Introduction
Hepatocellular carcinoma (HCC) is one of the leading
causes of cancer-associated mortality worldwide (1), and the third most lethal cancer type in
China, accounting for >40% of global HCC-associated deaths
(2). Despite recent advances in the
diagnosis and treatment of HCC, it remains a highly lethal disease
and the survival of most patients remains poor with no significant
reduction in the mortality rate over the past decades. Even
following radical resection of small HCC (tumor sizes <3 cm),
the 5-year postoperative recurrence and metastasis rate remains as
high as 43.5% (3). The majority of
HCC-associated deaths results from cancer metastasis. In addition,
the heterogeneities of HCC make the problem even worse in clinical
oncology (4). It has generally been
recognized that cancer invasion, including HCC invasion, is not
merely a local problem involving cells in the local tumor
microenvironment, but the result of the activity of numerous
oncogenic somatic immune cells cancer cells throughout the body
(5). With this regard, a fundamental
importance lies in changes in the tumor microenvironment (5). The dynamic evolution of cancer cells
within and at the borders of the tumor microenvironment is crucial
in every aspect of tumor progression (5). The role of the tumor microenvironment,
including stromal cells and the extracellular matrix (ECM), varies
from ECM remodeling to determining the polarity of tissue by
linearization of interstitial collagens at tumor invasion front
(6), to the recruitment of immune
cells as it has been reported that collagen degradation products
serve as chemotactic stimuli for monocytes (7), for the acquisition of features defined
as ‘cancer hallmarks’ to generate a physiologically dysfunctional
microenvironment. Therefore, rich information hidden in the tumor
microenvironment should be systematically investigated to gain a
complete understanding of cancer invasion-associated biology.
The ECM has been traditionally regarded as a
physical scaffold that holds cells and tissues together, and its
remodeling is a common feature of diverse pathological processes,
including tissue fibrosis and cancer (8,9). The
correlation between tissue fibrosis and cancer has attracted
increasing attention from clinicians (10). During cancer invasion, ECM scaffolds
undergo considerable structural remodeling, characterized by
increased deposition of fibronectin, proteoglycans and collagens,
and enhanced matrix cross-linking (11), accompanied with tumor angiogenesis, as
well as recruitment and conversion of immune cells (12). The balance of ECM degradation and
re-deposition is disrupted, which actively induces
epithelial-mesenchymal transition (EMT) of cancer cells (13,14).
Consequently, the cancer cells are able to migrate from the ECM and
develop metastases through stromal cells and immune responses
(15), by participating in signaling
transduction between the ECM and cancer cells (16), in which the collagen degradation
products recruit monocytes, and immune cells aid collagen
reorganization to improve cancer cell penetration (17). In addition, angiogenesis, the
formation of tumor neo-vasculature, is essential for any malignant
tumor type, including HCC, as tumors must recruit vessels to secure
oxygen and nutrient supply, and to remove waste products (18,19).
Macrophages also have important roles in cancer cell invasion and
metastasis by regulating the immune response of the body to cancer
cells and secrete various cytokines (20). A study by Ruffell et al
(21) demonstrated that macrophages
promote cancer invasion in Hodgkin's lymphoma, while other studies
indicated that macrophages inhibit colon cancer invasion (22) or that they have no influence on the
prognosis of cancer patients (23).
The present study aimed to assess the density of
macrophages, tumor neo-vessels and the expression of α-SMA in tumor
stroma in order to investigate the heterogeneity of the tumor
microenvironment in depth, in an attempt to provide a guide for
improving the individual treatment strategy for patients with
HCC.
Materials and methods
Patients and specimens
A total of 3 separate HCC tissue sections (4 µm)
from 101 patients with HCC were collected from Zhejiang Cancer
Hospital (Hangzhou, China) between January 2012 and December 2014.
The study protocol was approved by the Institutional Ethics
Committee of Zhejiang Cancer Hospital (Hangzhou, China) and the
patients provided written informed consent regarding the use of
their tissues. Major clinicopathological features of these patients
are listed in Table I. All of the
cases underwent curative radical surgery without neo-adjuvant
radiotherapy or chemotherapy. Tumor staging was based on the
tumor-nodes-metastasis (TNM) classification system of the American
Joint Committee on Cancer staging criteria (version 7) (24). The median follow-up was 41 months.
Disease-free survival (DFS) was defined as the interval from the
date of surgery to recurrence, and if no recurrence was identified,
patients were censored on the date of death or the last follow-up.
Evidence of disease recurrence was based on the following criteria:
The concentration of AFP (>200 ng/ml), local recurrence
identified on ultrasonography, computed tomography (CT) or magnetic
resonance imaging; peritoneal dissemination on ultrasonography or
CT scan with positive peritoneal cytology; lung metastasis on chest
radiography; and bone metastasis on radiography or bone scan on
emission computed tomography.
 | Table I.Major demographic and
clinicopathological characteristics of hepatocellular carcinoma
cases (n=101). |
Table I.
