay I, Jansen B and Borner C: Bcl-2 prolongs cell
survival after Bax-induced release of cytochrome c. Nature.
391:496–499. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo B, Zhai D, Cabezas E, Welsh K,
Nouraini S, Satterthwait AC and Reed JC: Humanin peptide suppresses
apoptosis by interfering with Bax activation. Nature. 423:456–461.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arafat W, Zhou T, Naoum GE and Buchsbaum
DJ: Targeted radiotherapy potentiates the cytotoxicity of a novel
anti-human DR5 monoclonal antibody and the adenovirus encoding
soluble TRAIL in prostate cancer. J Egypt Natl Canc Inst.
27:205–215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Torricelli C, Salvadori S, Valacchi G,
Souček K, Slabáková E, Muscettola M, Volpi N and Maioli E:
Alternative pathways of cancer cell death by rottlerin: Apoptosis
versus autophagy. Evid Based Complement Alternat Med.
2012:9806582012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Murphy M, Mabruk MJ, Lenane P, Liew A,
McCann P, Buckley A, Billet P, Leader M, Kay E and Murphy GM: The
expression of p53, p21, Bax and induction of apoptosis in normal
volunteers in response to different doses of ultraviolet radiation.
Br J Dermatol. 147:110–117. 2002. View Article : Google Scholar : PubMed/NCBI
|