Introduction
Gastric cancer (GC) is the most common
gastrointestinal malignant tumor, although its incidence rates vary
across different countries and regions. A high incidence of GC is
observed in individuals between 40 and 60 years of age (1). Each year, ~900,000 new cases of GC are
diagnosed, while ~700,000 patients succumb to GC globally (2), with half of these mortalities occurring
in Asia. The incidence rate in males is approximately two-fold
higher than that in females (3). The
pathogenesis of GC is unclear and lacks specific clinical
manifestations, and therefore the rate of early diagnosis is low
(4). Regional variations in GC
incidence reflect differences in dietary patterns, food storage,
and the availability of fresh produce, as well as the prevalence of
Helicobacter pylori infection (5). Surgical resection remains the
first-choice treatment modality for GC (6). However, even with a combination of
surgical and chemotherapeutic treatments, the 5-year overall
survival rate for patients with advanced-stage GC is ≤20% (2).
The MYC proto-oncogene protein (hereafter MYC)
serves key roles in the proliferation (7), cell cycle (8), differentiation and apoptosis of cells
(9,10). However, abnormal expression of MYC has
been implicated in almost all human tumors (11). Activation of the MYC gene is
frequently associated with the progression of tumors, poor patient
prognosis and malignant properties, including increased mobility,
and invasive and metastatic capacities (12). The expression and functional
regulation of the MYC gene involves a variety of mechanisms, one of
which is based on the MYC-binding protein (MYCBP) signaling pathway
(13). The MYCBP gene encodes a
protein of ~11 kDa which, through its C-terminal structure, can
bind the MYC N-terminal region, thereby activating MYC to promote
tumorigenesis (14). MYCBP has been
identified as a target of β-catenin/T cell factor (TCF)/lymphoid
enhancer-binding factor (LEF) transcriptional regulation in colon
carcinoma (15).
Recently, researchers have demonstrated that 30–50%
of GC cases are associated with the abnormal activation of the
Wnt/β-catenin signaling pathways (16,17). The
TCF/LEF axis serves a crucial role in the Wnt/β-catenin signaling
pathway (18). A previous study
observed that the transcription of a series of target genes within
the Wnt/β-catenin signaling pathway was activated following the
formation of a TCF/LEF/β-catenin complex in the nucleus, which
regulated cellular biological activities and promoted the migratory
and invasive abilities of tumor cells (19). Recent studies have also indicated that
MYCBP is associated with tumorigenesis in a variety of tumor types,
including colon cancer and glioma (20,21).
However, the potential role of MYCBP in promoting the growth of GC
has, to the best of our knowledge, not been reported. In the
present study, the role of MYCBP as a potential biomarker for the
diagnosis and prognosis of GC was evaluated.
Materials and methods
Cell culture
The human GC SGC-7901, MKN-45, AGS and BGC-823 cell
lines, and the human gastric mucosal epithelial GES-1 cell line
were provided by the Cell Bank of the Shanghai Institute of Cell
Biology (Shanghai, China). All cells were cultured in RPMI 1640
medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
containing 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) and antibiotics (100 U/ml streptomycin
and 100 U/ml penicillin; Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA), and maintained at 37°C under 5% CO2 and
100% humidity atmosphere. Cells were passaged at 80% confluency
using 0.02% EDTA/0.25% trypsin for 3–5 min.
Clinical samples
A total of 77 fresh specimens from patients via GC
resection (aged 42–78 years, median age of 64 years, 45 male and 32
female patients) were acquired from Zhejiang Provincial People's
Hospital (Hangzhou, China) between January and December 2015, and
stored at −80°C. Paired adjacent non-cancerous tissues were also
obtained from patients. None of the patients had received
radiotherapy or chemotherapy prior to surgery. The diagnosis of all
gastric cancer patients depended on the results of pathological
sections. All samples were verified by three pathologists at the
Department of Pathology, Zhejiang Provincial People's Hospital.
Tumor grade was determined according to various classifications of
tumors [Tumor-Node-Metastasis (22)].
Written informed consent was obtained from all patients prior to
participation and the study was approved by the Ethics Committee of
Zhejiang Provincial People's Hospital. All procedures performed
involving human participants were conducted in accordance with the
ethical standards of the institutional and/or national research
committee, and with The 1964 Helsinki Declaration and its later
amendments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cell lines and
fresh specimens using TRIzol reagent (Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
cDNA was prepared using a Superscript III cDNA Synthesis kit
(Takara Bio, Inc., Otsu, Japan) following the manufacturer's
protocol. qPCR was performed using FastStart Essential DNA Green
Master mix (Roche Diagnostics, Basel, Switzerland) with
miRNA-specific primers. GAPDH was used as the endogenous control.
The specific primers used were as follows: GAPDH forward,
5′-TGAAGGTCGGAGTCAACGG-3′ and reverse, 5′-CTGGAAGATGGTGATGGGATT-3′;
MYCBP were forward, 5′-TGGCACCTGTTGGAGACTATG-3′ and reverse,
5′-CACCAGCCATAGCCACATTC-3′. PCR thermocycling conditions were as
follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec,
58°C for 10 sec and 72°C for 10 sec. At the end of the PCR cycles,
melting curve analysis was performed. MYCBP expression levels in
tumor tissues and cell lines were compared with those in the
matched normal tissues and normal gastric cells, respectively, and
relative expression levels were calculated using the
2−ΔΔCq method (23).
MYCBP small interfering RNA (siRNA)
and LEF-1 siRNA transfection
MKN-45 cells exhibited a relatively high level of
MYCBP expression compared with the normal gastric cell line GES-1
and the other GC cell lines, and thus were used in the transfection
assays. The MKN-45 cells were cultured in 6-well plates (plated at
5.0×105 cells/well) for 24 h prior to transfection.
