Introduction
Breast cancer is the most common malignancy and
second leading cause of cancer-associated mortality in females
globally (1,2). Breast cancer incidence and mortality
rates are high in western countries, while the incidence of breast
cancer exhibits an increasing trend in developing countries
(1). In China in 2010, there were
208,000 cases of breast cancer, with 55,500 mortalities (3,4). The
staging of breast cancer is based on the tumor-node-metastasis
system, and distinct stages of disease are associated with various
prognosis (5). Tumor size and
axillary node status are considered risk factors for breast cancer
distant metastases (5), whereas the
status of regional lymph nodes is critical in defining the staging,
treatment and prognosis of breast cancer (6). Therefore, it is imperative to determine
the nature of differential protein expression between primary
breast tumor (PBT) and paired metastatic lymph node (PMLN) tissues.
It is reported that Serpin H1 (7),
cluster of differentiation 146 (8)
and Ku86 (9) expression may be
correlated with breast cancer. However, the molecular mechanisms
underlying breast cancer metastasis remain poorly understood.
Various proteins are essential for the normal
functions of organisms and the proteome, the entirety of the
proteins expressed by a genome of a cell or an organ, alters
according to time and environment (10). Therefore, an improved understanding of
the processes underlying breast cancer metastasis may be achieved
by investigating the differences in protein expression between
paired primary breast cancerous tissue and metastasis tissue. A
previous study implemented the isobaric tags for relative and
absolute quantitation (iTRAQ) proteomic technique to directly
analyze the differences in protein expression between these paired
tissue types (11). This
high-throughput, sensitive and accurate protein detection method
identified numerous proteins that may be associated with breast
cancer metastasis (11). This
previous study demonstrated that protein S100-A8 exhibited a
1.72-fold change between PBT and PMLN, suggesting a potential role
for this protein in breast cancer metastasis (11).
Protein S100-A8 is a member of the S100 family of
leukocyte proteins, and is a calcium-binding protein (12). The majority of S100 proteins exist as
homodimers; however, a number assemble as heterodimers, including
S100-A8/S100-A9, encoded by genes clustered on the 1q21 human
chromosomal region (13). Protein
S100-A8 has previously been identified as a pro-inflammatory factor
in arthritis and autoimmune disease (12). As an inflammatory protein, it may
serve a role in tumor-stromal interactions (14), and elevated levels of protein S100-A8
have been observed in numerous types of malignancy, including
cutaneous squamous cell carcinoma (12), gastric adenocarcinoma (15) and kidney cancer (16). In addition, a previous study
demonstrated a correlation between the expression levels of protein
S100-A8 and colorectal carcinoma progression, during which this
protein may contribute to colorectal carcinoma cell survival and
migration via the Wnt/β catenin signaling pathway (17). To the best of our knowledge, the
present study is the first to report differences in the expression
levels of protein S100-A8 between fresh PBT and fresh PMLN tissues,
screened using the iTRAQ proteomic technique. Furthermore,
immunohistochemistry (IHC) was utilized to identify variations in
protein S100-A8 expression levels in non-metastatic breast tumor
(NMBT), PBT, PMLN and benign breast disease (BBD) FFPE tissue. In
the present study, further analysis of the correlation between
S100-A8 protein expression levels and the clinicopathological
factors or overall survival rate of breast cancer was
performed.
Materials and methods
Study subjects and clinical data
A total of 54 paired fresh primary tumor and
metastatic lymph node tissues were collected from Hunan Cancer
Hospital and The Affiliated Cancer Hospital of Xiangya School of
Medicine, Central South University (Changsha, China) between
November 2013 and March 2014. All the female patients were
initially diagnosed with breast cancer, and did not receive
radiotherapy or chemotherapy prior to surgery (modified radical
mastectomy). Each axillary lymph node tissue sample was divided
into two parts: The first was used for H&E staining, and the
second was stored in liquid nitrogen. A total of 23 paired axillary
lymph nodes were identified to be positive.
A total of 207 tissue samples were obtained from the
female patients with breast disease who underwent surgery
(including radical mastectomy, modified radical mastectomy and
local excision) in Hunan Cancer Hospital (Changsha, China) between
May 1996 and March 2008 and used for immunohistochemical staining.
Among them, 15 patients were diagnosed with BBD, including breast
fibrocystic changes, atypic proliferation and fibroadenoma, and 192
patients were diagnosed with breast cancer with (n=109) or without
(n=83) regional lymph node metastases. All tissue slides were
examined by two pathologists in the Pathology Department of Hunan
Cancer Hospital. The pathological parameters of the patients are
summarized in Table I. This study was
approved by the Research Ethics Committee of Hunan Cancer Hospital
and informed consent was obtained from all of the patients.
 | Table I.Clinicopathological characteristics
of 192 patients with breast cancer. |
Table I.
Clinicopathological characteristics
of 192 patients with breast cancer.
| Parameters | Number |
|---|
| Median age, years
(range) | 45 (30–73) |
| Median follow up
period, months (range) | 80 (8–210) |
| Histological type,
n (%) |
|
|
Ductal | 156 (81.3) |
|
Lobular | 36 (18.8) |
| Histological grade,
n (%) |
|
|
I–II | 35 (18.2) |
|
III | 157 (81.8) |
| Nodal status, n
(%) |
|
|
Negative | 83 (43.2) |
|
Positive | 109 (56.8) |
| Tumor size, cm; n
(%) |
|
| ≤2 | 58 (30.2) |
|
2–5 | 111 (57.8) |
|
>5 | 20 (10.4) |
|
Unknown | 3 (0.5) |
| Clinical stage, n
(%) |
|
| I | 29 (15.1) |
| II | 98 (51.0) |
|
III | 62 (32.3) |
|
Unknown | 3 (0.5) |
| ER, n (%) |
|
|
Negative | 90 (46.9) |
|
Positive | 102 (53.1) |
| PR, n (%) |
|
|
Negative | 78 (40.6) |
|
Positive | 114 (59.4) |
| Cerb B-2, n
(%) |
|
|
Negative | 58 (30.2) |
|
Positive | 124 (64.6) |
|
Unknown | 10 (5.2) |
| Menstrual history,
n (%) |
|
|
Premenopause | 126 (65.6) |
|
Postmenopause | 66 (34.4) |
iTRAQ proteomic analysis
The iTRAQ proteomic analysis was performed using the
Fitgene iTRAQ Proteomics Platform (http://www.fitgene.com) according to the
manufacturer's protocol. The tissue was placed in liquid nitrogen
and ground it until it broke down. A total of 50 mg smashed tissue
was added into a 1.5 ml eppendorf tube with 300 µl
radioimmunoprecipitation assay lysate by pipette and agitated
repeatedly. The lysate cells release a viscous material which was
sonicated using ultrasound (5%, 1 sec on, 1 sec off, 10 sec on
ultrasound, cooled on ice and repeated 8 times), and then
centrifuged (4°C, 14,000 × g, 20 min) to obtain the supernatant.
