Introduction
Ovarian cancer (OC) is the most common type of
malignant tumor of the female reproduction system and the leading
cause for mortality from gynecological cancer worldwide (1). It is usually characterized by indistinct
symptoms and rapid progression, therefore, the majority of patients
with OC are diagnosed at an advanced stage and the prognosis is
relatively poor (2). In recent years,
the overall survival (OS) rate of patients with OC has improved to
some extent due to the development and combinations of treatments,
including surgery, chemotherapy and molecularly targeted therapy.
However, the prognosis for patients with OC remains relatively
poor, which is likely attributable to drug resistance (3,4).
Therefore, the identification of a novel method for the prevention
of OC drug resistance is necessary.
Rab23, a member of the Rab GTPase family, was first
isolated from brain tissue in 1994 (5). Its abberant expression has been reported
in various different types of tumors; however, its role in tumor
progression and its association with prognosis are controversial
topics. Jian et al (6)
reported that Rab23 could promote squamous cell carcinoma cell
migration and invasion via the integrin β1/Rac1 pathway. Wang et
al (7) reported that Rab23 was
overexpressed in human astrocytoma and promoted cell migration and
invasion through the regulation of Rac1. However, Denning et
al (8) demonstrated that Rab23
exhibited low expression and served an inhibitory role in thyroid
cancer progression. Furthermore, Liu et al (9) reported that Rab23 expression could
inhibit proliferation and promote apoptosis in breast cancer.
However, to the best of our knowledge, the expression levels and
function of Rab23 in OC has not yet been reported.
In the present study, immunohistochemistry (IHC)
analysis demonstrated that Rab23 was highly expressed in OC tissues
and positively associated with poor OS and disease-free survival
(DFS) time. Cell-level experiments indicated that Rab23 was highly
expressed in OC cells and could promote the cisplatin (DDP)
resistance of OC cells. Investigation into the potential molecular
mechanisms behind this effect revealed that the sonic hedgehog
(Shh)-Gli family zinc finger 1 (Gli1)-ATP-binding cassette
sub-family G member 2 (ABCG2) pathway was potentially responsible
for DDP resistance induced by Rab23.
Materials and methods
Specimens
In total, 74 primary OC specimens were obtained from
patients who accepted surgery between January 2008 and December
2010 at Rizhao People's Hospital (Rizhao, China). Following
surgery, tissues were immersed in 10% paraformaldehyde at room
temperature for 4 h and subsequently embedded in paraffin. Patients
were aged between 34 and 71 years of age, with a median age of 51
years. Patients who had received chemotherapy, radiotherapy or
immunomodulatory therapy prior to surgery were excluded from the
study. The present study was approved by the review board and
ethics committee of Rizhao People's Hospital.
Immunohistochemistry (IHC)
Tissues were cut into 4-µm-thick sections and
incubated with a rabbit anti-human Rab23 primary antibody (cat. no.
ab192420; dilution, 1:300; Abcam, Cambridge, UK) at 4°C overnight.
Normal rabbit IgG (cat. no. ab172730; dilution, 1:300; Abcam) was
used as a negative control. Sections were then incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG (cat. no.
KIT-7710; working dilution; Fuzhou Maixin Biotech. Co., Ltd.,
Fuzhou, China) and stained with 3,3′-diaminobenzidine (1 mg/ml) for
20 sec at room temperature; cell nuclei were stained using 10%
hematoxylin for 14 sec at room temperature. The staining score was
calculated by two independent pathologists simultaneously, at ×400
magnification in 5 random fields of view. The proportion score
represented the percentage of stained tumor cells among total cells
in each field of view: 0, <10%; 1, 10–25%; 2, 26–75% and 3,
>75%. The intensity score represented the overall staining
intensity: 0, none; 1, weak; 2, intermediate, and 3, strong. The
expression score of Rab23 was calculated as the product of
proportion score and intensity score. Scores ≥4 were classified as
high expression and <4 as low expression.
Cell culture
The IOSE80 human normal ovarian cell line, and OC
cell lines A2780 and SKOV-3, were purchased from the American Type
Culture Collection (Manassas, VA, USA). All cell lines were
cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS) (Invitrogen; Thermo Fisher Scientific Inc.). All cells were
cultured in 5% CO2 at 37°C. GANT61 (cat. no.
