Introduction
Due to its increasing rates of incidence and
mortality, cancer is the leading cause of death and a major public
health concern, with >1.6 million new diagnoses and 1.2 million
cancer-associated deaths occurring each year in China (1). Breast cancer is one of the most common
and aggressive malignancies and accounts for ~20% of cancer-related
deaths in women, according to WHO statistics. Breast cancer is also
the most prevalent cancer among Chinese women, and its incidence
continues to increase by ~5% each year. Despite tremendous advances
in diagnostic and therapeutic methods in recent years, breast
cancer remains a complex malignancy in terms of clinical treatment
(2,3).
The majority of patients with breast cancer tend to be diagnosed in
the later stages of disease due to a lack of early specific
symptoms or biomarkers. Therefore, improving early diagnosis and
targeted therapies is particularly important for breast cancer.
Accumulating evidence has confirmed that the invasion or metastasis
of breast cancer cells is a major factor that causes breast
cancer-related death.
The breast cancer suppressor candidate-1 (BCSC-1)
gene is a recently identified tumor suppressor gene that is located
at chromosome 11q23 and can encode a 786-amino-acid protein with an
86-kDa molecular weight (4). Previous
studies have shown that BCSC-1 expression is relatively low in
different types of tumor cells, including nasopharyngeal carcinoma,
cervical carcinoma and melanoma cells (5–7). In our
previous study, we also detected low expression of BCSC-1 in liver
cancer, lung cancer and esophageal squamous cell carcinoma (ESCC)
(8). These findings indicate that low
expression of BCSC-1 may promote tumorigenesis. However, the
relationship between BCSC-1 and human breast cancer remains
unclear.
Accumulating evidence has also confirmed that matrix
metalloproteinases (MMPs) play an important role in tumor invasion
and metastasis. As an important member of the MMP family, MMP-14
was the first membrane-type MMP (MT1-MMP) to be identified. MMP-14
is widely expressed on the surfaces of a large variety of cancer
cells, such as gastric, lung or liver cancer cells, and can insert
itself into the membrane via a transmembrane domain (9). MMP-14 is also involved in a variety of
biological processes, such as basement membrane remodeling, tumor
cell proliferation and invasion, angiogenesis and fibrous tissue
expansion. In particular, MMP-14 can be produced by tumor cells and
does not require additional activation because of its capacity to
be presented on the cell surface in an active form. A previous
study demonstrated that MMP-14 expression in tumor cells
significantly correlated with enhanced cell migration capacity and
poor patient prognosis (10–12).
Recent reports regarding BCSC-1 and MMP-14, and
particularly their relationship with one another in human tissues,
are rare. In the present study, we investigated BCSC-1 and MMP-14
expression in breast cancer tissues by immunohistochemistry, and
then verified this expression at the mRNA and protein levels using
reverse transcription-quantitative PCR (RT-qPCR) and western
blotting. The associations of these proteins with the clinical and
pathological characteristics of breast cancer patients were also
evaluated. The aim of the study was to investigate the prognostic
value of BCSC-1 and MMP-14 in breast cancer.
Materials and methods
Tissue microarrays and clinical tissue
specimens
A breast cancer tissue microarray consisting of 69
breast cancer cases and 3 normal breast tissues cases was purchased
from Alenabio (Xian, China; no. BC08013a). A total of 127 patients
with pathologically and clinically confirmed breast cancer, who
were treated at the Breast Surgery Center of Weifang People's
Hospital (Weifang, China), were included in this study for further
verification of the tissue microarray results and other analyses.
The present study was approved by the Ethics Committee of Weifang
People's Hospital and written informed consent was obtained from
all patients. The breast cancer tissues and paired adjacent normal
breast tissues were obtained before patients received any other
treatments. All samples were stored at −80°C prior to use for RNA
isolation or protein extraction. Tissue samples collected from the
breast cancer patients were fixed in formalin and embedded in
paraffin. Approximately 5-µm-thick, paraffin-embedded sections were
deparaffinized in xylene and rehydrated in graded ethanol. The
tissue microarray and clinical tissue specimens were placed in EDTA
buffer at 100°C for 20 min for antigen retrieval, and then
incubated at 4°C overnight with mouse anti-human BCSC-1 antibody
(cat. no. ab64977; 1:500; Abcam, Cambridge, MA, USA) and mouse
anti-human MMP-14 antibody (cat. no. ab51074; 1:500; Abcam). The
peroxidase-conjugated anti-mouse secondary antibody (cat. no.