The prepared lysates (200 µg) were prepared with 4 µl reducing
reagent (dithiotreitol; United States Biological, Salem, MA, USA)
for 1 h at 60°C and then blocked with 2 µl cysteine
Triethylammonium bicarbonate (TEAB; Sigma-Aldrich; Merck KgaA,
Darmstadt, Germany) for 10 min at room temperature. Following
centrifugation (14,000 × g, 20 min, room temperature) and solution
collection, 100 µl 1M TEAB was added and discarded and the solution
at the bottom of the tube following centrifugation (14,000 × g, 20
min) was collected, which was repeated 3 times. Subsequently,
trypsin (trypsin to protein mass ratio, 1:100) was added and after
2 h trypsin was added again (trypsin to protein mass ratio, 1:50).
1M TEAB was added to make the volume of solution to 50 µl and the
cell samples were stored overnight at 37°C. The digested peptide
solution was mixed, centrifuged (14,000 × g, 4°C, 20 min), 50 µl 1
M TEAB was added, and sample were centrifuged again (14,000 × g,
4°C, 20 min) to obtain 100 µl digested sample. A total of 50 µl
prepared protein sample and iTRAQ reagents were mixed according to
the manufacturer's protocol, and the Dionex Ultimate 3000 system
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), was used to
analyze the labeled protein sample. Protein identification and
quantification were achieved by searching the UniProt database
(18). Proteomics profiling and
database searching based on TripleTOF® 5600+ System
(SCIEX, Framingham, MA, USA) and 6 ProteinPilot 4.0 (SCIEX) were
performed according to the manufacturer's protocol.
To ensure the reliability and stability of the
reported data, the following steps were performed for data quality
control. First, prior to database searching, ‘Run False Discovery
Rate Analysis’ was selected in the software ProteinPilot
(ProteinPilot™ Software, version 4.5) for FDR control (SCIEX).
Second, according to the SCIEX manufacturer's protocol, the
software was set up as Unused ≥1.3 to ensure credibility ≥95%.
Third, results identified by the reverse database were removed.
Fourth, PMLN proteins with abnormal levels of quantified expression
were excluded, specifically, when the expression of protein S100-A8
in PMLN was <0.05-fold or >20-fold compared with that in PBT.
Finally, proteins with abnormal quantification between technical
repetition and biological repetition were removed. A >1.5-fold
change of protein expression was considered to indicate a
significant difference between fresh PBT and fresh PMLN tissue.
Gene ontology (GO) analysis of protein S100-A8 was performed,
including biological process (GO ID: 0034641, 0009058, 0007165,
0006950, 0008219, 0002376, 0009056, 0042592, 0007010, 0048870,
0040011, 0051604, 0007155, 0040007), cellular component (GO ID:
0005634, 0005737, 0005829, 0043226, 0005576, 0005886, 0005856,
0005615) and molecular function (GO ID: 0043167, 0008092, 0008289),
by searching GO browsers.
IHC staining
Paraffin-embedded tissue (4 µm thick, fixed in 10%
neutral formalin for 12–24 h at room temperature) sections were
heated at 60°C for 2 h, and then dewaxed by soaking in
dimethylbenzene for 10 min twice, followed by rinsing in a graded
ethanol series for 5 min at each concentration. Subsequently, the
slides were immersed in citrate retrieval solution (trisodium
citrate 3 g, citric acid 0.4 g, pH 6.0, 1,000 ml; Fuzhou Maixin
Biotech Co., Ltd., Fuzhou, China), heated using a microwave and
cooled to room temperature. A solution of 3%
H2O2 was added to the tissue samples for 15
min at room temperature, and the slides were subsequently treated
with ultra V block (TL-125-QHD; Thermo Fisher Scientific, Inc.) for
15 min at room temperature to block nonspecific binding.
Subsequently, an anti-protein S100-A8 antibody (1:100; ab92331;
Abcam, Cambridge, UK) was added to the tissue sections, and the
slides were incubated at 4°C overnight. The following day, sections
were warmed at room temperature for 1 h and incubated with primary
antibody amplifier (TL-125-QHD) for 15 min at room temperature.
Following this, the tissue samples were incubated with HRP polymer
(TL-125-QHD) for 15 min at room temperature. Next,
3,3′-Diaminobenzidine (DAB; ready-to-use; cat no. TL-125-QHD;
Thermo Fisher Scientific, Inc.) was used to stain the tissue
sections for a few seconds at room temperature and evaluated by
light microscopy (Olympus Corporation, Tokyo, Japan). Finally, the
tissue sections were washed with water and stained with
hematoxylin. Negative controls were performed by replacing DAB with
PBS.
Evaluation of immunostaining
IHC staining was evaluated by two independent
pathologists (Pathology Department of Hunan Cancer Hospital and The
Affiliated Cancer Hospital of Xiangya School of Medicine, Central
South University, Changsha, China). A total immunostaining score
was calculated as the proportion score × the intensity score. The
proportion score described the estimated fraction of
positive-stained tumor cells as follows: 0, 0–4%; 1, 5–25%; 2,
26–50%; 3, 51–75%; 4, >75%. The intensity score represented the
estimated staining intensity as follows: 0, no or marginal
staining; 1, weak; 2, moderate; 3, intense. The total score ranged
from 0–12, with 0 representing negative, 1–4 representing weak
positive, 6–8 indicating moderate positive and 9–12 describing an
intense positive score (19,20).
