Introduction
Phosphaturic mesenchymal tumor (PMT) is a
polymorphous group of extremely rare mesenchymal tumors that often
result in hypophosphatemic osteomalacia/rickets, caused by the
overproduction of the phosphaturic hormone fibroblast growth factor
23 (FGF23) (1–7). PMTs occur more frequently in middle-aged
adults, although the age of the patients can range from 3 to 73
years, and are originated predominantly from soft tissue or bone
involving a single site (8,9). The vast majority of PMTs are benign,
small, subcutaneous and undetectable on physical examination
(10–12). The female to male ratio in patients
with PMT is 1:1.2. The most common subtype of PMT is the mixed
connective tissue, which accounts for >70% of cases (13).
Historically, it has been documented that many kinds
of mesenchymal neoplasms have been classically associated with
tumor-induced osteomalacia (TIO) (14,15). TIO,
also known as oncogenic osteomalacia, is a rare paraneoplastic
syndrome in which vitamin D-resistant osteomalacia occurs due to
the presence of a tumor (16).
Therefore, in addition to distinguishing the four different
histological subtypes of PMTs previously described (17), it is more important to discriminate
this neoplasm from other vascular tumors preoperatively.
Furthermore, these tumors frequently occur in variable odd
locations making discovery very difficult. As a consequence, these
tumors remain a largely misdiagnosed, ignored or unknown entity by
most radiologists and clinicians.
Owing to the rarity of PMT, our knowledge of imaging
features of this entity has come mostly from the context of case
reports or very small case series in the endocrinology, osteology,
nuclear medicine, and pathology literature. There have been >300
cases of PMTs described in the literature so far (16). While many of these studies have
focused on the pathologic characterization and whole-body imaging
modalities of choice in the investigation of PMTs, to the best of
our knowledge, there have been no prior reports on the computed
tomography (CT) and magnetic resonance imaging (MRI) features of
PMTs in the radiology literature. Based on clinical and laboratory
examination, musculoskeletal radiologists can originally make the
