Introduction
Socioeconomic and demographic factors, including
marital status, have a marked effect on the survival of patients
with various diseases. Compared with married patients with cancer,
patients with cancer who have never married, including divorced,
separated or widowed, exhibit shorter overall survival (OS) and
higher mortality rates (1–4). Previous studies have described the
importance of marital status on the outcomes of patients with
different types of cancer (4–7). However, the influence of marital status
on the survival rates of patients with prostate cancer (PCa)
remains controversial.
Two large-scale population-based studies, based on
the Surveillance, Epidemiology and End Results (SEER) database,
indicated that patients who were separated/divorced/widowed (SDW)
exhibited higher cancer-specific and overall mortality rates
(8,9).
Similarly, another study (10)
consisting of 3,570 patients with PCa treated in three prospective
Radiation Therapy Oncology Group clinical trials revealed that the
survival rates of unmarried individuals were significantly
decreased compared with married individuals. However, a study by
Schiffmann et al (11) using
the Martini-Klinik Prostate Cancer database indicated that married
men with PCa did not have a significantly higher OS rate compared
with SDW men over follow-up period of 48 months.
It has been demonstrated that the cancer-specific
mortality rate for PCa differs significantly between married and
SDW individuals. However, there are no significant differences in
mortality rates between married individuals and those that have
never been married [hazard ratio (HR), 1.03; 95% confidence
interval (CI), 0.91–1.17] (8). Li
et al (7) revealed that
widowed patients had the lowest survival rate among patients with
colorectal cancer (HR, 1.49, 95% CI, 1.45–1.53). Furthermore, no
clear differences were observed between divorced/separated and
married patients with pancreatic cancer at any stage in
cause-special survival (CSS) (12).
All of these indicate that the heterogeneity of unmarried
individuals may somewhat influence the predictive value. However,
previous studies (8–11) compared the survival outcomes of
patients with PCa in terms of marital status without
differentiating separated, divorced and widowed status. Several of
these studies (9–11) did not even distinguish between
patients that had never married and those that were SDW. In
addition, the proportion of married individuals in the population,
the prostate specific antigen (PSA) based screening strategy,
mortality rate, surgical equipment, as well as the guidelines,
including AUA (13) and EAU (14,15), have
changed, which may affect the impact of marital status on patient
outcomes (16,17).
In the United States, PCa is the most commonly
diagnosed type of non-skin cancer and the second leading cause of
cancer-associated mortality in men (18). Marriage remains an important part of
many people's lives, therefore, data from the SEER database was
used to determine the aspects of marital status that affect OS and
CSS in patients with PCa who underwent radical prostatectomy
(RP).
Patients and methods
Patients
The current SEER database (November 2015 submission;
http://seer.cancer.gov/), consisting of 18
population-based cancer registries, represents ~28% of the entire
population in the United States. The SEER*Stat (National Cancer
Institute SEER*Stat software, version 8.3.2; http://www.seer.cancer.gov/seerstat/) was used to
identify a cohort of patients with PCa with known marital status
who underwent RP between 2004 and 2009. The inclusion criteria were
as follows: i) Diagnosis of PCa confirmed by histology; ii) age at
diagnosis, >18 years old; iii) presence of a single PCa or PCa
as the first of ≥2 primary cancers; and iv) the cause of mortality
and survival time were documented. Additionally, only patients with
prostate adenocarcinomas, including 8,140, 8,141, 8,143, 8,255,
8,260, 8,310 and 8,323, as classified by the International
Classification of Diseases for Oncology, 3rd edition morphology
codes system (19) were included in
the present study. Patients reported by a death certificate or
autopsies were excluded. Data regarding patients included in the
SEER database contain no identifiers and are publicly available for
cancer-based analyses. Therefore, extra informed consent from
patients and ethics committee approval were not required.
Patient clinicopathological
characteristics
Clinicopathological parameters, including age at
diagnosis, ethnicity, year of diagnosis, grade, SEER historic stage
A, sequence number, Gleason score (GS), marital status, cause of
mortality and survival months were extracted from the SEER
database. According to the SEER staging system, patients were
categorized as localized or regional, or distant or unstaged.
Within the SEER database, marital status was recorded at the time
of diagnosis. Patients were categorized as married, divorced,
widowed, separated or never married. In the present study,
separated and divorced individuals in the separated and divorced
groups were combined into a divorced/separated group.