Statistical analysis
The age and follow-up time of patients is presented
as the median (range). Patients were divided into positive and
negative groups according to the total immunostaining score for
protein S100-A8. All statistical analyses were performed using SPSS
version 20.0 (IBM SPSS, Armonk, NY, USA). McNemar's test was used
to examine the difference in S100-A8 protein expression between the
positive rate of the PBT and PMLN FFPE tissues. Additionally, the
χ2 test was utilized to analyze the difference in the
positive rate of protein S100-A8 expression between the BBD, NMBT,
PBT and PMLN tissues. Associations between protein S100-A8 positive
expression and the clinicopathological characteristics of 192
patients were also examined using the χ2 test. The age
and Survival rates of patients with breast cancer with protein
S100-A8 positive or negative expression were calculated by using
Kaplan-Meier analysis, and were examined using the log-rank test.
The Cox proportional hazards model was utilized to determine the
association between protein S100-A8 and breast cancer prognosis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
iTRAQ proteomic analysis and GO
analysis of protein S100-A8
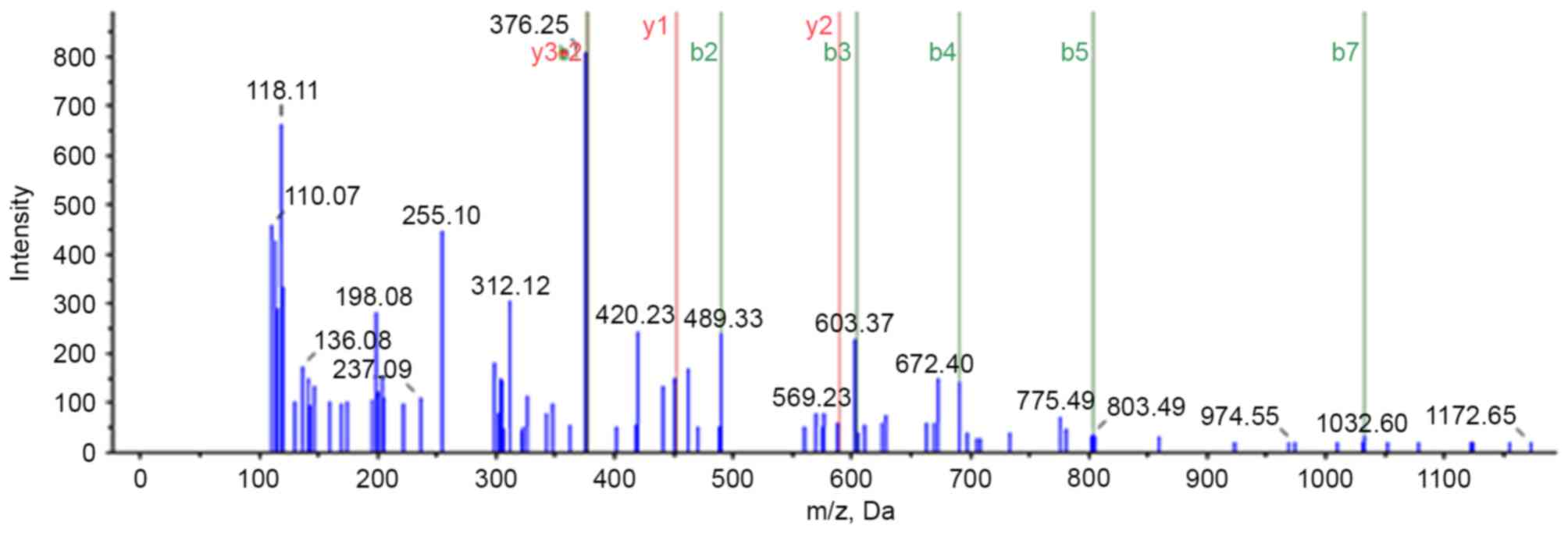
A previous study utilized the iTRAQ proteomic method
to analyze differences in protein expression between PBT and PMLN
tissues (11). A total of 4,837
proteins were identified, of which 643 were differentially
expressed proteins, including 402 upregulated and 241
downregulated, in PMLN tissue, compared with PBT tissue. Protein
S100-A8 was observed upregulated 1.72-fold in PMLN tissue, compared
to PBT tissue. Further mass spectrometry analysis identified
S100-A8 protein (Fig. 1). Based on
the GO analysis performed in the current study (Table II), protein S100-A8 is involved in
various biological processes, including cellular nitrogen compound
metabolism, biosynthetic processes and signal transduction.
Furthermore, GO analysis (http://www.uniprot.org/uniprot/P05109) revealed that
it is an important constituent of numerous cellular components,
including the nucleus, cytoplasm, cytosol, organelles,
extracellular regions, plasma membrane and cytoskeleton. The
molecular functions of protein S100-A8 include ion binding,
cytoskeleton protein binding and lipid binding. This may suggest
that protein S100-A8 serves a role in breast cancer metastasis,
although further studies are required to elucidate this
process.
 | Table II.Biological properties of protein
S100-A8. |
Table II.
Biological properties of protein
S100-A8.