diagnosis of PMTs given the typical CT and MRI findings, but
unfortunately the diagnosis has appeared to be often delayed in the
radiology community.
To overcome this diagnostic problem, the current
study discusses three cases of PMT-MCT in the soft tissue using
routine MRI examination. All three patients presented with
nonspecific clinical presentation, including diffuse pain, myalgia,
muscle spasms, muscle weakness and fatigue. In the current case
report, the CT and MRI findings of the three patient cases, for
diagnosing the soft-tissue tumor, are described. Furthermore, a
comprehensive review of the clinicopathological features of PMTs
that have been previously reported in the medical literature is
discussed.
Case report
Case 1
A 59-year-old male presented with a five-year
history of fatigue and generalized muscle pain (Table I). He was admitted to the First
Affiliated Hospital of Fujian Medical University (Fuzhou, China) in
August 2016. The long-term muscle pain became increasingly worse
with temporary relief from physiotherapy and analgesics. Routine
blood test was performed at the time of admission with the results
of hypophosphatemia, normal calcium and parathyroid hormone (PTH)
levels (Table II). Slightly elevated
cystatin-C and an elevation of alkaline phosphatase (ALP) were
noted (Table II). The preoperative
bone metabolism markers were presented in Table III. A clinical diagnosis of
hypophosphatemic osteomalacia of unknown etiology was considered
and treatment with oral phosphorus and high-dose vitamin D was
initiated. A whole-body Tc bone scintigraphy scan was performed as
a first step and no lesions were identified. Additional whole-body
18F-fludeoxyglucose (FDG) positron emission tomography
(PET)/CT scan was ordered to detect the causative tumor. A
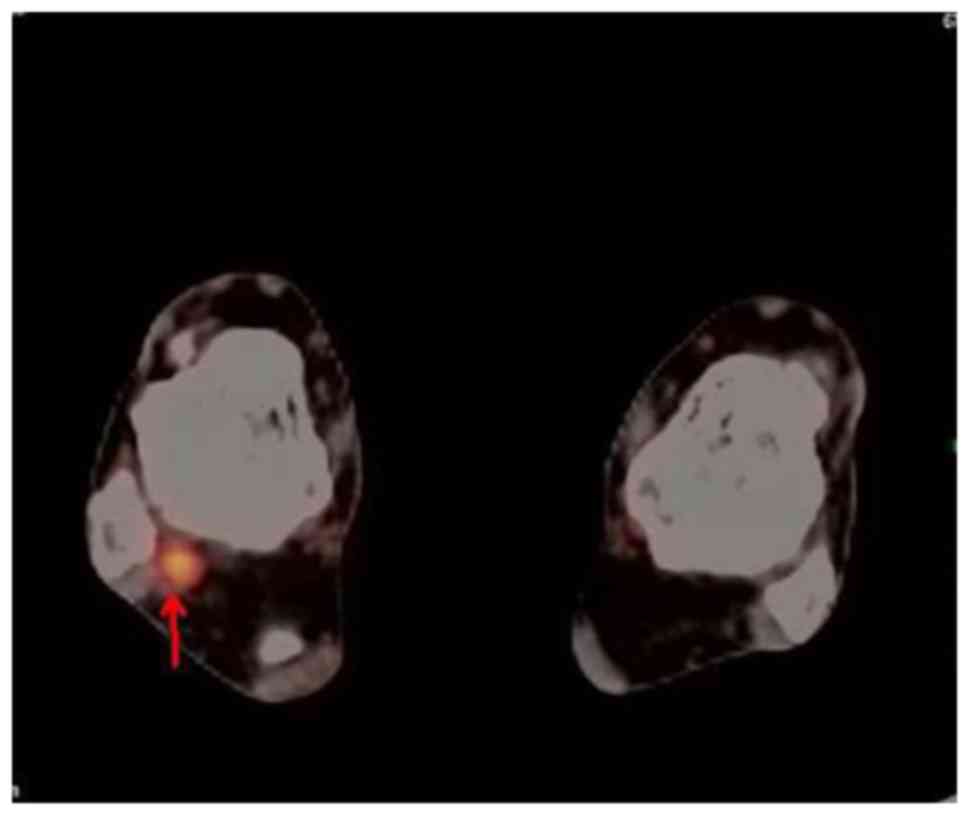
promising focus of intense FDG uptake was detected in the right
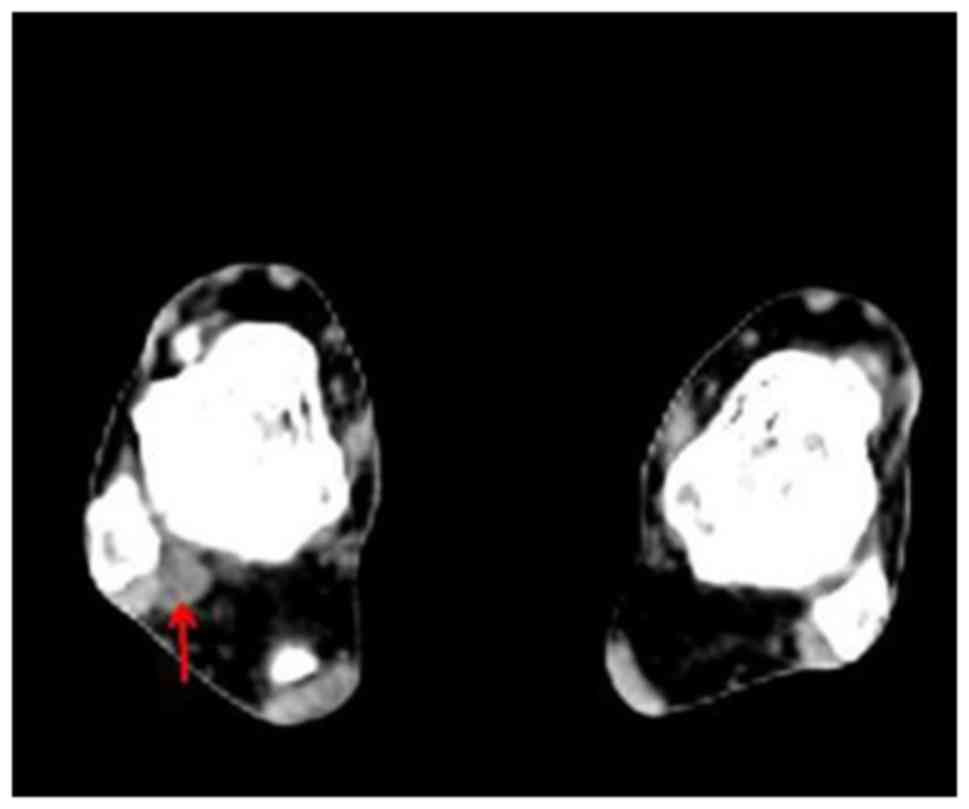
ankle (Fig. 1). The CT scan revealed
a small round homogeneous soft-tissue mass, which was located
between the distal end of the tibia and fibula and thought to be a
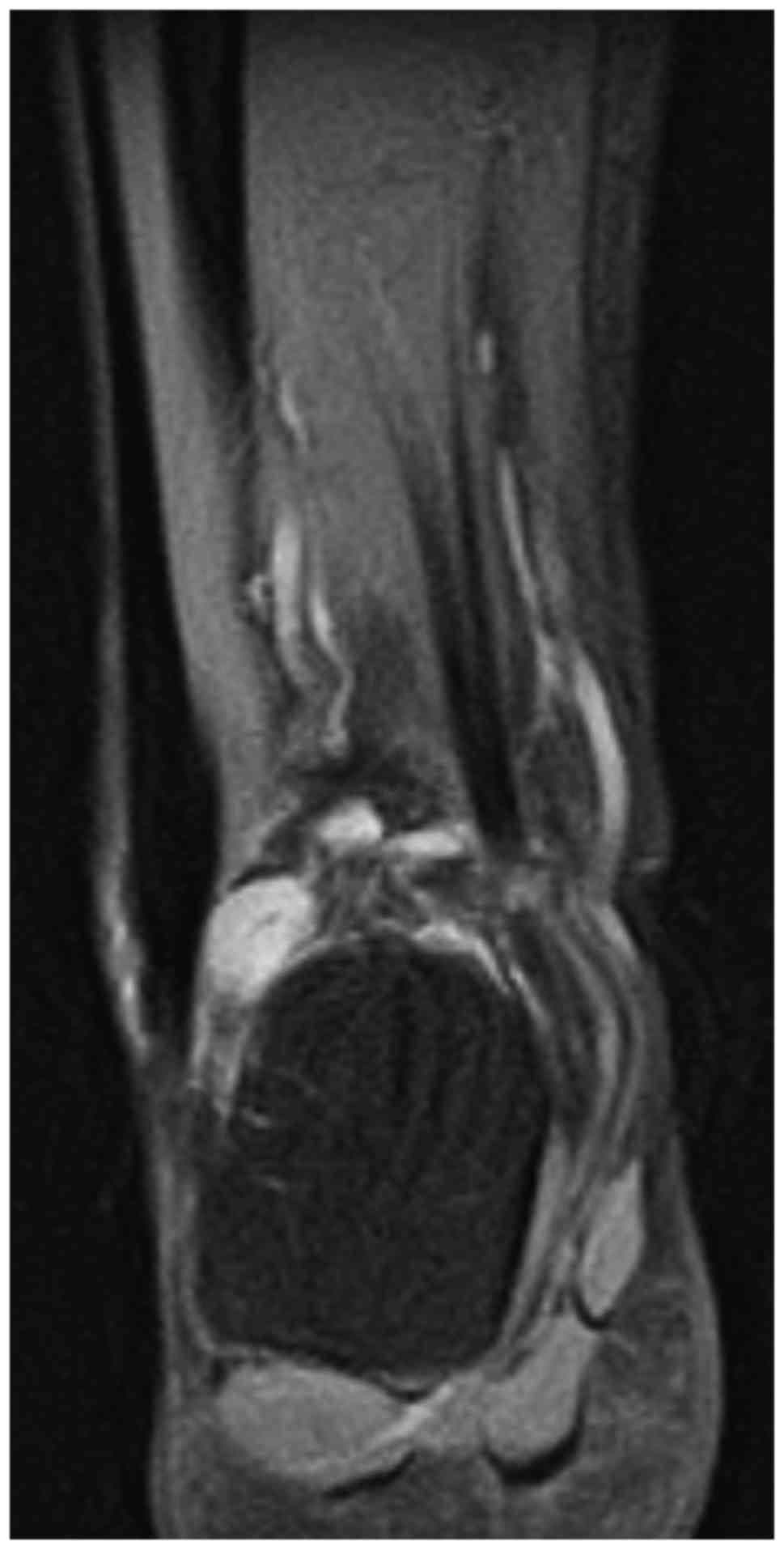
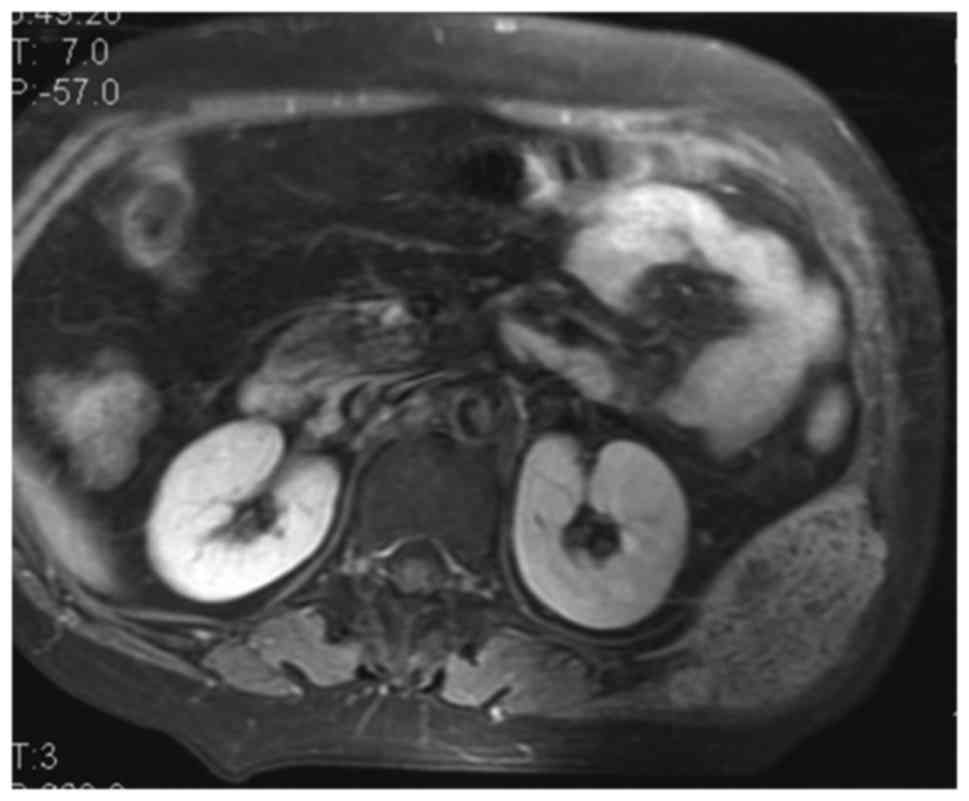