500579-04-4, Selleck Chemicals, Houston, TX, USA) is an inhibitor,
which can block the hedgehog signaling pathway through inhibiting
Gil1. This in turn reduces the drug resistance ability of cells. In
the present study, GANT61 was used to inhibit Gli1.
Stable transfection
pcDNA3.1/Rab23 or empty plasmids (Shanghai
GenePharma Co., Ltd., Shanghai, China) were transfected into A2780
cells to produce A2780-Rab23 cells, and p-GPU6/Rab23-shRNA or empty
plasmids (Shanghai GenePharma Co., Ltd.) were transfected into
SKOV-3 cells, using Lipofectamine 2000® (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. Cells were then cultured in 1.0 µg/ml
puromycin for 3 weeks and monoclones were selected. Five weeks
following transfection, Rab23 expression was detected by RT-PCR and
western blotting.
Silencing ABCG2 in OC cells
ABCG2 siRNA
(5′-CCAGAACUAACGCAUUCAAdTdAdAdTGGUCUUGAUUGCGUAAGUU-3′; Shanghai
GenePharma Co., Ltd.) was transfected into A2780-Rab23 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. A negative siRNA was as used as a control.
At 48 h, ABCG2 expression was detected by reverse
transcription-quantitative polymerase chain reaction (RT-PCR) and
western blotting.
RT-PCR
Total RNA was extracted using TRIzol (Life
Technologies; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. cDNA was synthesized from the total RNA
using the PrimeScript RT-PCR kit (Takara Biotechnology Co., Ltd.,
Dalian, China). The polymerase chain reaction mixture was as
follows: 1.2 µl cDNA, 1.8 µl forward primer, 1.4 µl reverse primer,
5.6 µl double distilled dH2O was mixed. The thermocycling
conditions were as follows: 94°C for 5 min, then 30 cycles of 94°C
for 30 sec, 54°C for 30 sec, 72°C for 30 sec and 72°C for 5 min.
The products were electrophoretically separated on agarose gel
(1.0%). Bands were analyzed using Labworks software (version 4.0;
UVP LLC, Upland, CA, USA). The primer sequences used were as
follows: Rab23, forward, 5′-AGGCACTGGCAAAAAGGTTA-3′, and reverse,
5′-TAGACCACCTTCAGTGAGGC-3′; ABCG2, forward,
5′-CTGAGATCCTGAGCCTTTGG-3′, and reverse,
5′-TGCCCATCACAACATCATCT-3′. GAPDH was used as an internal control
and the primer sequence was as follows: GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse,
5′-GGGGTCGTTGATGGCAACA-3′.
Western blot analysis
Total protein concentration was measured using the
BCA kit (Pierce; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Radioimmunoprecipitation assay buffer
(P0013B; Beyotime Institute of Biotechnology, Haimen, China)
containing 1% protease inhibitor was used for protein extraction.
In total, 250 µg protein was loaded and separated using SDS-PAGE
(10% gel) and transferred onto nitrocellulose membranes. Blocking
was performed using TBS with Tween 20 containing 5% non-fat dried
milk for 1 h at room temperature. The membranes were incubated with
the primary antibodies (Table I) at
4°C overnight. The membranes were then incubated with
peroxidase-conjugated goat anti-rabbit-IgG (dilution 1:4,000;
Abcam, Cambridge, UK) at room temperature for 1 h. Immunoreactive
signals were detected using enhanced chemiluminescence reagent
(Pierce; Thermo Fisher Scientific, Inc.). The bands were analyzed
using Image-Pro (v5.1; Media Cybernetics, Inc., Rockville, MD,
USA).
 | Table I.Primary antibodies used in western
blotting. |
Table I.
