Introduction
Steviol glycosides, a family of popular natural
non-nutritive sweeteners from leaves of Stevia rebaudiana
bertoni, are metabolized in human colon by colon bacteria. The
colonic metabolite of the primary steviol glycosides, including
rebaudioside A and stevioside, is steviol. In the colon, portions
of this steviol is absorbed and then undergoes glucuronidation in
the liver, while the remaining is identified in feces (1,2). At
present, a few of studies have been reported about the cytotoxicity
of steviol on human resource cells.
Steviol exhibits a kaurene diterpenoid structure,
similar to that of gibberellin (3).
Its rearrangement product isosteviol and steviol itself have been
used as starting reagents for synthetic medicines (4). The acceptable daily intake of steviol is
4 mg/kg body weight/day (5) and its
median lethal dose (LD50) value is 15 g/kg body weight
in rats and mice, irrespective of the gender (3). During the metabolism of steviol or
steviol glycosides, steviol is not detectable in blood, and half
maximal inhibitory concentration (IC50) value of steviol
is much higher than that of current chemotherapy agents such as
5-fluorouracil (5-FU) and doxorubicin (6). Therefore, if steviol could efficiently
inhibit cancer cells with clear mechanism, it could be expected as
a chemotherapy agent applied in large doses.
High-dose chemotherapy and chemoresistance are the
typical features of osteosarcoma treatment, which is a primary
malignant bone cancer with high morbidity (7,8). Patients
with metastasis exhibit a 5-year survival rate of only 20%
(9,10). Efficient treatment of osteosarcomas
requires systemic chemotherapy prior and subsequent to surgery
(11). The majority of chemotherapy
regimens applied in OS are based on the following drugs: High-dose
methotrexate with leucovorin rescue (12), doxorubicin (Adriamycin®,
ADM), cisplatin, and ifosfamide (13). These regimens are associated with
marked short- and long-term collateral toxic effects (14), including acute alopecia,
myelosuppression, mucositis and nausea (15). In addition, rare ADM-regimen cases of
toxic mortalities caused by early or late cardiac failure have been
identified, which were due to ADM toxicity and sepsis following
febrile neutropenia (16).
Therefore, many efforts have been made to develop
novel drugs to increase the number of options for chemotherapy in
OS, such as: Rapamycin (17);
ampelopsin (18); JQ1 (a BET protein
inhibitor) in combination with rapamycin (19); and few other small molecules (20). However, only a small number of studies
have been conducted on the use of natural medicines such as
evodiamine (21), riccardin D
(22) and piperine (8).
The anticancer activity of steviol has not been well
examined. Boonkaewwan and Burodom (22) suggested that unpurified steviol did
not present cytotoxicity on Caco-2 cells at 0.1–100 µmol/l, but it
suppressed lipopolysaccharide (LPS)-mediated tumor necrosis
factor-α, interleukin (IL)-1β and IL-6 release, and attenuated the
production of LPS-induced pro-inflammatory cytokines. However,
higher steviol dosage (200–800 µmol/l) decreased cell viability of
T84, Caco-2 and HT29 cells (23).
Steviol also inhibited renal cyst growth in a mouse model of
polycystic kidney disease (24). A
two-stage carcinogenesis model on mouse skin demonstrated that
steviol markedly inhibited the promotion and initiation stages of
lymphoblastoid cells (25). These
results suggest that steviol may be a potential chemotherapy agent
for cancer treatment.
At present, with the exception of some
aforementioned studies investigated the inhibition of the
proliferation of cancer cells by steviol, including lymphoblastoid
cells (25), none have explored its
possible molecular mechanisms. Our preliminary study indicated an
anti-cancer activity of steviol on human osteosarcoma U2OS cells
(data not shown). Therefore, the present study focused on the in
vitro anti-proliferative effects of steviol on human
osteosarcoma U2OS cells and the potential molecular mechanisms
involved.
Materials and methods
Materials and chemicals
Steviol (Sigma-Aldrich, Shanghai, China 99% purity
as determined by high performance liquid chromatography);
doxorubicin (AMD) was purchased from Shanghai Aladdin Bio-Chem
Technology Co., Ltd. (Shanghai, China). 5-FU (biological-grade
reagent, BR), dimethyl sulfoxide (DMSO, BR),
Na2CO3, NaHCO3, NaCl, KCl,
Na2HPO4·12H2O,
NaH2PO4·2H2O, EDTA disodium, SDS,
glycocoll, bromoxylenol blue, ammonium persulphate, tris
(hydroxymethyl) methyl aminomethane (BR), Ponceau (BR),
N,N,N,N-tetramethylethylenediamine (TEMED, 99%), xylene brilliant
cyanin G (BS, G250), and phenylmethylsulfonyl fluoride (PMSF, 99%)
were purchased from Sinopharm Chemical Reagent Co., Ltd.,
(Shanghai, China). Trypsin-EDTA solution, propidium iodide (PI),
Triton X-100, endonuclease (RNase A),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
penicillin-streptomycin solution (100X), BeyoECL Plus,
polyvinylidene fluoride, radioimmunoprecipitation assay (RIPA)
lysis buffer and
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
(JC-1) were purchased from Beyotime Institute of Biotechnology Co.,
Ltd. (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM)
and fetal bovine serum (FBS) were purchased from Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). All antibodies were
purchased from Cell Signaling Technologies, Inc. (Danvers, MA,
USA): Primary antibodies against cyclin-dependent kinase inhibitor
1 rabbit mAb (p21, 21 kDa; cat. no. 2947S; 1:1,000 dilution), tumor
protein 53 rabbit mAb (p53, 53 kDa; cat. no. 2527S; 1:1,000
dilution), Cyclin D1 rabbit mAb (36 kDa; cat. no. 2978T; 1:1,000
dilution), Cyclin E rabbit mAb (48 kDa; cat. no. 20808S; 1:1,000
dilution), cyclin-dependent kinase 2 (CDK2; cat. no. 78B2) rabbit
mAb (33 kDa; cat. no. 2546S; 1:1,000 dilution), B-cell lymphoma 2
(Bcl-2) mouse mAb (26 kDa; cat. no. 15071S; 1:1,000 dilution),
Bcl-2 X associated protein (Bax) rabbit mAb (20 kDa; cat. no.