| Name | Accession
number | Molecular weight,
Da | Isoelectric
point | Biological
processes | Cellular
component | Molecular
function |
|---|
| Protein
S100-A8 |
sp|P05109|S10A8-HUMAN | 10834.51 | 6.9781 | Cellular nitrogen
compound metabolic process | Nucleus | Ion binding |
|
|
|
|
|
| Cytoplasm | Cytoskeleton
protein binding |
|
|
|
|
| Biosynthetic
process | Cytosol |
|
|
|
|
|
| Signal
transduction | Organelle |
|
|
|
|
|
| Response to
stress | Extracellular
region |
|
|
|
|
|
| Cell death | Plasma
membrane |
|
|
|
|
|
| Immune system
process | Cytoskeleton |
|
|
|
|
|
| Catabolic
process | Extracelluar
space |
|
|
|
|
|
| Homeostatic
process |
|
|
|
|
|
|
| Cytoskeleton
organization |
|
|
|
|
|
|
| Cell motility |
|
|
|
|
|
|
| Locomotion |
|
|
|
|
|
|
| Protein
maturation |
|
|
|
|
|
|
| Cell adhesion |
|
|
|
|
|
|
| Growth |
|
|
High expression levels of protein
S100-A8 were correlated with metastatic lymph nodes in breast
cancer
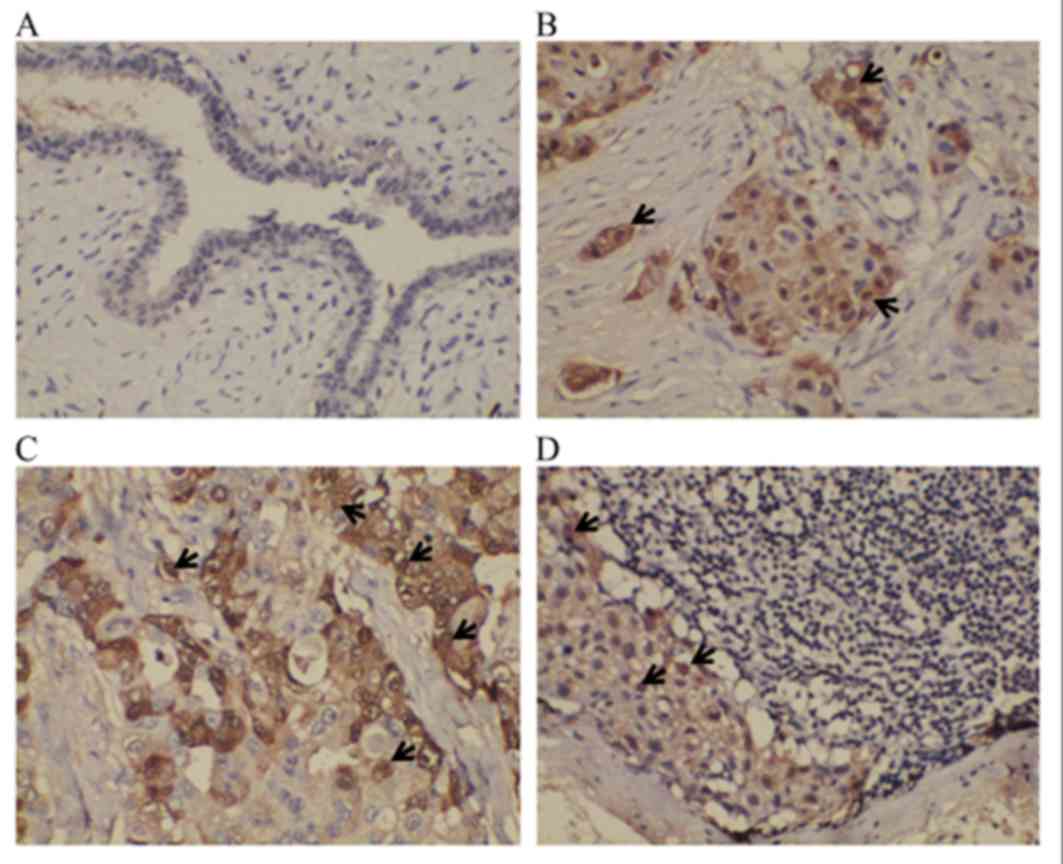
IHC was utilized to examine the positive expression
of protein S100-A8 in the NMBT, PBT and PMLN tissues, as well as
the BBD control tissues. It was demonstrated that S100-A8 was
located primarily in the cytoplasm of cancerous cells, whilst the
nuclei of certain cancerous cells also exhibited sporadic,
low-moderate expression of this protein (Fig. 2). In the BBD tissue, epithelial cells
exhibited low or negative expression of protein S100-A8, whereas
the staining of protein S100-A8 in the PBT tissue was more intense,
compared with NMBT and PMLN tissues (Fig.
2). The majority of tissue types exhibited negative expression
of protein S100-A8, and moderate or intense expression of protein
S100-A8 was observed in BBD and NMBT tissues (Table III). A number of the PBT and PMLN
tissues exhibited moderate staining (Table III). The positive expression
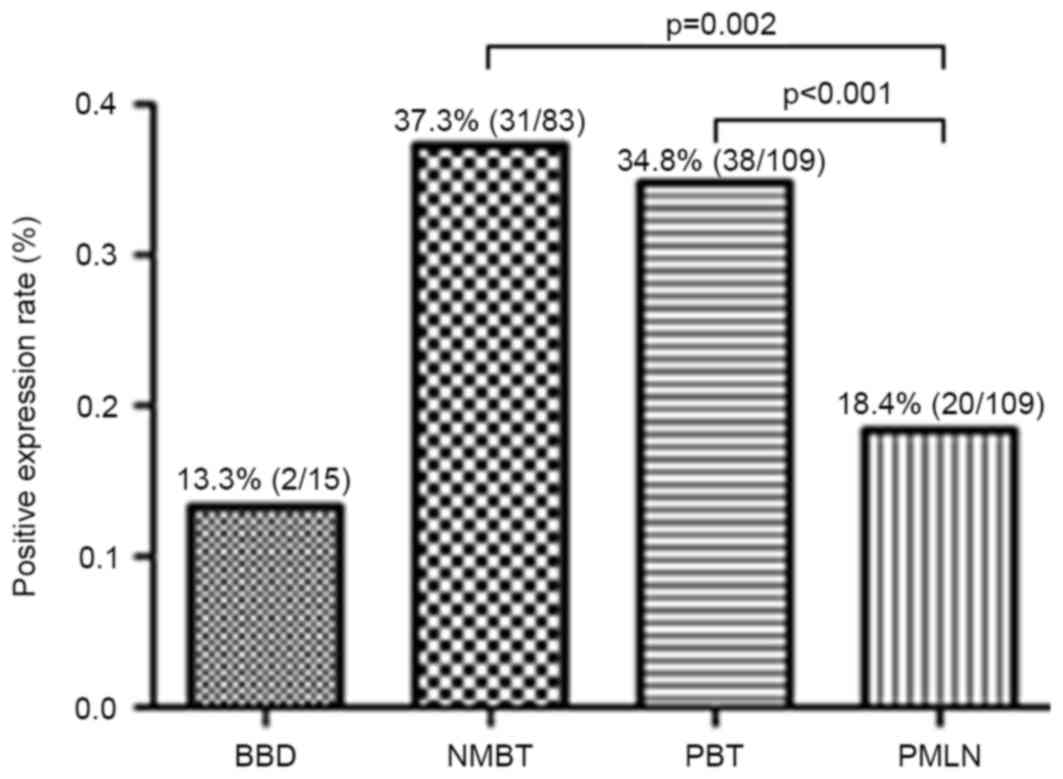
percentages of protein S100-A8 in NMBT, PBT, PMLN and BBD control
tissues were 37.3 (31/83), 34.8 (38/109), 18.4 (20/109) and 13.3%
(2/15), respectively (Fig. 3).