Statistical analysis
Two-sided χ2 tests were used to compare
patient baseline characteristics in different marital status
groups. As to follow-up time, one-way analysis of variance and a
post hoc test by Dunnett's test were used to compare the
difference. Survival curves were generated using Kaplan-Meier
estimates and differences in the survival rates were assessed using
the log-rank test. The impact of marital status and other
clinicopathological parameters on survival outcomes were evaluated
by building multivariable Cox regression models. The primary
endpoint of the present study was CSS. PCa-associated mortality was
treated as an event and mortality from other causes was treated as
a censored observation. The secondary endpoint was OS. All
statistical analyses were performed using the statistical software
package SPSS for Windows, version 19 (SPSS, Inc., Chicago, IL,
USA). All P-values were two tailed and P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
In total, 95,847 men with a histologically confirmed
diagnosis of PCa who underwent RP between 2004 and 2009 were
included in the present study. Of these, 78,244 (81.6%) were
married, 9,072 (9.5%) were single, 6,627 (6.9%) were
divorced/separated and 1,903 (2.0%) were widowed (Table I). The mean follow-up time was 78.3
(22.8) months. Men in the married group had the longest follow-up
time compared with the other three groups (all P<0.05). The
widowed group contained a significantly higher (P<0.05) number
of elderly patients (≥66 years old), a higher proportion of
patients with distant disease and a higher proportion of patients
with a high GS (Gleason score, ≥8).
 | Table I.Baseline demographic and tumor
characteristics of patients with PCa included in the SEER database
between 2004–2009. |
Table I.
Baseline demographic and tumor
characteristics of patients with PCa included in the SEER database
between 2004–2009.
| Patient
characteristics | Total | Married, n (%) | Never married, n
(%) | Divorced/separated, n
(%) | Widowed, n (%) | P-value |
|---|
| Cases | 95,846 | 78,244 | 9,072 | 6,627 | 1,903 |
|
| Age (years) |
|
|
|
|
| <0.001 |
|
≤50 |
6,277 | 4,703 (6.0) | 1,078 (11.9) | 468 (7.1) | 28 (1.5) |
|
|
51–65 | 64,529 | 52,525 (67.1) | 6,320 (69.7) | 4,793 (72.3) | 891 (46.8) |
|
|
≥66 | 25,040 | 21,016 (26.9) | 1,674 (18.5) | 1,366 (20.6) | 984 (51.7) |
|
| Ethnicity |
|
|
|
|
| <0.001 |
|
White | 79,211 | 66,002 (84.4) | 6,605 (72.8) | 5,086 (76.7) | 1,518 (79.8) |
|
|
African-American | 11,431 | 7,785 (9.9) | 2,049 (22.6) | 1,305 (19.7) | 292 (15.3) |
|
|
Othera |
5,204 | 4,457 (5.7) | 418 (4.6) | 236 (3.6) | 93 (4.9) |
|
| Grade |
|
|
|
|
| <0.001 |
|
Well/moderately | 39,512 | 32,435 (41.5) | 3,774 (41.6) | 2,594 (39.1) | 709 (37.3) |
|
|
Poorly/undifferentiated | 56,200 | 45,710 (58.4) | 5,281 (58.2) | 4,022 (60.7) | 1,187 (62.4) |
|
|
Unknown | 134 | 99 (0.1) | 17 (0.2) | 11 (0.2) | 7 (0.4) |
|
| SEER historic stage
A |
|
|
|
|
| 0.021 |
|
Localized/regional | 95,187 | 77,706 (99.3) | 9,004 (99.3) | 6,593 (99.5) | 1,884 (99.0) |
|
|
Distant | 146 | 120 (0.2) | 10 (0.1) | 8 (0.1) | 8 (0.4) |
|
|
Unstaged | 513 | 418 (0.5) | 58 (0.6) | 26 (0.4) | 11 (0.6) |
|
| Sequence
number |
|
|
|
|
| <0.001 |
| One
primary only | 88,407 | 72,070 (92.1) | 8,466 (93.7) | 6,162 (93.0) | 1,709 (89.8) |
|
| 1st of
2 or more primaries |
7,439 | 6,174 (7.9) | 606 (6.7) | 465 (7.0) | 194 (10.2) |
|
| GS |
|
|
|
|
| <0.001 |
| ≤6 | 36,893 | 30,386 (38.8) | 3,468 (38.2) | 2,400 (36.2) | 639 (33.6) |
|
| 7 | 45,581 | 37,074 (47.4) | 4,367 (48.1) | 3,216 (48.5) | 924 (48.6) |
|
| ≥8 |
9,483 | 7,670 (9.8) | 845 (9.3) | 714 (10.8) | 254 (13.3) |
|
|
Unknown |
3,889 | 3,114 (4.0) | 392 (4.3) | 297 (4.5) | 86 (4.5) |
|
| Follow-up
(months)b | 78.3 | 78.7 | 76.6 | 76.8 | 77.0 | <0.001 |
Marital status and OS
The 8-year OS was 93% in the married group, 91% in
the never married group, 88% in the divorced/separated group and
86% in the widowed group (P<0.001). Several other covariates,
including an age ≥66 years old (P<0.001), African-American
ethnicity (P<0.001), poorly or undifferentiated tumor grade
(P<0.001), distant disease (P<0.001), high GS (P<0.001)
and the presence of ≥2 primary types of cancer (P<0.001) were
identified as significant risk factors for poor OS following
univariate analysis. These covariates were further validated as
independent prognostic factors using multivariate analysis
(Table II). Compared with the
married group, men in the three unmarried groups tended to exhibit
worse OS (never married group: HR, 1.39, 95% CI, 1.27–1.51;
divorced/separated group: HR, 1.82, 95% CI, 1.67–1.98; widowed
group: HR, 1.69, 95% CI, 1.47–1.93), following adjustment for age,
ethnicity, tumor grade, tumor stage, GS and sequence number
(Table II).