5023S; 1:1,000 dilution), Caspase 3 rabbit mAb (35 kDa; cat. no.
9665S; 1:1,000 dilution), Survivin rabbit mAb (16 kDa,; cat. no.
2808S; 1:1,000 dilution), β-Actin rabbit mAb (45 kDa; cat. no.
4970S; 1:1,000 dilution) and horseradish peroxidase-conjugated
secondary antibodies (anti-rabbit IgG, HRP-linked antibody; cat.
no. 7074; anti-mouse IgG, HRP-linked antibody; cat. no. 7076). All
other reagents were of analytical grade and used as purchased,
unless otherwise stated.
Cell culture
The human osteosarcoma U2OS cell line was purchased
from Shanghai Institute of Biochemistry and Cell Biology, Chinese
Academy of Science (Shanghai, China). Cells were maintained in DMEM
supplemented with 10% FBS, 1% glutamine (200 mmol/l), penicillin
(100 IU/ml), and streptomycin (100 mg/l) in a humidified 5%
CO2 atmosphere at 37°C prior to use.
MTT assay on cell proliferation
The effect of steviol on carcinoma cell
proliferation was evaluated with an MTT assay (26,27). The
cells in the logarithmic growth phase were digested with 0.25%
trypsin and adjusted to 5,000 cells/well using DMEM complete
medium. Prior to the steviol treatment, 100 µl cell suspension was
pipetted into each well in 96-well plates and cultured for 24 h at
37°C in 5% CO2. Subsequently, cells were incubated with
steviol at 37°C in 5% CO2 for 48 h. The culture medium
was then removed and 100 µl MTT reagent (0.5 mg/ml in culture
medium) was added. Following an additional 4 h of incubation, the
MTT/medium was removed and 150 µl of DMSO was added to dissolve the
formazan crystals. Absorbance of the solution was recorded at 570
nm to calculate the inhibition rate on cell growth. Chemotherapy
agents 5-FU and ADM were employed as positive controls. Cells
without drug treatment were used as the control. All measurements
were performed in triplicate. The inhibition rate was calculated as
following:
inhibition rate(%)=A570of control–A570of
sampleA570of control×100
Colony formation assay
U2OS cells were plated in 6-well plates at a density
of 1,000/well. Following 12 h of incubation, the cells were treated
with 0, 5, 10 and 20 µg/ml of steviol. Following 14 days of
incubation, the media were removed, and the cells were washed three
times with PBS prior to the addition of 1 ml methanol to each well
for 15 min at 37°C. The cells were then washed three times with PBS
for about 1 min each and incubated with 0.1% crystal violet for 10
min at 37°C. Finally, the plates were washed five times and cell
colonies were counted with microscopy.
Cell cycle analysis
U2OS cells were plated at 2×105/well in
6-well plates and treated with steviol (0, 50, 100 and 200 µg/ml).
After 48 h, the cells were then harvested with trypsin, washed
three times for 1 min each, resuspended in cold PBS and fixed in
4°C 70% ethanol for 4 h, and storage at −20°C overnight. Next, the
cells were washed three times for 1 min each and resuspended in PBS
containing 40 µg/ml PI and 0.1 mg/ml RNase (cat. no. C1052;
Beyotime Institute of Biotechnology Co., Ltd), and then incubated
for 30 min at room temperature. PI-stained cells were analyzed
using a flow cytometer and ModFit LT 5.0 software (Verity Software
House, Inc., Topsham, ME, USA).
Mitochondrial membrane potential
detection and Hoechst 33342 staining assay
Mitochondrial membrane potential detection was
conducted with a JC-1 assay (cat. no. C2006; Beyotime Institute of
Biotechnology Co., Ltd). Briefly, following steviol treatment (0,
50, 100 and 200 µg/ml for 48 h at 37°C), cells were cultured in
24-well plates and incubated with JC-1 staining solution (5 µg/ml;
cat. no. C2006; Beyotime Institute of Biotechnology Co., Ltd.) for
20 min at 37°C. Cells were then rinsed twice with JC-1 staining
buffer. Cells on chamber slides were scanned with a fluorescence
microscope (magnification, ×400).
Hoechst 33342 staining assay
U2OS cells were seeded onto chamber slides in 6-well
plates at a density of 1×105 cells/well for 24 h of
incubation in DMEM supplemented with 10% of FBS and incubated at
37°C in 5% CO2. The medium was removed and replaced with
medium containing steviol for 24 h at 37°C in 5% CO2.
Subsequent to the removal of the medium, the cells were washed
three times for 2 min each with ice-cold PBS and then fixed with
formalin (4%, w/v) for 15 min at 37°C. Cell nuclei were
counterstained with Hoechst 33342 (10 mg/ml in PBS) for 10 min at
37°C, and then observed and imaged using fluorescence microscopy
(magnification, ×400).