Compared with PMLN, the positive expression rate of protein S100-A8
in NMBT (P=0.002) and PBT (P<0.001) tissues was significantly
higher. However, no significant differences were observed between
any other types of tissue used in the study (BBD vs. NMBT, P=0.063;
BBD vs. PBT, P=0.140; BBD vs. PMLN, P=1.000; PBT vs MNBT, P=0.643)
(Fig. 3).
 | Table III.Expression of protein S100-A8 in
NMBT, PBT, PMLN and control BBD tissues. |
Table III.
Expression of protein S100-A8 in
NMBT, PBT, PMLN and control BBD tissues.
|
| Protein S100-A8
expression |
|---|
|
|
|
|---|
|
| None, TIS (0) | Weak, TIS
(1–4) | Moderate, TIS
(6,8) | Intense, TIS
(9,12) |
|---|
|
|
|
|
|
|
|---|
| Variables | n | % | n | % | n | % | n | % |
|---|
| BBD | 13 | 86.7 | 2 | 13.3 | 0 | 0 | 0 | 0 |
| NMBT | 52 | 62.7 | 31 | 37.3 | 0 | 0 | 0 | 0 |
| PBT | 71 | 65.1 | 36 | 33.0 | 2 | 1.8 | 0 | 0 |
| PMLN | 89 | 81.7 | 17 | 15.6 | 3 | 2.8 | 0 | 0 |
Correlations between protein S100-A8
expression levels and clinicopathological features
The association between protein S100-A8 and the
clinicopathological features of 192 patients with breast cancer was
further analyzed. The results indicated that the expression of this
protein was associated with breast cancer histological type
(P=0.022) and estrogen receptor expression (ER; P=0.009; Table IV). No significant correlation was
observed between protein S100-A8 expression and patient age at
diagnosis, histological grade, nodal status, tumor size, clinical
stage, progesterone receptor (PR) expression, c-erbB2 expression,
menstrual history (P>0.05; Table
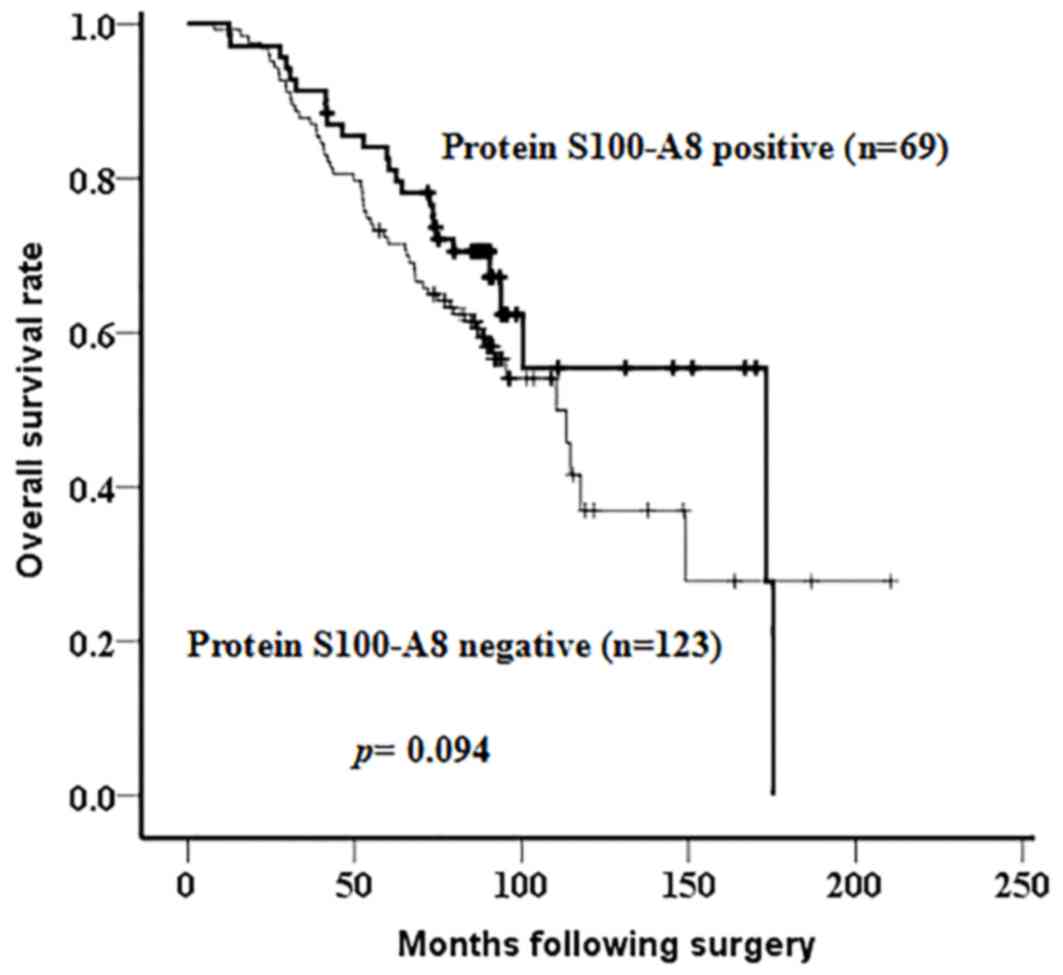
IV). Additionally, Kaplan-Meier analysis, followed by a
log-rank test, was applied to identify the prognostic role of
protein S100-A8. Although there was no significant difference
observed in the overall survival rate between patients with breast
cancer with negative or positive expression of protein S100-A8
(P=0.094), a possible association between the positive expression
of protein S100-A8 and an increased survival time following surgery
was observed (Fig. 4). According to
the Cox proportional hazards model, histological grade (P=0.031)
and nodal status (P=0.001) were risk factors for poor prognosis of
patients with breast cancer (Table
V).
 | Table IV.Association between protein S100-A8
expression levels and the clinicopathological characteristics of
192 patients with breast cancer. |
Table IV.