Major demographic and
clinicopathological characteristics of hepatocellular carcinoma
cases (n=101).
| Item | Value |
|---|
| Age (years) | 51 (19–75) |
| Sex |
|
|
Male | 90 (89.1) |
|
Female | 11 (10.9) |
| Tumor size
(cm) |
|
| ≤5 | 26 (25.7) |
|
>5 | 75 (74.3) |
| Tumor number |
|
|
Single | 86 (85.1) |
|
Multiple | 15 (14.9) |
| AFP (ng/ml) |
|
|
≤200 | 54 (53.5) |
|
>200 | 47 (46.5) |
| Liver
cirrhosis |
|
| No | 58 (57.4) |
|
Yes | 43 (42.6) |
| Venous
infiltration |
|
| No | 53 (52.5) |
|
Yes | 48 (47.5) |
| TNM stage |
|
| Early
(Stage I, II) | 47 (46.5) |
|
Advanced (Stage III, IV) | 54 (53.5) |
| Tumor
recurrence |
|
| No | 37 (36.6) |
|
Yes | 64 (63.4) |
| Distant
metastasis |
|
| No | 46 (45.5) |
|
Yes | 55 (54.5) |
| DFS (Median,
range) | 5.0 (0–54.0) |
Immunohistochemical staining
Immunohistochemical staining of macrophages marked
by CD68, tumor neo-vessels marked by CD105 and α-SMA were performed
using the streptavidin-biotin peroxidase complex method. In brief,
tissue slides were first deparaffinized in xylene, ethanol and
water, and then the slides were treated with 0.01 M citrate buffer
(pH 6.0) and heated in a microwave oven at 98°C for 10 min. For
staining, endogenous peroxidase activity was blocked by immersing
samples in 3% H2O2 in methanol for 10 min at
37°C to prevent any nonspecific binding. After blocking with 2%
bovine serum albumin (Shanghai Ruji Biotechnology, Shanghai,
China), the slides were incubated with primary antibodies to CD68
(cat. no. MA1-38069; dilution, 1/300; Affinity BioReagents, Golden,
CO, USA), CD105 (ab-169545; dilution, 1/300; Abcam, Cambridge, MA,
USA) and α-SMA (ab-5694; dilution, 1/300; Abcam) for 90 min at
37°C, and then incubated with the corresponding secondary antibody
for 15 min at 37°C, and finally incubated with peroxidase-labeled
streptavidin (cat. no., 091014395C; dilution, 1:250; Maixin
Biotechnology, Fuzhou, China) for 15 min. The reaction products
were visualized with diaminobenzidine (DAKO, Glostrup, Denmark).
All slides were counterstained with hematoxylin for 2 min at room
temperature. As a negative control, the primary antibody was
replaced with Tris-buffered saline on sections.
Quantification of immunohistochemical
staining
The slides were examined under an Olympus BX51
microscope equipped with an Olympus DP72 camera (Olympus Optical
Co., Ltd., Tokyo, Japan) and the CRi Nuance multispectral imaging
system (Cambridge Research & Instrumentation, Inc., Woburn, MA,
USA). Positive staining was indicated by brownish granules. A
spectral cube for each image, which contains the complete spectral
information at 10 nm wavelength intervals from 420 to 720 nm, was
collected using the CRi Nuance multispectral imaging system. For
each cube identical settings were used to avoid selection bias.
Positive signal unmixing was performed using the software package
within the Nuance system as previously described (25). Then, after obtaining the images of
signal unmixing, the infiltrating macrophages marked by CD68 and
tumor neo-vessels marked by CD105 were counted in five high-power
fields selected at the tumor site, and the mean cells counts were
documented. The thickness of infiltrating stroma marked by α-SMA
was measured to evaluate the depth between tumor nests. For
determining the density of infiltrating macrophages, neo-vessels
density and thickness of the stroma, the cutoff value to classify
subgroups was 270, 215 and 238.6 µm respectively (26), according to the mean value.
Statistical analysis
Statistical analyses were performed with SPSS
software version 21.0 (IBM Corp., Armonk, NY, USA). For categorical
data, χ2 test was performed. The Kaplan-Meier method was
used to estimate survival curves for DFS and the log-rank test was
used to assess the difference in survival and recurrence rates
between subgroups. The Cox regression model was used to perform
univariate and multivariate analyses. Logistic regression was used
to assess the influence of binary factors. A two-sided P<0.05
was considered to indicate a statistically significant
difference.