Primary antibodies used in western
blotting.
| Gene | Company | Catalogue number | Dilution |
|---|
| Rab23 | Abcam, Cambridge,
UK | ab192420 | 1:300 |
| ATP-binding cassette
sub-family G member 2 | Abcam, Cambridge,
UK | ab24115 | 1:400 |
| Sonic hedgehog | Cell Signaling
Technology, Danvers, MA, USA | 2207 | 1:400 |
| Gli family zinc
finger 1 | Cell Signaling
Technology, Danvers, MA, USA | 3538 | 1:300 |
| GAPDH | Abcam, Cambridge,
UK | ab9485 | 1:500 |
Drug resistance assay
Cells were plated into 96-well plates in triplicate
at 8×103 cells per well. After 24 h, cells were cultured
in medium containing different concentrations of DDP (5, 10, 20, 40
and 80 µmol/l) for 48 h. Cells were then incubated with MTT for 4
h, then formazan was dissolved with dimethyl sulfoxide. Viability
was analyzed by the absorbance at 490 nm. The experiment was
repeated at least 3 times. The IC50 was then calculated
from the survival curves constructed.
Statistical analysis
All data are expressed as the mean ± standard
deviation. SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA) was
used for all statistical analysis. Survival curves were generated
by Kaplan-Meier analysis and were compared with the log-rank test.
Differences between groups were analyzed using one-way analysis of