 | Table II.Multivariate analysis evaluating the
influence of marital status on OS and CSS. |
Table II.
Multivariate analysis evaluating the
influence of marital status on OS and CSS.
|
|
| Multivariate
analysis |
|
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Patient
characteristics | 8-year OS | HR | 95% CI | P-value | 8-year CSS (%) | HR | 95% CI | P-value |
|---|
| Age (years) |
|
|
| <0.001 |
|
|
| <0.001 |
|
≤50 | 96 | Reference |
| 99 | Reference |
|
|
51–65 | 94 | 1.65 | (1.41–1.93) |
| 98 | 1.07 | (0.81–1.40) |
|
|
≥66 | 87 | 2.90 | (2.47–3.40) |
| 97 | 1.46 | (1.10–1.93) |
|
| Ethnicity |
|
|
| <0.001 |
|
|
| <0.001 |
|
White | 92 | Reference |
| 98 | Reference |
|
|
African-American | 89 | 1.54 | (1.43–1.65) |
| 98 | 1.19 | (1.01–1.40) |
|
|
Othera | 94 | 0.85 | (0.75–0.96) |
| 99 | 0.56 | (0.41–0.75) |
|
| Grade |
|
|
| <0.001 |
|
|
| <0.001 |
|
Well/moderately | 94 | Reference |
| 99 | Reference |
|
|
Poorly/undifferentiated | 91 | 1.16 | (1.04–1.30) |
| 97 | 1.58 | (1.18–2.10) |
|
|
Unknown | 73 | 2.29 | (1.47–3.55) |
| 90 | 4.79 | (2.34–9.77) |
|
| SEER historic stage
A |
|
|
| <0.001 |
|
|
| <0.001 |
|
Localized/regional | 92 | Reference |
| 98 | Reference |
|
|
Distant | 68 | 4.80 | (3.60–6.40) |
| 75 | 11.27 | (8.03–15.82) |
|
|
Unstaged | 86 | 1.47 | (1.11–1.95) |
| 96 | 1.75 | (1.00–3.06) |
|
| Sequence
number |
|
|
| <0.001 |
|
|
| <0.001 |
| One
primary only | 94 | Reference |
| 98 | Reference |
|
| 1st of
2 or more primaries | 69 | 5.76 | (5.45–6.09) |
| 96 | 1.95 | (1.68–2.27) |
|
| GS |
|
|
| <0.001 |
|
|
| <0.001 |
| ≤6 | 94 | Reference |
| 99 | Reference |
|
| 7 | 92 | 1.10 | (0.98–1.24) |