Results
Major clinicopathological features of
101 HCC cases
Among the 101 HCC cases included in this study, 90
(89.1%) were males and 11 (10.9%) were females, ranging in age from
19 to 75 years (median age, 51 years). The major demographic and
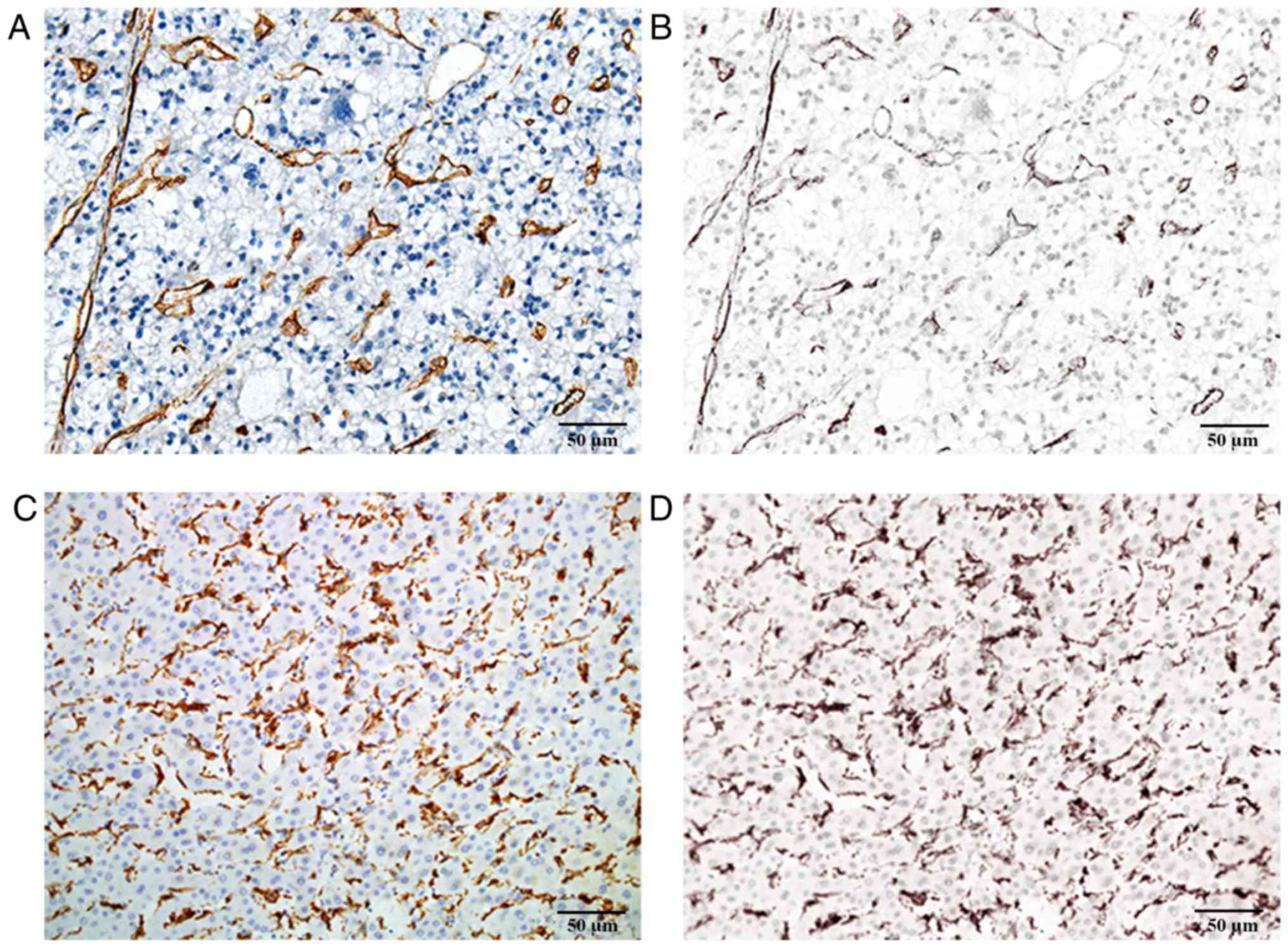
clinicopathological characteristics are presented in Table I. Immunohistochemical analysis was
performed on all HCC tissue sections and the corresponding images
of signal unmixing for tumor neo-vessels and macrophages were
obtained with the CRi Nuance multispectral imaging system (Fig. 1).
Association between tumor neo-vessels,
macrophages, α-SMA and clinicopathological features
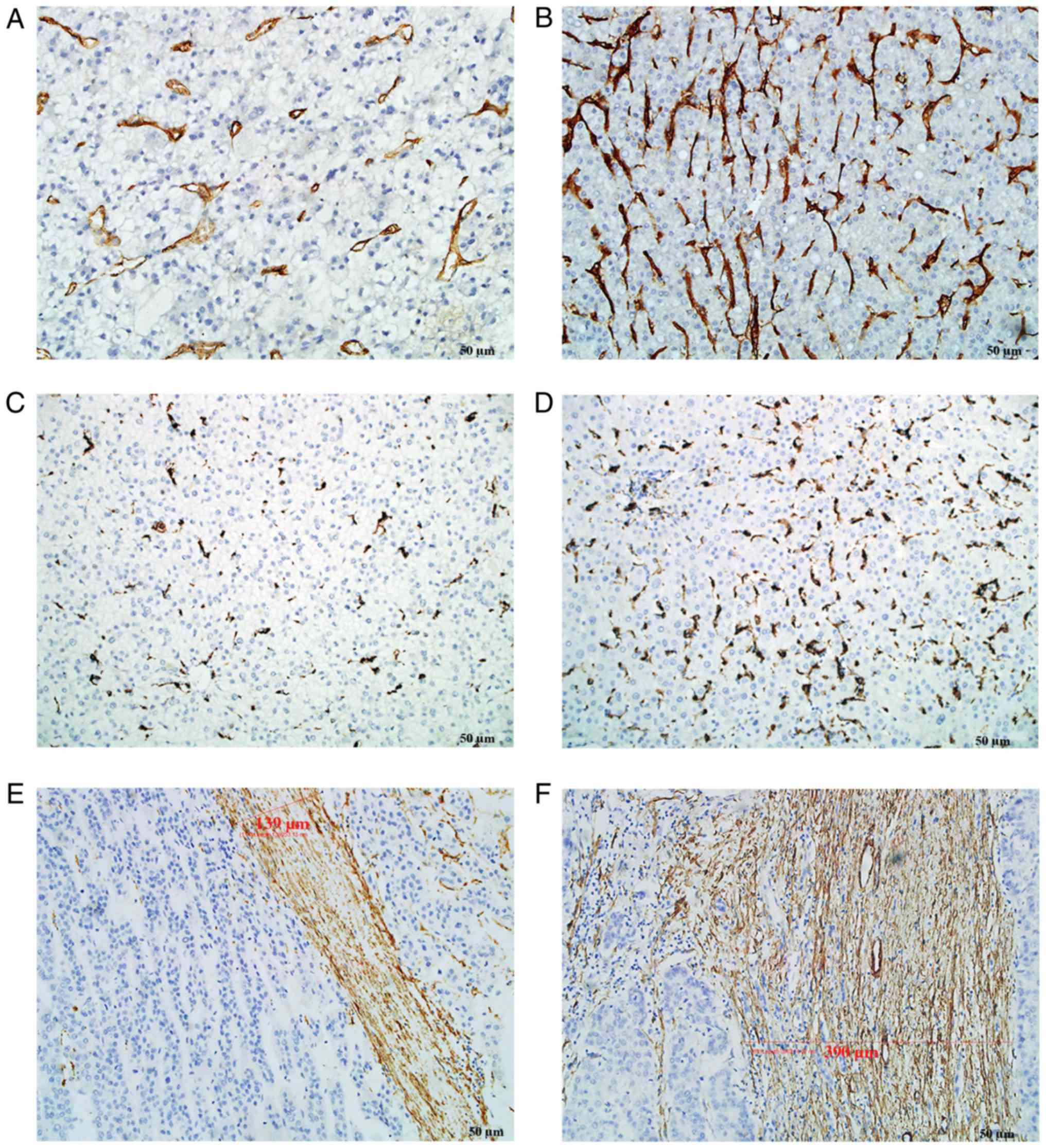
CD105, CD68 and α-SMA staining was mainly located in
the cytoplasm or on the cell membrane. Tumor neo-vessels (stained
by CD105) and macrophages (stained by CD68) were distributed in
tumor nests as well as in tumor stroma. α-SMA staining was mainly
located in the tumor stroma. The majority of the cancer cells were
negative for α-SMA staining, although sporadic positive staining on
these cells was also observed. However, all above components,
including tumor neo-vessels, macrophages and α-SMA, were
heterogeneously expressed in the tumor microenvironment (Fig. 2). In certain cases, only few tumor
neo-vessels and macrophages were present compared with other cases
with a rich distribution in tumor tissues. The amount of α-SMA
distributed in the stroma was also different among the
patients.
The quantitative data of tumor neo-vessels,
macrophage density and stromal thickness are presented in Table II. For infiltrating macrophage
density, neo-vessel density and thickness of the stroma, the cutoff
value to classify subgroups was 270, 215 and 238.6 µm respectively.
In the high and low tumor neo-vessel subgroups, 24 (52.2%) and 16
(36.4%) of cases, respectively, had a high macrophage density. In
the high and low α-SMA subgroups, 29 (58.0%) and 19 (40.4%) of
cases, respectively, had a high tumor neo-vessel density, and 23
(48.9%) and 17 (39.5%) of cases, respectively, had a high
macrophage density. Of note, the tumor neo-vessel, macrophage and
α-SMA densities were not significantly correlated with any of the
clinicopathological features, as summarized in Table II, which indicated that these key
components of the tumor microenvironment were independent from
clinical features including tumor size, tumor number, TNM stage and
venous infiltration.
 | Table II.Association between the density of
tumor neo-vessels, macrophages and α-SMA expression, and
clinicopathological features. |