Subsequently, MYCBP siRNA (20 µmol/l; Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) and LEF-1 siRNA (20 µmol/l; Guangzhou RiboBio
Co., Ltd.) were individually transfected into the MKN-45 cells.
Negative control siRNA (Guangzhou RiboBio Co., Ltd.) was used to
establish a negative control group in parallel. The specific
sequences used were as follows: MYCBP siRNA forward,
5′-AAUCCAAAGCACUGUUAGGUU-3′ and reverse,
5′-CCUAACAGUGCUUUGGAUUUU-3′; LEF-1 siRNA forward,
5′-ACUUGAUGUCAGCUAAAUCGC-3′ and reverse,
5′-GAUUUAGCUGACAUCAAGUCU-3′; NC siRNA forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. The two negative controls have the
same siRNA sequence. Transfections were performed with
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol.
Transwell assay
At 24 h after transfection, GC cells were used in
migration and invasion assays. Transwell migration assays were
performed using a Costar Transwell assay kit (Corning Incorporated,
Corning, NY, USA), whereas invasion assays were performed using
invasion chambers (Corning Incorporated) pre-coated with Matrigel.
Cells (1.5×105 per well for invasion assays and
8.0×104 per well for migration assays) were seeded in
the upper chamber with FBS-free RPMI-1640 medium, and RPMI-1640
medium containing 30% FBS was added to the lower chamber. After 24
or 48 h of incubation at 37°C in an atmosphere of 5%
CO2, non-migrated or non-invaded cells were removed from
the upper surfaces of the transwell membranes with a cotton swab,
and the migrated or invaded cells on the lower membrane surfaces
were fixed by 95% ethyl alcohol for 10 min at room temperature and
stained with hematoxylin and eosin for 15 min at room temperature
prior to imaging and counting under high-power magnification (×200;
Olympus IX71 live cell imaging fluorescence microscope; Olympus,
Tokyo, Japan).
Cell cycle and apoptosis assay
At 24 h following transfection with MYCBP siRNA,
LEF-1 siRNA or control siRNA, MKN45 cells were washed with PBS and
fixed with 70% ethanol for >12 h at 4°C. Following
centrifugation (100 × g) at room temperature for 3 min, cells were
incubated with 500 µl propidium iodide (PI; Beyotime Institute of
Biotechnology, Haimen, China) staining solution for 30 min in the
dark. The cell cycle distribution was analyzed by flow cytometry
(FACSCalibur flow cytometer with CellQuest software (version. 5.1;
BD Biosciences, Franklin Lakes, NJ, USA). Additionally, an Annexin
V/PI Apoptosis Detection kit (Beyotime Institute of Biotechnology)
was used to assess cell apoptosis. Briefly, Annexin V and PI were
used to label early and late apoptotic cells, respectively and,
following staining for 15 min at room temperature, the cells were
analyzed with the FACSCalibur flow cytometer and CellQuest software
to detect apoptotic cells.
Western blot analysis
According to the results of the prediction of the
genes interaction on the Genecards, MYCBP may be a downstream gene
of LEF-1 (http://www.genecards.org/). This
prediction was investigated by western blotting. In brief, MKN-45
GC cells transfected with LEF-1, MYCBP or control siRNA (Guangzhou
RiboBio, Co., Ltd., Guangzhou, China) were harvested, washed and
lysed with Radioimmunoprecipitation Assay buffer (Beyotime
Institute of Biotechnology). Total protein concentration was
measured using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). Equivalent quantities (30–50 µg per lane)
of protein were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene fluoride microporous membranes (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 5% non-fat milk in
Tris-buffered saline for 2 h at room temperature, and then
incubated overnight at 4°C with primary antibodies against LEF-1
(1:1,000; cat. no. 14972-1-AP; ProteinTech Group, Inc., Chicago,
IL, USA), MYCBP (1:1,000; cat. no. 12022-1-AP; ProteinTech Group,
Inc.), MYC (1:1,000; cat. no. 10828-1-AP; ProteinTech Group, Inc.)
and GAPDH (1:1,000; cat. no. 10828-1-AP; ProteinTech Group, Inc.)
at the dilutions specified by the manufacturer. The membranes were
washed three times with TBS with Tween-20 (1:1,000) and incubated
with corresponding horseradish peroxidase (HRP)-conjugated
secondary antibodies (cat. no. HA1001; HuaBio, Hangzhou, China) at
1:1,000 dilution for 1 h. Bound secondary antibodies were detected
using an enhanced chemiluminescence system (Wuhan Sanying
Biotechnology, Wuhan, China).
Statistical analysis
Statistical analysis was performed using SPSS
version 22.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference. The MYCBP
levels determined by qPCR were expressed as the mean ± standard
deviation. The means of normally distributed results were compared
by either paired Student t-tests or one-way analysis of variance,
as appropriate.