| 99 | 1.53 | (1.12–2.10) |
|
| ≥8 | 83 | 2.30 | (2.03–2.62) |
| 90 | 11.42 | (8.31–15.70) |
|
|
Unknown | 90 | 1.35 | (1.14–1.61) |
| 96 | 3.79 | (2.61–5.52) |
|
| Marital status |
|
|
| <0.001 |
|
|
| <0.001 |
|
Married | 93 | Reference |
| 98 | Reference |
|
| Never
married | 91 | 1.39 | (1.27–1.51) |
| 98 | 1.20 | (1.00–1.44) |
|
|
Divorced/separated | 88 | 1.82 | (1.67–1.98) |
| 97 | 1.61 | (1.34–1.93) |
|
|
Widowed | 86 | 1.69 | (1.47–1.93) |
| 97 | 1.13 | (0.81–1.58) |
|
Marital status and CSS
Compared with the married group, men in the
divorced/separated and widowed groups had a worse CSS (P<0.001,
P=0.001, respectively), while men in the never married group
exhibited a similar CSS (P=0.134) according to log-rank test
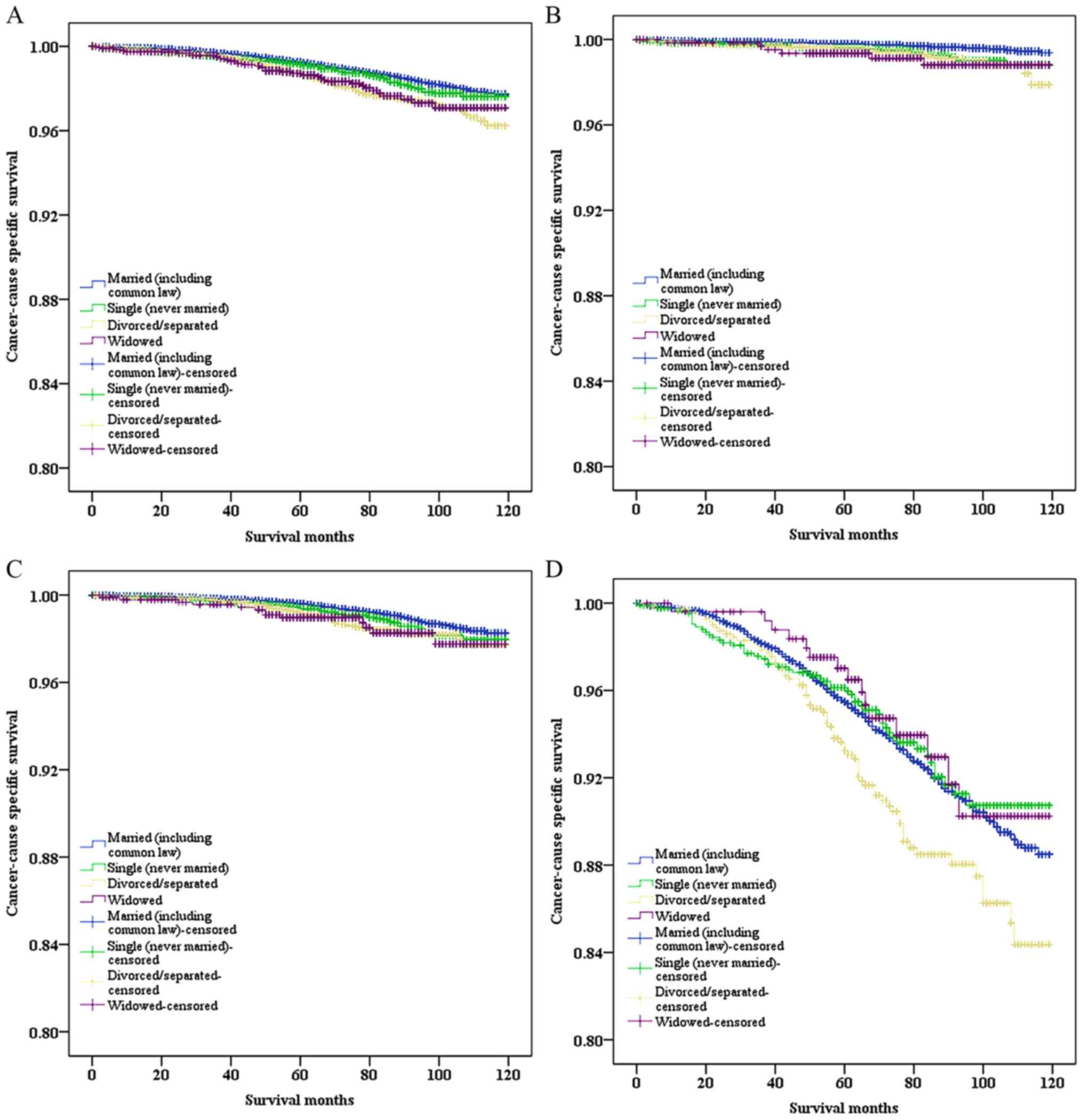
(Fig. 1A). Cox regression
multivariate analysis of CSS was performed and revealed that the
marital status and several other covariates remained as independent
prognostic factors (Table II).
Compared with young men and non-African-American men, elderly men
and African-American men had poorer CSS (HR, 1.46, 95% CI,
1.10–1.93; HR, 1.19, 95% CI, 1.01–1.40, respectively). GS≥8 and
combining with distant disease are predictive factors of worse
cancer-specific mortality (HR, 11.42, P<0.001; HR.11.27,
P<0.001, respectively). In terms of the marital status, men in
the divorced/separated group (HR, 1.61, 95% CI, 1.34–1.93), but not
in the never married or widowed group (HR, 1.20, 95% CI, 1.00–1.44;
HR, 1.13, 95% CI, 0.81–1.58), had a poorer CSS, when compared with
men in the married group, following adjustment for age, ethnicity,
grade, stage, GS and sequence number.