Table II.
Association between the density of
tumor neo-vessels, macrophages and α-SMA expression, and
clinicopathological features.
|
| Tumor neo-vessel
density | Macrophage
density | α-SMA
expression |
|---|
|
|
|
|
|
|---|
| Feature | Low (n=49) | High (n=49) | χ2 | P-value | Low (n=51) | High (n=40) | χ2 | P-value | Low (n=48) | High (n=52) | χ2 | P-value |
|---|
| Age (years) |
|
| 0.511 | 0.634 |
|
| 1.000 | 0.102 |
|
| 4.408 | 0.060 |
|
<60 | 39 (52.0) | 36 (48.0) |
|
| 38 (55.1) | 31 (44.9) |
|
| 32 (42.1) | 44 (57.9) |
|
|
|
≥60 | 10 (43.5) | 13 (56.5) |
|
| 13 (59.1) | 9 (40.9) |
|
| 16 (66.7) | 8 (33.3) |
|
|
| Gender |
|
| 0.102 | 0.749 |
|
| 0.071 | 0.789 |
|
| 0.670 | 0.529 |
|
Male | 44 (50.6) | 43 (49.4) |
|
| 45 (55.6) | 36 (44.4) |
|
| 44 (49.4) | 45 (50.6) |
|
|
|
Female | 5 (45.5) | 6 (54.5) |
|
| 6 (60.0) | 40 (40.0) |
|
| 4 (36.4) | 7 (63.6) |
|
|
| Tumor size
(cm) |
|
| 2.631 | 0.164 |
|
| 0.679 | 0.467 |
|
| 1.322 | 0.265 |
| ≤5 | 9 (36.0) | 16 (64.0) |
|
| 14 (63.6) | 8 (36.4) |
|
| 15 (57.7) | 11 (42.3) |
|
|
|
>5 | 40 (54.8) | 33 (45.2) |
|
| 37 (53.6) | 32 (46.4) |
|
| 33 (44.6) | 41 (55.4) |
|
|
| Tumor number |
|
| 1.968 | 0.261 |
|
| 0.633 | 0.539 |
|
| 0.545 | 0.568 |
|
Single | 39 (47.0) | 44 (53.0) |
|
| 43 (54.4) | 36 (45.6) |
|
| 40 (46.5) | 46 (53.5) |
|
|
|
Multiple | 10 (66.7) | 5 (33.3) |
|
| 8 (66.7) | 4 (33.3) |
|
| 8 (57.1) | 6 (42.9) |
|
|
| AFP (ng/ml) |
|
| 1.027 | 0.418 |
|
| 0.038 | 0.845 |
|
| 3.344 | 0.075 |
|
≤200 | 24 (45.3) | 29 (54.7) |
|
| 27 (55.1) | 22 (44.9) |
|
| 30 (56.6) | 23 (43.4) |
|
|
|
>200 | 25 (55.6) | 20 (44.4) |
|
| 24 (57.1) | 18 (42.9) |
|
| 18 (38.3) | 29 (61.7) |
|
|
| Liver
cirrhosis |
|
| 0.377 | 0.682 |
|
| 0.091 | 0.832 |
|
| 2.425 | 0.156 |
| No | 27 (47.4) | 30 (52.6) |
|
| 29 (54.7) | 24 (45.3) |
|
| 24 (41.4) | 34 (58.6) |
|
|
|
Yes | 22 (53.7) | 19 (46.3) |
|
| 22 (57.9) | 16 (42.1) |
|
| 24 (57.1) | 18 (42.9) |
|
|
| Venous
infiltration |
|
| 0.368 | 0.686 |
|
| 2.724 | 0.139 |
|
| 0.334 | 0.689 |
| No | 24 (47.1) | 27 (52.9) |
|
| 23 (47.9) | 25 (52.1) |
|
| 24 (45.3) | 29 (54.7) |
|
|
|
Yes | 25 (53.2) | 22 (46.8) |
|
| 28 (65.1) | 15 (34.9) |
|
| 24 (51.1) | 23 (48.9) |
|
|
| TNM stage |
|
| 1.027 | 0.418 |
|
| 2.247 | 0.145 |
|
| 1.054 | 0.324 |
|
Early | 20 (44.4) | 25 (55.6) |
|
| 20 (47.6) | 22 (52.4) |
|
| 20 (42.6) | 27 (57.4) |
|
|
|
Advanced | 29 (54.7) | 24 (45.3) |
|
| 31 (63.3) | 18 (36.7) |
|
| 28 (52.8) | 25 (47.2) |
|
|
| Tumor
recurrence |
|
| 0.180 | 0.832 |
|
| 0.437 | 0.661 |
|
| 0.109 | 0.837 |
| No | 18 (52.9) | 16 (47.1) |
|
| 20 (60.6) | 13 (39.4) |
|