Association between protein S100-A8
expression levels and the clinicopathological characteristics of
192 patients with breast cancer.
|
|
| S100-A8 protein
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | N | No | Yes | P-value |
|---|
| Age at diagnosis,
years |
|
|
|
|
|
<50 | 114 | 76 | 38 | 0.363 |
|
≥50 | 78 | 47 | 31 |
|
| Histological
type |
|
|
|
|
|
Ductal | 156 | 94 | 62 | 0.022a |
|
Lobular | 36 | 29 | 7 |
|
| Histological
grade |
|
|
|
|
|
I–II | 35 | 20 | 15 | 0.345 |
|
III | 157 | 103 | 54 |
|
| Nodal status |
|
|
|
|
|
Negative | 83 | 52 | 31 | 0.722 |
|
Positive | 109 | 71 | 38 |
|
| Tumor size, cm |
|
|
|
|
| ≤2 | 58 | 40 | 18 | 0.630 |
|
2–5 | 111 | 69 | 42 |
|
|
>5 | 20 | 12 | 8 |
|
|
Unknown | 3 |
|
|
|
| Clinical stage |
|
|
|
|
| I | 29 | 20 | 9 | 0.313 |
| II | 98 | 66 | 32 |
|
|
III | 62 | 35 | 27 |
|
|
Unknown | 3 |
|
|
|
| ER |
|
|
|
|
|
Negative | 90 | 49 | 41 | 0.009a |
|
Positive | 102 | 74 | 28 |
|
| PR |
|
|
|
|
|
Negative | 78 | 48 | 30 | 0.547 |
|
Positive | 114 | 75 | 39 |
|
| Cerb B-2 |
|
|
|
|
|
Negative | 58 | 42 | 16 | 0.118 |
|
Positive | 124 | 75 | 39 |
|
|
Unknown | 10 |
|
|
|
| Menstrual
history |
|
|
|
|
|
Premenopause | 126 | 84 | 42 | 0.299 |
|
Postmenopause | 66 | 39 | 27 |
|
 | Table V.Cox regression analysis of risk
factors for short overall survival time of breast cancer
patients. |
Table V.
Cox regression analysis of risk
factors for short overall survival time of breast cancer
patients.
| Variables | B | SE | Wald | Sig. | Exp(B) (95%
CI) |
|---|
| Nodal status | 0.917 | 0.273 | 11.243 | 0.001a | 2.501
(1.464–4.275) |
| Histological
grade | 0.924 | 0.428 | 4.649 | 0.031a | 2.518
(1.088–5.830) |
Discussion
Breast cancer is a significant global health burden,
causing a substantial number of annual mortalities (2). This disease is hormone dependent, and
mortality rates decreased following linking hormone therapy with
breast cancer (21). However, this
form of cancer is also a systemic disease and ~10–15% of patients
with aggressive disease develop distant metastases within three
years of the initial primary tumor diagnosis (5).
Metastasis remains a key risk factor for lowering
the overall survival rate in breast cancer, with the regional
axillary lymph nodes becoming involved first during this process
(22). A previous study demonstrated
that, among patients with stage 0–II breast cancer, local
recurrences were 2.8 times less frequent in radically treated
patients, compared with conservatively treated patients, for whom
lymph node recurrences were twice as common (23). Accurate and effective evaluation of
the status of axillary lymph nodes is of prognostic significance in
patients with breast cancer (22).
The S100 protein family is exclusive to vertebrates,
and is comprised of 10–20 kDa acidic proteins that have previously
been associated with inflammation and cancer (24). The genes encoding ≥16 of the S100
family members, including S100-A8, are clustered on the human
chromosomal region 1q21, which frequently undergoes chromosomal
rearrangement during tumor development (25). Additionally, protein S100-A8 has been
implicated in tumor cell proliferation and metastasis regulation
(25–27). Protein S100-A8, also known as
myeloid-related protein 8, is a small intracellular calcium-binding
protein that has also been reported to be expressed and secreted in
the extracellular matrix of prostate cancer cells (28). Katanov et al (29) emphasized the important role of
inflammation in breast cancer stroma, and suggested that nuclear
factor (NF)-κB may be a potential inhibitory target in
tumor-adjacent stromal cells. Additionally, protein S100-A8 has
been identified to be a novel member of the NF-κB signaling pathway
(30), participating in the
development and progression of laryngeal squamous cell carcinoma
via its interaction with human leukocyte antigen B (31). A previous study also demonstrated that
zinc may promote and impede tumorigenesis by activating and
suppressing the interaction between protein S100-A8 and the
receptor for advanced glycation endproducts, as well as downstream
NF-signaling (32).
In a previous study, the protein expression profiles
in primary tumor and paired metastatic lymph node tissues were
compared using the iTRAQ proteomic method, during which protein
S100-A8 was identified to be upregulated (1.72-fold) in PMLN,
compared with PBT (11). This
suggested a potential association between protein S100-A8 and
breast cancer metastasis (11).
Therefore, IHC staining was applied to analyze the differences in
S100-A8 protein expression in NMBT, PBT, PMLN and BBD control
tissues. However, IHC staining exhibited decreased protein S100-A8
expression levels in PMLN, compared with PBT. The contrast between
these two results is potentially due to the limited number of fresh
tissue samples used in the current study. Furthermore, PMLN with
the largest breast cancer metastases, or where lymph tissue was
completely replaced by metastatic tissue, were preferentially
selected. Although there is a discrepancy between the iTRAQ
proteomic analysis and the IHC staining results, the IHC sample
size was larger, and the expression levels of protein S100-A8
exhibited a significant difference between PBT and PMLN tissues in
iTRAQ analysis and IHC staining. These results may suggest that
protein S100-A8 is a potential candidate metastatic marker in
breast cancer.