PMT (Fig. 2). Subsequent MRI revealed
a small subcutaneous tumor (1.2×1.1×0.9 cm), which appeared
isointense to the muscles on T1-weighted imaging, slightly
hyperintense on T2-weighted imaging, markedly hyperintense on short
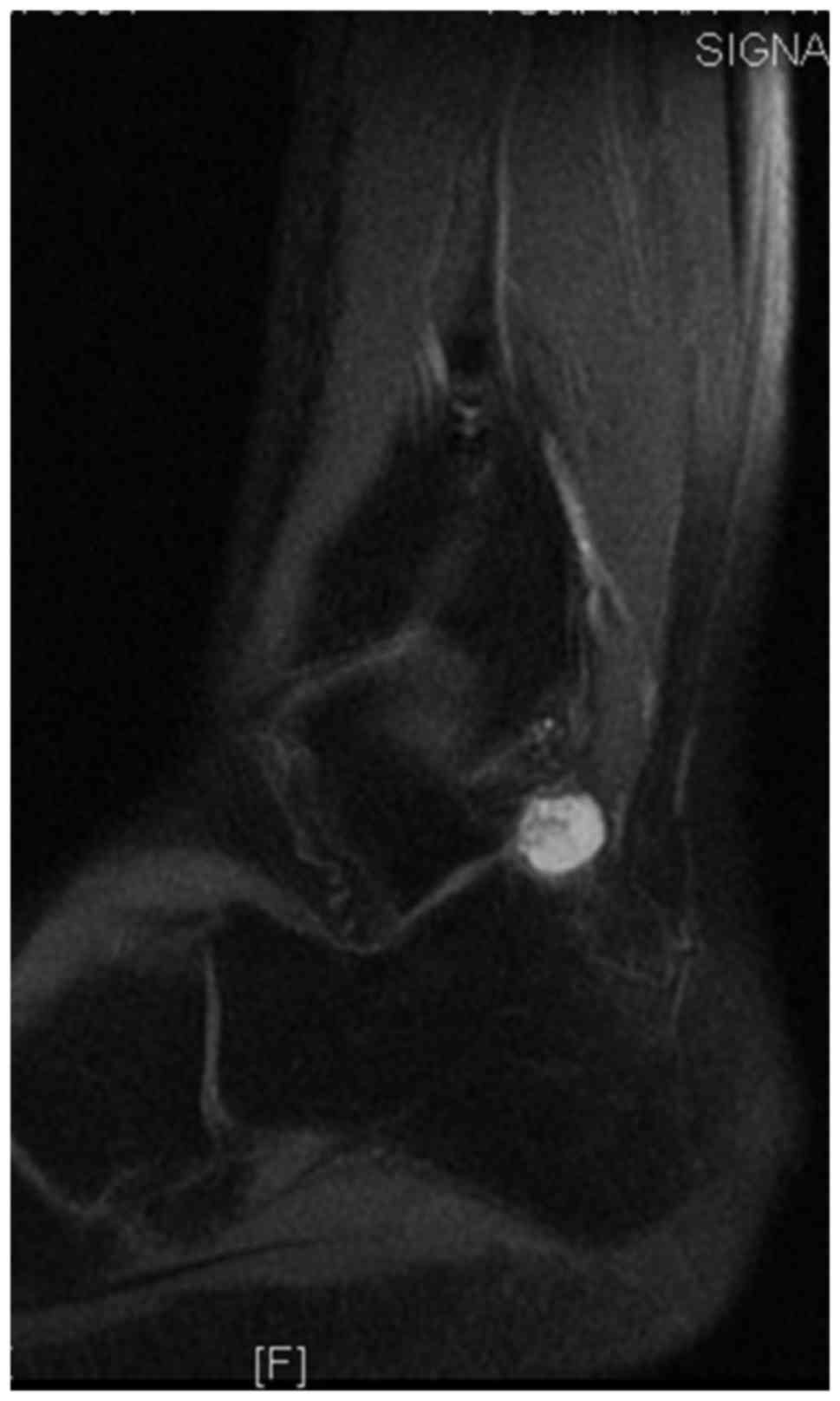
tau inversion recovery (STIR) coronal imaging (Fig. 3), and displayed homogeneous
enhancement on postcontrast T1-weighted fat-suppression imaging
(Fig. 4). The tumor was radically
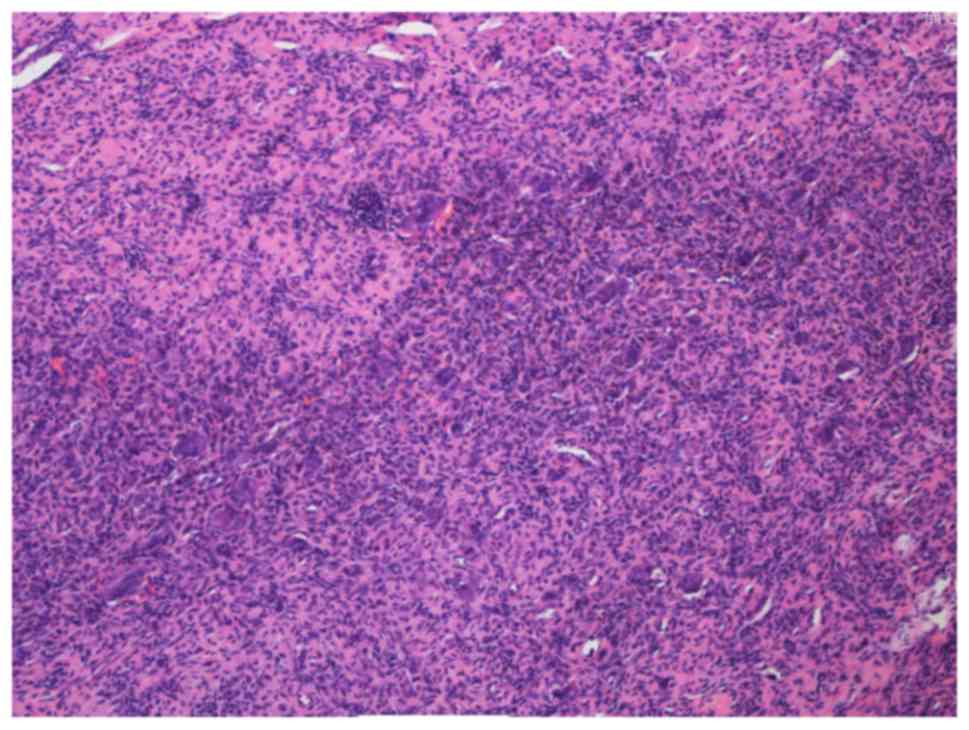
resected and diagnosed as PMT-MCT pathologically (Fig. 5; Table
IV). Table V lists the levels of
postoperative bone mentalism markers and ALP at 8 months following
surgery. Muscle pain was relieved quickly after the operation, and
serum phosphorus levels gradually recovered to normal.
 | Table I.Patient demographics and phosphaturic
mesenchymal tumor diagnostic characteristics. |
Table I.
Patient demographics and phosphaturic
mesenchymal tumor diagnostic characteristics.
| Patient | Age at time of
diagnosis (years) | Sex | Tumor location
(soft tissue) | Size (cm) | Presenting
symptom | Time to diagnosis
(years) | Pathologic
fracture |
|---|
| Case 1 | 59 | Male | Right ankle
joint | 1.2×1.1×0.9 | Fatigue,
generalized muscle pain | 5 | No |
| Case 2 | 52 | Female | Left posterior
chest | 14.0×9.0×8.0 | Left posterior
chest pain | 10 | No |
| Case 3 | 59 | Female | Left posterior
chest | 1.5×1.5×0.5 | Left chest noticed
muscle spasms with pain | 1 | No |
 | Table II.Preoperative laboratory values. |
Table II.
Preoperative laboratory values.
| Patient | Serum phosphate
(mmol/l) | Calcium
(mmol/l) | PTH (pmol/l) | CRP (mg/l) | ALP (U/l) | ESR (mm/h) | Cystatin-C
(mg/l) | GFR (ml/min) | Urine Bence-Jones
protein | Treatment | Postoperative serum
phosphate (mmol/l) |
|---|
| Case 1 | 0.46 | 2.20 | 4.36 | 1.87 | 294 | 17 | 1.12 | 125.96 | Negative | Surgery | 1.28 |
| Case 2 | 0.38 | 2.31 | 5.32 | 2.65 | 334 | 3 | 0.56 | 175.70 | Negative | Surgery | 1.1 |
| Case 3 | 0.52 | 2.17 | 5.83 | 3.41 | 282 | 22 | n/a | 81.42 | Negative | Surgery | 0.82 |
| Normal range | 0.81–1.55 | 2.03–2.54 | 1.6–6.9 | 0–8 | 45–125 | 0–15 | 0.40–1.10 | >80 |
|
| 0.81–1.55 |
 | Table III.Preoperative bone mentalism
markers. |
Table III.