Results
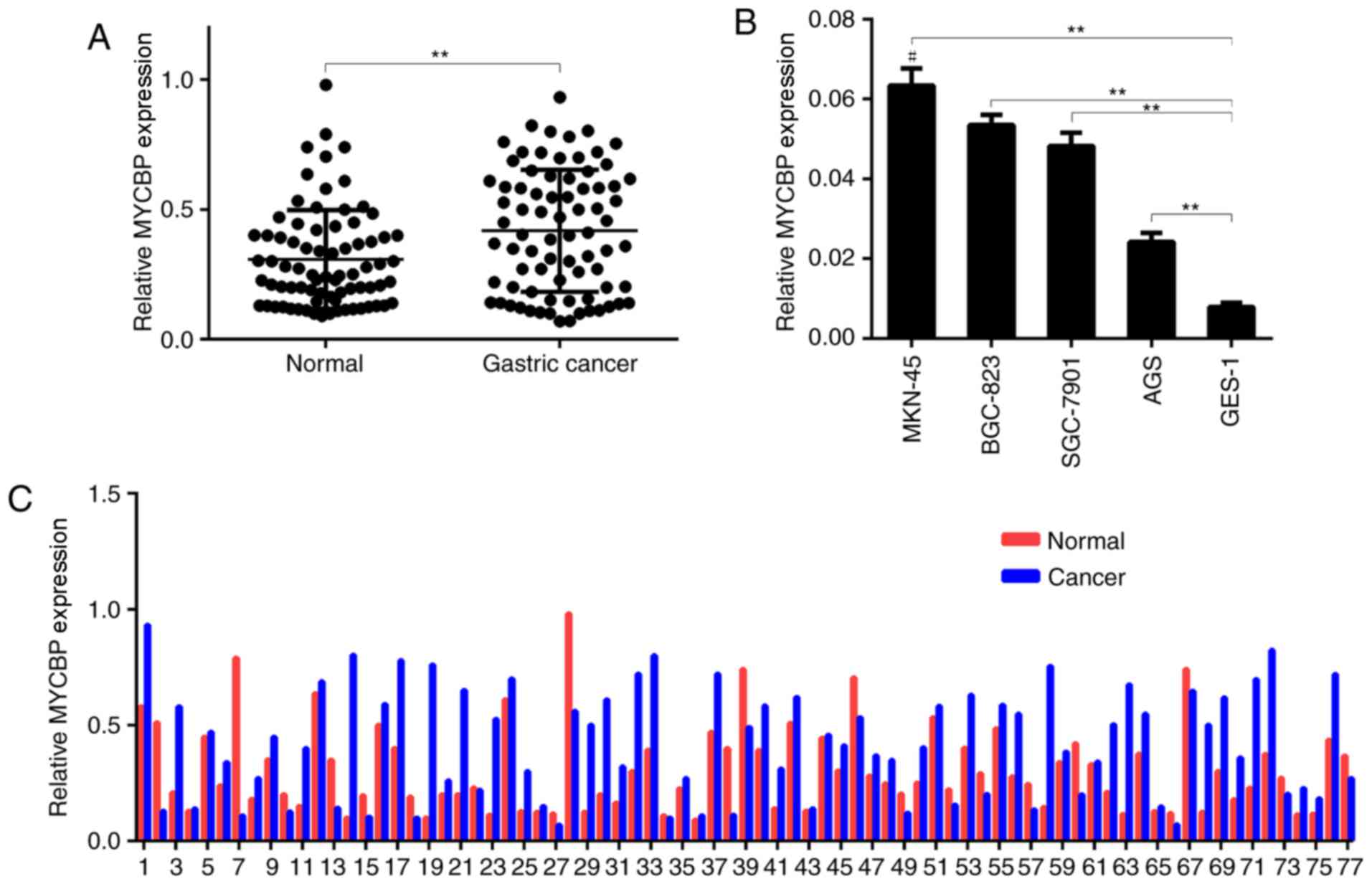
MYCBP expression in GC tissues
The expression of MYCBP in 77 GC and matched
adjacent normal gastric mucosal tissue samples was detected by
RT-qPCR. The expression of MYCBP in the GC tissues was
significantly higher than that in the adjacent normal gastric
mucosal tissues (0.4171±0.027 vs. 0.3076±0.022, P<0.01; Fig. 1A and 1C), and the relative MYCBP
overexpression rate was 71.4%. Furthermore, in GC cells in
vitro, the expression levels of MYCBP were significantly higher
in the four GC cell lines (SGC-7901, MKN-45, BGC-823 and AGS) than
in the GES-1 normal gastric mucosal cell line (Fig. 1B). As shown in Fig. 1C, the expression of MYCBP in each
paired samples is different. Among the GC cell lines, MKN-45
exhibited a relatively higher level of MYCBP expression.