| 17 (45.9) | 20 (54.1) |
|
|
|
Yes | 31 (48.4) | 33 (51.6) |
|
| 31 (53.4) | 27 (46.6) |
|
| 31 (49.2) | 32 (50.8) |
|
|
| Distant
metastasis |
|
| 0.164 | 0.840 |
|
| 2.247 | 0.145 |
|
| 0.511 | 0.113 |
| No | 22 (47.8) | 24 (52.2) |
|
| 20 (47.6) | 22 (52.4) |
|
| 18 (39.1) | 28 (60.9) |
|
|
|
Yes | 27 (51.9) | 25 (48.1) |
|
| 31 (63.3) | 18 (36.7) |
|
| 30 (55.6) | 24 (44.4) |
|
|
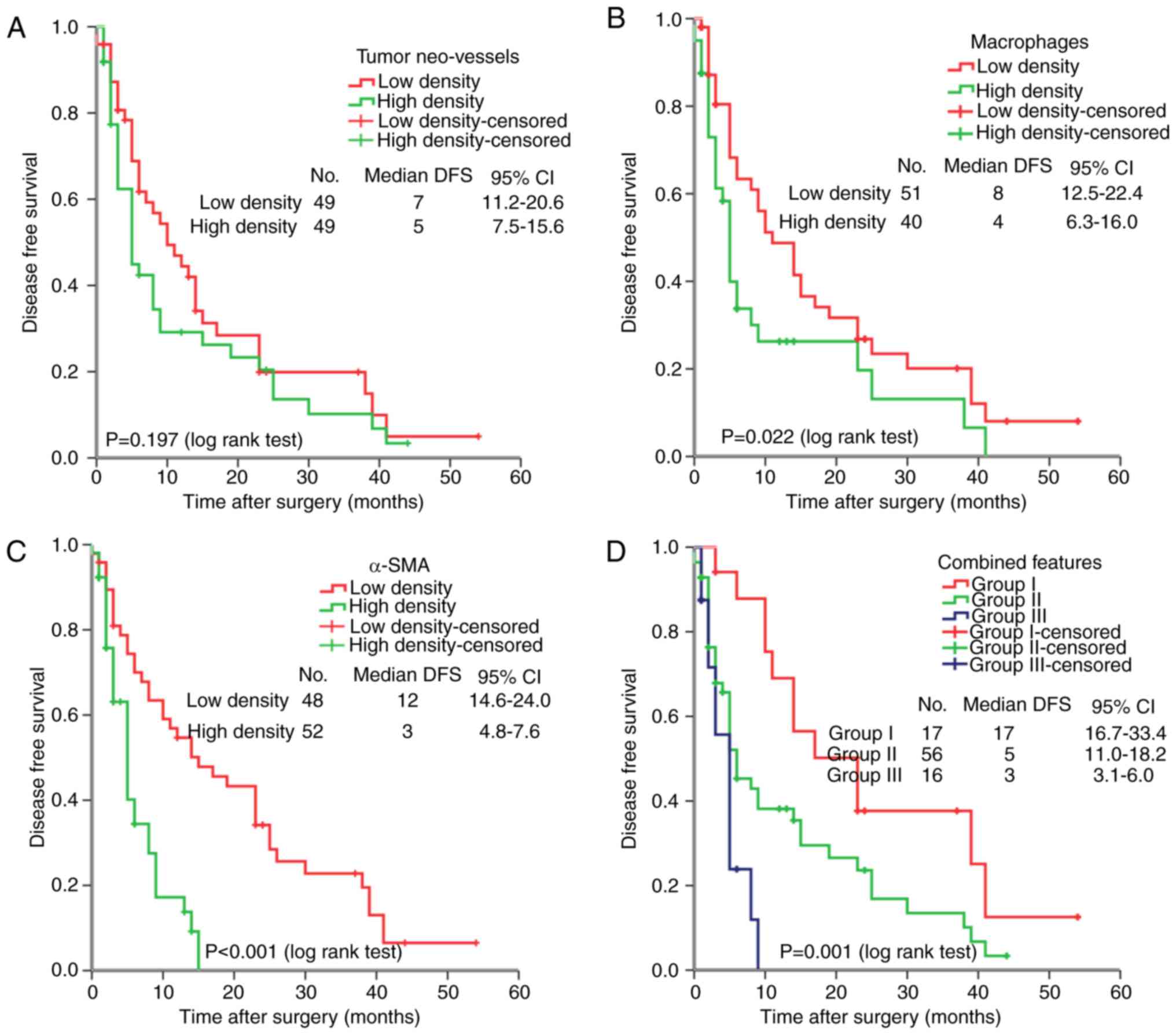
Survival analysis
For the 101 HCC cases, the median DFS was 5.0 months
(range, 22 days-54.0 months). As expected, several clinical factors
were associated with the DFS of HCC patients, including tumor size,
venous infiltration and tumor recurrence (P<0.05 for all).
Furthermore, tumor macrophage and α-SMA densities were negatively
associated with DFS (P=0.022 and P<0.001, respectively). In
addition, the combined features based on the above three key
components in the tumor microenvironment were explored to reveal
the association between key stromal factors with HCC prognosis.
According to the expression levels of the three key stromal
components, patients were divided into three subgroups according to
the density of tumor neo-vessels, macrophages and α-SMA: Group I,
all three components were expressed at a low level; group II, 1 or
2 components were expressed at a high level; group III, three
components were expressed at a high level. Combined analysis
indicated that patients in group III had a worse DFS than those in
groups I and II (P=0.001; Fig.
3D).
Multivariate analysis
In univariate analysis, traditional
clinicopathological features (including tumor size, venous
infiltration and recurrence status), macrophage density and α-SMA
were associated with DFS. In addition, analysis of the combined key
stromal components of tumor neo-vessels, macrophages and α-SMA
indicated that the mortality risk in combined group III was
significantly increased (P=0.001).
As presented in Table
III, in the multivariate analysis Model 1, all factors were
integrated into a multivariate Cox proportional hazards analysis.
Liver cirrhosis status, tumor recurrence status, macrophage density
and α-SMA density were independent prognostic factors for DFS
(P<0.05 for all). In Model 2, factors exhibiting significance
according to a univariate analysis were integrated into a
multivariate Cox proportional hazards analysis in order to avoid
multicollinearity among those variables. Tumor recurrence status,
macrophage density and α-SMA density were independent prognostic
factors for DFS (P<0.05 for all).
 | Table III.Multivariate analyses of factors
associated with DFS. |
Table III.