SP-0022; ZSGB-BIO, Beijing, China) was then added at a 1:1,000
dilution, followed by the addition of DAB substrate, according to
the manufacturer's instructions. The degree of immunostaining was
then observed and scored. The staining was graded as follows: 0,
absent; 1, weak; 2, moderate; and 3, strong. The percentage of
positively stained cells was graded as follows: 0, no staining; 1,
0–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. The final score was
calculated by multiplying the staining intensity and the percentage
of positive cells, and total final scores were classified as
follows: A score of ≤2 was negative (−); 3–4 was weak (+); 6–8 was
moderate (++) and 9–12 was strong (+++). Scores of (−) and (+) were
regarded as low expression, while (++) and (+++) were regarded as
high expression.
RNA extraction and RT-qPCR
Total RNA from the frozen tissue specimens was
extracted using TRIzol reagent (cat. no. 9108), and cDNA was
synthesized from 0.5 µg of RNA using RT reagents (cat. no. DRR036s;
both Takara, Dalian, China). qPCR was performed according to the
manufacturer's protocol. SYBR Premix Ex Taq™ (cat. no.
DRR820s; Takara) was used for qPCR in a LightCycler 480 instrument
(Roche, Basel, Switzerland). The PCR conditions were as follows:
Initial denaturation at 95°C for 30 sec; followed by 35 cycles of
denaturation at 94°C for 30 sec, annealing at 50°C for 30 sec, and
extension at 70°C for 30 sec. The relative mRNA expression level
was determined by normalizing the cycle threshold (Cq) of the gene
of interest to that of β-actin using the 2−ΔΔCt formula.
The following specific primer pairs were used in the present study:
BCSC-1 forward, 5′-TGCTTCTGCCCCATTGAAGA-3′ and reverse,
5′-CTGTGCTGGTCCTTGTGAC-3′; MMP-14 forward,
5′-CGAGGTGCCCTATGCCTAC-3′ and reverse, 5′-CTCGGCAGAGTCAAAGTGG-3′;
β-actin forward, 5′-CCTAGAAGCATTTGCGGTGG-3′ and reverse,
5′-GAGCTACGAGCTGCCTGACG-3′
Western blot analysis
The breast cancer samples were prepared by
homogenizing frozen tissues, and proteins were extracted using cell
lysis buffer (Beyotime Biotechnology, Haimen, China) containing
protease inhibitors. The mixture was incubated on ice for 30 min
and cell debris was removed by centrifugation at 12,000 × g for 5
min. The protein concentrations were measured using BCA kits
(Pierce Biotechnology, Inc., Rockford, IL, USA). Subsequently, 40
µg of protein per lane was separated by SDS-PAGE and subsequently
transferred onto nitrocellulose membranes (Millipore, Bedford, MA,
USA). The proteins were blocked and incubated with goat anti-human
BCSC-1 (cat. no. sc-137568; 1:1,000; Santa Cruz Biotechnology,
Santa Cruz, CA, USA), mouse anti-human MMP-14 (cat. no. ab51074;
1:1,000 dilution; Abcam) and mouse anti-human β-actin (cat. no.
AA128; 1:1,000 dilution; Beyotime Biotechnology) antibodies
overnight at 4°C. After washing with TBST buffer, the blots were
incubated with the corresponding horseradish peroxidase-conjugated
secondary antibodies (cat. nos. A0181 and A0216; 1:1,000 dilution;
Beyotime Biotechnology) and visualized using ECL detection reagent
(Sangon Biotech, Shanghai, China).
Statistical analysis
All data were analyzed using SPSS 10.0 software
(SPSS, Inc., Chicago, IL, USA). χ2 and Fisher's exact
tests were used to analyze the associations between BCSC-1 and
MMP-14 expression and the clinicopathological characteristics of
the patients. Spearman's rank correlation was used to analyze the
correlation between BCSC-1 and MMP-14 expression levels. P<0.05
was considered to indicate a statistically significant
difference.