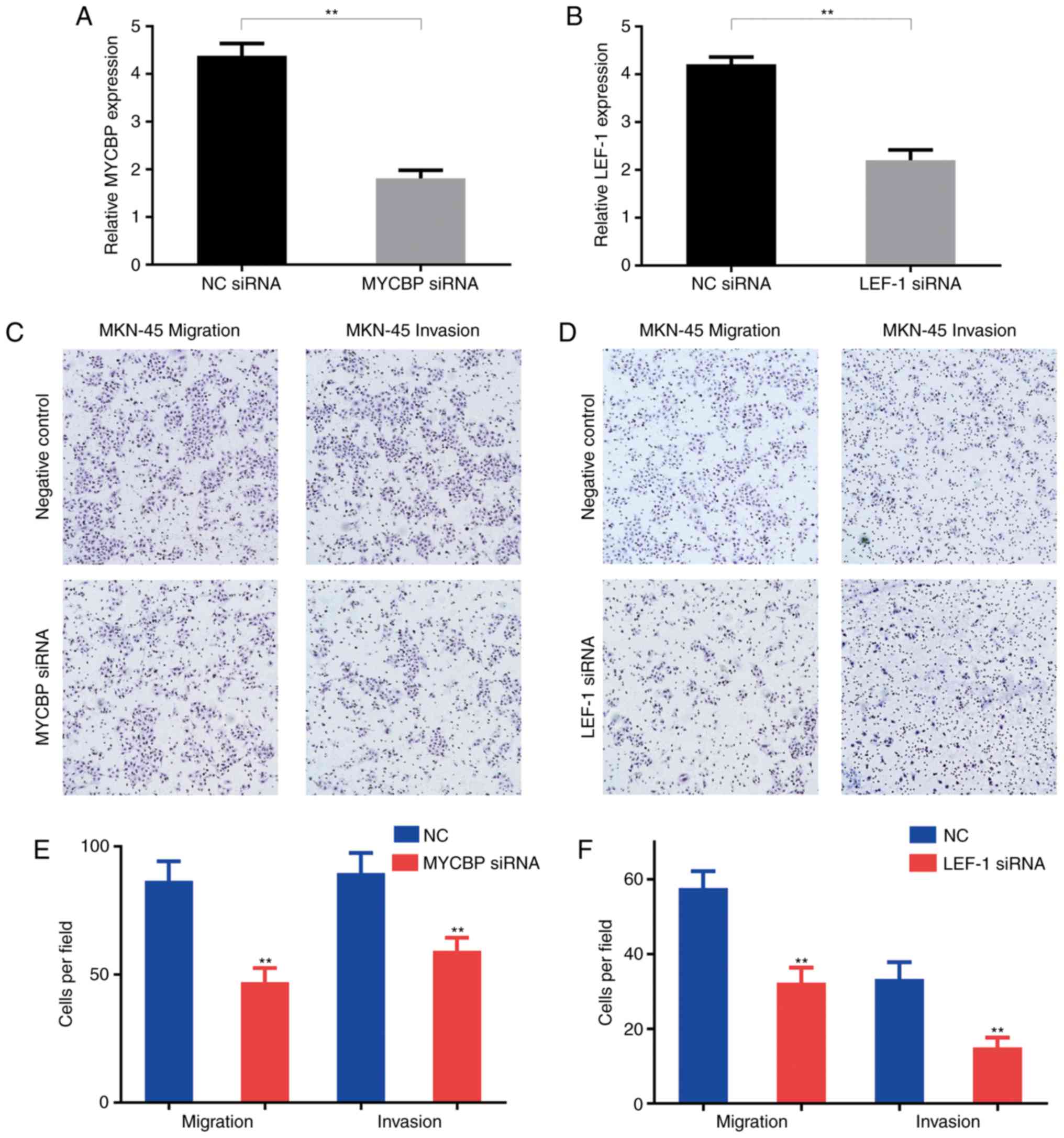
MYCBP and LEF-1 affect the invasion
and migration capacities of GC cells
To investigate the role of MYCBP in the development
and progression of GC, MYCBP or LEF-1 siRNA were transfected into
the GC cell line MKN-45, and the effects on cell migration and
invasion capacities were investigated using transwell assays
(Fig. 2). The expression of MYCBP and
LEF-1incells transfected with MYCBP siRNA and LEF-1 siRNA was
observed to be significantly inhibited (P<0.01; Fig. 2A and B). It was observed that the
numbers of MKN-45 cells invading and migrating across the transwell
membrane were significantly reduced by transfection with the MYCBP
siRNA or LEF-1 siRNA (P<0.01; Fig.