Determination of apoptotic percentage
in cells
Apoptotic percentage in cells was tested using
Annexin V-fluorescein isothiocyanate (FITC)/PI double-labeled flow
cytometry. U2OS cells were plated at a density of
2×105/well in a 6-well flask and exposed to 0, 50, 100
and 200 µg/ml of steviol for 24 h at 37°C in 5% CO2.
Followed by 24 h of incubation at 37°C, cells of
2×106/well were collected, centrifuged (1,000 × g for 3
min at room temperature), and resuspended in 100 µl Annexin
blinding buffer, then stained with Annexin V-FITC (5 µl) and
propidium iodide (PI; 1 µl) for 15 min of incubation at room
temperature. Cells were detected with a FACS Calibur flow cytometer
subsequent to the addition of 400 µl Annexin-blinding buffer.
Western blot analysis
Steviol-treated cells were digested with 0.25%
trypsin and 0.2% EDTA, washed with cold (4°C) PBS three times for 1
min each, suspended in ice-cold RIPA lysis buffer containing 1 mM
PMSF and incubated on ice for 30 min. The suspension was then
centrifuged at 12,000 × g for 5 min at 4°C. The protein
concentration of lysates was measured with the Bradford method.
Equivalent amounts of protein (40 µg) were separated using a 10%
gel and SDS-PAGE and then transferred to a polyvinylidene
difluoride membrane. Membranes were blocked for 1 h at room
temperature using TBST containing 5% w/v non-fat milk, and probed
with primary antibodies overnight at 4°C following washing three
times for 5 min each. The membranes were incubated with secondary
antibody for 1 h at room temperature. Protein bands were detected
using the ChemiDoc MP imaging system (Image Lab 4.1, Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are expressed as the means ± standard deviation
from experiments performed in triplicate. Multi-group comparisons
of the means were carried out by one-way analysis of variance test
with post hoc contrasts by Student-Newman-Keuls test. All
statistical analyses were performed using SPSS software (SPSS
Statistics 20.0; IMB Corp, Armonk, NY, USA). P-values were
two-tailed; P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibition of steviol on the viability
of U2OS cells
For comparison, two common chemotherapy agents ADM
(LD50, 570 mg/kg, oral, mouse) and 5-FU
(LD50, 115 mg/kg, oral, mouse) were used as positive
contrasts in this experiment. Fig. 1
indicated that steviol presented similar inhibition rates as 5-FU,
but weaker compared with ADM; they all inhibited U2OS cell
viability in time- and dose-dependent manners. The IC50
values for steviol, ADM and 5-FU were 200, 1.2 and 250 µg/ml,
respectively, in U2OS cells after 24 h. Moreover, as demonstrated
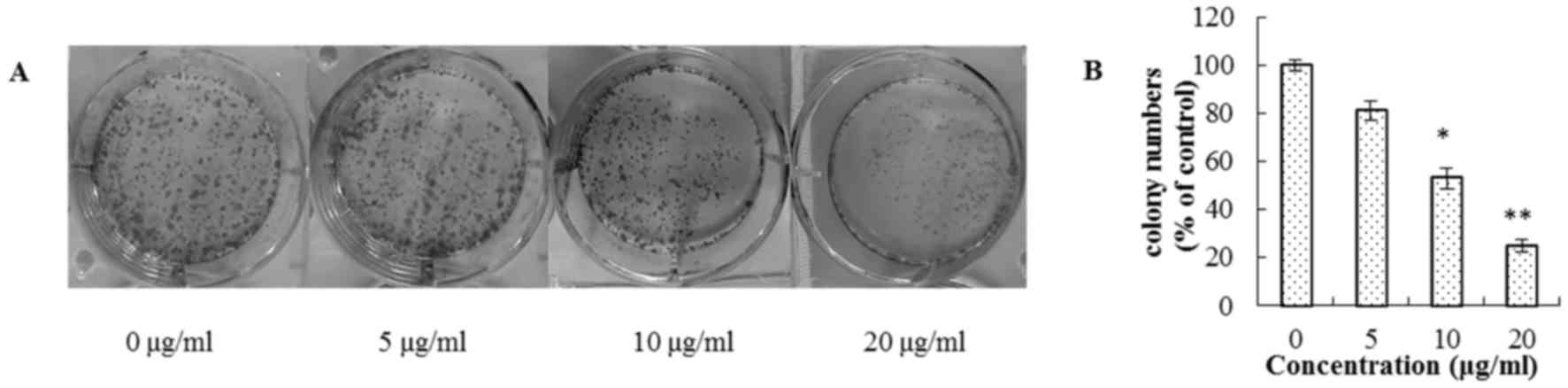
in Fig. 2, a progressive inhibition
of colony formation was observed with increasing steviol
concentrations. Furthermore, microscopic observations of the cell
morphology and numbers suggested cell apoptosis, which was examined
in subsequent experiments.