variance followed by Dunnett's post-hoc test. IC50 was
calculated using regression analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Rab23 is relatively highly expressed
in OC and associated with a reduced OS and DFS rate
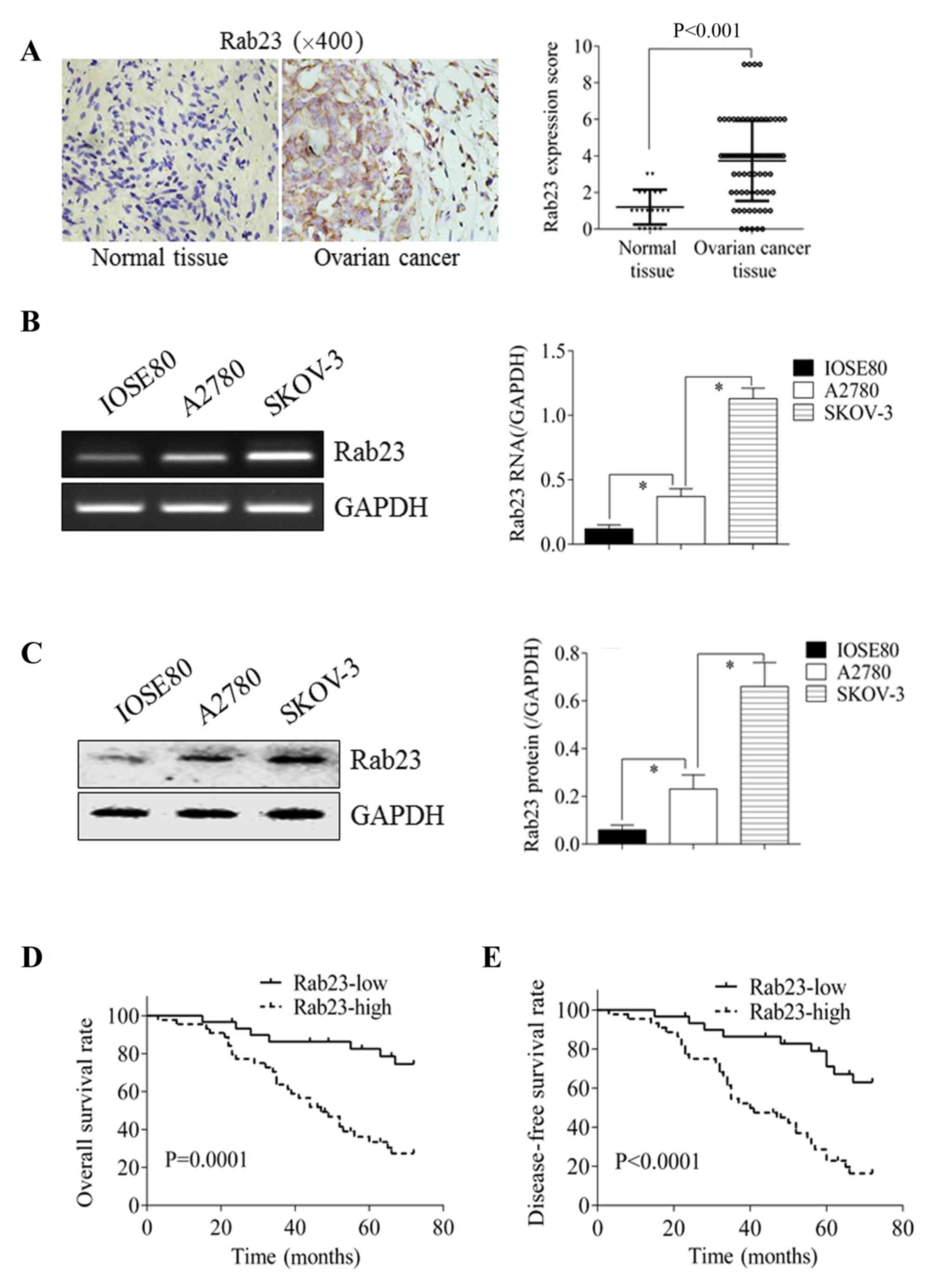
Rab23 expression was detected using the IHC staining
of 74 OC tissues and 20 normal ovarian control tissues. As
demonstrated in Fig. 1A, Rab23
expression in OC tissue was significantly increased compared with
normal ovarian tissue (P<0.001). Cell line experiments also
demonstrated that Rab23 expression was higher in the OC cell lines,
A2780 and SKOV-3, than in the normal ovarian cell line, IOSE80, at
the mRNA (Fig. 1B) and protein
(Fig. 1C) levels (P<0.05).
The 74 OC tissues were divided into low (n=30) and
high (n=44) Rab23 expression groups. In the low Rab23 expression
group, 7 mortalities occurred during the follow-up period, an OS
rate of 76.67%; in the high Rab23 expression group, 30 mortalities
occurred, an OS rate of 31.82%. In addition, 10 patients exhibited
disease progression in the low Rab23 expression group, a DFS rate
of 66.67%; in the high Rab23 expression group, 34 patients
exhibited disease progression, a DFS rate of 22.73%. Using
Kaplan-Meier survival curves and the log-rank test, it was