2C-F) when compared with those in the negative control group.
These data indicated that MYCBP and LEF-1 affected the invasion and
migration capacities of the GC cells.
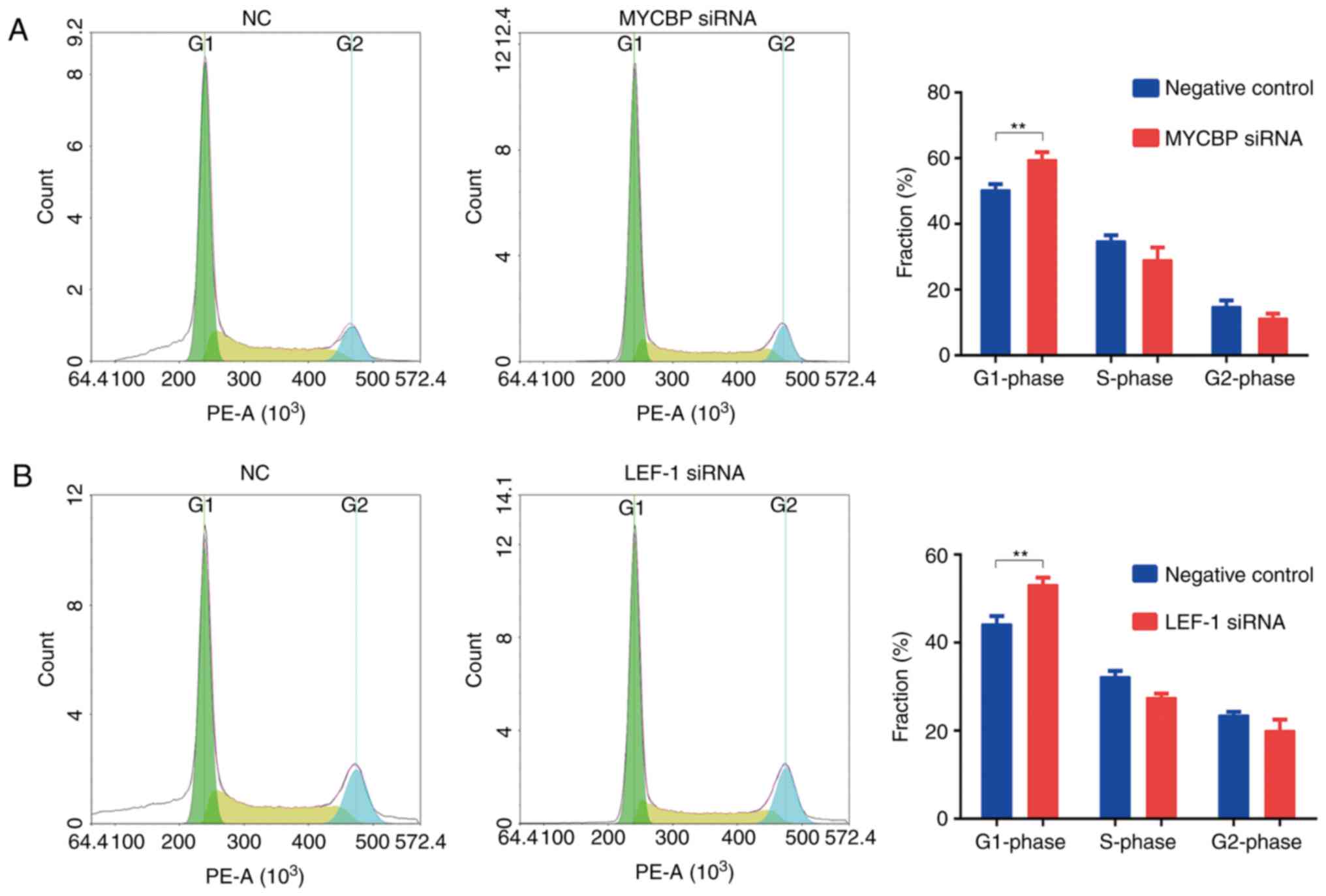
Effects of MYCBP and LEF-1 on GC cell
cycle and apoptosis
To investigate the role of MYCBP in GC further, the
cell cycle distribution and apoptosis of MKN-45 cells was assessed
following transfection with the MYCBP and LEF-1 siRNAs. According
to the cell cycle assay, MKN-45 cells transfected with MYCBP or
LEF-1 siRNA were markedly arrested in the G1 phase, with
percentages of G1-phase cells of 59.6±2.3 and 53.2±1.6%,
respectively, when compared with the control group (50.4±1.8 and
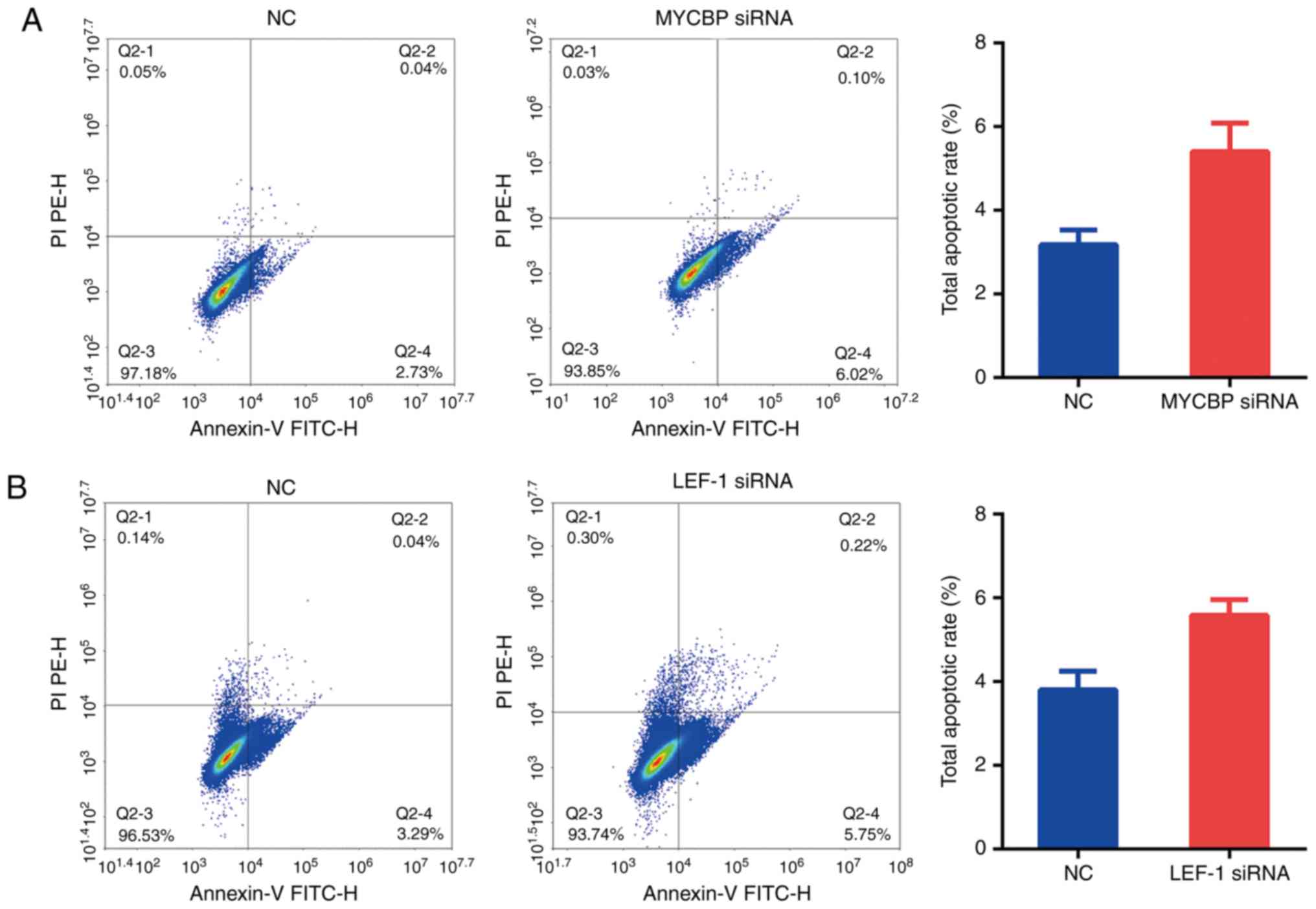
44.2±1.8%, respectively; Fig. 3).