Considering the effect of cancer stage and GS on
CSS, subgroup analyses were performed to assess if they influenced
the effect of marital status on CSS. The results demonstrated that
marital status was not a valid risk factor among men with distant
disease according to univariate and multivariate analyses (P=0.146
and P=0.187, respectively). However, among men diagnosed with
localized/regional disease, marital status significantly affected
CSS (P<0.001; Table III).
Furthermore, marital status remained an independent prognostic
factor in each GS group, as determined by univariate and
multivariate analyses (Fig. 1B-D and
Table IV). It was observed that the
HR of CSS among divorced/separated men decreased when GS increased
(GS≤6: HR, 2.5, 95% CI, 1.54–4.04; GS=7: HR, 1.71, 95% CI,
1.24–2.36; GS≥8: HR, 1.50, 95% CI, 1.17–1.93; all P<0.05).
 | Table III.Univariate and multivariate analysis
of marital status on prostate cancer-cause specific survival based
on different SEER historic stages. |
Table III.
Univariate and multivariate analysis
of marital status on prostate cancer-cause specific survival based
on different SEER historic stages.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Marital status | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
|
Localized/regional |
|
| <0.001 |
|
| <0.001 |
|
Married | Reference |
| Reference |
|
| Never
married | 1.14 | (0.95–1.38) |
| 1.20 | (0.99–1.44) |
|
|
Divorced/separated | 1.73 | (1.45–2.08) |
| 1.66 | (1.39–1.99) |
|
|
Widowed | 1.50 | (1.06–2.11) |
| 1.17 | (0.83–1.65) |
|
| Distant |
|
| 0.187 |
|
| 0.146 |
|
Married | Reference |
| Reference |
|
| Never
married | 2.95 | (1.13–7.68) |
| 2.64 | (0.96–7.23) |
|
|
Divorced/separated | 0.61 | (0.60–4.41) |
| 0.42 | (0.05–3.40) |
|
|
Widowed | 1.25 | (0.30–5.25) |
| 0.72 | (0.15–3.40) |
|
 | Table IV.Univariate and multivariate analysis
of marital status on prostate cancer-cause specific survival in
terms of different GS range. |
Table IV.
Univariate and multivariate analysis
of marital status on prostate cancer-cause specific survival in
terms of different GS range.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Marital status | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| GS≤6 |
|
| <0.001 |
|
| <0.001 |
|
Married | Reference |
| Reference |
|
| Never
married | 2.13 | (1.37–3.33) |
| 2.17 | (1.39–3.41) |
|
|
Divorced/separated | 2.54 | (1.57–4.11) |
| 2.50 | (1.54–4.04) |
|
|
Widowed | 2.80 | (1.23–6.39) |
| 1.75 | (0.76–4.14) |
|
| GS=7 |
|
| 0.002 |
|
| 0.002 |
|
Married | Reference |
| Reference |
|
| Never
married | 1.32 | (0.97–1.81) |
| 1.37 | (1.00–1.88) |
|
|
Divorced/separated | 1.67 | (1.21–2.29) |
| 1.71 | (1.24–2.36) |
|
|
Widowed | 1.73 | (0.99–3.01) |
| 1.45 | (0.83–2.52) |
|
| GS≥8 |
|
| 0.009 |
|
| 0.02 |
|
Married | Reference |
| Reference |
|
| Never
married | 0.92 | (0.69–1.22) |
| 0.91 | (0.68–1.22) |
|
|
Divorced/separated | 1.45 | (1.13–1.86) |
| 1.50 | (1.17–1.93) |
|
|
Widowed | 0.86 | (0.52–1.44) |
| 0.85 | (0.51–1.42) |
|
Discussion
The effect of marital status on the survival of
patients with PCa who have undergone RP has been studied using SEER
and the Martini-Clinic Prostate Cancer database (8,11).
However, the results of these investigations have not been
consistent and no studies have been conducted focusing on the
heterogeneity of unmarried patients. Therefore, the present study
was performed to assess the effect of marital status on the
outcomes of men with PCa. Multivariate analyses of 95,847 men
revealed that marital status was an independent prognostic factor
of OS and CSS. Compared with married men, divorced or separated men
with PCa had a higher GS at diagnosis (HR, 1.12, 95% CI, 1.03–1.22)
and those patients exhibited the worst survival outcomes
independent of age, ethnicity, grade, stage or sequence number. Due
to the distributional discrepancies of GS, a grade-by-grade
comparison was performed. Interestingly, the HR of CSS for divorced
or separated men decreased when GS increased, compared with married
men (GS≤6: HR, 2.5; GS=7: HR: 1.71; GS≥8: HR: 1.50,
respectively).
Several hypotheses have been proposed to explain the
inferior survival outcomes of unmarried individuals.