Preoperative bone mentalism
markers.
| Patient | Total 25-hydroxy
vitamin D (ng/ml) | Osteocalcin
(ng/ml) | Beta-CrossLaps
(ng/ml) | TPINP (ng/ml) |
|---|
| Case 1 | 35.09 | 33.09 | 0.95 | 103.60 |
| Case 2 | 5.6 | 19.77 | 0.599 | 39.61 |
| Normal range | 20–70 (<5 marked
decrease; 5–10 modest decrease; 10–20 slight decrease; >200
intoxication) | 14–46 | 0–0.704 | 16.89–65.491
(pre-menopause, 5.13–58.59; post-menopause with HRT treatment,
14.28–58.92; post-menopause without HRT treatment,
20.25–76.31) |
 | Table IV.Pathological features and imaging
modalities. |
Table IV.
Pathological features and imaging
modalities.
|
|
|
Immunohistochemical findings |
|
|---|
|
|
|
|
|
|---|
| Patient | Histopathological
diagnosis | BCL2 | Vimentin | CD68 | CD56 | SMA | CK | P63 | EMA | KI67 | S-100 | Imaging |
|---|
| Case 1 | PMT-MCT | + | + | + | + | − | − | − | − | + 5% | − | Tc bone
scintigraphy+PET/CT+MRI |
| Case 2 | PMT-MCT |
| + | + | + | − | − |
| − | + 5% | − | Tc bone
scintigraphy+CT+MRI |
| Case 3 | PMT-MCT | + | + | + | + | + | − | + |
| 2% |
| Tc bone
scintigraphy+CT+MRI |
 | Table V.Postoperative (8 months) bone
mentalism marker and ALP levels. |
Table V.
Postoperative (8 months) bone
mentalism marker and ALP levels.
| Patient | Total 25-hydroxy
vitamin D (ng/ml) | Osteocalcin
(ng/ml) | beta-CrossLaps
(ng/ml) | TPINP (ng/ml) | ALP (U/l) |
|---|
| Case 1 | 28.27 | 35.12 | 0.65 | 38.27 | 199 |
| Case 2 | 9.25 | 17.54 | 0.43 | 39.61 | 223 |
| Case 3 | − | − | − | − | 207 |
| Normal range | 20–70 (<5 marked
decrease; 5–10 modest decrease; 10–20 slight decrease; >200
intoxication) | 14–46 | 0–0.70 | 16.89–65.491
(pre-menopause, 5.13–58.59; post-menopause with HRT treatment,
14.28–58.92; post-menopause without HRT treatment,
20.25–76.31) | 45–125 |
Case 2
A 52-year-old female presented with left posterior
chest pain for the duration of ten years. She was admitted to the
First Affiliated Hospital of Fujian Medical University (Fuzhou,
China) in July 2008. Further physical examination revealed a large
left chest wall tumor involving the 9–11th ribs. The serum bone
metabolism markers were presented in Table III. A whole-body Tc bone
scintigraphy scan was used to identify a potential tumor of
osteomalacia. An area of abnormal absence of uptake in the 10th rib
and slight increased uptake in the 9th and 11th rib were observed.
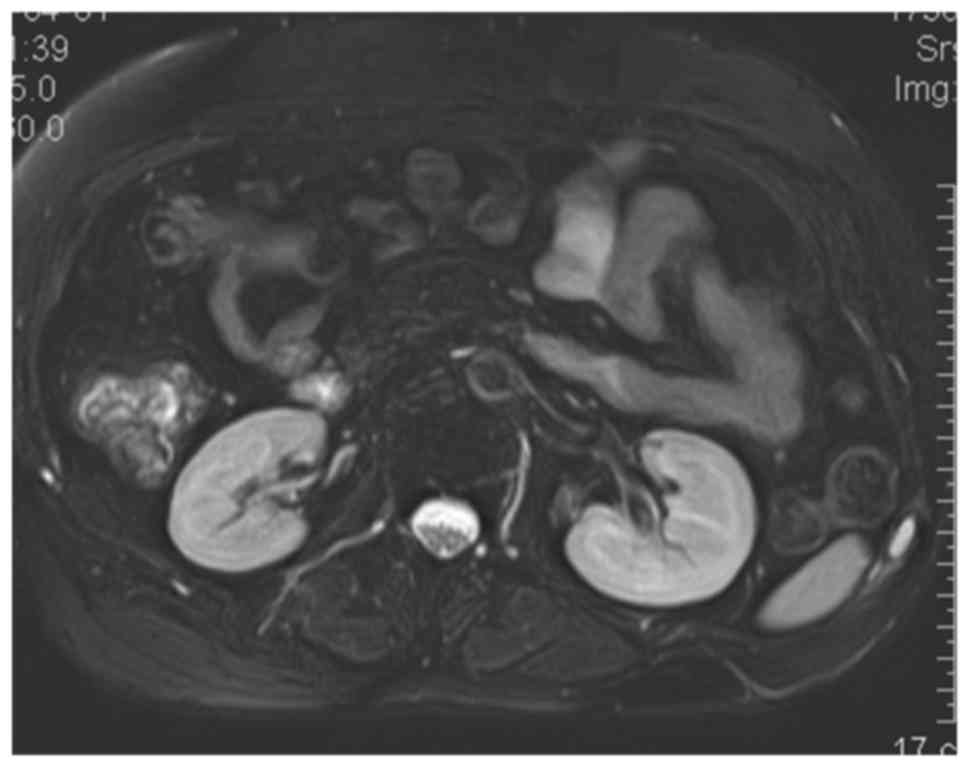
The patient was subsequently referred for plain CT and
contrast-enhanced MRI scans for evaluation. The axial CT of the
chest revealed a large poorly circumscribed mass (14×9×8 cm). The
lesion appeared heterogeneously hypointense on T1WI, hyperintense
on fat-saturated T2WI containing prominent vascular flow voids, and
marked heterogeneous enhancement on fat-saturated T1-weighted
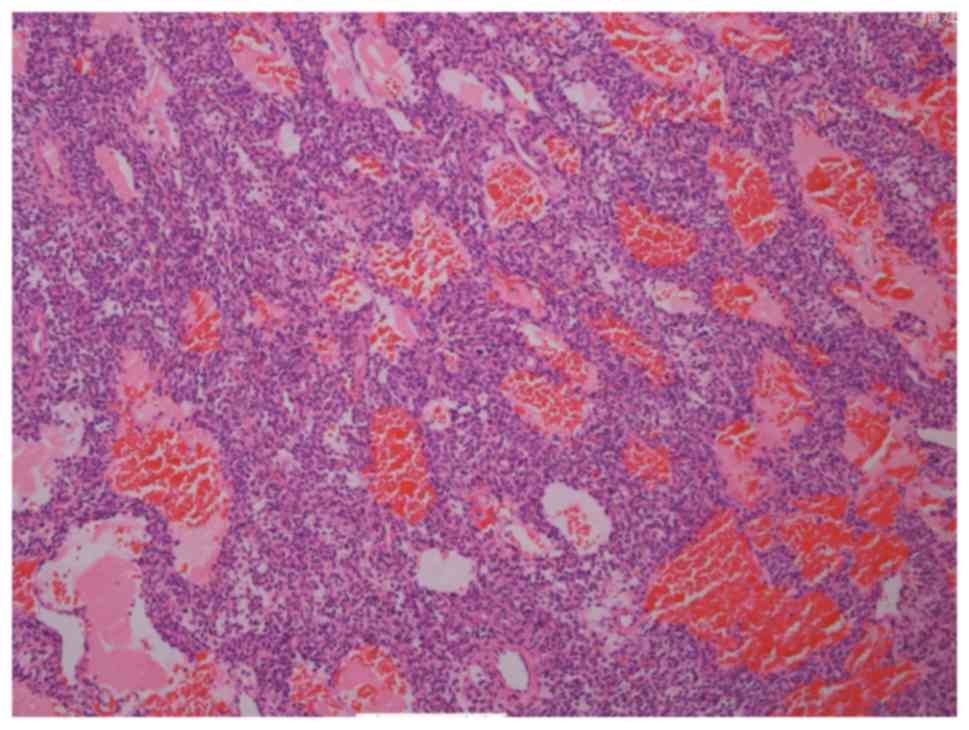
sequences (Fig. 6). Surgical
resection of the lesion was conducted, and the pathologic findings
confirmed a PMT-MCT with no evidence of malignancy (Fig. 7; Table
IV). Table V lists the levels of
postoperative bone mentalism markers and ALP at 8 months following
surgery. The patient recovered well during the postoperative
period, and the chest pain diminished over time. Furthermore,
preoperative serum phosphate levels were marked low at 0.38 mmol/l
(normal range, 0.81–1.55 mmol/l) and the levels normalized to 1.1
mmol/l at 2 months after the surgery (Table II).
