Introduction
Alternative splicing produces multiple mRNA splice
variants from the same gene; thus, a limited number of genes can
encode a variety of different proteins (1). Specific splice variants have been
reported to serve significant roles in the development, clinical
diagnosis and treatment of cancer (2). Human estrogen receptor-α (hER-α) is a
widely accepted predictive marker of the effectiveness of endocrine
(anti-estrogen) therapy in patients with breast cancer (3). In general, ER-α-positive patients
respond effectively to anti-estrogens, including tamoxifen, whereas
ER-α-negative patients do not (4,5). Despite
this general pattern, a proportion of ER-α-negative patients with
breast cancer are responsive to anti-estrogen treatment (6). It is possible that ER-α is expressed in
these patients, but that its pre-mRNA undergoes alternative
splicing resulting in the expression of variant isoforms, the
protein products of which cannot be detected using commercially
available ER-α antibodies. These variants may be induced during the
formation and progression of breast cancer, influencing the
behavior of breast cancer cells via uncharacterized mechanisms, and
potentially promoting the progression of breast cancer to more
aggressive phenotypes, including loss of responsiveness to
anti-estrogen treatment (7,8).
In the present study, a novel 30 kDa hER-α splice
variant (ER-α30), was identified, which is encoded by a distinct
ER-α mRNA and enhanced the malignant biological behaviors of human
breast cancer MDA-MB-231 cells.
Materials and methods
Clinical breast tumor tissues
Breast tumor tissues were collected from 33 female
patients of breast invasive ductal carcinoma treated at the
Affiliated Hospital of Guilin Medical University (Guangxi, China)
between August 2013 and June 2014. The age of patients ranged from
37–81, with an average age of 56 years. The specimens were obtained
during surgical resection, cut into 0.3–0.5 cm2 sections
and stored in liquid nitrogen prior to experimentation. No patients
had received chemotherapy or radiotherapy prior to surgery. The
tumor stage was pathologically determined according to the American
Joint Committee on Cancer staging system (9,10). ER-α66,
progesterone receptor (PR) and Erb-B2 receptor tyrosine kinase 2
(Her-2) expression statuses were determined by immunohistochemistry
analysis in the hospital's pathology department. The present study
was approved by the Human Ethics Committee of the Affiliated
Hospital of Guilin Medical University (Guangxi, China) and informed
consent was obtained from all patients.
ER-α30 cloning and expression in
breast cancer tissue
Total RNA was extracted from 300–500 mg breast tumor
tissues using TRIzol (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacturer's instructions. cDNA was then
synthesized using 3 µg total RNA and oligodT primers using a
RevertAid First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.), according to the manufactuerer's protocol. The
open-reading frame (ORF) of ER-α30 was amplified by semi-nested
reverse transcription-polymerase chain reaction (RT-PCR) in two
30-cycle reactions. The thermocycling conditions were as follows:
Round 1: 94°C for 5 min, then 30 cycles of 94°C for 30 sec, 58°C
for 30 sec and 72°C for 90 sec, completed at 72°C for 5 min; round
2, 94°C for 5 min, then 30 cycles of 94°C for 30 sec, 60°C for 30
sec and 72°C for 90 sec, completed at 72°C for 5 min under the
conditions recommended by the LA Taq™ kit (Takara
Biotechnology Co., Ltd., Dalian, China). Primers were designed and
synthesized by Shenggong, Biotechnology Co., Ltd. (Shanghai, China)
for exon 1 (forward, 5′-ATGACCATGACCCTCCACACCAAAG-3′) and exon 8
(outer reverse 1, 5′-GCAGCAGGGATTATCTGAACCG-3′ and inner reverse 2,
5′-GGAATGCGATGAAGTAGAGCC-3′), respectively, with the cDNAs used as
a template for round 1 and the product of round 1 used as the
template for round 2. Hypoxanthine phosphoribosyl transferase was
used as an internal control (forward primer,
5′-GCTTTCCTTGGTCAGGCAGTA-3′ and reverse primer,
5′-CGATGTCAATAGGACTCCAGATGT-3′). The RT-PCR product was then,
sequenced by Shenggong, Biotechnology Co., Ltd., and homology was
analyzed using the National Centre for Biotechnology Information
Basic Local Alignment Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The
association between ER-α30 expression status and clinical
characteristics, including age, tumor size, tumor stage, lymph
nodal status, ER-α66, PR, and Her-2 status, was analyzed.
Cell culture
The breast cancer MDA-MB-231 cell line [ER-α66(−),
PR(−), Her-2(−)] were acquired from the Cell Bank of the Type
Culture Collection of the Chinese Academy of Sciences (KunMing,
China), and MCF-7 cell line [ER-α66(+)] were provided as a gift
from Professor Yiming Tao (Department of Biotechnology, Guilin
Medical University, Guilin, China). All cells were cultured in
DMEM-F12 (Gibco; Thermo Fisher Scientific, Inc.) containing 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin at 37°C and 5%
CO2.