Results
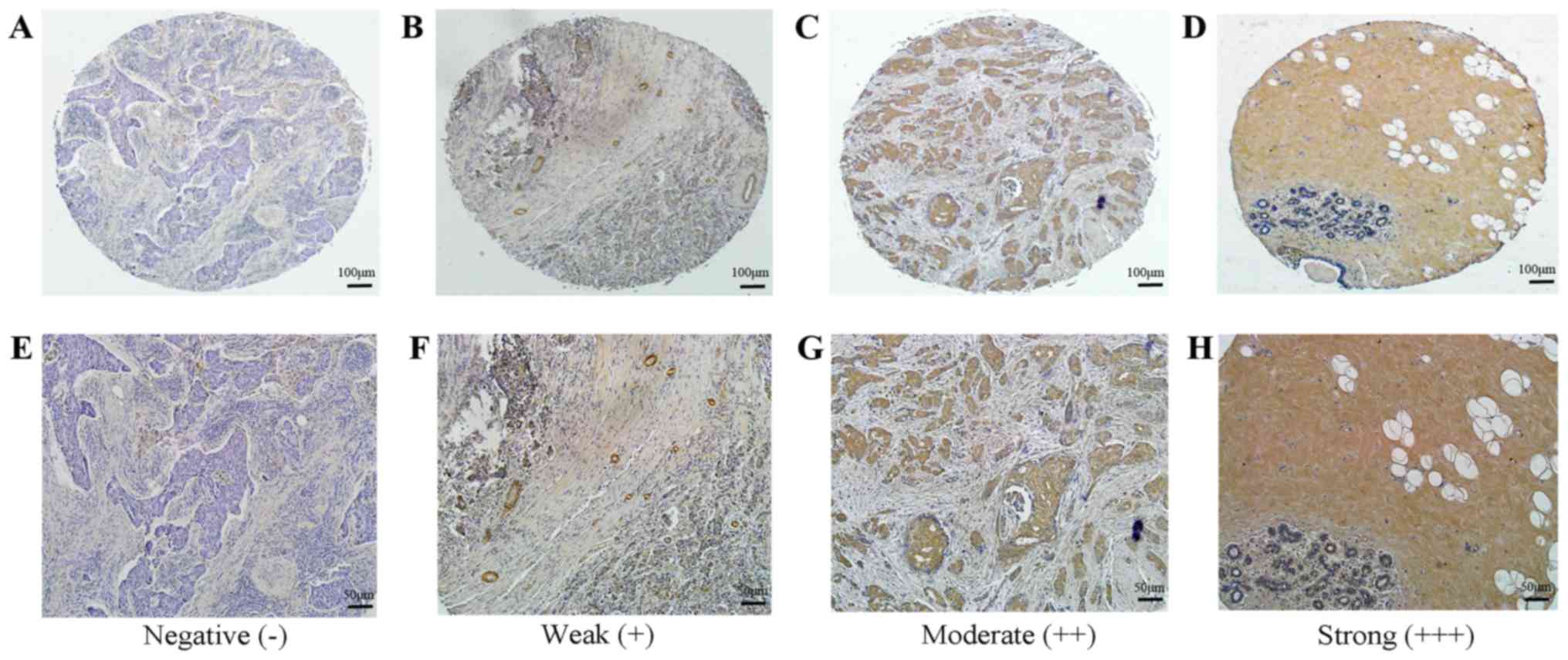
Expression of BCSC-1 and MMP-14 in
breast cancer tissue microarrays and clinical tissue specimens
To investigate the function of BCSC-1 and MMP-14 in
breast cancer, we evaluated their expression in a breast cancer
tissue microarray using immunohistochemistry firstly. For BCSC-1,
42 breast cancer tissues exhibited low expression and 27 breast
cancer tissues exhibited high expression, while all 3 normal tissue
samples exhibited high expression (Fig.