Case 3
A 59-year-old female presented with left chest
muscle spasms with pain which worsened progressively for the
duration of one year. She was admitted to the First Affiliated
Hospital of Fujian Medical University (Fuzhou, China) in April
2016. On admission, no significant abnormality was observed on
physical examination. No muscle atrophy was found initially.
Laboratory data disclosed hypophosphatemia with a serum phosphorus
level of 0.68 mmol/l (normal range, 0.81–1.55 mmol/l), with normal
serum PTH and calcium levels (Table
II). Urinary excretion of phosphorus over 24 h increased to
8.91 mmol/l (normal range, 0.96–1.62 mmol/l).
Radiographs of her bones revealed diffused
demineralization and DEXA bone densitometry scan revealed marked
decrease of bone density in both lumbar spine (0.751 g/cm2;
T-score, −3.6) and left femoral neck (0.439 g/cm2;
T-score, −4.5). A whole-body Tc bone scintigraphy scan was
performed and it demonstrated a focal area of increased uptake in
the 10th rib. The patient began taking phosphorus and high-dose
vitamin D orally. One month later, her condition did not improve
considerably. Extensive radiology evaluation including CT and MR
scan was performed. Axial CT scan revealed a small soft-tissue mass
(1.5×1.5×0.5 cm) in the lower posterior chest wall. The neoplasm
appeared homogeneously isointense on T1WI, hyperintense on
fat-saturated T2WI (Fig. 8), and
marked homogeneous enhancement on fat-saturated T1-weighted
sequences. Subsequent surgical resection was conducted, and the
neoplasm was removed and the surgical specimen was pathologically
diagnosed to be a PMT-MCT (Fig. 9;
Table IV). Four days after the
operation serum phosphorus levels normalized to 0.82 mmol/l, and
one month after the operation muscle pain was completely relieved.
Table V lists the levels of
postoperative ALP at 8 months after surgery.
Discussion
Clinicopathological features and tumor
detection
The clinical symptoms of PMTs are nonspecific and
typically include, but not limited to, diffused pain, muscle
weakness, pathologic bone fracture, motor weakness, skeletal
deformities, height loss and generalized debilitated state
secondary to osteomalacia (18–24). In
addition, patients often present with a firm, slow-growing,
unpalatable soft-tissue mass when the tumors arise within the
extremities (25–27). Approximately 53% of reported cases
occur in the bone, ~45% in soft tissue, and ~2% in the skin
(28–32). The typical biochemical parameters are
hypophosphatemia resulting from renal phosphate wasting, an
inappropriately low serum 1,25-dihydroxyvitamin D3,
normal 25-hydroxyvitamin D3, normal or slightly low
serum calcium and elevated ALP levels (8,25,33–35). The
clinical and biochemical characteristics of the patients presented
in the current report were similar to those observed in previously
published literature, in which the tumor was situated elsewhere in
the body. In case 1, a slightly elevated serum Cystatin-C was also
observed, associated with renal tubule damage.
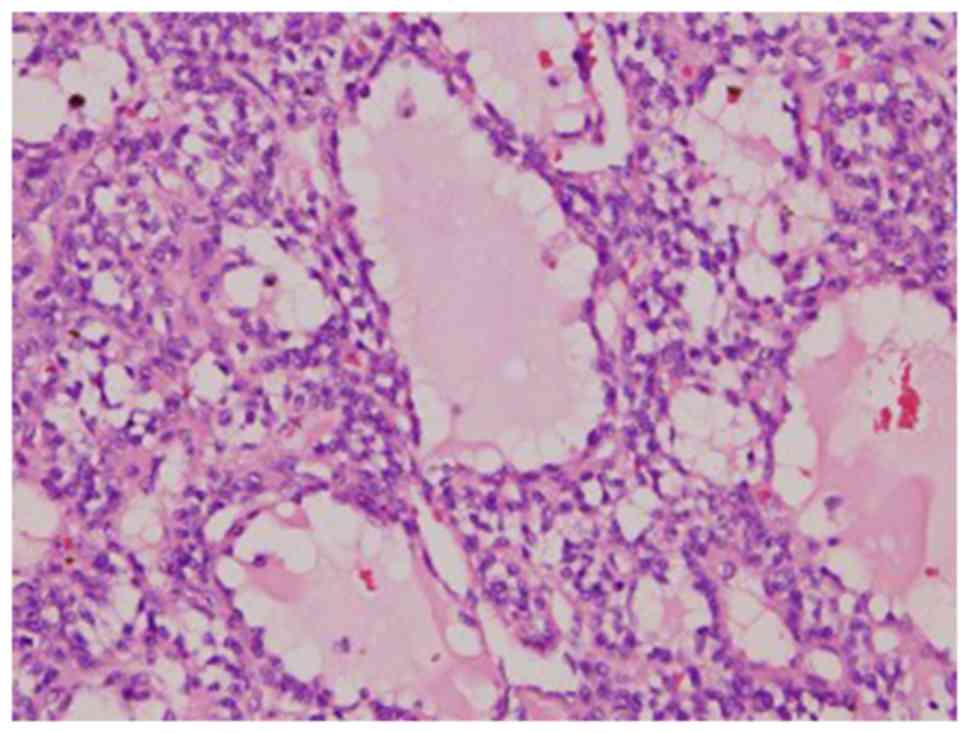
Pathologically, four morphologic patterns of PMTs
have been described and widely accepted: primitive-appearing mixed
connective tissue, osteoblastoma-like, nonossifying fibroma-like,
and ossifying fibroma-like (10,11,17,36,37).
It is now believed that the latter three variants may reflect
different bone-specific reaction patterns considered within the
spectrum of PMT-MCTs (38–40). The classic microscopic
characterization of a PMT-MCT is that of a variably prominent
vascular proliferation of plump oval, spindled to stellate cells
with generally low nuclear grade, and very low or usually absent
mitotic activity. The spindled cells are typically embedded
distinctively within a ‘grungy’, calcified, myxoid to chondromyxoid
matrix and often include other findings, including osteoclast-like
giant cells, mature fat cells, chondroid or osteoid-like matrix,
poorly developed cartilage or bone, areas of hemorrhage and
microcysts. Histopathology analysis reveals that pancytokeratin,
desmin, S-100 and CD34 are not expressed in the tumor cells
(13,27,31,41,42),
similar to what was observed in the present cases.
In order to identify the causative tumor of TIO,
multiple noninvasive imaging techniques, such as ultrasonography,
whole body CT and MRI, 18F-FDG PET/CT, 201Tl
scintigraphy, 99mTc-MIBI SPECT, 99mTc bone
scintigraphy, 111In-pentetreotide or octreotide
scintigraphy, 99mTc-HYNIC-TOC scan, and
68Ga-DOTATATE PET/CT have been pursued (23,43–54).