Divorced/separated men are more likely to be diagnosed with high
grade PCa compared with married men (HR, 1.12, 95% CI: 1.03–1.22,
P=0.007). Furthermore, patients with PCa and high GS have poorer
PCa-related survival outcomes (20,21).
However, this does not explain why the married and widowed
patients, had a similar CSS. Psychosocial factors may account for
such a difference in CSS; for example, married men may be
encouraged by their spouses to choose a more curative therapy
(8,22,23).
It is known that men with cancer exhibit more severe
psychological distress than those with other chronic health
conditions (24). Separated and
divorced individuals have a higher risk of distress [odds ratio
(OR), 5.25; OR, 2.79, respectively], whereas married are less
likely to experience distress, depression and anxiety (24,25). In
addition, being married is associated with higher perceived
tangible and emotional support (both P<0.001), which may
influence follow-up care use by fostering a sense of obligation to
manage one's health and encouraging access to medical care
(26). Married men may also benefit
from increased financial resources and better access to health
insurance.
Psychosocial support may predict the prognosis of
men with cancer (27), Garssen
(28) summarized the results of 70
prospective studies and revealed that a low level of social support
and chronic depression can predict unfavorable prognosis.
Neurohormones and neurotransmitters (cortisol and norepinephrine),
the vagal nerve and inflammation, DNA damage, interleukin-1 and
oxytocin may be specific mediators in this potential association
either by directly affecting tumorigenesis or indirectly affecting
other factors, such as cellular immunity (27). It has been demonstrated that the
activity of natural killer cells is low when an individual
perceives a lack of social support (29), which may result in a failure to treat
and control cancer (30).
In the present study, as well as 88,407 patients
with a single primary PCa, 7,439 patients with >1 primary cancer
were also included. Among these patients, PCa was the first
diagnosed type of cancer. In the multivariate analysis, it was
observed that novel-onset cancer was associated with poorer OS and
CSS (HR, 5.76; HR, 1.95, respectively), following adjustment for
age, ethnicity, marital status, GS, stage and grade.
Compared with that in the married group, a higher
proportion of patients in the widowed group had distant disease and
a high GS (HR, 2.28, P=0.027; HR, 1.23, P=0.005, respectively);
however they had a similar CSS when compared with the married
group. By contrast, the results of previous studies investigating
two other types of solid cancer demonstrated that widowed patients
exhibited significantly poorer CSS (7,12). Two
possible reasons may explain this discrepancy. Firstly, 1,765 of
13,370 patients with pancreatic cancer and 21,279 of 112,776
patients with colorectal cancer were widowed in these two studies.
However, in the present study, widowed men occupied a smaller
fraction of the total cases (1,903/95,846) and only 37 widowed men
succumbed from PCa with a mean of 77 months' of follow-up, which
made it hard to obtain positive observations. Secondly, ‘widowed’
in the present study only referred to widowed men, which was
different from the definition of ‘widowed’ in the other studies
investigating pancreatic and colorectal cancer. Therefore, the CSS
of widowed patients with PCa should be further analyzed in a
large-scale population.
The current study demonstrated that, among men with
localized or regional PCa, marital status was an independent
prognostic factor of CSS (P<0.001). However, among those
diagnosed with distant PCa, there was no difference in CSS among
men with different marital status, as determined by univariate and
multivariate analyses (P=0.146 and P=0.187, respectively).
This may have been due to the small sample size, which only
accounted for 0.152% of the total patients, which may make it more
difficult to detect the effect of marital status on CSS in patients
with PCa.
Although all data were derived from a large
population-based study, several potential limitations should also
be taken into consideration. Firstly, PSA levels in men diagnosed
between 2004 and 2009 are currently being removed and reviewed for
potential error. PSA was introduced as a component of staging in
the 7th edition of American Joint Committee on Cancer (AJCC) in
2010 (31). It was difficult to
convert from AJCC 6th to AJCC 7th; therefore, SEER historic stage
A, which categorizes cancer as localized or regional, distant or
unstaged, was used instead of the AJCC staging system. The SEER
database does not collect information concerning education,
insurance and income status, diet, or family status, all of which
may influence the effect of the marital status on patient survival
(9,32–35).
Furthermore, the quality of marriage is hard to document, and the
marital status may change over time. As the current study did not
identify whether unmarried men lived with their partners, a more
concrete conclusion could not be reached. Thirdly, information
regarding comorbidities, including diabetes, hypertension,
cardiovascular disease and several other chronic illnesses, which
have been proven to exhibit an impact on cancer survival (36–38), were
not included in the SEER database. Lack of these covariates may
partial bias the observations of the present study.