However, the difference in the rate of cell apoptosis between
groups was not statistically significant (Fig. 4).
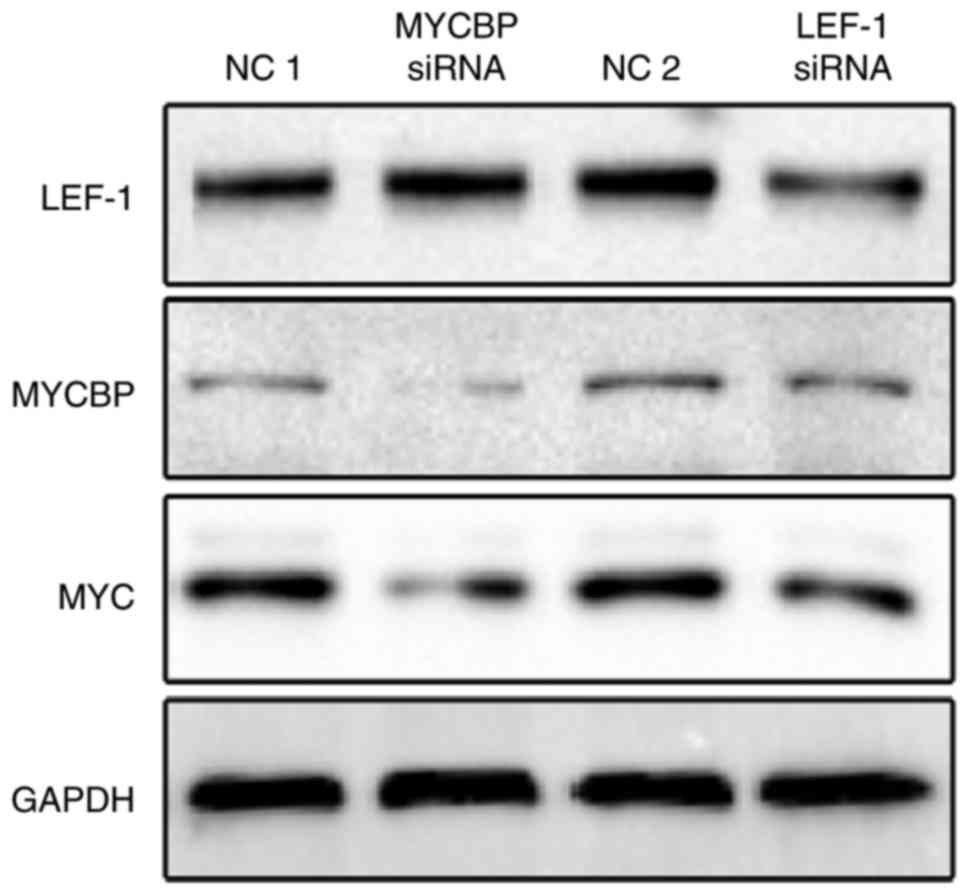
MYCBP may be a direct target of
LEF-1
MYCBP was predicted to be a downstream target of
LEF-1 (http://www.genecards.org/). To verify
the results, western blotting was used to detect the expression of
MYCBP following downregulation of LEF-1 expression. As shown in
Fig. 5, in the MKN-45 cells,
downregulation of LEF-1 with siRNA caused a marked reduction in
MYCBP expression at the protein level. These findings indicated
that MYCBP may be one of the direct targets of LEF-1.
Discussion
Recent studies indicated that MYCBP is abnormally
expressed during tumorigenesis in numerous types of cancer. Jung
and Kim (15) reported that the
upregulation of MYCBP in colon carcinoma cells may have
co-activating effects on MYC. Wang et al (20) reported that MYCBP regulated multiple
biological processes within glioma cells, which is associated with
malignant characteristics, including cancer cell proliferation,
transformation and metastasis. Additionally, Furusawa et al
(24) suggested that MYCBP is a
trigger in the tumorigenesis of chronic myeloid leukemia. However,
the potential role and molecular mechanisms of MYCBP in GC remains
unclear.
In the present study, MYCBP was significantly
overexpressed in GC tissues compared with adjacent normal gastric
mucosal tissues, which indicated that MYCBP might serve an
oncogenic role in human GC. Additionally, the function of MYCBP in
the GC cell line MKN-45 was investigated, and it was demonstrated
that MYCBP downregulation suppressed the migratory and invasive
capacities of the MKN-45 cells in vitro, thus indicating
that MYCBP might serve a key role in the metastasis of GC.
MYCBP is an 11 kDa protein that binds to the
N-terminal region of MYC and stimulates the activation of
E-box-dependent transcription by MYC (25). Overexpression of MYC has been
suggested to serve key roles in cell migration and invasion by
promoting the expression of target genes (26). Previous studies have been performed to
inhibit the expression of MYCBP, and thus the MYC pathway, and
observe the resultant inhibition of cancer cell migration and
invasion (20,27). Furthermore, the overexpression of
MYCBP facilitated Hedgehog signaling responses, whereas the
knockdown of MYCBP compromised Hedgehog signaling responses
(28). It has been suggested that
abnormal Hedgehog signaling activity serves important roles in the
development and progression of cancer, by promoting cell
proliferation, mobility and invasiveness (29). Accordingly, blocking Hedgehog
signaling has been demonstrated to inhibit the invasion and
metastasis of cancer cells (30). As
listed in GeneCards®: The Human Gene Database
(http://www.genecards.org/), MYCBP is a
member and positive regulator of the Notch signaling pathway.