Plasmid preparation and
transfection
A 1,002-bp fragment from the PCR experiment was
amplified and cloned into the corresponding sites of the expression
vector, pEGFP-N1 (provided as a gift from Professor Heling Su,
Department of Biotechnology, Guilin Medical University, Guilin,
China) [with deleted enhanced green fluorescent protein (EGFP)],
with HindIII and BamHI. The cloning was then verified
by sequencing (Shenggong, Biotechnology Co., Ltd., Shanghai,
China). Transfection was performed using Lipofectamine®
2000 (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. MDA-MB-231 cells were seeded in a 6-well
plate 24 h prior to transfection with the plasmids. Cells were
washed twice with PBS and then cultured in 2 ml antibiotic-free
medium containing either pEGFP-N1/ER-α30 or the empty vector
pEGFP-N1 plasmid with Lipofectamine® 2000 (3 µg plasmid,
5 µl Lipofectamine, and 1.2×105 cells/well). After 48 h,
stably transfected cells were selected using 600 µg/ml geneticin
(G418; Thermo Fisher Scientific, Inc.) for 2–3 weeks. The remaining
surviving individual clones were pooled and expanded for further
analysis.
Western blot analysis
Stably transfected MDA-MB-231 cells and MCF-7 cells
[acted as the positive control of ER-α66(+) without transfection]
were lysed in radioimmunoprecipitation assay buffer (Beyotime
Biotechnology Co., Ltd., Shanghai, China) containing 1 mmol/l
phenylmethanesulfonyl fluoride. Protein quantification was
performed according to the manufacturer's instructions of an
Enhanced BCA Protein assay kit (Beyotime Biotechnology Co., Ltd.).
A total of 20 µg protein was separated by SDS-PAGE using a 5%
stacking gel and a 10% separating gel. The proteins were electro
transferred onto a polyvinylidene difluoride membrane and blocked
at room temperature in 3% bovine serum albumin (Roche Diagnostics,
Basel, Switzerland) in tris-buffered saline and 0.5% Tween-20
(TBST) for 30 min. The membrane was then incubated with anti-ER-α
(dilution, 1:100; cat. no. sc-7207, H-184; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA, provided as a gift from
Professor Joshua Liao), and β-actin (dilution, 1:1,000; cat. no.
AA128; Beyotime Biotechnology Co., Ltd.) primary antibodies
overnight at 4°C. Finally, the membrane was incubated with
horseradish peroxidase-conjugated goat anti-rabbit (for ER-α, cat.
no. CW0103; CW, Biotechnology Co., Ltd., Beijing, China) or goat
anti-mouse (for β-actin, cat. no. CW0102; CW, Biotechnology Co.,
Ltd.) secondary antibody (dilution, 1:10,000) at room temperature
for 1 h. The protein bands were visualized using the BeyoECL Plus
kit (Beyotime Biotechnology Co., Ltd.).
MTT assay
Transfected cells were seeded into 96-well plates at
a density of 400 cells/well (5 wells were seeded for each
experimental group) and maintained in serum-free medium (DMEM-F12,
containing 100 U/ml penicillin and 100 µg/ml streptomycin) for 24 h
(day 1), then replaced with fresh complete medium (DMEM-F12,
containing 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin)
and cultured for 5 days (day 2–6). Cell proliferation was evaluated
daily by adding 10 µl 5 mg/ml MTT, and incubation for 4 h at 37°C.
The supernatant was then removed and the purple formazan crystals
were dissolved in 100 µl DMSO at room temperature for 15 min.
Finally, absorbance was determined using a spectrophotometer,
measured at 570 nm. The relative proliferation rate was calculated
by dividing the optical density value on each of the 5 days by the
value on day 1.
Monolayer colony formation assay
A total of 200 transfected cells were seeded in
triplicate in 6-well plates. The cells maintained in complete
medium, refreshed every 3 days. After 2 weeks, the medium was
removed and the cells were fixed at room temperature for 10 min
using 100% methanol, dyed at room temperature for 20 min with 0.1%
crystal violet and washed with PBS. Visible colonies were counted
to calculate the colony formation rate (%) according to the
following formula: Number of colonies/200 × 100.
Transwell migration and invasion
assay
Transfected cells were incubated in serum-free DMEM
for 24 h. For the migration assay (without Matrigel), 100 µl
serum-free cell suspension containing 2×104 cells was
directly added into the upper chambers of a Transwell plate (8 µm,
24-well plate; Corning Incorporated, Corning, NY, USA). For
invasion studies, Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA) was thawed overnight at 4°C and diluted with serum-free medium
at a ratio of 1:8. The polycarbonate membranes of the Transwell
inserts were coated with 50 µl diluted Matrigel and incubated at
37°C for 1 h. Next, 100 µl serum-free cell suspension containing
4×104 cells was added to each upper chamber, and 500 µl
DMEM-F12 containing 10% FBS was added to each lower chamber.