determined that the differences in the OS (P=0.0001; Fig. 1D) and DFS rates (P<0.0001; Fig. 1E) were significant.
Rab23 promotes ABCG2 expression and
DDP resistance
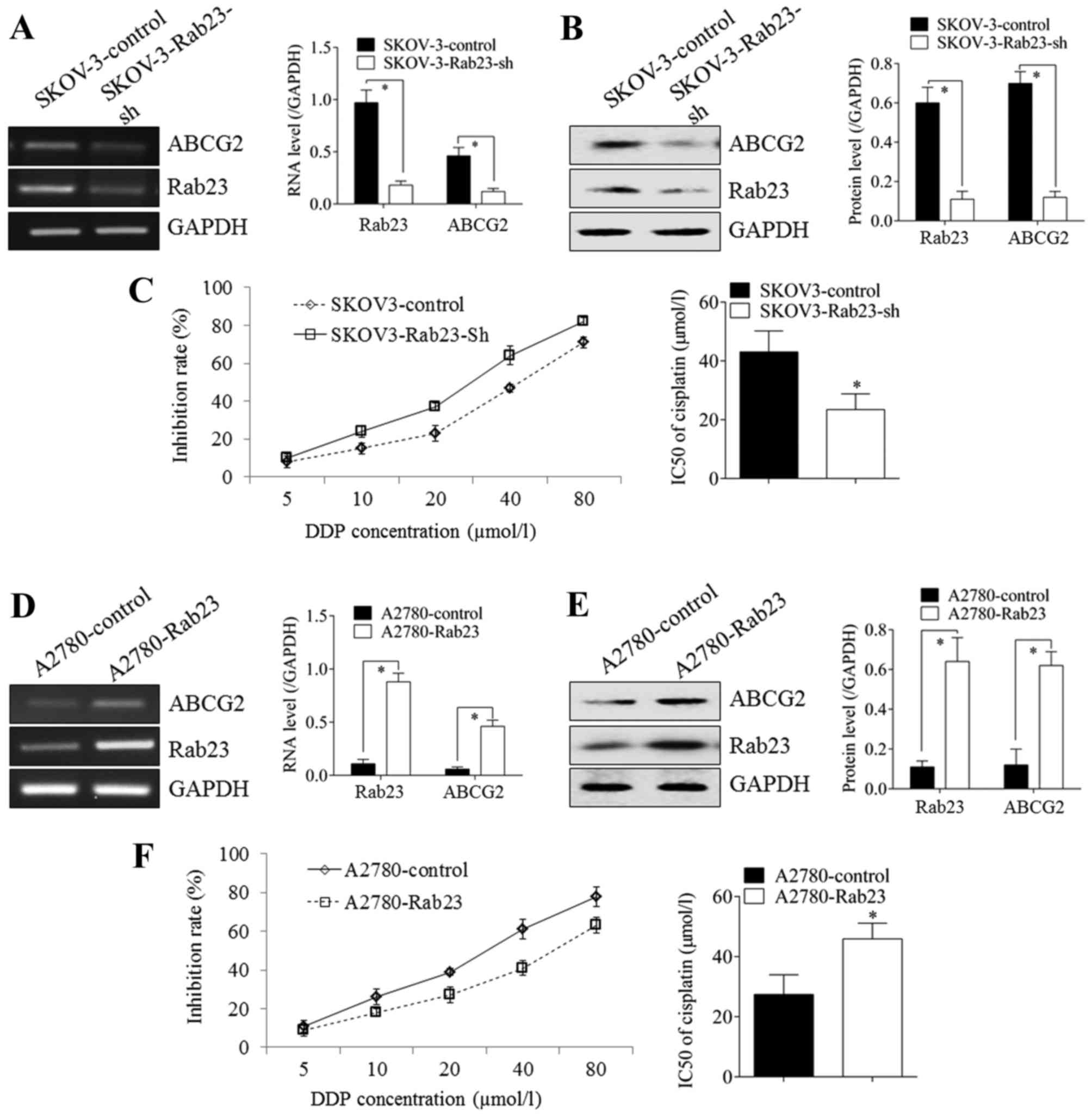
Silencing Rab23 in SKOV-3 cells caused the
downregulation of ABCG2 expression at the mRNA (Fig. 2A) and protein (Fig. 2B) levels. The growth inhibition rate
of SKOV-3 cells treated with DDP was significantly higher in
Rab23-silenced SKOV3 cells compared with control cells; the
IC50 decreased from 43.09±7.12 to 26.46±5.38 µmol/l
(Fig. 2C). Rab23 overexpression in
A2780 cells resulted in the upregulation of ABCG2 expression at the
mRNA (Fig. 2D) and protein (Fig. 2E) levels. The growth inhibition rate
of A2780 cells treated with DDP was significantly lower in
RAb23-overexpressing A2780 cells compared with control cells; the
IC50 increased from 27.42±6.54 to 45.92±5.23 µmol/l
(Fig. 2F; P<0.05).
Shh-Gli1-ABCG2 is associated with the
DDP resistance induced by Rab23 expression
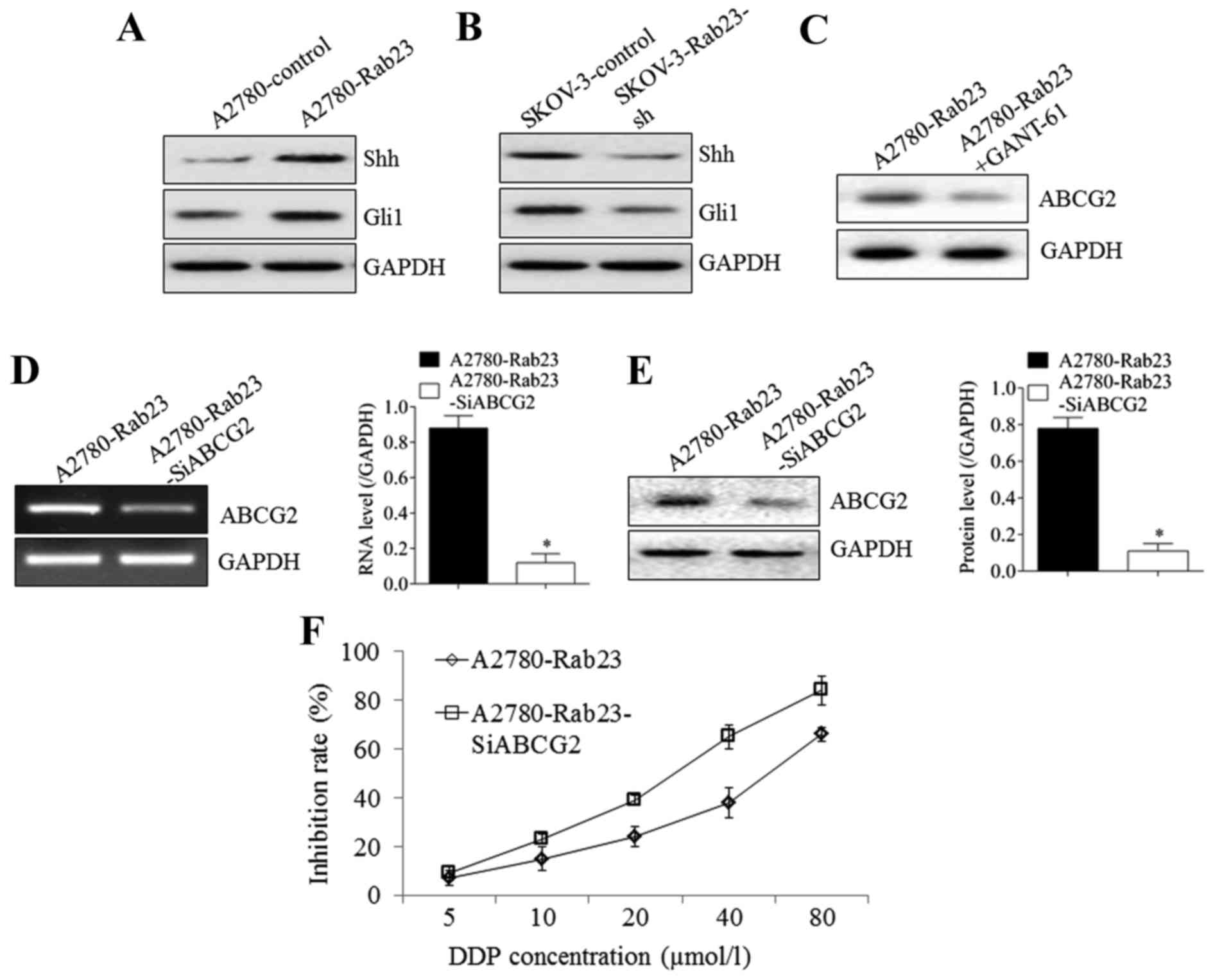
Rab23 overexpression in A2780 cells caused the Shh
and Gli1 protein expression to increase compared with empty control
plasmid-transfected A2780 cells (Fig.
3A). The silencing of Rab23 in SKOV-3 cells resulted in the
downregulation of Shh and Gli1 protein expression compared with
empty control plasmid-transfected SKOV-3 cells (Fig. 3B). Furthermore, the addition of the