Previous studies concerning several cancer types demonstrated that
blocking the Notch signaling pathway led to decreased expression
and diminished bioactivity of urokinase-type plasminogen activator,
which contributed to inhibited cancer cell migration, invasion and
apoptosis (31,32). A previous study confirmed that MYCBP
acted downstream of the vascular endothelial growth factor
receptor, and also indicated that when binding of MYCBP to
cortactin was inhibited, tumor invasion, metastasis and
angiogenesis were blocked (33).
Notably, the present results illustrated that
transfection with MYCBP siRNA markedly induced G1 phase
arrest in GC cells when compared with the control group. Recent
research indicated that MYC also served a role in the cell cycle
transition from G1 to S phase (34). The regulatory mechanisms underlying
the cell cycle are complex, and any single pathway does not
represent the full function of MYC. Hermeking et al
(35) identified cyclin-dependent
kinase 4 (CDK4) as a target of MYC, which functionally inactivates
the products of the tumor suppressor genes RB and p16, providing a
link between MYC and the CDK4/cyclin D1/retinoblastoma protein/p16
pathway, and may account for the lack of genetic alterations to
retinoblastoma protein and p16 in certain types of cancer.
To investigate the possible function of MYCBP and to
reveal its underlying molecular mechanism, the identification of
regulatory targets is required. In MKN-45 cells in the present
study, the downregulation of LEF-1 expression caused a reduction in
the MYCBP protein level. This indicated that MYCBP may be one of
the targets of LEF-1. LEF-1 has previously been reported to be
expressed excessively and to function as an oncogene in numerous
types of cancer (36). Furthermore,
the TCF/LEF axis serves a crucial role in the Wnt/β-catenin
signaling pathway, and the Wnt signaling pathway has been shown to
be involved in a number of cellular functions, including cell
proliferation, survival, and differentiation (37,38).
Western blotting was used to detect the expression of MYCBP protein
following the exogenous regulation of LEF-1 expression. On the
basis of these results, MYCBP may be a target of LEF-1, which
itself is an important factor of the Wnt signaling pathway.
In summary, the present study demonstrated that
MYCBP is upregulated in GC tissues and cell lines, and is
associated with the metastatic capacity of GC cells, potentially
via interaction with LEF-1. These findings indicate that MYCBP may
function as a novel tumor promoter in GC, and may be a potential
biomarker for the diagnosis of GC. To the best of the authors'
knowledge, the present research is the first study to demonstrate
that MYCBP is a downstream gene of LEF-1 of the Wnt signaling
pathway in GC.
Acknowledgements
The present study was funded by the Medicine and
Health Research Foundation of Zhejiang Province (grant no.
2017KY018) and the Zhejiang Provincial Natural Science Foundation
of China (grant no. LY18H160043).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Casaretto L, Sousa P and Mari J:
Chemotherapy versus support cancer treatment in advanced gastric
cancer: A meta-analysis. Braz J Med Biol Res. 39:431–440. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Zhan Z, Wu J, Zhang C, Yang Y,
Tong S, Sun Z, Qin L, Yang X and Dong W: Association among
polymorphisms in EGFR gene exons, lifestyle and risk of gastric
cancer with gender differences in Chinese Han subjects. Plos One.
8:e592542013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie Y, Zhi X, Su H, Wang K, Yan Z, He N,
Zhang J, Chen D and Cui D: A novel electrochemical microfluidic
chip combined with multiple biomarkers for early diagnosis of
gastric cancer. Nanoscale Re Lett. 10:4772015. View Article : Google Scholar
|
|
5
|
Buiatti E, Palli D, Decarli A, Amadori D,
Avellini C, Bianchi S, Bonaguri C, Cipriani F, Cocco P, Giacosa A,
et al: A case-control study of gastric cancer and diet in Italy:
II. Association with nutrients. Int J Cancer. 48:369–374. 2010.
View Article : Google Scholar
|
|
6
|
Shi Y and Zhou Y: The role of surgery in
the treatment of gastric cancer. J Surg Oncol. 101:687–692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dang CV: The interplay between MYC and HIF
in the Warburg effect. Ernst Schering Found Symp Proc. pp. 1–53.
2007;
|
|
8
|
Rabbitts PH, Watson JV, Lamond A, Forster
A, Stinson MA, Evan G, Fischer W, Atherton E, Sheppard R and
Rabbitts TH: Metabolism of c-myc gene products: c-myc mRNA and
protein expression in the cell cycle. Embo J. 4:2009–2015.
1985.PubMed/NCBI
|
|
9
|
Pelengaris S and Khan M: The many faces of
c-MYC. Arch Biochem Biophys. 416:129–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okuyama H, Endo H, Akashika T, Kato K and
Inoue M: Downregulation of c-MYC protein levels contributes to
cancer cell survival under dual deficiency of oxygen and glucose.
Cancer Res. 70:10213–10223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mai S and Mushinski JF: c-MYC-induced
genomic instability. J Environ Pathol Toxicol Oncol. 22:179–199.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu D, Wu J, Tang X, Hu F, Yang Y, Du J, Ye
S and Zhang R: Molecular cloning and tissue distribution of a
Schistosoma japonicum gene encoding AMY-1. Mol Med Rep.