Following incubation for 12 h, the supernatant was removed from the
upper chamber and cells unable to penetrate the membrane were
carefully removed with a cotton swab. The Transwell membrane was
then fixed at room temperature for 15 min with 4% paraformaldehyde,
washed with PBS and dyed at room temperature for 20 min with 0.1%
crystal violet. Images from 5 fields on each membrane were captured
at ×40 magnification using a light microscope and the number of
penetrated cells fixed to the lower surface of the membrane was
counted. Independent experiments were repeated ≥3 times using 3
Transwell inserts for each repeat.
Statistical analysis
SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) was used to perform all statistical analysis. The
association between ER-α30 expression and clinicopathological
characteristics was analyzed using Fisher's exact test. The results
of the colony formation, migration and invasion assays are
presented as the mean ± standard error of the mean of repeated
independent experiments. Comparisons between 2 groups were analyzed
by Student's t-test subsequent to Levene's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cloning of ER-α30
The ER-α30 ORF was amplified by semi-nested RT-PCR
using RNA from clinical breast cancer tumor tissue. A 1,002-bp PCR
fragment was purified, cloned and successfully sequenced. The
sequence of exons 1, 2, 3 and 8 were identical to the DNA sequence
of the ER-α66 genomic sequence. However, the fragment contained
only partial segments of exons 4 and 6, and was completely lacking
exons 5 and 7. The first 24 bp of exon 4 were directly spliced to
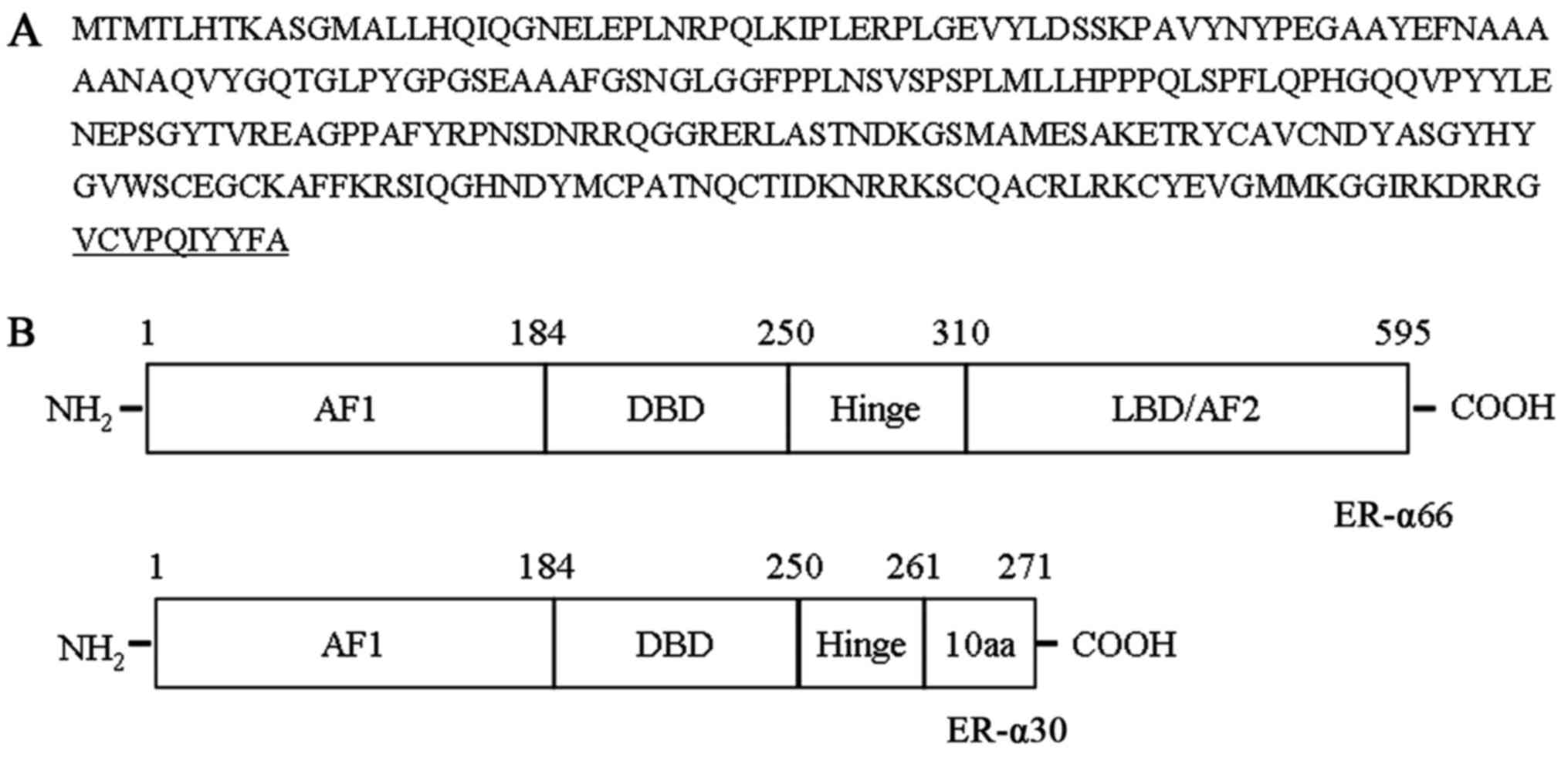
the last 44 bp of exon 6. The cDNA sequence encoded a protein 271
amino acids in length with a predicted molecular weight of 30 kDa
(Fig. 1A). ER-α30 differed from
ER-α66 as it lacked a ligand-binding domain (LBD) and a
ligand-dependent transcriptional activation domain (AF2). It
retained the N-terminal transcriptional activation domain (AF1),
the DNA-binding domain (DBD), a partial hinge domain and contained