Gli1 inhibitor, GANT-61, to Rab23-overexpressing A2780
(A2780-Rab23) cells resulted in a significant decrease in ABCG2
expression compared with untreated A2780-Rab23 cells (Fig. 3C). Silencing ABCG2 in A2780-Rab23
cells (Fig. 3D and E) significantly
reduced the inhibition rate induced by Rab23 overexpression
compared with un-transfected A2780-Rab23 cells (P<0.05); the
IC50 declined from 51.66±8.32 to 25.61±6.17 µmol/l
(Fig. 3F). These results suggest that
the Shh-Gli1-ABCG2 pathway was induced by Rab23 to promote drug
resistance.
Discussion
As a member of the Ras-related small GTPase family,
Rab23 has been studied in various types of tumor, and whether it
has a negative or positive role in tumor progression is a
controversial issue (6–9). In recent years, tumor-promoting roles
for Rab23 have been reported in several types of tumors. In 2015,
Cai et al (10) reported that
Rab23 activated the invasion and motility of pancreatic duct
adenocarcinoma cells. In 2017, Chang et al (11) reported that the downregulation of
Rab23 in prostate cancer could inhibit tumor growth in vitro
and in vivo. However, the role of Rab23 in ovarian cancer
remains unknown. In the present study, the relatively high
expression of Rab23 was demonstrated in OC tissue and cell lines.