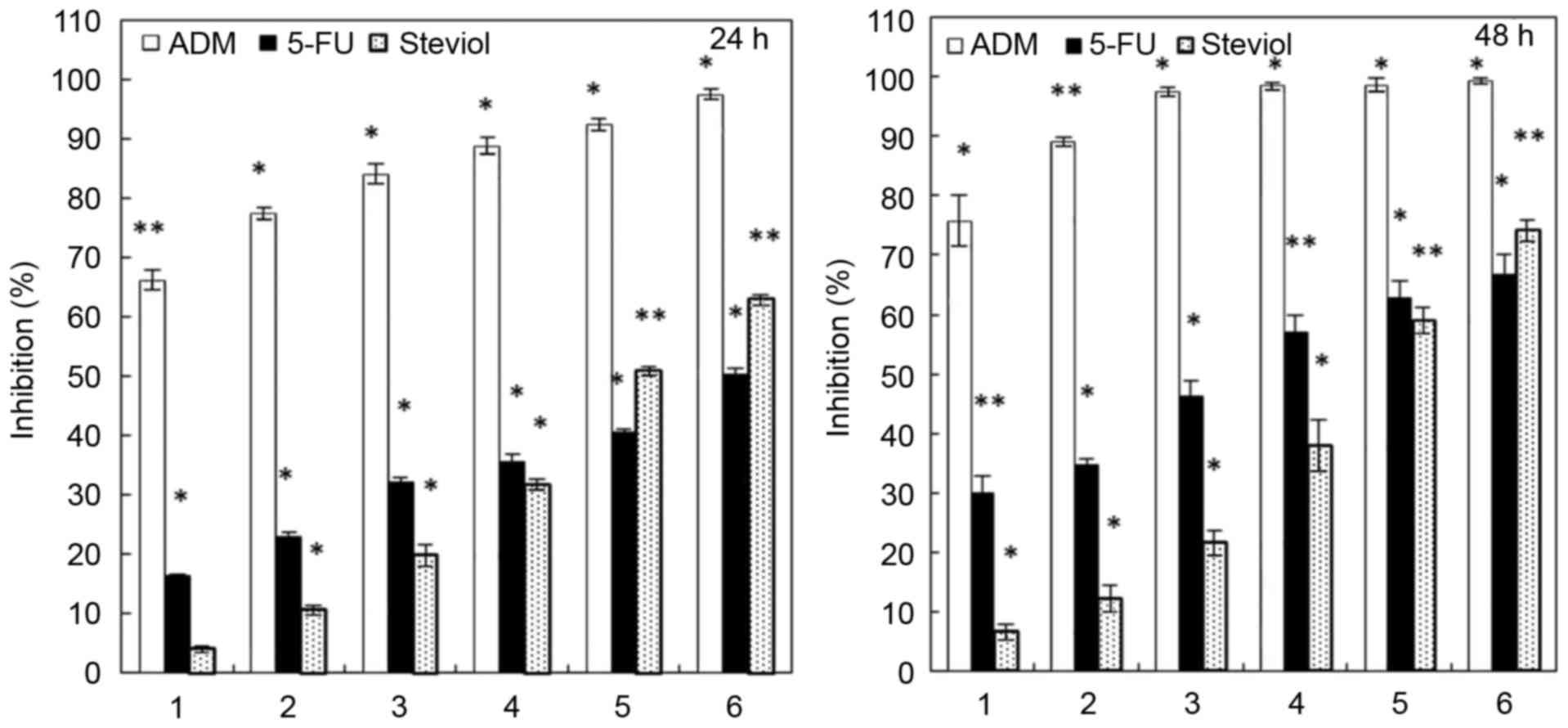 | Figure 1.Inhibition of U2OS proliferation by
steviol at 24 and 48 h. Numbers 1–6 represent the sample
concentrations (µg/ml): ADM: 1, 2, 5, 10, 20, 25; 5-FU and steviol:
25, 50, 100, 150, 200, 250, respectively. *P<0.05 and
**P<0.01 vs. untreated cells. |
Steviol causes G1 phase arrest and
apoptosis in U2OS cells
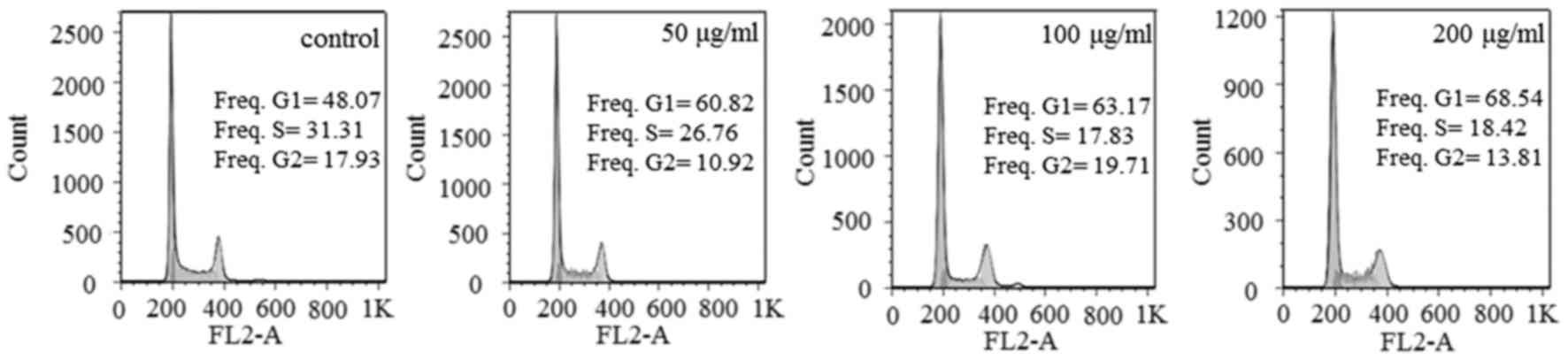
Flow cytometry analysis was performed to determine
the cell cycle distribution and population of dead cells in
steviol-treated U2OS cells. Exposure to steviol resulted in a
marked increase in the percentages of U2OS cells in G1 phase and
decrease of the S and G2 populations in the cell cycle (Fig. 3; Table
I). Therefore, steviol may cause G1 arrest in cell cycle of
U2OS cells. The mechanism will be discussed subsequently.
 | Table I.Regulation of steviol on cell cycle
progression of U2OS cells. |
Table I.