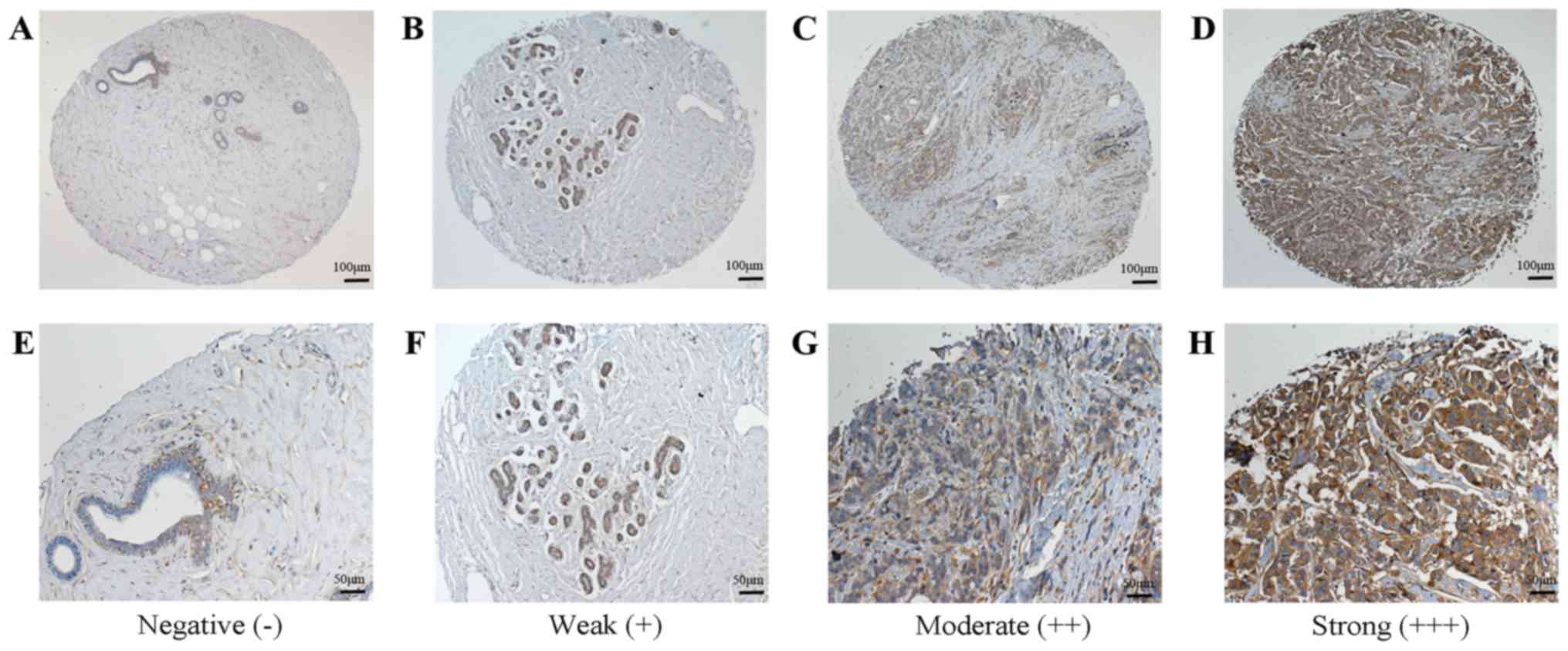
1). For MMP-14, 12 breast cancer tissues exhibited low
expression and 57 breast cancer tissues exhibited high expression,
while 2 of the normal tissue samples exhibited low expression and 1
exhibited high expression (Fig. 2).
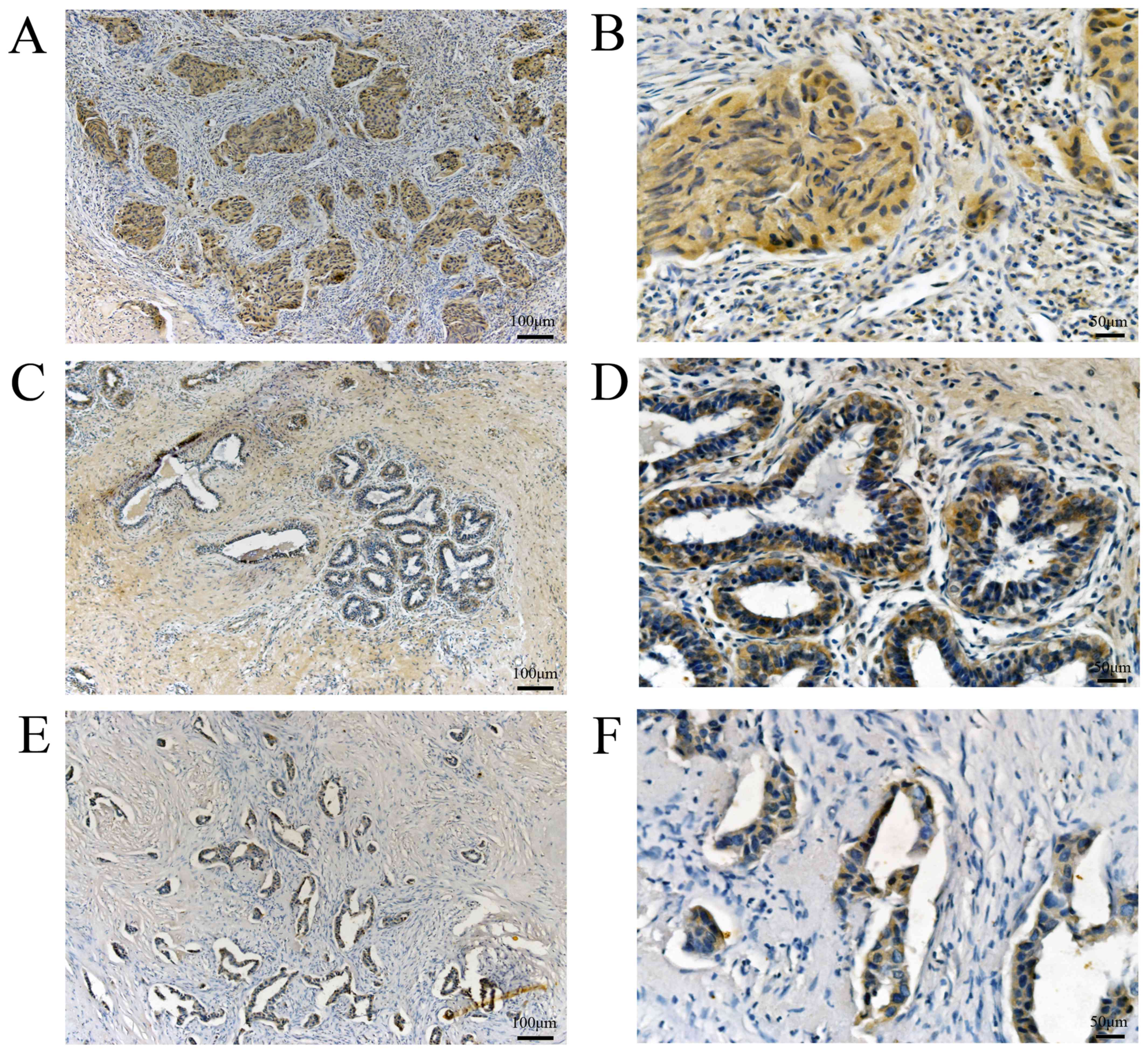
For further confirmation, we collected 127 pathologically and
clinically confirmed breast cancer specimens from patients treated
at the Breast Surgery Center of Weifang People's Hospital, for