Survival analysis indicated that Rab23 was positively associated
with the poor OS and DFS rates of OC patients. This is consistent
with the studies by Cai et al (10) on pancreatic duct adenocarcinoma, and
by Chang et al (11) on
prostate cancer. However, the results are conflicting with studies
in thyroid and breast cancer (8,9). This may
be associated with the different origins of the tumors.
ABCG2, also known as breast cancer resistance
protein, is a member of the ABC family of efflux proteins and can
transport substrates out of cells, resulting in drug resistance
(12,13). Thus, ABCG2 is considered an important
target for drug resistance research, and ABCG2 inhibitors are being
assessed in clinical studies. In the present study, the silencing
or overexpression of Rab23 induced the down- or upregulation of
ABCG2 expression, respectively, at the mRNA and protein levels. DDP
is one of the most commonly used chemotherapy drugs in multiple
types of tumors, and functions by inducing cancer cell death via
the activation of the DNA damage response, leading to cell cycle
arrest and the induction of mitochondrial apoptosis (14,15). In
OC, DDP is used as a first line chemotherapy drug (16). In the present study, it was
demonstrated that the DDP resistance of OC diminished when Rab23
was silenced. Overall, these results indicate that Rab23 functions
in the drug resistance mechanism of OC cells.
The Shh signaling pathway is an important regulator
of human cell proliferation, differentiation and embryonic
development (17). The dysregulated
activation of the Shh signaling pathway in cancer cells is
associated with tumor occurrence, drug resistance and poor cancer
prognosis (18). Gli1 activates the
target genes downstream of the Shh pathway (19,20). It
has been reported that Rab23 is associated with the Shh signaling
pathway, but whether this correlation is positive or negative is
still under debate. Chi et al (21) reported that Rab23 could negatively
regulate Gli1 in a SUFU-dependent manner. However, Fuller et
al (22) reported that Rab23
promotes nodal signaling in vertebrate left-right patterning
independent of the Shh signaling pathway. In the present study, it
was demonstrated that Rab23 upregulated ABCG2 expression via the
Shh-Gli signal pathway in OC cells. The differences in the results
regarding the effect of Rab23 on Shh signaling between studies may
be attributable to the different types of cell under
investigation.
In conclusion, the present study demonstrates that
Rab23 may function in the drug resistance of OC, and presents a
novel potential molecular mechanism for this effect. The present
study provides experimental evidence to support the further
investigation of Rab23 as a target to inhibit drug resistance and
improve the prognosis of patients with OC.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huo J, Bian XH, Huang Y, Miao ZC and Song
LH: Inhibitory effect and mechanism of metformin on human ovarian
cancer cells SKOV-3 and A2780. Eur Rev Med Pharmacol Sci.
21:484–489. 2017.PubMed/NCBI
|
|
2
|
Li R, Dong T, Hu C, Lu J, Dai J and Liu P:
Salinomycin repressed the epithelial-mesenchymal transition of
epithelial ovarian cancer cells via downregulating Wnt/b-catenin
pathway. Onco Targets Ther. 10:1317–1325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perroud HA, Scharovsky OG, Rozados VR and
Alasino CM: Clinical response in patients with ovarian cancer
treated with metronomic chemotherapy. Ecancermedicalscience.
11:7232017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HH, Bellat V and Law B: Chemotherapy
induces adaptive drug resistance and metastatic potentials via
phenotypic CXCR4-expressing cell state transition in ovarian
cancer. PLoS One. 12:e01710442017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Y, Ng F and Tang BL: Rab23 activities
and human cancer-emerging connections and mechanisms. Tumour Biol.
37:12959–12967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jian Q, Miao Y, Tang L, Huang M, Yang Y,
Ba W, Liu Y, Chi S and Li C: Rab23 promotes squamous cell carcinoma
cell migration and invasion via integrin b1/Rac1 pathway.