In the present study, the correlation between
protein S100-A8 expression and the clinicopathological features of
207 patients with breast cancer was analyzed. The results
demonstrated that protein S100-A8 expression levels were
significantly associated with the histological type (P=0.022) and
ER positive expression (P=0.009) of breast cancer tumors.
Concordantly, Parris et al (33) proposed a combined predictive model for
breast cancer outcomes, containing a four-marker panel
(α-2-glycoprotein 1, zinc-binding, prolactin-inducible protein,
S100-A8 and ubiquitin conjugating enzyme E2 C), which suggested a
significant correlation with histological grade, tumor inflammatory
cell infiltration, ER and PR status.
In addition, the present study applied Kaplan-Meier
analysis to analyze the impact of altered S100-A8 expression levels
on the prognosis of patients with breast cancer. Although no
significant association was observed between the overall survival
rate of patients with breast cancer and the expression of this
protein (P=0.094), positive S100-A8 expression was revealed to
potentially contribute to a longer overall survival time for
patients with breast cancer. This protein has also been reported to
be a survival-associated factor in oropharyngeal squamous cell
carcinoma (34). However, protein
S100-A8 was also demonstrated to be a marker of poor prognosis for
invasive ductal carcinoma of the breast (35). The aforementioned four-marker panel
proposed by Parris et al (33)
may be used to predict an unfavorable clinical outcome of patients
with breast cancer. The present study implemented the Cox
proportional hazards model, which demonstrated that protein S100-A8
is not an independent risk factor for prognosis in breast
cancer.
S100-A8 also assembles heterodimers with S100-A9,
which increases matrix metalloproteinase (MMP)-2 or MMP-12
expression and activity to promote tumor cell migration and
invasion (36,37). This suggests that the interaction of
S100-A8 within heterodimers is able to affect its activity during
the process of breast cancer progression. Therefore, further
studies examining the role of S100-A8 in breast cancer metastasis,
and using a larger number of tissue specimens, are required.
In conclusion, a number of proteins associated with
breast cancer metastasis were identified using iTRAQ. Protein
S100-A8 presented a positive association with breast cancer
metastasis, which may provide a potential biomarker for predicting
breast cancer lymph node metastasis. As the exact association of
this protein with the prognosis of patients with breast cancer, and
the mechanism underlying the process by which it may promote tumor
cell migration and invasion is unclear, further studies are
required to investigate the function of S100-A8 in this complex
metastatic network. This may provide a novel and effective
prognostic biomarker, aiding the treatment of breast cancer.
Acknowledgements
This study was supported by the Hunan Province
Science and Technology Project (grant no. 2014FJ6090). The authors
would like to thank the proteomic technique platform Fitgene
Biotechnology Co., Ltd., (Guangzhou, China) for providing technical
support and professional advice regarding iTRAQ proteomic
technology.
Glossary
Abbreviations
Abbreviations:
|
iTRAQ
|
isobaric tags for relative and
absolute quantitation
|
|
PBT
|
primary breast tumor
|
|
PMLN
|
paired metastatic lymph node
|
|
iTRAQ
|
isobaric tags for relative and
absolute quantitation
|
|
NMBT
|
non-metastatic breast tumor
|
|
BBD
|
benign breast disease
|
|
FFPE
|
formalin fixed paraffin-embedded
|
|
IHC
|
immunohistochemistry
|
|
TIS
|
total immunostaining score
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Libson S and Lippman M: A review of
clinical aspects of breast cancer. Int Rev Psychiatry. 26:4–15.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng H, Zheng R, Zhang S, Zou X and Chen
W: Female breast cancer statistics of 2010 in China: Estimates
based on data from 145 population-based cancer registries. J Thorac
Dis. 6:466–470. 2014.PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pinheiro DJ, Elias S and Nazário AC:
Axillary lymph nodes in breast cancer patients: Sonographic
evaluation. Radiol Bras. 47:240–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hill JJ, Tremblay TL, Pen A, Li J,
Robotham AC, Lenferink AE, Wang E, O'Connor-McCourt M and Kelly JF:
Identification of vascular breast tumor markers by laser capture
microdissection and label-free LC-MS. J Proteome Res. 10:2479–2493.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng Q, Zhang P, Wu Z, Xue P, Lu D, Ye Z,
Zhang X, Huang Z, Feng J, Song L, et al: Quantitative proteomics
reveals ER-α involvement in CD146-induced epithelial-mesenchymal
transition in breast cancer cells. J Proteomics. 103:153–169. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lagadec C, Romon R, Tastet C, Meignan S,
Com E, Page A, Bidaux G, Hondermarck H and Le Bourhis X: Ku86 is
important for TrkA overexpression-induced breast cancer cell
invasion. Proteomics Clin Appl. 4:580–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen ZC: Advances in cancer proteomics
study. Ai Zheng. 23:113–117. 2004.(In Chinese). PubMed/NCBI
|
|
11
|
Zeng L, Zhong J, He G, Li F, Li J, Zhou W,
Liu W, Zhang Y, Huang S, Liu Z and Deng X: Identification of
nucleobindin-2 as a potential biomarker for breast cancer
metastasis using iTRAQ-based quantitative proteomic analysis. J
Cancer. 8:3062–3069. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi DK, Li ZJ, Chang IK, Yeo MK, Kim JM,
Sohn KC, Im M, Seo YJ, Lee JH, Kim CD and Lee Y:
Clinicopathological roles of S100A8 and S100A9 in cutaneous
squamous cell carcinoma in vivo and in vitro. Arch Dermatol Res.
306:489–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foell D and Roth J: Proinflammatory S100
proteins in arthritis and autoimmune disease. Arthritis Rheum.
50:3762–3771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basso D, Bozzato D, Padoan A, Moz S,
Zambon CF, Fogar P, Greco E, Scorzeto M, Simonato F, Navaglia F, et
al: Inflammation and pancreatic cancer: Molecular and functional
interactions between S100A8, S100A9, NT-S100A8 and TGFβ1. Cell
Commun Signal. 12:202014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi JH, Shin NR, Moon HJ, Kwon CH, Kim
GH, Song GA, Jeon TY, Kim DH, Kim DH and Park DY: Identification of
S100A8 and S100A9 as negative regulators for lymph node metastasis
of gastric adenocarcinoma. Histol Histopathol. 27:1439–1448.