BCSC-1, 82 breast cancer tissues exhibited low expression and 45
breast cancer tissues exhibited high expression, whereas 40
adjacent normal breast tissues exhibited low expression and 87
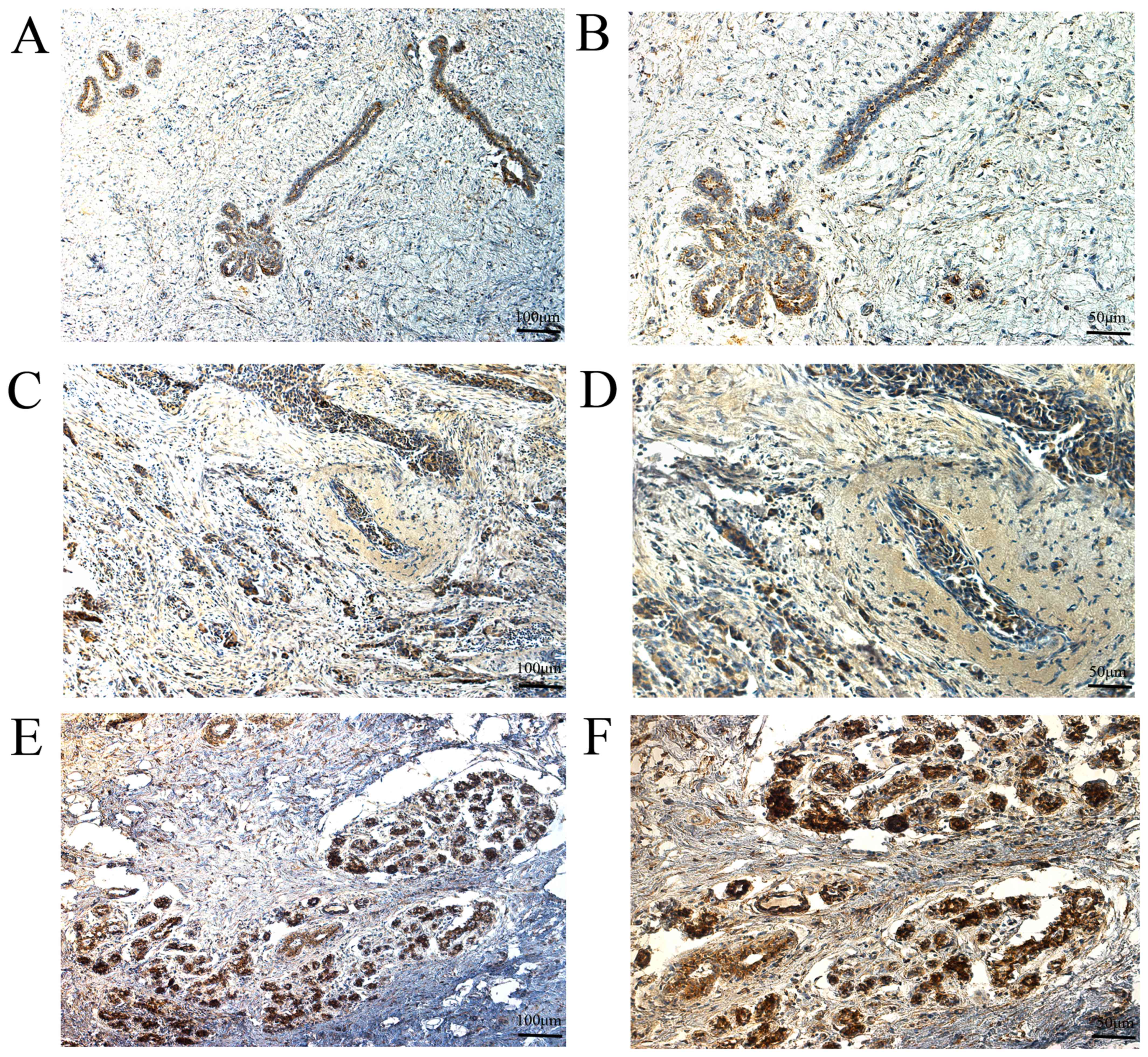
normal breast tissues exhibited high expression (Fig. 3). For MMP-14,56 breast cancer tissues
exhibited low expression and 71 breast cancer tissues exhibited
high expression, whereas 92 adjacent normal breast tissues
exhibited low expression and 35 adjacent normal breast tissues
exhibited high expression (Fig. 4).