4:1267–1271. 2011.PubMed/NCBI
|
|
14
|
Taira T, Maëda J, Onishi T, Kitaura H,
Yoshida S, Kato H, Ikeda M, Tamai K, Iguchi-Ariga SM and Ariga H:
AMY-1, a novel C-MYC binding protein that stimulates transcription
activity of C-MYC. Genes Cells. 3:549–565. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung HC and Kim K: Identification of MYCBP
as a beta-catenin/LEF-1 target using DNA microarray analysis. Life
Sci. 77:1249–1262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiurillo MA: Role of the Wnt/β-catenin
pathway in gastric cancer: An in-depth literature review. World J
Exp Med. 5:84–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu C, Zhuang Y, Jiang S, Liu S, Zhou J, Wu
J, Teng Y, Xia B, Wang R and Zou X: Interaction between
Wnt/β-catenin pathway and microRNAs regulates
epithelial-mesenchymal transition in gastric cancer (Review). Int J
Oncol. 48:2236–2246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sawa M, Masuda M and Yamada T: Targeting
the Wnt signaling pathway in colorectal cancer. Expert Opin Ther
Targets. 20:419–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Yan X, Ji LY, Ji XT, Wang P, Guo
SW and Li SZ: miR-139 functions as an antioncomir to repress glioma
progression through targeting IGF-1 R, AMY-1, and PGC-1β. Technol
Cancer Res Treat. 16:497–511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Byun E, Park B, Lim JW and Kim H:
Activation of NF-κB and AP-1 mediates hyperproliferation by
inducing β-catenin and c-Myc in Helicobacter pylori-infected
gastric epithelial cells. Yonsei Med J. 57:647–651. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Washington K: 7th Edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Furusawa M, Onishi T, Taira T,
Iguchi-Ariga SM and Ariga H: AMY-1 is a trigger for the erythrocyte
differentiation of K562 cells. Int J Oncol. 16:339–345.
2000.PubMed/NCBI
|
|
25
|
Sakamuro D and Prendergast GC: New
Myc-interacting proteins: A second Myc network emerges. Oncogene.
18:2942–2954. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dhru HD, McDonough Winslow WS, Armstrong
B, Tuncali S, Eschbacher J, Kislin K, Loftus JC, Tran NL and Berens
ME: Reciprocal activation of transcription factors underlies the
dichotomy between proliferation and invasion of glioma cells. Plos
One. 8:e721342013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang X, Hu C, Arnovitz S, Bugno J, Yu M,
Zuo Z, Chen P, Huang H, Ulrich B, Gurbuxani S, et al: miR-22 has a
potent anti-tumour role with therapeutic potential in acute myeloid
leukaemia. Nat Commun. 7:114522016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin C, Yao E, Wang K, Nozawa Y, Shimizu H,
Johnson JR, Chen JN, Krogan NJ and Chuang PT: Regulation of Sufu
activity by p66β and Mycbp provides new insight into vertebrate
Hedgehog signaling. Genes Dev. 28:2547–2563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liao X, Siu MK, Au CW, Wong ES, Chan HY,
Ip PP, Ngan HY and Cheung AN: Aberrant activation of hedgehog
signaling pathway in ovarian cancers: Effect on prognosis, cell
invasion and differentiation. Carcinogenesis. 30:131–140. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feldmann G, Dhara S, Fendrich V, Bedja D,
Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C,
Jimeno A, et al: Blockade of hedgehog signaling inhibits pancreatic
cancer invasion and metastases: A new paradigm for combination
therapy in solid cancers. Cancer Res. 67:2187–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimizu M, Cohen B, Goldvasser P, Berman
H, Virtanen C and Reedijk M: Plasminogen activator uPA is a direct
transcriptional target of the JAG1-Notch receptor signaling pathway
in breast cancer. Cancer Res. 71:277–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raghu H, Gondi CS, Dinh DH, Gujrati M and
Rao JS: Specific knockdown of uPA/uPAR attenuates invasion in
glioblastoma cells and xenografts by inhibition of cleavage and
trafficking of Notch-1 receptor. Mol Cancer. 10:1302011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashimoto A, Hashimoto S, Ando R, Noda K,
Ogawa E, Kotani H, Hirose M, Menju T, Morishige M, Manabe T, et al:
GEP100-Arf6-AMAP1-cortactin pathway frequently used in cancer
invasion is activated by VEGFR2 to promote angiogenesis. PLoS One.
6:e233592011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakamuro D and Prendergast GC: New
Myc-interacting proteins: A second Myc network emerges. Oncogene.
18:2942–2954. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hermeking H, Rago C, Schuhmacher M, Li Q,
Barrett JF, Obaya AJ, O'Connell BC, Mateyak MK, Tam W, Kohlhuber F,
et al: Identification of CDK4 as a target of c-MYC. Proc Natl Acad
Sci USA. 97:pp. 2229–2234. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Delaunay S, Rapino F, Tharun L, Zhou Z,
Heukamp L, Termathe M, Shostak K, Klevernic I, Florin A, Desmecht
H, et al: Elp3 links tRNA modification to IRES-dependent
translation of LEF1 to sustain metastasis in breast cancer. J Exp
Med. 213:2503–2523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schambony A and Wedlich D: Wnt-5A/Ror2
regulate expression of XPAPC through an alternative noncanonical
signaling pathway. Dev Cell. 12:779–792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Willert K and Jones KA: Wnt signaling: Is
the party in the nucleus? Genes Dev. 20:1394–1404. 2006. View Article : Google Scholar : PubMed/NCBI
|