Regulation of steviol on cell cycle
progression of U2OS cells.
|
| Cell cycle
stage |
|---|
|
|
|
|---|
| Dosage (µg/ml) | G1 (%) | S (%) | G2 (%) |
|---|
| Control
Steviol | 48.07 | 31.31 | 17.93 |
| 50 | 60.82 | 26.76 | 10.92 |
|
100 | 63.17 | 17.83 | 19.71 |
|
200 | 68.54 | 18.42 | 13.81 |
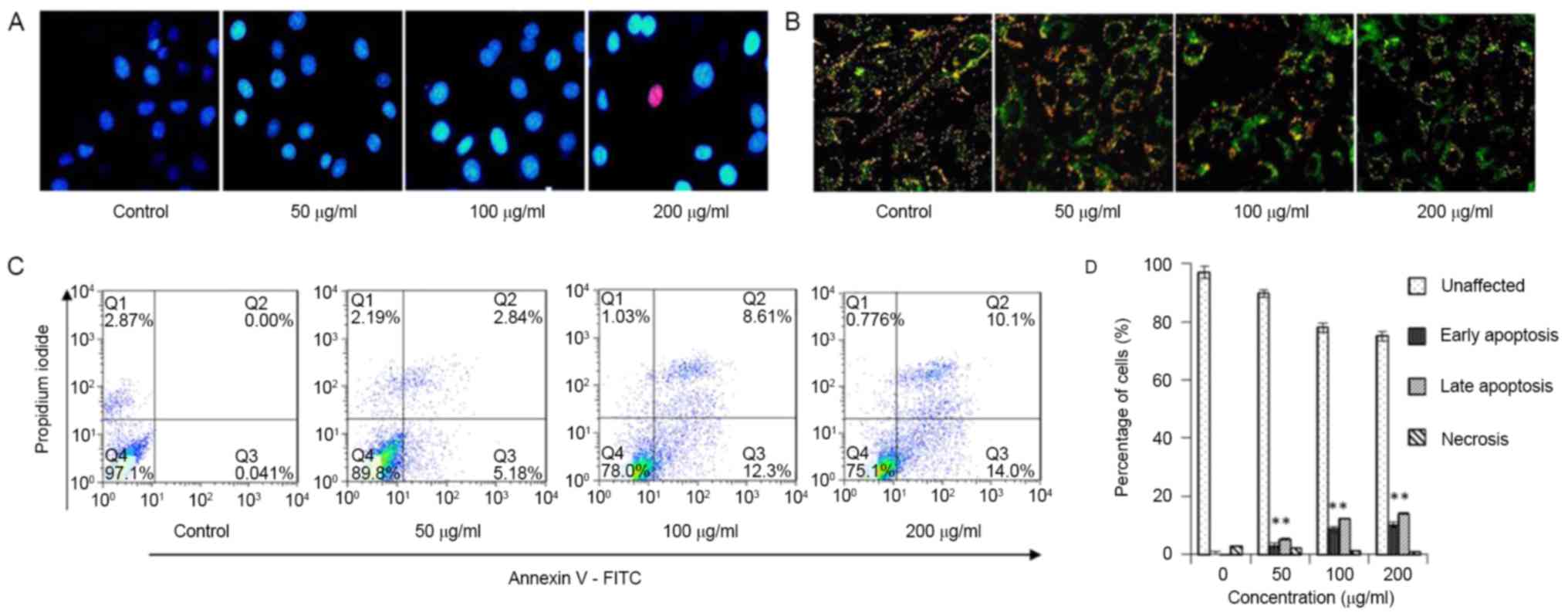
To further identify cell apoptosis, Hoechst staining
(Fig. 4A) and JC-1 staining (Fig. 4B) were employed, followed by Annexin
V-FITC/PI double-labeled flow cytometry (Fig. 4C and D). As steviol concentration
increased, the green fluorescence intensity increased (Fig. 4B). All results indicate a
concentration-dependent effect on cell apoptosis. The total
apoptosis ratio increased from 0.04 to 24.10% with the increasing
concentration of steviol up to 200 µg/ml in 48 h (Fig. 4C). These results suggest that steviol
inhibits the viability of U2OS cell through cell cycle arrest at
the G1 phase and by stimulating apoptosis.