The results of clinical tissue specimens were in accordance with
those obtained from the tissue microarrays. In general, for BCSC-1,
124 (63.27%) breast cancer tissues exhibited low expression and 72
(36.73%) breast cancer tissues exhibited high expression, whereas
40 (30.77%) adjacent normal breast tissues exhibited low expression
and 90 (69.23%) normal breast tissues exhibited high expression.
For MMP-14, 68 (34.69%) breast cancer tissues exhibited low
expression and 128 (65.31%) breast cancer tissues exhibited high
expression, whereas 94 (72.31%) adjacent normal breast tissues
exhibited low expression and 36 (27.69%) adjacent normal breast
tissues exhibited high expression, the expression level of BCSC-1
and MMP-14 differed significantly between breast cancer and normal
breast tissues, the expression level of BCSC-1 in breast cancer was
significantly lower than of normal breast tissues, the expression
level of MMP-14 in breast cancer was significantly higher than in
the normal breast tissues (P<0.05). These results suggest that
BCSC-1 and MMP-14 may be involved in the pathogenesis of breast
cancer (Table I).
 | Table I.Expression of BCSC-1 and MMP-14 in
breast cancer tissues compared with normal breast tissues. |
Table I.
Expression of BCSC-1 and MMP-14 in
breast cancer tissues compared with normal breast tissues.
|
|
| BCSC-1 |
| MMP-14 |
|
|---|
|
|
|
|
|
|
|
|---|
| Sample | No. | Low (%) | High (%) | P-value | Low (%) | High (%) | P-value |
|---|
| Breast cancer | 196 | 124 (63.27) | 72 (36.73) | <0.01a | 68 (34.69) | 128 (65.31) | <0.01a |
| Adjacent normal
tissues | 130 | 40 (30.77) | 90 (69.23) |
| 94 (72.31) | 36 (27.69) |
|
Association of BCSC-1 and MMP-14
expression with the clinicopathological features of breast cancer
patients
The associations between BCSC-1 and MMP-14
expression (low expression or high expression) and the
clinicopathological features of breast cancer were evaluated in the
present study. The results indicated that neither BCSC-1 or MMP-14
expression status was associated significantly with patient age,
histological type or clinical stage (P>0.05), but that both were
closely associated with tumor differentiation, lymph node
metastasis and distant metastasis (P<0.05), implying that BCSC-1