Multivariate analyses of factors
associated with DFS.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
factor | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Age (<60 vs. ≥60
years) | 0.284 | 1.014 | 0.988–1.040 |
|
|
|
| Sex (female vs.
male) | 0.381 | 0.588 | 0.180–1.926 |
|
|
|
| Tumor size (≤5 vs.
>5) | 0.979 | 0.989 | 0.442–2.216 | 0.181 | 1.552 | 0.815–2.956 |
| Tumor number
(single vs. multiple) | 0.402 | 0.558 | 0.142–2.188 |
|
|
|
| AFP (≤200 vs.
>200) | 0.088 | 1.742 | 0.921–3.293 |
|
|
|
| Liver cirrhosis (no
vs. yes) | 0.020 | 0.514 | 0.294–0.899 |
|
|
|
| Venous infiltration
(no vs. yes) | 0.141 | 0.277 | 0.050–1.530 | 0.696 | 1.114 | 0.649–1.910 |
| TNM stage (early
vs. advanced)a | 0.098 | 4.955 | 0.745–32.937 |
|
|
|
| Tumor recurrence
(no vs. yes) | <0.001 | 28.416 | 7.979–101.205 | <0.001 | 17.035 | 5.644–51.415 |
| Distant metastasis
(no vs. yes) | 0.411 | 0.755 | 0.386–1.476 |
|
|
|
| Tumor neo-vessel
density (low vs. high) | 0.794 | 1.000 | 0.997–1.002 |
|
|
|
| Macrophage density
(low vs. high) | 0.008 | 1.004 | 1.001–1.007 | 0.025 | 1.870 | 1.082–3.233 |
| α-SMA expression
(low vs. high) | 0.005 | 1.004 | 1.001–1.007 |
|
|
|
Discussion
It has generally been recognized that cancer
invasion, including HCC invasion, is not merely a local problem,
but a multifactor and multistep continuum, with a variety of
molecular dysfunction and cell signaling dysregulation. The genetic
or epigenetic changes of cancer cells are the ‘initial factors’ of
carcinogenesis, and the responses of stromal cells in the tumor
microenvironment may ‘promote’ or ‘regulate’ cancer invasion and
metastasis, which ultimately results in an altered tumor
microenvironment favoring cancer invasion and progression (27). The temporal-spatial evolution of the
microenvironment and the interplay between cancer cells and their
microenvironment are critical in every aspect of tumor development,
including cancer cell dormancy, proliferation, invasion and
migration (5). Currently, the
importance of the tumor microenvironment has been increasingly
appreciated, from the physical structures to chemical components,
particularly the role of macrophages and tumor neo-vessels and the
structure of tumor stroma. Inflammation and tumor angiogenesis are
two indispensable factors for cancer invasion (28). The tumor mass relies on blood vessels
to gain nutrients and dispose of waste in order to keep growing
(29). Typical features of tumor
neo-vessels, including irregular shape, disordered structure, high
permeability and immaturity contribute to the spreading of tumor
cells to distant organs (30).