To conclude, the results of the current study
support the hypothesis that marital status is an independent
prognostic factor of OS and CSS. Divorced or separated men are more
likely to exhibit a higher GS PCa at diagnosis and the poorest
survival outcomes independent of age, ethnicity, grade, stage and
sequence number. This association between marital status and poor
outcomes was maintained in each GS group. Psychosocial factors may
be the main reasons for the poorer survival outcomes of divorced or
separated men. Although patients in the widowed group had a higher
proportion of patients with distant disease and high GS, they had a
similar CSS compared with the married group. Considering the low
proportion of widowed men included in the current study, a
large-scale study involving widowed patients with PCa is required.
Further understanding of the potential associations among the
marital status, psychosocial factors, survival outcomes may help in
identifying sound strategies of treating men with PCa.
Acknowledgements
The present study used the linked SEER database. The
authors acknowledge the efforts of the SEER program tumor
registries in the creation of the SEER database. Besides, the
authors would like to thank Lu Feng for revising the study. The
present study was supported by Jiangsu Provincial Commission of
Health and Family Planning (grant no. H201550).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaplan RM and Kronick RG: Marital status
and longevity in the United States population. J Epidemiol
Community Health. 60:760–765. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu YR and Goldman N: Mortality
differentials by marital status: An international comparison.
Demography. 27:233–250. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kravdal O: The impact of marital status on
cancer survival. Soc Sci Med. 52:357–368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aizer AA, Chen MH, McCarthy EP, Mendu ML,
Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE,
et al: Marital status and survival in patients with cancer. J Clin
Oncol. 31:3869–3876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hellenthal NJ, Chamie K, Ramirez ML and
deVere White RW: Sociodemographic factors associated with
nephrectomy in patients with metastatic renal cell carcinoma. J
Urol. 181:1013–1019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pruthi RS, Lentz AC, Sand M, Kouba E and
Wallen EM: Impact of marital status in patients undergoing radical
cystectomy for bladder cancer. World J Urol. 27:573–576. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Q, Gan L, Liang L, Li X and Cai S: The
influence of marital status on stage at diagnosis and survival of
patients with colorectal cancer. Oncotarget. 6:7339–7347.
2015.PubMed/NCBI
|
|
8
|
Abdollah F, Sun M, Thuret R, Abdo A,
Morgan M, Jeldres C, Shariat SF, Perrotte P, Montorsi F and
Karakiewicz PI: The effect of marital status on stage and survival
of prostate cancer patients treated with radical prostatectomy: A
population-based study. Cancer Causes Control. 22:1085–1095. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tyson MD, Andrews PE, Etzioni DA, Ferrigni
RG, Humphreys MR, Swanson SK and Castle EK: Marital status and
prostate cancer outcomes. Can J Urol. 20:6702–6706. 2013.PubMed/NCBI
|
|
10
|
Du KL, Bae K, Movsas B, Yan Y, Bryan C and
Bruner DW: Impact of marital status and race on outcomes of
patients enrolled in radiation therapy oncology group prostate
cancer trials. Support Care Cancer. 20:1317–1325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schiffmann J, Beyer B, Tennstedt P, Boehm
K, Mehring G, Schlomm T, Salomon G, Karakiewicz P and Graefen M:
Oncological outcome after radical prostatectomy: Marital status
does not make a difference. Int J Urol. 22:484–489. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang XD, Qian JJ, Bai DS, Li ZN, Jiang GQ
and Yao J: Marital status independently predicts pancreatic cancer
survival in patients treated with surgical resection: An analysis
of the SEER database. Oncotarget. 7:24880–24887. 2016.PubMed/NCBI
|
|
13
|
Sanda MG, Cadeddu JA, Kirkby E, Chen RC,
Crispino T, Fontanarosa J, Freedland SJ, Greene K, Klotz LH,
Makarov DV, et al: Clinically localized prostate cancer:
AUA/ASTRO/SUO Guideline. Part I: Risk stratification, shared
decision making, and care options. J Urol. Dec 14–2017.(Epub ahead
of print).
|
|
14
|
Mottet N, Bellmunt J, Bolla M, Briers E,
Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau
S, et al: EAU-ESTRO-SIOG guidelines on prostate cancer. Part.
1:Screening, diagnosis, and local treatment with curative intent.
Eur Urol 71: 618–629. 2017.
|
|
15
|
Cornford P, Bellmunt J, Bolla M, Briers E,
De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, et al:
EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of
relapsing, metastatic, and castration-resistant prostate cancer.
Eur Urol. 71:630–642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kravdal H and Syse A: Changes over time in
the effect of marital status on cancer survival. BMC Public Health.