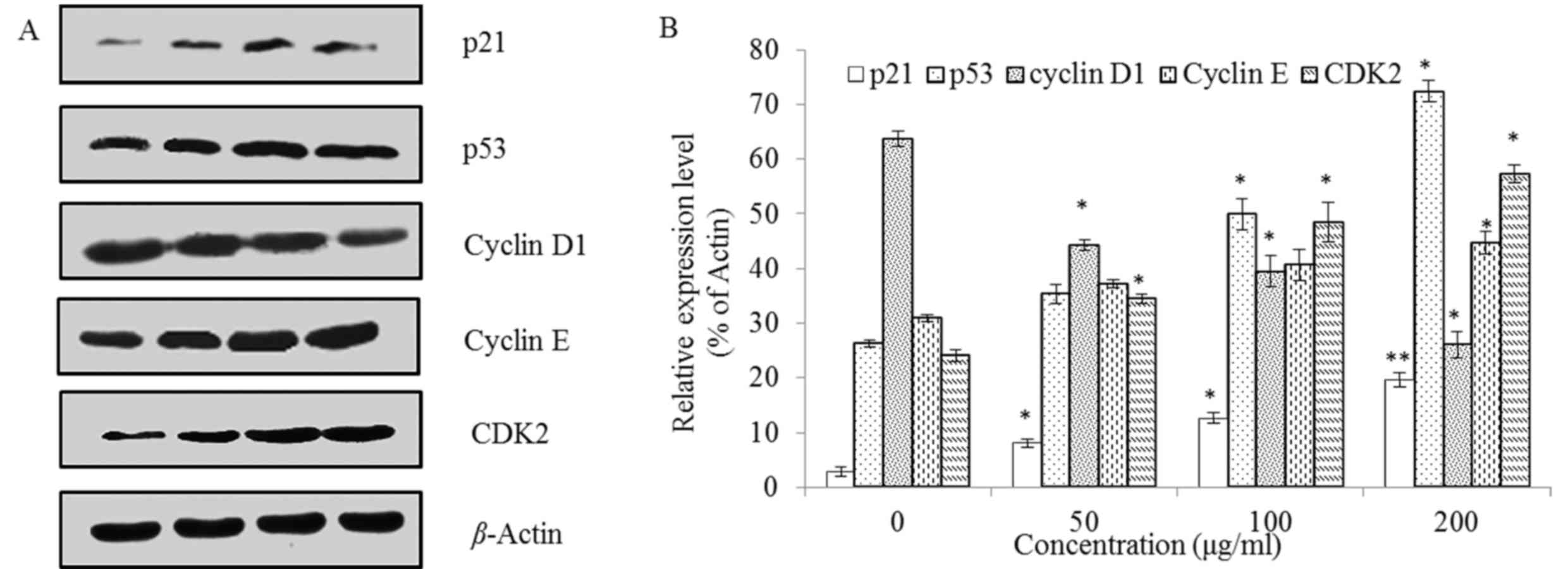
Apoptotic pathway
Firstly, to investigate the mechanism of
steviol-mediated G1 arrest, the levels of G1 regulation-associated
proteins p21, p53, Cyclin D1, Cyclin E, and CDK2 were assessed. As
demonstrated in Fig. 5, the
expression of p21, p53, Cyclin E and CDK2 was upregulated, whereas
Cyclin D1 was downregulated, and all were concentration
dependent.
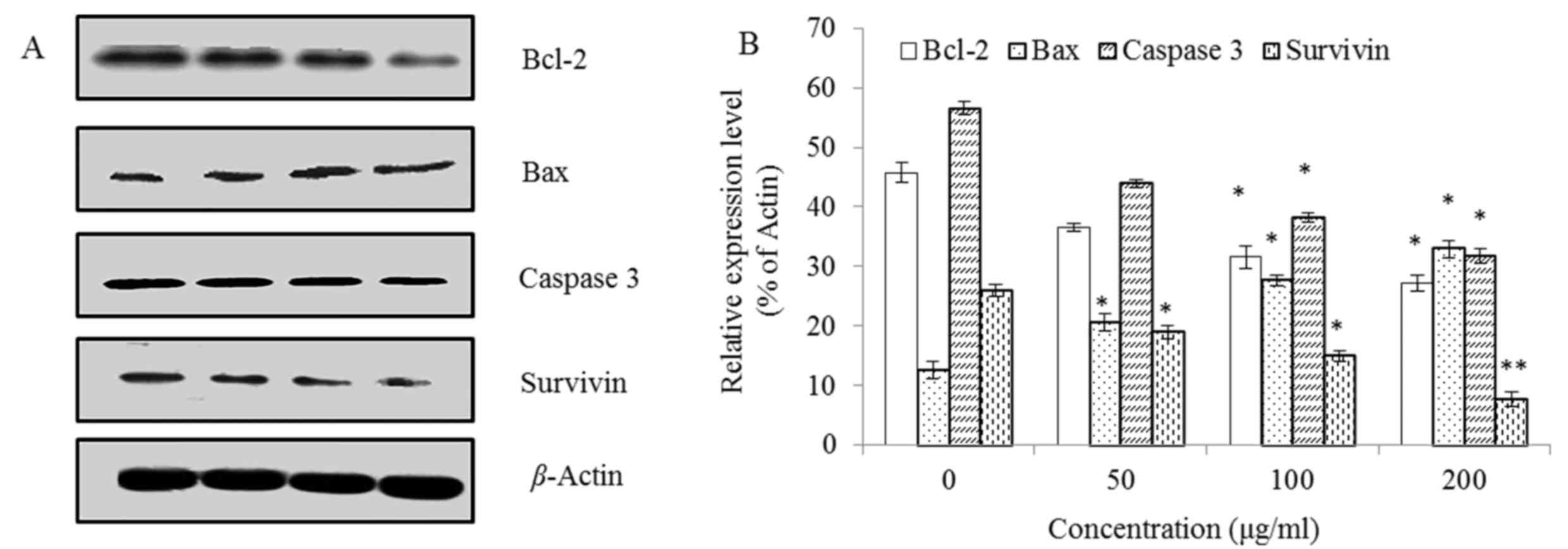
Subsequently, the apoptosis-associated proteins
Bcl-2, Bax, Caspase 3 and Survivin in the steviol treated U2OS
cells were detected using western blotting.