However, the present study demonstrated that tumor neo-vessels are
not significantly associated with the prognosis of HCC patients. It
is therefore suggested that other key components in the tumor
microenvironment, e.g. macrophages, have presumed the significant
role of tumor neo-vessels in the process of cancer invasion and
metastasis. Tumor-associated macrophages have crucial roles in
tumor angiogenesis, including prompting the production of vascular
endothelial cells, boosting vessel sprouting and accelerating
cancer invasion and metastasis (31).
The results of the present study indicated that macrophages indeed
have a profound impact on HCC progression and are independent
prognostic factors for HCC. In addition, macrophages synthesize
other stromal matrix components, including hyaluronic acid, matrix
metalloproteinase and fibroblast-activated proteins, all of which
may contribute to cancer invasion and metastasis (32). In the present study, it was also
confirmed that the density of tumor stroma marked by α-SMA was
highly associated with HCC prognosis and was an independent
prognostic factor for HCC patients. Numerous other studies have
reported that fibroblasts promote lymph node metastasis (33,34). It
has been suggested that the biological characteristics of
fibroblasts in normal tissues are exceptionally distinguished from
those in cancer tissues (35). The
majority of fibroblasts are located at the invasion frontier, the
interface between tumor mass and surrounding stroma, or near the
tumor neo-vessels in the tumor microenvironment, regulating the
growth and invasion of cancer cells by synthetizing and remodeling
ECM, participating in the process of tumor angiogenesis and
downregulating the of the anti-tumor immune response (36). In the present study, increased of
fibroblast density indicated thicker stroma and worse prognosis. It
was previously reported that the stiffness of the stroma is closely
associated with the risk of carcinogenesis (37) and fibrosis is relevant to poor
prognosis of cancer patients (38).
Tumors are normally characterized as lumps of great toughness,
which prompted oncologists to investigate the concept that tumor
progression may be eased by decreasing the toughness of tissues
(39). Others have confirmed that
only a slight change in the toughness of stroma or in mechanical
signals affects the biological behavior of cells (40). Thus, the significance of research into
the tumor microenvironment is particularly prominent.
In conclusion, key components in the tumor
microenvironment may interact with cancer cells, and together
accelerate the cancer invasion and metastasis. The present study
implied that tumor-associated macrophages and the expression of
α-SMA are intimately linked with the prognosis of HCC patients.
However, further research using larger amounts of samples is still
required to confirm and interpret relevant mechanisms in more
depth.
Acknowledgements
This study was supported by the Zhejiang Medical and
Health Science and Technology Project (grant no. 2016KYA048), the
Zhejiang Natural Science Foundation (grant no. LQ17H180003), and
the Postdoctoral Program of Zhejiang Province and the Chinese
Postdoctoral Fund (grant no. 520000-X91601).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye
QH, Wang L, Zhou J, Qiu SJ, Li Y, et al: A decade's studies on
metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol.
130:187–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marusyk A and Polyak K: Tumor
heterogeneity: Causes and consequences. Biochim Biophys Acta.
1805:105–117. 2010.PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paszek MJ, Zahir N, Johnson KR, Lakins JN,
Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M,
Boettiger D, et al: Tensional homeostasis and the malignant
phenotype. Cancer Cell. 8:241–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weathington NM, van Houwelingen AH,
Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G,
Nijkamp FP and Blalock JE: A novel peptide CXCR ligand derived from
extracellular matrix degradation during airway inflammation. Nat
Med. 12:317–323. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schäfer M and Werner S: Cancer as an
overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell
Biol. 9:628–638. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Araya J and Nishimura SL: Fibrogenic
reactions in lung disease. Annu Rev Pathol. 5:77–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin LJ and Boyd NF: Mammographic
density. Potential mechanisms of breast cancer risk associated with
mammographic density: Hypotheses based on epidemiological evidence.
Breast Cancer Res. 10:2012008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Egeblad M, Rasch MG and Weaver VM: Dynamic
interplay between the collagen scaffold and tumor evolution. Curr
Opin Cell Biol. 22:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polyak K, Haviv I and Campbell IG:
Co-evolution of tumor cells and their microenvironment. Trends
Genet. 25:30–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friedl P, Locker J, Sahai E and Segall JE:
Classifying collective cancer cell invasion. Nat Cell Biol.