11:8042011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jaffe DH, Manor O, Eisenbach Z and Neumark
YD: The protective effect of marriage on mortality in a dynamic
society. Ann Epidemiol. 17:540–547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fritz A, Percy C, Jack A, Shanmugaratnam
K, Sobin L, Parkin DM and Whelan S: International Classification of
Diseases for Oncology. 3rd. World Health Organization; Geneva:
2000
|
|
20
|
Tefilli MV, Gheiler EL, Tiguert R, Sakr W,
Grignon DJ, Banerjee M, Pontes JE and Wood DP Jr: Should Gleason
score 7 prostate cancer be considered a unique grade category?
Urology. 53:372–377. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohno Y, Ohori M, Nakashima J, Okubo H,
Satake N, Hashimoto T and Tachibana M: Association between
preoperative serum total cholesterol level and biochemical
recurrence in prostate cancer patients who underwent radical
prostatectomy. Mol Clin Oncol. 4:1073–1077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Denberg TD, Beaty BL, Kim FJ and Steiner
JF: Marriage and ethnicity predict treatment in localized prostate
carcinoma. Cancer. 103:1819–1825. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Denberg TD, Glodé LM, Steiner JF, Crawford
ED and Hoffman RM: Trends and predictors of aggressive therapy for
clinical locally advanced prostate carcinoma. BJU Int. 98:335–340.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaiser NC, Hartoonian N and Owen JE:
Toward a cancer-specific model of psychological distress:
Population data from the 2003–2005 National Health Interview
Surveys. J Cancer Surviv. 4:291–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DiMatteo MR, Lepper HS and Croghan TW:
Depression is a risk factor for noncompliance with medical
treatment: Meta-analysis of the effects of anxiety and depression
on patient adherence. Arch Intern Med. 160:2101–2107. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Forsythe LP, Alfano CM, Kent EE, Weaver
KE, Bellizzi K, Arora N, Aziz N, Keel G and Rowland JH: Social
support, self-efficacy for decision-making, and follow-up care use
in long-term cancer survivors. Psychooncology. 23:788–796. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gidron Y and Ronson A: Psychosocial
factors, biological mediators, and cancer prognosis: A new look at
an old story. Curr Opin Oncol. 20:386–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Garssen B: Psychological factors and
cancer development: Evidence after 30 years of research. Clin
Psychol Rev. 24:315–338. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Levy SM, Herberman RB, Whiteside T, Sanzo
K, Lee J and Kirkwood J: Perceived social support and tumor
estrogen/progesterone receptor status as predictors of natural
killer cell activity in breast cancer patients. Psychosom Med.
52:73–85. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Antoni MH, Lutgendorf SK, Cole SW, Dhabhar
FS, Sephton SE, McDonald PG, Stefanek M and Sood AK: The influence
of bio-behavioural factors on tumour biology: Pathways and
mechanisms. Nat Rev Cancer. 6:240–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang M, Kenfield SA, Van Blarigan EL,
Batista JL, Sesso HD, Ma J, Stampfer MJ and Chavarro JE: Dietary
patterns after prostate cancer diagnosis in relation to
disease-specific and total mortality. Cancer Prev Res (Phila).
8:545–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kenfield SA, DuPre N, Richman EL, Stampfer
MJ, Chan JM and Giovannucci EL: Mediterranean diet and prostate
cancer risk and mortality in the health professionals follow-up
study. Eur Urol. 65:887–894. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fossati N, Nguyen DP, Trinh QD, Sammon J,
Sood A, Larcher A, Guazzoni G, Montorsi F, Briganti A, Menon M and
Abdollah F: The impact of insurance status on tumor characteristics
and treatment selection in contemporary patients with prostate
cancer. J Natl Compr Canc Netw. 13:1351–1358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mahal BA, Aizer AA, Ziehr DR, Hyatt AS,
Lago-Hernandez C, Chen YW, Choueiri TK, Hu JC, Sweeney CJ, Beard
CJ, et al: The association between insurance status and prostate
cancer outcomes: Implications for the affordable care act. Prostate
Cancer Prostatic Dis. 17:273–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Polesel J, Gini A, Dal Maso L, Stocco C,
Birri S, Taborelli M, Serraino D and Zucchetto A: The impact of
diabetes and other metabolic disorders on prostate cancer
prognosis. J Diabetes Complications. 30:591–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Armenian SH, Xu L, Ky B, Sun C, Farol LT,
Pal SK, Douglas PS, Bhatia S and Chao C: Cardiovascular disease
among survivors of Adult-onset cancer: A community-based
retrospective cohort study. J Clin Oncol. 34:1122–1130. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zist A, Amir E, Ocana AF and Seruga B:
Impact of comorbidity on the outcome in men with advanced prostate
cancer treated with docetaxel. Radiol Oncol. 49:402–408. 2015.
View Article : Google Scholar : PubMed/NCBI
|