a unique 10 amino acid domain at the C-terminus (Fig. 1B).
ER-α30 expression is inversely
associated with ER-α66 and PR expression
A total of 33 breast tumor specimens were analyzed
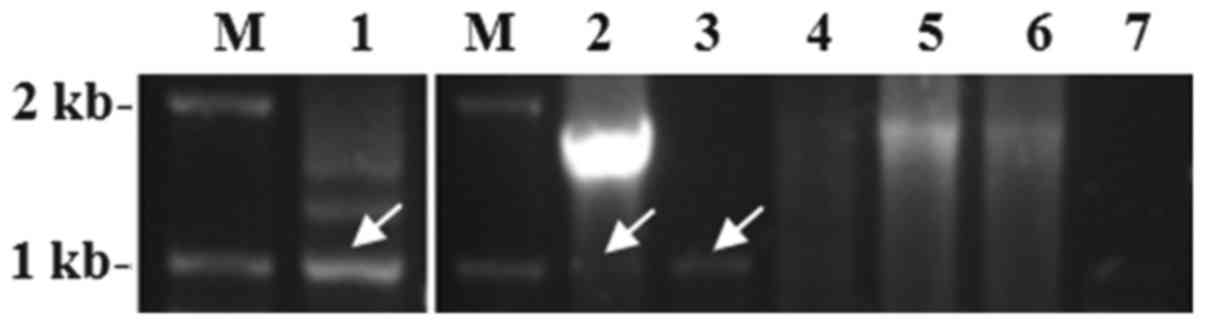
using semi-nested RT-PCR and sequence analysis, revealing that the
1,002-bp PCR fragment was amplified in 11 tumor tissues but not in
the remaining 22 tumor tissues, indicating that 11 specimens
(33.3%) expressed ER-α30 mRNA (Fig.
2). It was also demonstrated that ER-α30 expression was
negatively associated with ER-α66 and PR expression status
(Table I). The ER-α30 expression
frequency of ER-α66 and PR-negative tumors was higher than that for
ER-α66 and PR-positive tumors (60 vs. 11.1%, and 55.6 vs. 6.7%,
respectively). No associations were identified between ER-α30
expression and other clinical characteristics, including age,
menopausal status, tumor size, tumor stage, lymph node status, or
Her-2 status.
 | Table I.Association between ER-α30 expression
and clinical characteristics. |
Table I.
Association between ER-α30 expression
and clinical characteristics.
|
|
| ER-α30 expression
status |
|---|
|
|
|
|
|---|
| Characteristic | Patients, n | Positive | Negative |
|---|
| Age, years |
|
|
|
| ≥50 | 22 | 8 | 14 |
|
<50 | 11 | 3 | 8 |
| Menopausal
status |
|
|
|
|
Premenopausal | 12 | 4 | 8 |
|
Postmenopausal | 21 | 7 | 14 |
| Tumor size, cm |
|
|
|
| ≤2 | 12 | 4 | 8 |
|
>2 | 21 | 7 | 14 |
| Tumor stage |
|
|
|
| I | 2 | 0 | 2 |
| II | 16 | 5 | 11 |
| III | 15 | 6 | 9 |
| Lymph node
status |
|
|
|
| 0 | 14 | 5 | 9 |
| ≥1 | 19 | 6 | 13 |
| ER-α66
statusa |
|
|
|
|
Positive | 18 | 2 | 16 |
|
Negative | 15 | 9 | 6 |
| PR
statusa |
|
|
|
|
Positive | 15 | 1 | 14 |
|
Negative | 18 | 10 | 8 |
| Her-2 status |
|
|
|
|
Positive | 22 | 9 | 13 |
|
Negative | 11 | 2 | 9 |
ER-α30 ORF encodes a 30 kDa protein
recognized by antibodies targeting the N-terminal AF1 of
ER-α66
To confirm whether the cloned ER-α30 ORF expresses
the predicted protein, it was transfected into the human breast
cancer MDA-MB-231 cell line using the pEGFP-N1 expression vector.