2012.PubMed/NCBI
|
|
16
|
Mirza Z, Schulten HJ, Farsi HM,
Al-Maghrabi JA, Gari MA, Chaudhary AG, Abuzenadah AM, Al-Qahtani MH
and Karim S: Impact of S100A8 expression on kidney cancer
progression and molecular docking studies for kidney cancer
therapeutics. Anticancer Res. 34:1873–1884. 2014.PubMed/NCBI
|
|
17
|
Duan L, Wu R, Ye L, Wang H, Yang X, Zhang
Y, Chen X, Zuo G, Zhang Y, Weng Y, et al: S100A8 and S100A9 are
associated with colorectal carcinoma progression and contribute to
colorectal carcinoma cell survival and migration via Wnt/b-catenin
pathway. PLoS One. 26:e620922013. View Article : Google Scholar
|
|
18
|
The UniProt Consortium, . UniProt: The
universal protein knowledgebase. Nucleic Acids Res. 45:D158–D169.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang X, Xu X, Ma J, Xue X, Li Z, Deng P,
Zhang S, Zhi Y, Chen J and Dai D: NDRG1 expression is related to
the progression and prognosis of gastric cancer patients through
modulating proliferation, invasion and cell cycle of gastric cancer
cells. Mol Biol Rep. 41:6215–6223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gastl G, Spizzo G, Obrist P, Dünser M and
Mikuz G: Ep-CAM overexpression in breast cancer as a predictor of
survival. Lancet. 356:1981–1982. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chlebowski RT, Kuller LH, Prentice RL,
Stefanick ML, Manson JE, Gass M, Aragaki AK, Ockene JK, Lane DS,
Sarto GE, et al: Breast cancer after use of estrogen plus progestin
in postmenopausal women. N Engl J Med. 360:573–587. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu D, Chen Y, Deng M, Xie G, Wang J,
Zhang L, Liu Q, Yuan P and Feng X: Lymph node ratio and breast
cancer prognosis: A meta-analysis. Breast Cancer. 21:1–9. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ursaru M, Jari I, Popescu R, Negru D, Naum
A and Scripcariu V: Multifactorial analysis of local and lymph node
recurrences after conservative or radical surgery for stage 0–II
breast cancer. Rev Med Chir Soc Med Nat Lasi. 118:1062–1067.
2014.
|
|
24
|
Foell D, Wittkowski H, Vogl T and Roth J:
S100 proteins expressed in phagocytes: A novel group of
damage-associated molecular pattern molecules. J Leukoc Biol.
81:28–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SK, Kim EJ, Leem SH, Ha YS, Kim YJ and
Kim WJ: Identification of S100A8-correlated genes for prediction of
disease progression in non-muscle invasive bladder cancer. BMC
Cancer. 10:212010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cross SS, Hamdy FC, Deloulme JC and Rehman
I: Expression of S100 proteins in normal human tissues and common
cancers using tissue microarrays: S100A6, S100A8, S100A9 and
S100A11 are all overexpressed in common cancers. Histopathology.
46:256–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salama I, Malone PS, Mihaimeed F and Jones
JL: A review of the S100 proteins in cancer. Eur J Surg Oncol.
34:357–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hermani A, Hess J, De Servi B, Medunjanin
S, Grobholz R, Trojan L, Angel P and Mayer D: Calcium-binding
proteins S100A8 and S100A9 as novel diagnostic markers in human
prostate cancer. Clin Cancer Res. 11:5146–5152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Katanov C, Lerrer S, Liubomirski Y,
Leider-Trejo L, Meshel T, Bar J, Feniger-Barish R, Kamer I,
Soria-Artzi G, Kahani H, et al: Regulation of the inflammatory
profile of stromal cells in human breast cancer: Prominent roles
for TNF-α and the NF-κB pathway. Stem Cell Res Ther. 6:872015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gebhardt C, Németh J, Angel P and Hess J:
S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol.
72:1622–1631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu WN, Guo Y, Huang DF, Shang C and Sun
KL: Novel partners of S100A8 identified in laryngeal cancer cell
lines. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 24:266–270.
2007.PubMed/NCBI
|
|
32
|
Taccioli C, Wan SG, Liu CG, Alder H,
Volinia S, Farber JL, Croce CM and Fong LY: Zinc replenishment
reverses overexpression of the proinflammatory mediator S100A8 and
esophageal preneoplasia in the rat. Gastroenterology. 136:953–966.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parris TZ, Kovács A, Aziz L, Hajizadeh S,
Nemes S, Semaan M, Forssell-Aronsson E, Karlsson P and Helou K:
Additive effect of the AZGP1, PIP, S100A8 and UBE2C molecular
biomarkers improves outcome prediction in breast carcinoma. Int J
Cancer. 134:1617–1629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Funk S, Mark R, Bayo P, Flechtenmacher C,
Grabe N, Angel P, Plinkert PK and Hess J: High S100A8 and S100A12
protein expression is a favorable prognostic factor for survival of
oropharyngeal squamous cell carcinoma. Int J Cancer. 136:2037–2046.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arai K, Takano S, Teratani T, Ito Y,
Yamada T and Nozawa R: S100A8 and S100A9 overexpression is
associated with poor pathological parameters in invasive ductal
carcinoma of the breast. Curr Cancer Drug Targets. 8:243–252. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwon CH, Moon HJ, Park HJ, Choi JH and
Park DY: S100A8 and S100A9 promotes invasion and migration through
p38 mitogen-activated protein kinase-dependent NF-κB activation in
gastric cancer cells. Mol Cells. 35:226–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Silva EJ, Argyris PP, Zou X, Ross KF and
Herzberg MC: S100A8/A9 regulates MMP-2 expression and invasion and
migration by carcinoma cells. Int J Biochem Cell Biol. 55:279–287.
2014. View Article : Google Scholar : PubMed/NCBI
|