and MMP-14 may participate in breast cancer metastasis (Table II).
 | Table II.Association of BCSC-1 and MMP-14
expression with the clinicopathological features of breast cancer
patients. |
Table II.
Association of BCSC-1 and MMP-14
expression with the clinicopathological features of breast cancer
patients.
|
|
| BCSC-1
expression |
| MMP-14
expression |
|
|---|
|
|
|
|
|
|
|
|---|
| Clinical feature | No. | Low (%) | High (%) | P-value | Low (%) | High (%) | P-value |
|---|
| Age, years |
|
|
| 0.085 |
|
| 0.111 |
|
<35 | 132 | 78 (59.09) | 54 (40.91) |
| 51 (38.64) | 81 (61.36) |
|
| ≥35 | 64 | 46 (71.88) | 18(28.12) |
| 17 (26.56) | 47(73.44) |
|
| Histological
type |
|
|
| 0.395 |
|
| 0.130 |
| Invasive
ductal carcinoma | 169 | 109 (64.50) | 60 (35.50) |
| 55 (32.54) | 114 (67.46) |
|
| Medullary
carcinoma | 27 | 15 (55.56) | 12 (44.44) |
| 13 (48.15) | 14 (51.85) |
|
| Clinical stage |
|
|
| 0.249 |
|
| 0.242 |
| I–II | 141 | 93 (65.96) | 48 (34.04) |
| 45 (31.91) | 96 (68.09) |
|
|
II–IV | 55 | 31 (56.36) | 24 (43.64) |
| 23 (41.82) | 32 (58.18) |
|
| Tumor
differentiation |
|
|
| 0.006a |
|
| 0.023a |
|
T1-T2 | 136 | 95 (69.85) | 41 (30.15) |
| 40 (29.41) | 96 (70.59) |
|
|
T3-T4 | 60 | 29 (48.33) | 31 (51.67) |
| 28 (46.67) | 32 (53.33) |
|
| Lymph node
metastasis |
|
|
| 0.017a |
|
| 0.042a |
|
N0-N1 | 155 | 105 (67.74) | 50 (32.26) |
| 48 (30.97) | 107 (69.03) |
|
|
N2-N3 | 41 | 19 (46.34) | 22 (53.66) |
| 20 (48.78) | 21 (51.22) |
|
| Distant
metastasis |
|
|
| a |
|
| 0.016a |
| M0 | 174 | 116 (66.67) | 58 (33.33) |
| 55 (31.61) | 119 (68.39) |
|
| M1 | 22 | 8 (36.36) | 14 (63.64) |
| 13 (59.09) | 9 (40.91) |
|
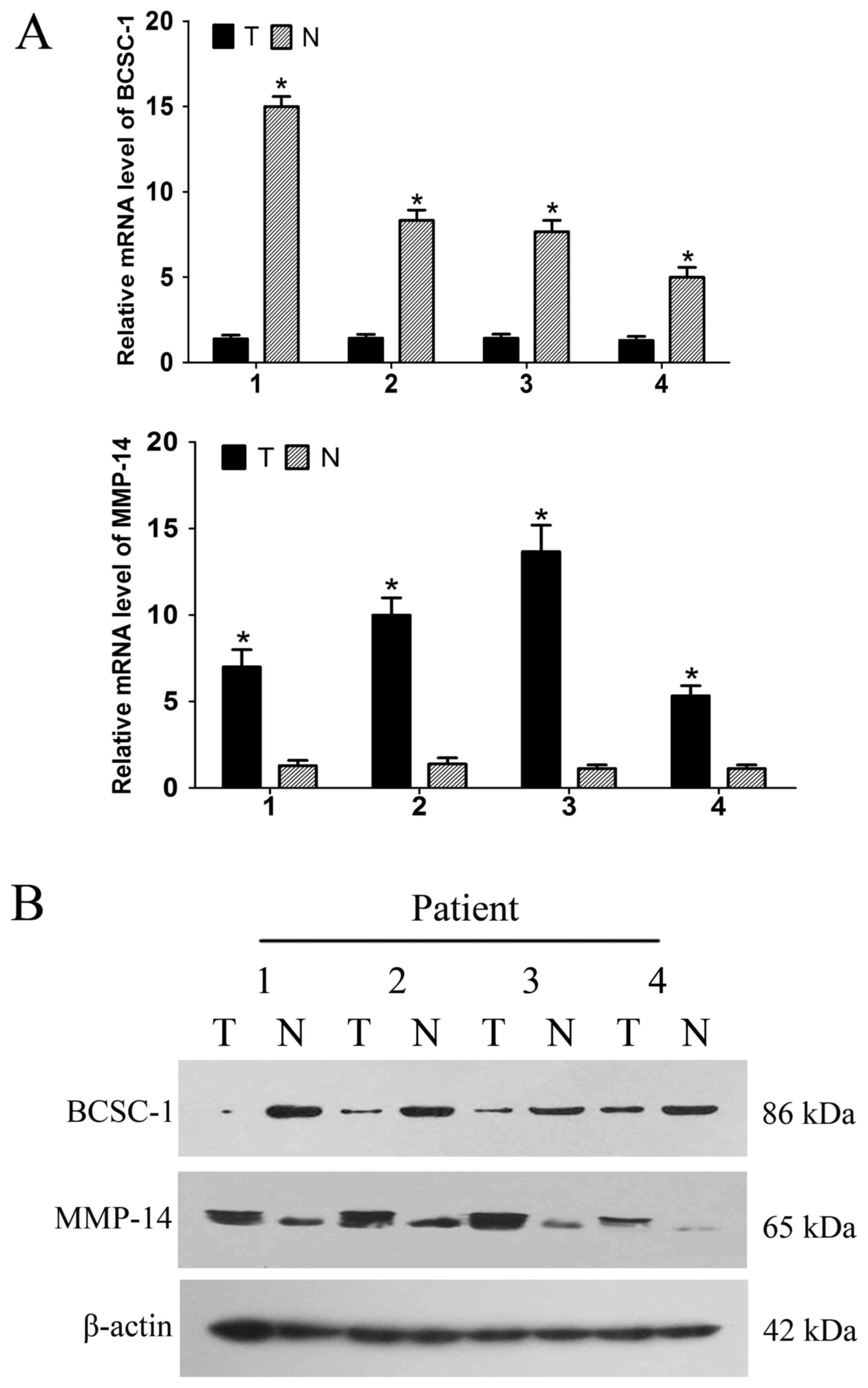
mRNA and protein expression levels of
BCSC-1 and MMP-14 in breast cancer
The mRNA and protein expression levels of BCSC-1 and
MMP-14 in the breast cancer tissues and adjacent normal tissues
were detected using RT-qPCR and western blot analysis. RT-qPCR
revealed low expression of BCSC-1 and high expression of MMP-14 in
breast cancer tissues compared with normal tissues; these findings
were recapitulated at the protein level using a western blot
analysis, and were consistent with those of the immunohistochemical
staining. This suggests that BCSC-1 and MMP-14 may be involved in
the pathogenesis of breast cancer (Fig.