Whole-cell protein extracts from MDA-MB-231/ER-α30 cells (stably
transfected with pEGFP-N1-ER-α30), MDA-MB-231/v cells (stably
transfected with the pEGFP-N1 empty vector), and MCF-7 cells
(control) were subjected to western blot analysis using a
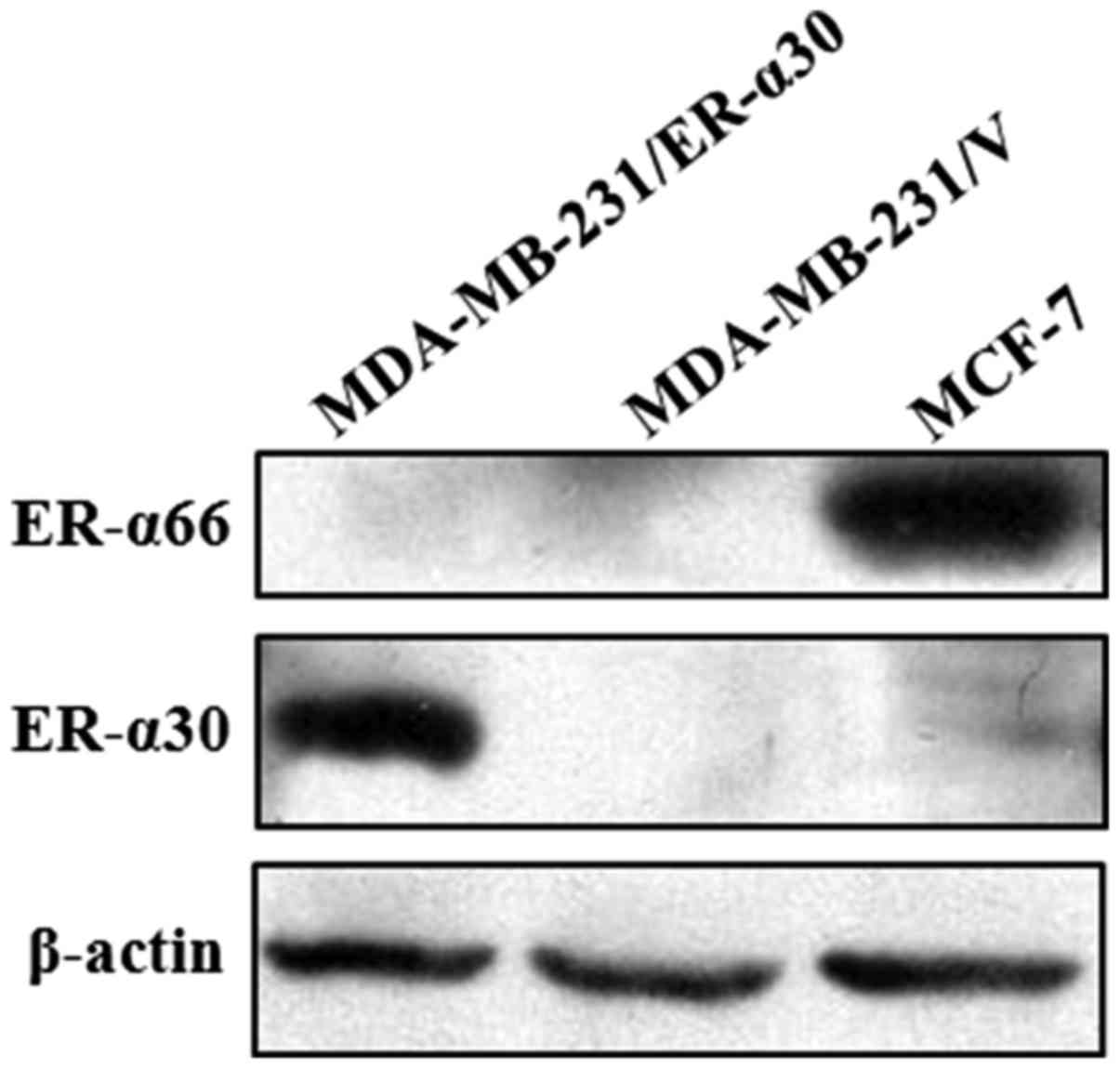
polyclonal antibody targeting amino acids 2–185 of ER-α66.