Recently, 68Ga-DOTANOC is firstly deemed as a
sufficiently sensitive and specific in detecting an occult tumor
(43,46). In China, however, since
somatostatin-based functional scans are not approved,
18F-FDG PET/CT and 99mTc bone scintigraphy
are usually used when diagnosing mesenchymal tumors (55).
CT and MR features and differential
diagnosis
The majority of the published literature on imaging
studies of PMTs is focused on the choice of optical imaging
modality in localizing the tumor, not on the imaging features of
PMTs. On CT scans, the tumor exhibits a round/oval, well-bordered,
isodense/hypodense soft tissue mass and displays a uniform
enhancement when the tumor is small (45,56),
similar to what was observed in the present cases. The typical MR
appearances of PMT-MCT are isointense relative to the muscles on
T1-weighted imaging and markedly hyperintense on T2-weighted
imaging, with markedly homogenous enhancement on post contrast
T1-weighted fat-suppression imaging. However, the variable tumor
sizes result in different MRI imaging features. PMT-MCT with small
tumor size displays homogenous signal intensity on both T2WI and
T1WI and uniform enhancement on post-contrast T1WI. In contrast, a
large tumor displays heterogeneous signal intensity on T2WI and
T1WI and heterogeneous enhancement on post-contrast T1WI. The areas
of heterogeneous low signals are consistent with vascular flow
voids within large tumors.
Case 2 had a large chest mass and the tumor
displayed an oval, poorly-bordered, isodense mass on CT scan,
heterogeneously decreased signal intensity on T1WI, increased
signal intensity on T2WI containing multiple abnormal tortuous
vascular flow voids with a vivid enhancement, validated by
pathology that the tumor contained prominently vascular components
with abundant giant cells. The lesions were clearly depicted as
high signal intensity on both STIR and diffusion weighted imaging
(DWI), however, the findings were considered nonspecific. Since DWI
may have resulted from T2 shine-through effect and has poor spatial
resolution, high solution STIR sequence should be the optimal
sequence in localizing the tumor (8,57–59).
In the present cases, the neoplasms occurred in soft
tissue and followed a benign clinical course. The differential
possibilities for PMT-MCTs include any soft tissue mass, such as
neurofibroma, hemangioendothelioma, fibroma, neurofibrosarcoma,
hemangioma, leiomyoma, giant cell tumor, giant cell reparative
granuloma, tenosynovitis, ganglion cyst, histiocytoma, desmoid
tumor and neuroma. On the basis of histologic and pathologic
findings, radiological features and the characteristic clinical
signs, the neoplasms were concluded to be PMT-MCTs, which show
similar morphology to those soft-tissue tumors previously
reported.
To the best of our knowledge, this is the first
report of radiological and histological description of a large
PMT-MCT (Case 2), which arises from chest wall and causes oncogenic
osteomalacia. Thus, it appears to be essential that this entity
should be considered among the chest wall tumors.
Treatment and prognosis
The PMT-MCT frequently has an infiltrating growth
pattern, as reported in the present cases, and this may explain the
high rates of local recurrence (44,60–64).
Early, complete surgical resection remains the definitive treatment
of PMT-MCTs. It has been previously reported that ~90% of patients
with PMT-MCTs are cured by excision (20,34,65–67).
All three of the current patients were treated with surgery, which
led to the normalization of serum phosphate levels and resolution
of symptoms. A key point of surgery is the total removal of the
tumor. Wide negative margins are essential for preventing local
recurrence of PMT-MCTs, even though bone resection may be required.
In addition, intralesional treatment is insufficient to control the
tumors occurring in bone and radical resection should be planned.
However, local recurrence and metastasis after surgical resection
have been described. Although malignant transformation of PMT-MCTs
is extremely rare (12), the patient
experienced multiple recurrence and developed histologic features
that were highly suggestive of high-grade status, hypercellularity,
and increased mitotic activity transformation of primary benign
tumors.
Medical therapy with phosphorus and calcitriol is
often given after incomplete resection of the tumor, resulting in
anatomic restrictions or medical comorbidities. Although
somatostatin receptors have been identified on PMT-MCTs, octreotide
therapy, which was based on decreasing FGF23 secretion, has not
been effective. Periodic measurement of serum phosphate and FGF23
levels has been advocated as a means to detect recurrences
(3,60,68).
The prognosis for most patients with PMT-MCTs is
good; however, a delay in the diagnosis and surgical treatment can
increase the overall mortality rates associated with this tumor
(69,70).
In conclusion, the present report suggested that
once patients have been diagnosed with oncogenic osteomalacia, a
whole-body screening scan, such as 68Ga-DOTANOC or
18F-FDG PET/CT, should be performed to search for the
underlying causative tumor, especially when this occurs in an odd
location. After identifying the tumor with functional imaging,
anatomic imaging analysis, such as CT or MRI, should be used to
investigate the radiological features of the tumor and define the
exact location.
Acknowledgements
This study was supported by the Science and
Technology Department of Fujian Province (grant no. 2016Y0039), the
Health and Planning Committee of Fujian Province (grant no.
2016013), the Natural Science Foundation of Fujian province (grant
no. 2015J01450) and the Fujian Provincial Department of Finance
(grant no. BPB-lym2014). The authors would like to thank Dr.
Kamisha Ramen (Fujian Medical University, Fuzhou, Fujian, China)
for her contribution to the language editing of the present
manuscript.
References
|
1
|
Shiba E, Matsuyama A, Shibuya R, Yabuki K,
Harada H, Nakamoto M, Kasai T and Hisaoka M: Immunohistochemical
and molecular detection of the expression of FGF23 in phosphaturic
mesenchymal tumors including the non-phosphaturic variant. Diagn
Pathol. 11:262016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakamura K, Ohishi M, Matsunobu T,
Nakashima Y, Sakamoto A, Maekawa A, Oda Y and Iwamoto Y:
Tumor-induced osteomalacia caused by a massive phosphaturic
mesenchymal tumor of the acetabulum: A case report. Mod Rheumatol.
4:1–5. 2016.(Epub ahead of print). View Article : Google Scholar
|
|
3
|
Hautmann AH, Schroeder J, Wild P, Hautmann
MG, Huber E, Hoffstetter P, Fleck M and Girlich C: Tumor-induced
osteomalacia: Increased level of FGF-23 in a patient with a
phosphaturic mesenchymal tumor at the tibia expressing periostin.
Case Rep Endocrino. 2014:7293872014.
|
|
4
|
Norden AG, Laing RJ, Rowe P, Unwin RJ,
Wrong O and Crisp AJ: Oncogenic osteomalacia, raised FGF-23 and
renal Fanconi syndrome. QJM. 107:139–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larsson T, Zahradnik R, Lavigne J,
Ljunggren O, Jüppner H and Jonsson KB: Immunohistochemical
detection of FGF-23 protein in tumors that cause oncogenic
osteomalacia. Eur J Endocrinol. 148:269–276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dupond JL, Magy N, Mahammedi M, Prie D,
Gil H, Meaux-Ruault N and Kantelip B: Oncogenic osteomalacia: The
role of the phosphatonins. Diagnostic usefulness of the Fibroblast
growth factor 23 measurement in one patient. Rev Med Interne.
26:238–241. 2005.(In French).
|
|
7
|
Nelson AE, Bligh RC, Mirams M, Gill A, Au
A, Clarkson A, Jüppner H, Ruff S, Stalley P, Scolyer RA, et al:
Clinical case seminar: Fibroblast growth factor 23: A new clinical
marker for oncogenic osteomalacia. J Clin Endocrinol Metab.
88:4088–4094. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qari H, Hamao-Sakamoto A, Fuselier C,
Cheng YS, Kessler H and Wright J: Phosphaturic mesenchymal tumor: 2
New oral cases and review of 53 cases in the head and neck. Head
Neck Pathol. 10:192–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okamiya T, Takahashi K, Kamada H, Hirato
J, Motoi T, Fukumoto S and Chikamatsu K: Oncogenic osteomalacia
caused by an occult paranasal sinus tumor. Auris Nasus Larynx.