Oncotarget. 7:5342–5352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang M, Dong Q and Wang Y: Rab23 is
overexpressed in human astrocytoma and promotes cell migration and
invasion through regulation of Rac1. Tumour Biol. 37:11049–11055.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denning KM, Smyth PC, Cahill SF, Finn SP,
Conlon E, Li J, Flavin RJ, Aherne ST, Guenther SM, Ferlinz A, et
al: A molecular expression signature distinguishing follicular
lesions in thyroid carcinoma using preamplification RT-PCR in
archival samples. Mod Pathol. 20:1095–1102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Zeng C, Bao N, Zhao J, Hu Y, Li C
and Chi S: Effect of Rab23 on the proliferation and apoptosis in
breast cancer. Oncol Rep. 34:1835–1844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai ZZ, Xu LB, Cai JL, Wang JS, Zhou B and
Hu H: Inactivation of Rab23 inhibits the invasion and motility of
pancreatic duct adenocarcinoma. Genet Mol Res. 14:2707–2715. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang J, Xu W, Liu G, Du X and Li X:
Downregulation of Rab23 in prostate cancer inhibits tumor growth in
vitro and in vivo. Oncol Res. 25:241–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishihashi K, Kawashima K, Nomura T,
Urakami-Takebayashi Y, Miyazaki M, Takano M and Nagai J: Cobalt
chloride induces expression and function of breast cancer
resistance protein (BCRP/ABCG2) in human renal proximal tubular
epithelial cell line HK-2. Biol Pharm Bull. 40:82–87. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Yunyun Z, Wang L, Chen X and Zhu
Z: ABCG2 confers promotion in gastric cancer through modulating
downstream CRKL in vitro combining with biostatistics mining.
Oncotarget. 8:5256–5267. 2017.PubMed/NCBI
|
|
14
|
Kuo WY, Hwu L, Wu CY, Lee JS, Chang CW and
Liu RS: STAT3/NF-kB-regulated lentiviral TK/GCV suicide gene
therapy for cisplatin-resistant triple-negative breast cancer.
Theranostics. 7:647–663. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maeda O, Matsuoka A, Miyahara R, Funasaka
K, Hirooka Y, Fukaya M, Nagino M, Kodera Y, Goto H and Ando Y:
Modified docetaxel, cisplatin and capecitabine for stage IV gastric
cancer in Japanese patients: A feasibility study. World J
Gastroenterol. 23:1090–1097. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie XQ, Zhao QH, Wang H and Gu KS:
Dysregulation of mRNA profile in cisplatin-resistant gastric cancer
cell line SGC7901. World J Gastroenterol. 23:1189–1202. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du Z, Zhou F, Jia Z, Zheng B, Han S, Cheng
J, Zhu G and Huang P: The hedgehog/Gli-1 signaling pathways is
involved in the inhibitory effect of resveratrol on human
colorectal cancer HCT116 cells. Iran J Basic Med Sci. 19:1171–1176.
2016.PubMed/NCBI
|
|
18
|
Merchant JL and Ding L: Hedgehog signaling
links chronic inflammation to gastric cancer precursor lesions.
Cell Mol Gastroenterol Hepatol. 3:201–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HJ, Wu Q, Li H, Bae GU, Kim AK and Ryu
JH: A sesquiterpene lactone from Siegesbeckia glabrescens
suppresses Hedgehog/Gli-mediated transcription in pancreatic cancer
cells. Oncol Lett. 12:2912–2917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khatra H, Kundu J, Khan PP, Duttagupta I,
Pattanayak S and Sinha S: Piperazic acid derivatives inhibit Gli1
in Hedgehog signaling pathway. Bioorg Med Chem Lett. 26:4423–4426.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chi S, Xie G, Liu H, Chen K, Zhang X, Li C
and Xie J: Rab23 negatively regulates Gli1 transcriptional factor
in a Su(Fu)-dependent manner. Cell Signal. 24:1222–1228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fuller K, O'Connell JT, Gordon J, Mauti O
and Eggenschwiler J: Rab23 regulates Nodal signaling in vertebrate
left-right patterning independently of the Hedgehog pathway. Dev
Biol. 391:182–195. 2014. View Article : Google Scholar : PubMed/NCBI
|