Visualization of the membrane revealed the presence of a 30 kDa
protein band in the MDA-MB-231/ER-α30 cells, but not in the
MDA-MB-231/v cells or the MCF-7 cells. Meanwhile, a 66 kDa protein
band was visualized in the MCF-7 cells but not the
MDA-MB-231/ER-α30 cells or the MDA-MB-231/v cells. These results
indicated that the isolated ORF expressed the predicted protein,
which was recognized by the anti-ER-α66 antibody (Fig. 3).
Overexpression of ER-α30 in MDA-MB-231
cells enhances cell proliferation, migration, and invasion
To investigate the effect of ER-α30 overexpression
on the proliferation ability of MDA-MB-231 cells, MTT and monolayer
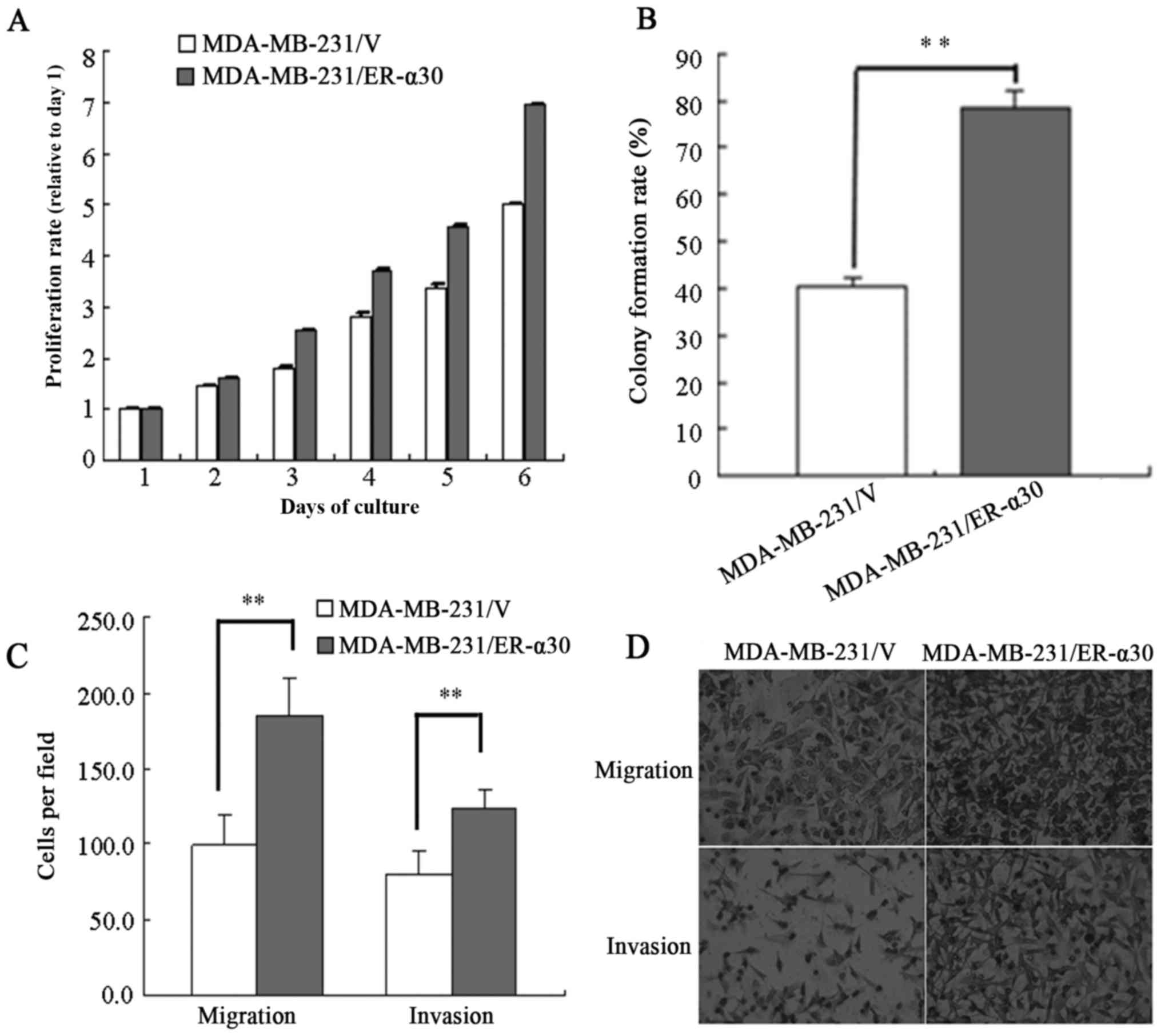
colony formation assays were performed. As demonstrated in Fig. 4A, MDA-MB-231/ER-α30 cells exhibited a
more rapid rate of proliferation than did MDA-MB-231/v control
cells. Furthermore, the monolayer colony-formation assay revealed
that MDA-MB-231/ER-α30 cells exhibited a greater colony-formation
ability than MDA-MB-231/v cells (78.5±3.5 vs. 40.3±1.7%; Fig. 4B). To determine whether ER-α30
expression modulated the migratory and invasive abilities of breast
cancer cells, a Transwell assay was performed. The
MDA-MB-231/ER-α30 cells exhibited a greater migratory ability than
the control cells (184.7±24.3 vs. 99.3±24.8 cells; Fig. 4C and D). The MDA-MB-231/ER-α30 cells
were also more invasive than the control cells (124.2±12.1 vs.