42:167–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ledford CK, Zelenski NA, Cardona DM,
Brigman BE and Eward WC: The phosphaturic mesenchymal tumor: Why is
definitive diagnosis and curative surgery often delayed? Clin
Orthop Relat Res. 471:3618–3625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiam P, Tan HC, Bee YM and Chandran M:
Oncogenic osteomalacia-hypophosphataemic spectrum from ‘benignancy’
to ‘malignancy’. Bone. 53:182–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morimoto T, Takenaka S, Hashimoto N, Araki
N, Myoui A and Yoshikawa H: Malignant phosphaturic mesenchymal
tumor of the pelvis: A report of two cases. Oncol Lett. 8:67–71.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
William J, Laskin W, Nayar R and De Frias
D: Diagnosis of phosphaturic mesenchymal tumor (mixed connective
tissue type) by cytopathology. Diagn Cytopathol. 40 Suppl
2:E109–E113. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guglielmi G, Bisceglia M, Scillitani A and
Folpe AL: Oncogenic osteomalacia due to phosphaturic mesenchymal
tumor of the craniofacial sinuses. Clin Cases Miner Bone Metab.
8:45–49. 2011.PubMed/NCBI
|
|
15
|
Chong WH, Molinolo AA, Chen CC and Collins
MT: Tumor-induced osteomalacia. Endocr Relat Cancer. 18:R53–R77.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaul M, Silverberg M, Dicarlo EF,
Schneider R, Bass AR and Erkan D: Tumor-induced osteomalacia. Clin
Rheumatol. 26:1575–1579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weidner N and Santa Cruz D: Phosphaturic
mesenchymal tumors. A polymorphous group causing osteomalacia or
rickets. Cancer. 59:1442–1454. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gatti AP, Tonello L, Neto JA, Teixeira UF,
Goldoni MB, Fontes PR, Sampaio JA, Lima LM and Waechter FL:
Oncogenic hypophosphatemic osteomalacia: From the first signal of
disease to the first signal of healthy. Int J Surg Case Rep.
30:130–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mok Y, Lee JC, Lum JH and Petersson F:
From epistaxis to bone pain-report of two cases illustrating the
clinicopathological spectrum of phosphaturic mesenchymal tumour
with fibroblast growth factor receptor 1 immunohistochemical and
cytogenetic analyses. Histopathology. 68:925–930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manara M and Sinigaglia L: ‘Slow and
steady wins the race’: The importance of perseverance in the
management of oncogenic osteomalacia. Endocrine. 57:1–2. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leow MK, Hamijoyo L, Liew H, Thirugnanam
U, Cheng MH, Loke KS, Teo MS, Chuah KL and Chng HH: Oncogenic
osteomalacia presenting as a crippling illness in a young man.
Lancet. 384:12362014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaniuka-Jakubowska S, Biernat W and
Sworczak K: Oncogenic osteomalacia and its symptoms:
Hypophosphatemia, bone pain and pathological fractures. Postepy Hig
Med Dosw (online). 66:554–567. 2012.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang CV, Conde SJ, Luvizotto RA, Nunes
VS, Bonates MC, Felicio AC, Lindsey SC, Moraes FH, Tagliarini JV,
Mazeto GM, et al: Oncogenic osteomalacia: Loss of hypophosphatemia
might be the key to avoid misdiagnosis. Arq Bras Endocrinol
Metabol. 56:570–573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogose A, Hotta T, Emura I, Hatano H, Inoue
Y, Umezu H and Endo N: Recurrent malignant variant of phosphaturic
mesenchymal tumor with oncogenic osteomalacia. Skeletal Radiol.
30:99–103. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pallavi R, Ravella PM, Gupta P and Popescu
A: A case of phosphaturic mesenchymal tumor. Am J Ther. 22:e57–e61.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fatani HA, Sunbuli M, Lai SY and Bell D:
Phosphaturic mesenchymal tumor: A report of 6 patients treated at a
single institution and comparison with reported series. Ann Diagn
Pathol. 17:319–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suryawanshi P, Agarwal M, Dhake R, Desai
S, Rekhi B, Reddy KB and Jambhekar NA: Phosphaturic mesenchymal
tumor with chondromyxoid fibroma-like feature: An unusual
morphological appearance. Skeletal Radiol. 40:1481–1485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akhter M, Sugrue PA, Bains R and Khavkin
YA: Oncogenic osteomalacia of the cervical spine: A rare case of
curative resection and reconstruction. J Neurosurg Spine.
14:453–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sidell D, Lai C, Bhuta S, Barnes L and
Chhetri DK: Malignant phosphaturic mesenchymal tumor of the larynx.
Laryngoscope. 121:1860–1863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hendry DS and Wissman R: Case 165:
Oncogenic osteomalacia. Radiology. 258:320–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shelekhova KV, Kazakov DV, Hes O, Treska V
and Michal M: Phosphaturic mesenchymal tumor (mixed connective
tissue variant): A case report with spectral analysis. Virchows
Arch. 448:232–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Folpe AL, Fanburg-Smith JC, Billings SD,
Bisceglia M, Bertoni F, Cho JY, Econs MJ, Inwards CY, Jan de Beur
SM, Mentzel T, et al: Most Osteomalacia-associated mesenchymal
tumors are a single histopathologic entity: An analysis of 32 cases
and a comprehensive review of the literature. Am J Surg Pathol.
28:1–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lopresti M, Daolio PA, Rancati JM, Ligabue
N, Andreolli A and Panella L: Rehabilitation of a patient receiving
a large-resection hip prosthesis because of a phosphaturic
mesenchyal tumor. Clin Pract. 5:8142015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Angeles-Angeles A, Reza-Albarrán A,
Chable-Montero F, Cordova-Ramón JC, Albores-Saavedra J and
Martinez-Benitez B: Phosphaturic mesenchymal tumors. Survey of 8
cases from a single mexican medical institution. Ann Diagn Pathol.
19:375–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Honda R, Kawabata Y, Ito S and Kikuchi F:
Phosphaturic mesenchymal tumor, mixed connective tissue type,
non-phosphaturic variant: Report of a case and review of 32 cases
from the Japanese published work. J Dermatol. 41:845–849. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deep NL, Cain RB, McCullough AE, Hoxworth
JM and Lal D: Sinonasal phosphaturic mesenchymal tumor: Case report
and systematic review. Allergy Rhinol (Providence). 5:162–167.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Papierska L, Ćwikła JB, Misiorowski W,
Rabijewski M, Sikora K and Wanyura H: Unusual case of phosphaturic
mesenchymal tumor. Pol Arch Med Wewn. 123:255–256. 2013.PubMed/NCBI
|
|
38
|
Gardner KH, Shon W, Folpe AL, Wieland CN,
Tebben PJ and Baum CL: Tumor-induced osteomalacia resulting from
primary cutaneous phosphaturic mesenchymal tumor: A case and review
of the medical literature. J Cutan Pathol. 40:780–784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Graham RP, Hodge JC, Folpe AL, Oliveira
AM, Meyer KJ, Jenkins RB, Sim FH and Sukov WR: A cytogenetic
analysis of 2 cases of phosphaturic mesenchymal tumor of mixed
connective tissue type. Hum Pathol. 43:1334–1338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bower RS, Daugherty WP, Giannini C and
Parney IF: Intracranial phosphaturic mesenchymal tumor, mixed
connective tissue variant presenting without oncogenic
osteomalacia. Surg Neurol Int. 3:1512012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Woo VL, Landesberg R, Imel EA, Singer SR,
Folpe AL, Econs MJ, Kim T, Harik LR and Jacobs TP: Phosphaturic
mesenchymal tumor, mixed connective tissue variant, of the
mandible: Report of a case and review of the literature. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 108:925–932. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reis-Filho JS, Paiva ME and Lopes JM:
Pathologic quiz case. A 36-year-old woman with muscle pain and
weakness. Phosphaturic mesenchymal tumor (mixed connective tissue
variant)/oncogenic osteomalacia. Arch Pathol Lab Med.