Fig. 6 reveals that
the expression level of Bax was increased whereas Bcl-2, Caspase 3
and Survivin were decreased in a concentration-dependent manner.
This indicates that the ratio of Bax/Bcl-2 was upregulated with
increasing concentrations of steviol, suggesting an activation of
mitochondrial apoptotic pathway.
Discussion
As aforementioned, steviol has not been studied
intensively as a potential anticancer agent, nor its mechanism of
inhibition in cancer cells. Boonkaewwan and Burodom (22) indicated that steviol decreased cell
viability at concentrations of 200–800 µmol/l, for example,
63.7–254.8 µg/ml in T84, Caco-2 and HT29 cells; the IC50
values were 400–800 µmol/l, similar to the results demonstrated in
the present study. The steviol used by Boonkaewwan and Burodom
(22) was 90% pure, obtained from the
oxidation of stevioside (23).
However, the effects of other drugs on the cell
cycle in U2OS cells have been studied: Riccardin D (RD), a
liverwort-derived product was identified to cause arrest of U2OS
cells in G1 phase, while p53 was not required in the apoptosis;
caspase-independent mechanisms were observed to be involved in
RD-mediated cell death (22). An
additional liverwort-derived product dihydroptychantol A induced
G2/M-phase cell cycle arrest and apoptosis in U2OS cells by
decreasing the expression of cyclin B1 (28). Cappadone et al (29) revealed that NSC743420, an indole
derivative, induced a cytostatic and differentiating effect in U2OS
cells, characterized by the cell cycle arrest in G0/G1 phase and
increased alkaline phosphatase activity. In the present study, as
indicated Figs. 3–6, the underlying mechanism of cell cycle
regulation induced by steviol, and the G1 phase-associated proteins
were identified along with the apoptosis-associated proteins.
Among these proteins, p21 is a cyclin-dependent
kinase inhibitors. As it well-known, cyclin D1 is a regulatory
subunit of cyclin-dependent kinases CDK4 and CDK6, dimerized with
CDK4/6 to regulate the G1/S phase transition and entry into the
S-phase. Cyclin D1-CDK4 also enables the activation of the cyclin
E-CDK2 complex by sequestering the CDK interacting protein/kinase
inhibitory protein family protein p21 (30). Therefore, all regulations presented in
Fig. 5 are coincident with Fig. 3. In conclusion, steviol induced G1
arrest of U2OS cells by suppressing the expression of Cyclin D1 and
upregulating the expression of p21, p53, Cyclin E, and CDK2.
The Bcl-2 family proteins serve crucial roles in
controlling the intrinsic (mitochondrial) apoptotic pathway, which
is one of the major mechanisms that induces apoptotic cell death
(31). It has been suggested that an
alteration in the balance between anti-apoptotic Bcl-2 and
pro-apoptotic Bax is a critical factor in regulation of the
susceptibility of cells to apoptosis (32).
Taken together, the results revealed that steviol
induced the mitochondrial apoptotic pathway in U2OS cells, and that
a Survivin and Caspase 3-independent mechanism was involved.
In conclusion, the present study indicates that
steviol possesses an anticancer activity in human osteosarcoma U2OS
cell line, similar to 5-FU or AMD. Steviol inhibits the
proliferation of U2OS by inducing G1 cell cycle arrest and
mitochondrial apoptosis, as demonstrated by the upregulation of the
Bax/Bcl-2 ratio, activation of p21, p53 and CDK2; a Survivin and
Caspase 3-independent mechanism may also be involved. These data
may suggest the steviol could be a potential anticancer drug as a
natural sweetener metabolite. However, additional studies are
required to verify the in vivo effects of steviol in
osteosarcoma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31772017; 31371837)
and the Project of Outstanding Scientific & Technological
Innovation Group of Jiangsu Province.
References
|
1
|
Renwick AG and Tarka SM: Microbial
hydrolysis of steviol glycosides. Food Chem Toxicol. 46 Suppl
7:S70–S74. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Purkayastha S, Pugh G Jr, Lynch B, Roberts
A, Kwok D and Tarka SM Jr: In vitro metabolism of rebaudioside B,
D, and M under anaerobic conditions: Comparison with rebaudioside
A. Regul Toxicol Pharmacol. 68:259–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toskulkac C, Chaturat L, Temcharoen P and
Glinsukon T: Acute toxicity of stevioside, a natural sweetener, and
its metabolite, steviol, in several animal species. Drug Chem
Toxicol. 20:31–44. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moons N, De Borggraeve W and Dehaen W:
Stevioside and steviol as starting materials in organic synthesis.
Curr Organic Chem. 16:1986–1995. 2012. View Article : Google Scholar
|
|
5
|
EFSA Panel on Food Additives and Nutrient
Sources (ANS), . Scientific Opinion on safety of steviol glycosides
for the proposed uses as a food additive. EFSA Journal. 8:15372010.