79.7±15.9, respectively; Fig. 4C and
D).
Discussion
In the present study, ER-α30, a novel splice variant
of hER-α, was cloned from clinical breast cancer tissue. In this
variant, the first 24 bp of exon 4 were directly spliced to the
last 44 bp of exon 6 of the hER-α genomic sequence. As exon 5 and 7
were completely deleted, the ORF of ER-α30 was predicted to encode
a truncated protein of 30 kDa. The protein differed from the
full-length hER-α (ER-α66) as it lacked a segment of the hinge
domain, the LBD and AF2, but possessed a unique C-terminal
10-amino-acid domain. The predicted truncated 30 kDa protein was
then overexpressed in MDA-MB-231 cells, as confirmed by a western
blot assay using an antibody directly targeting the N-terminal AF1
of ER-α66.
It is likely that ER-α30 has not been previously
identified in clinical specimens because the anti-ER-α66 antibody
that targets the LBD is not present in this variant (11–13). With
intact AF1 and DBD domains, ER-α30 may still function as a nuclear
transcription factor, similar to ER-α66, mediating the traditional
genomic signaling pathway. However, the C-terminal 10-amino-acid
domain that is substituted for the last 335 amino acids of the
LBD/AF2 domains indicates that ER-α30 may possess different
transcriptional activity from the full-length protein. For example,
it may exhibit a different ligand response profile to ER-a66.
Alternatively, it may function as a negative inhibitor of the
estrogen-signal response mediated through the ER-α66 AF2 domain,
similar to ER-α46, which also lacks the AF1 domain and strongly
represses AF1 activity of ER-α66 (14,15).
ER-α30 mRNA was expressed in 11/33 (33%) breast
cancer tissue specimens. As 2 adjacent normal breast tissues were
not collected and the quality of total RNA from 4 adjacent normal
tissues was too poor to be used for analysis, 5 adjacent normal
tissues from of the 11 ER-α30-positive patients were also analyzed.
Amplification of ER-α30 derived from the 5 adjacent normal breast
tissues was unsuccessful (data not shown). Therefore, we
hypothesized ER-α30 is mainly expressed in breast cancer tumor
tissue. Furthermore, the association analysis of ER-α30 expression
and clinical characteristics demonstrated that ER-α66- and
PR-negative breast tumor tissues exhibited expression of the ER-α30
variant more frequently than ER-α66- and PR-positive breast cancer
tumors. This indicates that ER-α30 may inhibit the expression of
ER-α66 and PR proteins, functioning as a negative regulation
variant. However, no significant associations between ER-α30
expression and other clinical characteristics were identified,
which is possibly due to the limited number of samples.
The ER-α30 expression status of the MDA-MB-231 cell
line was analyzed using semi-nested RT-PCR, which revealed that the
1,002-bp PCR fragment was not expressed in this cell line (data not
shown). ER-α30 overexpression induced in MDA-MB-231 cells was to
investigate the potential function of ER-α30. It was demonstrated
that ER-α30 overexpression promoted cell proliferation, migration,
and invasion, indicating that ER-α30 may bind DNA through the
retained DNA-binding domain to activate the transcription of
specific target genes through the retained AF1 domain. ER-negative
breast cancer exhibits higher rates of metastasis and recurrence
than ER-positive breast cancer (16).
The results of the present study indicated that ER-α30 may be
associated with ER-negative breast cancer, and thus that it may be
a potential biomarker for ER-negative breast cancer. ER-α30
expression was also analyzed in MCF-7 cells and negative results
were achieved (Fig. 3). This
conclusion would be strongly supported by performing a
gene-silencing assay in a cell line exhibiting default ER-α30
expression.