126:1245–1246. 2002.PubMed/NCBI
|
|
43
|
Singh D, Chopra A, Ravina M, Kongara S,
Bhatia E, Kumar N, Gupta S, Yadav S, Dabadghao P, Yadav R, et al:
Oncogenic osteomalacia: Role of Ga-68 DOTANOC PET/CT scan in
identifying the culprit lesion and its management. Br J Radiol.
90:201608112017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Basu S and Fargose P: 177Lu-DOTATATE PRRT
in recurrent skull-base phosphaturic mesenchymal tumor causing
osteomalacia: A potential application of PRRT beyond neuroendocrine
tumors. J Nucl Med Technol. 44:248–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kaneuchi Y, Hakozaki M, Yamada H, Hasegawa
O, Tajino T and Konno S: Missed causative tumors in diagnosing
tumor-induced osteomalacia with (18)F-FDG PET/CT: A potential
pitfall of standard-field imaging. Hell J Nucl Med. 19:46–48.
2016.PubMed/NCBI
|
|
46
|
Agrawal K, Bhadada S, Mittal BR, Shukla J,
Sood A, Bhattacharya A and Bhansali A: Comparison of 18F-FDG and
68Ga DOTATATE PET/CT in localization of tumor causing oncogenic
osteomalacia. Clin Nucl Med. 40:e6–e10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Breer S, Brunkhorst T, Beil FT, Peldschus
K, Heiland M, Klutmann S, Barvencik F, Zustin J, Gratz KF and
Amling M: 68Ga DOTA-TATE PET/CT allows tumor localization in
patients with tumor-induced osteomalacia but negative 111
In-octreotide SPECT/CT. Bone. 64:222–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jing H, Li F, Zhong D and Zhuang H: 99 m
Tc-HYNIC-TOC (99mTc-hydrazinonicotinyl-Tyr3-octreotide)
scintigraphy identifying two separate causative tumors in a patient
with tumor-induced osteomalacia (TIO). Clin Nucl Med. 38:664–667.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakanishi K, Sakai M, Tanaka H, Tsuboi H,
Hashimoto J, Hashimoto N and Tomiyama N: Whole-body MR imaging in
detecting phosphaturic mesenchymal tumor (PMT) in tumor-induced
hypophosphatemic osteomalacia. Magn Reson Med Sci. 12:47–52. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Andreopoulou P, Dumitrescu CE, Kelly MH,
Brillante BA, Cutler Peck CM, Wodajo FM, Chang R and Collins MT:
Selective venous catheterization for the localization of
phosphaturic mesenchymal tumors. J Bone Miner Res. 26:1295–1302.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hodgson SF, Clarke BL, Tebben PJ, Mullan
BP, Cooney WP III and Shives TC: Oncogenic osteomalacia:
Localization of underlying peripheral mesenchymal tumors with use
of Tc 99 m sestamibi scintigraphy. Endocr Pract. 12:35–42. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kimizuka T, Ozaki Y and Sumi Y: Usefulness
of 201Tl and 99mTc MIBI scintigraphy in a case of oncogenic
steomalacia. Ann Nucl Med. 18:63–67. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jan de Beur SM, Streeten EA, Civelek AC,
McCarthy EF, Uribe L, Marx SJ, Onobrakpeya O, Raisz LG, Watts NB,
Sharon M, et al: Localisation of mesenchymal tumours by
somatostatin receptor imaging. Lancet. 359:761–763. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Reubi JC, Waser B, Laissue JA and Gebbers
JO: Somatostatin and vasoactive intestinal peptide receptors in
human mesenchymal tumors: In vitro identification. Cancer Res.
56:1922–1931. 1996.PubMed/NCBI
|
|
55
|
Kong X, Liu Y, Li Y, Zhai Y and Wu Dan:
Imaging diagnosis of tumor-induced osteomalacia. Chin J Med
Imaging. 22:624–629. 2014.(In Chinese).
|
|
56
|
Cowan S, Lozano-Calderon SA, Uppot RN,
Sajed D and Huang AJ: Successful CT guided cryoablation of
phosphaturic mesenchymal tumor in the soft tissues causing
tumor-induced osteomalacia: A case report. Skeletal Radiol.
46:273–277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fukumoto S, Takeuchi Y, Nagano A and
Fujita T: Diagnostic utility of magnetic resonance imaging skeletal
survey in a patient with oncogenic osteomalacia. Bone. 25:375–377.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Avila NA, Skarulis M, Rubino DM and
Doppman JL: Oncogenic osteomalacia: Lesion detection by MR skeletal
survey. AJR Am J Roentgenol. 167:343–345. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Monappa V, Naik AM, Mathew M, Rao L, Rao
SK, Ramachandra L and PadmaPriya J: Phosphaturic mesenchymal tumour
of the mandible-the useful criteria for a diagnosis on fine needle
aspiration cytology. Cytopathology. 25:54–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ulas A, Dede DS, Sendur MA, Akinci MB and
Yalcin B: Expectations of response from octreotide therapy in
recurrent phosphaturic mesenchymal tumors-do they reflect reality?
Asian Pac J Cancer Prev. 15:10997–10998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dezfulian M and Wohlgenannt O: Revision
hip arthroplasty following recurrence of a phosphaturic mesenchymal
tumor. J Surg Case Rep. 9(pii): rjt0592013.
|
|
62
|
Allevi F, Rabbiosi D, Mandalà M and
Colletti G: Mesenchymal phosphaturic tumour: Early detection of
recurrence. BMJ Case Rep. 2014:bcr20132028272014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kimura S, Yanagisawa M, Fujita Y, Sakihara
S, Hisaoka M and Kurose A: A case of phosphaturic mesenchymal tumor
of the pelvis with vascular invasion. Pathol Int. 65:510–512. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Syed MI, Chatzimichalis M, Rössle M and
Huber AM: Recurrent phosphaturic mesenchymal tumour of the temporal
bone causing deafness and facial nerve palsy. J Laryngol Otol.
126:721–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Okiror L, Khalil H, Vaiyapuri S and Kalkat
M: Complete resection of a large phosphaturic mesenchymal tumour by
chest wall resection and reconstruction. Gen Thorac Cardiovasc
Surg. 64:355–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Farmakis SG and Siegel MJ: Phosphaturic
mesenchymal tumor of the tibia with oncogenic osteomalacia in a
teenager. Pediatr Radiol. 45:1423–1426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tarasova VD, Trepp-Carrasco AG, Thompson
R, Recker RR, Chong WH, Collins MT and Armas LA: Successful
treatment of tumor-induced osteomalacia due to an intracranial
tumor by fractionated stereotactic radiotherapy. J Clin Endocrinol
Metab. 98:4267–4272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chong WH, Andreopoulou P, Chen CC,
Reynolds J, Guthrie L, Kelly M, Gafni RI, Bhattacharyya N, Boyce
AM, El-Maouche D, et al: Tumor localization and biochemical
response to cure in tumor-induced osteomalacia. J Bone Miner Res.
28:1386–1398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Imanishi Y, Hashimoto J, Ando W, Kobayashi
K, Ueda T, Nagata Y, Miyauchi A, Koyano HM, Kaji H, Saito T, et al:
Matrix extracellular phosphoglycoprotein is expressed in causative
tumors of oncogenic osteomalacia. J Bone Miner Metab. 30:93–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jiang Y, Xia WB, Xing XP, Silva BC, Li M,
Wang O, Zhang HB, Li F, Jing HL, Zhong DR, et al: Tumor-induced
osteomalacia: An important cause of adult-onset hypophosphatemic
osteomalacia in China: Report of 39 cases and review of the
literature. J Bone Miner Res. 27:1967–1975. 2012. View Article : Google Scholar : PubMed/NCBI
|