View Article : Google Scholar
|
|
6
|
Ge Y, Wang Y, Pang L, Zhang L, Zhai Y and
Zhou H: Proliferation, apoptosis, and invasion effects of mistletoe
alkali on human osteosarcoma U2OS in vitro. Int Surg. April
25–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Zhu X, Li H, Li B, Sun L, Xie T,
Zhu T, Zhou H and Ye Z: Piperine inhibits proliferation of human
osteosarcoma cells via G2/M phase arrest and metastasis by
suppressing MMP-2/-9 expression. Int Immunopharmacol. 24:50–58.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ottaviani G and Jaffe N: The Epidemiology
of Osteosarcoma = Pediatric and Adolescent Osteosarcoma. Jaffe N,
Bruland OS and Bielack S: Springer US; pp. 3–13. 2010
|
|
10
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: an analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rejniak KA, Lloyd MC, Reed DR and Bui MM:
Diagnostic assessment of osteosarcoma chemoresistance based on
Virtual Clinical Trials. Med Hypotheses. 85:348–354. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-Where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: State of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hattinger CM, Pasello M, Ferrari S, Picci
P and Serra M: Emerging drugs for high-grade osteosarcoma. Expert
Opin Emerg Drugs. 15:615–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Janeway KA and Grier HE: Sequelae of
osteosarcoma medical therapy: A review of rare acute toxicities and
late effects. Lancet Oncol. 11:670–678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muñoz A, Alfaro J, Pardo N, García-Miguel
P, Quintero V, Gros L, Melero C, Antuña MJ, Ocete G, de Las Heras
J, et al: Long-term results of the Spanish Protocol SO-95 for the
treatment of non-metastatic high-grade osteosarcoma of the
extremities in children. Clin Transl Oncol. 11:387–392. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao S, Lu N, Chai Y and Yu X: Rapamycin
inhibits tumor growth of human osteosarcomas. J Buon. 20:588–594.
2015.PubMed/NCBI
|
|
18
|
Lu M, Huang W, Bao N, Zhou G and Zhao J:
The flavonoid ampelopsin inhibited cell growth and induced
apoptosis and G0/G1 arrest in human osteosarcoma MG-63 cells in
vitro. Pharmazie. 70:388–393. 2015.PubMed/NCBI
|
|
19
|
Lee DH, Qi J, Bradner JE, Said JW, Doan
NB, Forscher C, Yang H and Koeffler HP: Synergistic effect of JQ1
and rapamycin for treatment of human osteosarcoma. Int J Cancer.
136:2055–2064. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maugg D, Rothenaigner I, Schorpp K,
Potukuchi HK, Korsching E, Baumhoer D, Hadian K, Smida J and
Nathrath M: New small molecules targeting apoptosis and cell
viability in osteosarcoma. PLoS One. 10:e01290582015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng ZJ, Wu N, Liu Y, Shu KJ, Zou X, Zhang
RX, Pi CJ, He BC, Ke ZY, Chen L, et al: Evodiamine inhibits the
proliferation of human osteosarcoma cells by blocking PI3K/Akt
signaling. Oncol Rep. 34:1388–1396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Ji Y, Hu Z, Jiang H, Zhu F, Yuan H
and Lou H: Riccardin D induces cell death by activation of
apoptosis and autophagy in osteosarcoma cells. Toxicol in Vitro.
27:1928–1936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boonkaewwan C, Ao M, Toskulkao C and Rao
MC: Specific immunomodulatory and secretory activities of
stevioside and steviol in intestinal cells. J Agric Food Chem.
56:3777–3784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuajit C, Muanprasat C, Gallagher AR,
Fedeles SV, Kittayaruksakul S, Homvisasevongsa S, Somlo S and
Chatsudthipong V: Steviol retards renal cyst growth through
reduction of CFTR expression and inhibition of epithelial cell
proliferation in a mouse model of polycystic kidney disease.
Biochem Pharmacol. 88:412–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takasaki M, Konoshima T, Kozuka M, Tokuda
H, Takayasu J, Nishino H, Miyakoshi M, Mizutani K and Lee KH:
Cancer preventive agents. Part. 8:Chemopreventive effects of
stevioside and related compounds. Bioorg Med Chem 17: 600–605.
2009.
|
|
26
|
Shen J, Park HS, Xia YM, Kim GS and Cui
SW: The polysaccharides from fermented Ganoderma lucidum mycelia
induced miRNAs regulation in suppressed HepG2 cells. Carbohydr
Polym. 103:319–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plumb JA: Cell sensitivity assays: The MTT
assay = Cancer Cell Culture. Springer; pp. 165–169. 2004
|
|
28
|
Li X, Wu WK, Sun B, Cui M, Liu S, Gao J
and Lou H: Dihydroptychantol A a macrocyclic bisbibenzyl
derivative, induces autophagy and following apoptosis associated
with p53 pathway in human osteosarcoma U2OS cells. Toxicol Appl
Pharmacol. 251:146–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cappadone C, Stefanelli C, Malucelli E,
Zini M, Onofrillo C, Locatelli A, Rambaldi M, Sargenti A, Merolle
L, Farruggia G, Graziadio A, et al: p53-dependent and
p53-independent anticancer activity of a new indole derivative in
human osteosarcoma cells. Biochem Biophys Res Commun. 467:348–353.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martin S, Reutelingsperger C, McGahon AJ,
Rader JA, van Schie RC, LaFace DM and Green DR: Early
redistribution of plasma membrane phosphatidylserine is a general
feature of apoptosis regardless of the initiating stimulus:
Inhibition by overexpression of Bcl-2 and Abl. J Exp Med.
182:1545–1556. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zinkel S, Gross A and Yang E: BCL2 family
in DNA damage and cell cycle control. Cell Death Differ.
13:1351–1359. 2006. View Article : Google Scholar : PubMed/NCBI
|