In summary, a novel hER-α splice variant, ER-α30,
was identified in the present study, and its function was
preliminarily investigated. However, further characterization and
validation is required, as are further studies to determine the
significance of ER-α30 in human breast cancer. Future investigation
should include the development of an antibody targeted to the
unique 10-amino-acid domain of ER-α30 to characterize the
expression pattern of ER-α30 in normal and breast cancer tissues,
the mutual regulation between ER-α30 and ER-α66, and the mechanism
by which ER-α30 enhances malignant biological behaviors. These
studies will provide novel insights into the complex biological
aspects of breast cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the Guangxi Provincial
Natural Science Foundation of China (grant no.
2013GXNSFBA019189).
Availability of data and materials
The data generated in this study are available from
the corresponding upon reasonable request.
Authors' contribution
HZ performed the majority of the experiments and
wrote the manuscript. YH and YM participated in experiments and
sample collection. HS, YT and DJL provided materials and methods
support. YL and ZF designed the experiments and contributed to the
manuscript writing.
Ethics approval and consent to
participate
The present study was approved by the Human Ethics
Committee of the Affiliated Hospital of Guilin Medical University
(Guangxi, China) and informed consent was obtained from all
patients.
Consent for publication
Patients provided written informed consent for the
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Venables JP, Klinck R, Koh C, Gervais-Bird
J, Bramard A, Inkel L, Durand M, Couture S, Froehlich U, Lapointe
E, et al: Cancer-associated regulation of alternative splicing. Nat
Struct Mol Biol. 16:670–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J and Weiss WA: Alternative splicing
in cancer: Implications for biology and therapy. Oncogene. 34:1–14.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishunina TA and Swaab DF: Estrogen
receptor-alpha splice variants in the human brain. Gynecol
Endocrinol. 24:93–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dunnwald LK, Rossing MA and Li CI: Hormone
receptor status, tumor characteristics, and prognosis: A
prospective cohort of breast cancer patients. Breast Cancer Res.
9:R62007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goldhirsch A, Glick JH, Gelber RD, Coates
AS, Thürlimann B and Senn HJ Panel members: Meeting highlights:
International expert consensus on the primary therapy of early
breast cancer 2005. Ann Oncol. 16:1569–1583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang B, Warner M and Gustafsson JÅ:
Estrogen receptors in breast carcinogenesis and endocrine therapy.
Mol Cell Endocrinol. 418(Pt 3): 1–244. 2015.PubMed/NCBI
|
|
7
|
Li G, Zhang J, Jin K, He K, Zheng Y, Xu X,
Wang H, Wang H, Li Z, Yu X, et al: Estrogen receptor-α36 is
involved in development of acquired tamoxifen resistance via
regulating the growth status switch in breast cancer cells. Mol
Oncol. 7:611–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Penot G, Le Péron C, Mérot Y,
Grimaud-Fanouillère E, Ferrière F, Boujrad N, Kah O, Saligaut C,
Ducouret B, Métivier R and Flouriot G: The human estrogen
receptor-alpha isoform hERalpha46 antagonizes the proliferative
influence of hERalpha66 in MCF7 breast cancer cells. Endocrinology.
146:5474–5484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr; American
Society of Clinical Oncology, : American Society of Clinical
Oncology 2007 update of recommendations for the use of tumor
markers in breast cancer. J Clin Oncol. 25:5287–5312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singletary SE, Allred C, Ashley P, Bassett
LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, et
al: Revision of the american joint committee on cancer staging
system for breast cancer. J Clin Oncol. 20:3628–3636. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Denger S, Reid G, Kos M, Flouriot G,
Parsch D, Brand H, Korach KS, Sonntag-Buck V and Gannon F: ERalpha
gene expression in human primary osteoblasts: Evidence for the
expression of two receptor proteins. Mol Endocrinol. 15:2064–2077.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Haynes MP and Bender JR: Plasma
membrane localization and function of the estrogen receptor alpha
variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA.
100:pp. 4807–4812. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: Identification, cloning, and expression of human
estrogen receptor-alpha36, a novel variant of human estrogen
receptor-alpha66. Biochem Biophys Res Commun. 336:1023–1027. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flouriot G, Brand H, Denger S, Metivier R,
Kos M, Reid G, Sonntag-Buck V and Gannon F: Identification of a new
isoform of the human estrogen receptor-alpha (hER-alpha) that is
encoded by distinct transcripts and that is able to repress
hER-alpha activation function 1. EMBO J. 19:4688–4700. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Márquez DC and Pietras RJ:
Membrane-associated binding sites for estrogen contribute to growth
regulation of human breast cancer cells. Oncogene. 20:5420–5430.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parl FF, Schmidt BP, Dupont WD and Wagner
RK: Prognostic significance of estrogen receptor status in breast
cancer in relation to tumor stage, axillary node metastasis, and
histopathologic grading. Cancer. 54:2237–2242. 1984. View Article : Google Scholar : PubMed/NCBI
|