14:777–783. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barker HE, Cox TR and Erler JT: The
rationale for targeting the LOX family in cancer. Nat Rev Cancer.
12:540–552. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nerenberg PS, Salsas-Escat R and Stultz
CM: Collagen-a necessary accomplice in the metastatic process.
Cancer Genomics Proteomics. 4:319–328. 2007.PubMed/NCBI
|
|
16
|
Torzilli PA, Bourne JW, Cigler T and
Vincent CT: A new paradigm for mechanobiological mechanisms in
tumor metastasis. Semin Cancer Biol. 22:385–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang M, Yuan J, Peng C and Li Y: Collagen
as a double-edged sword in tumor progression. Tumour Biol.
35:2871–2882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kappler M, Taubert H and Eckert AW: Oxygen
sensing, homeostasis, and disease. N Engl J Med. 365:1845–1846.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giraldo NA, Becht E, Remark R, Damotte D,
Sautès-Fridman C and Fridman WH: The immune contexture of primary
and metastatic human tumours. Curr Opin Immunol. 27:8–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruffell B, Affara NI and Coussens LM:
Differential macrophage programming in the tumor microenvironment.
Trends Immunol. 33:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH,
Ding Y, Zhou QM, Zhang X, Pang ZZ and Wan DS: The density of
macrophages in the invasive front is inversely correlated to liver
metastasis in colon cancer. J Transl Med. 8:132010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Azambuja D, Natkunam Y, Biasoli I, Lossos
IS, Anderson MW, Morais JC and Spector N: Lack of association of
tumor-associated macrophages with clinical outcome in patients with
classical Hodgkin's lymphoma. Ann Oncol. 23:736–742. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edge SB: AJCC Cancer stagin Manual. 7th.
New York, NY: Springer. Liver; pp. 201–210. 2007
|
|
25
|
Taylor CR and Levenson RM: Quantification
of immunohistochemistry-issues concerning methods, utility and
semiquantitative assessment II. Histopathology. 49:411–424. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olsson PO, Kalamajski S, Maccarana M,
Oldberg Å and Rubin K: Fibromodulin deficiency reduces collagen
structural network but not glycosaminoglycan content in a syngeneic
model of colon carcinoma. PLoS One. 12:e01829732017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoon DS, Kitago M, Kim J, Mori T, Piris A,
Szyfelbein K, Mihm MC Jr, Nathanson SD, Padera TP, Chambers AF, et
al: Molecular mechanisms of metastasis. Cancer Metastasis Rev.
25:203–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Squadrito ML and De Palma M: Macrophage
regulation of tumor angiogenesis: Implications for cancer therapy.
Mol Aspects Med. 32:123–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Semenza GL: Oxygen sensing, homeostasis,
and disease. N Engl J Med. 365:537–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muz B, de la Puente P, Azab F and Azab AK:
The role of hypoxia in cancer progression, angiogenesis,
metastasis, and resistance to therapy. Hypoxia (Auckl). 3:83–92.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mantovani A and Allavena P: The
interaction of anticancer therapies with tumor-associated
macrophages. J Exp Med. 212:435–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Erler JT, Bennewith KL, Cox TR, Lang G,
Bird D, Koong A, Le QT and Giaccia AJ: Hypoxia-induced lysyl
oxidase is a critical mediator of bone marrow cell recruitment to
form the premetastatic niche. Cancer Cell. 15:35–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang P, Hong JW, Ubukata H, Liu G, Katano
M, Motohashi G, Kasuga T, Watanabe Y, Nakada I and Tabuchi T:
Myofibroblasts correlate with lymphatic microvessel density and
lymph node metastasis in early-stage invasive colorectal carcinoma.
Anticancer Res. 25:2705–2712. 2005.PubMed/NCBI
|
|
34
|
Kellermann MG, Sobral LM, da Silva SD,
Zecchin KG, Graner E, Lopes MA, Kowalski LP and Coletta RD: Mutual
paracrine effects of oral squamous cell carcinoma cells and normal
oral fibroblasts: Induction of fibroblast to myofibroblast
transdifferentiation and modulation of tumor cell proliferation.
Oral Oncol. 44:509–517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Castells M, Thibault B, Delord JP and
Couderc B: Implication of tumor microenvironment in
chemoresistance: Tumor-associated stromal cells protect tumor cells
from cell death. Int J Mol Sci. 13:9545–9571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cirri P and Chiarugi P:
Cancer-associated-fibroblasts and tumour cells: A diabolic liaison
driving cancer progression. Cancer Metastasis Rev. 31:195–208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Provenzano PP, Inman DR, Eliceiri KW,
Knittel JG, Yan L, Rueden CT, White JG and Keely PJ: Collagen
density promotes mammary tumor initiation and progression. BMC Med.
6:112008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baba Y, Iyama K, Ikeda K, Ishikawa S,
Hayashi N, Miyanari N, Sado Y, Ninomiya Y and Baba H: The
expression of type IV collagen alpha6 chain is related to the
prognosis in patients with esophageal squamous cell carcinoma. Ann
Surg Oncol. 15:555–565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Susic D: Cross-link breakers as a new
therapeutic approach to cardiovascular disease. Biochem Soc Trans.
35:853–856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McDaniel SM, Rumer KK, Biroc SL, Metz RP,
Singh M, Porter W and Schedin P: Remodeling of the mammary
microenvironment after lactation promotes breast tumor cell
metastasis. Am J Pathol. 168:608–620. 2006. View Article : Google Scholar : PubMed/NCBI
|