5).
Correlation analysis of BCSC-1 and
MMP-14 expression
Among the 124 cases with low expression of BCSC-1,
27 cases had low expression of MMP-14 and 97 cases had high
expression of MMP-14. Among the 72 cases with high expression of
BCSC-1, 41 cases had low expression of MMP-14 and 31 cases had high
expression of MMP-14. Spearman's rank correlation analysis
indicated that there was a negative correlation between BCSC-1 and
MMP-14 expression (r=−0.356, P<0.01) (Table III).
 | Table III.Correlation between the expression of
BCSC-1 and MMP-14. |
Table III.
Correlation between the expression of
BCSC-1 and MMP-14.
|
|
| MMP-14 |
|
|
|---|
|
|
|
|
|
|
|---|
| BCSC-1 | No. | Low | High | r | P-value |
|---|
| Low | 124 | 27 | 97 | −0.356 |
<0.01a |
| High | 72 | 41 | 31 |
|
|
Discussion
BCSC-1 is a newly discovered cancer-suppressor gene.
Martin et al (4) first
reported that 33 of 41 tumor cell lines exhibited low expression of
the BCSC-1 gene based on northern blot analysis. Meanwhile, the
tumorigenicity of human lung cancer H460 cell xenografts in nude
mice was significantly decreased after transfection with the BCSC-1
gene. We also found that the tumorigenicity and metastatic
capability of the human nasopharyngeal carcinoma cell line CNE-2L2
were decreased significantly with ectopic expression of BCSC-1
gene, while the expression levels of E-cadherin, alpha-catenin and
p53 were increased and cell-cycle arrest was induced (4,5). The
malignancy and proliferative capability of human lung cancer
NCI-H446 cells were also inhibited by ectopic expression of the
BCSC-1 gene; this inhibitory effect was related to cell-cycle
arrest and increased expression of the adhesion molecule CD44. We
also found that the migratory and invasive abilities of the human
breast cancer cell lines MCF-7 and MDA-MB-231 were reduced
significantly by transfection with the BCSC-1 gene and that the
expression level of ICAM-1 in the two cell lines was significantly
altered, suggesting that BCSC-1 may play an important role in the
expression of ICAM-1 (13). However,
little has been established regarding BCSC-1 in breast cancer
patients until now.
MMP-14 (also known as MT1-MMP) is a membrane-type
MMP that contains a membrane structural domain and is fixed on the
membrane surface. MMP-14 can not only directly degrade
extracellular matrix (ECM) components, such as collagen or
fiber-binding proteins, but also can activate other MMP members,
such as MMP-2 and MMP-9. MMPs participate in many
pathophysiological processes, such as fibrous tissue expansion,
tumorigenesis or inflammation (14,15). In
addition, increased MMP-14 expression has been observed in the
human stomach, lung and neuroblastoma tumor cells and has been
correlated with poor patient prognosis (16–18). A
meta-analysis of 1,918 cancer patients in 11 studies indicated that
MT1-MMP is a prognosticator for poor cancer survival; MMP-14
expression was significantly associated with poor overall survival
outcome in patients with lung cancer, gastric cancer or glioma
(19). In recent years, studies have
confirmed that MMP-14 participates in the formation of invadopodia,
which have been regarded as key structures that regulate the
metastatic potential of many tumor types and the
epithelial-mesenchymal transition process. Inhibiting the activity
of MMP-14 can significantly inhibit the activity of invadopodia
(20–22). The inhibition of MMP-14 activity by
NEDD9-depletion-mediated TIMP2 overexpression can down-regulate the
invasive activity of MCF-7 and BT-549 in vitro (23). Ampuja et al confirmed that
MMP-14 inhibition can down-regulate the invasive activity of
MDA-MB-231 and MDA-MB-361 breast cancer cells in a 3D environment
(24). Blocking the activity of
MMP-14 with an anti-MMP-14 inhibitory antibody (DX-2400) can
inhibit the tumorigenic ability of the murine breast carcinoma cell
lines 4T1 and E0771 (25).
Furthermore, the inhibition of MMP-14 by miR-886-5p can
downregulate the invasiveness of MCF-7 cells in vitro
(26). These experiments indicated
that MMP-14 may be a potential therapeutic target for breast
cancer. Although the correlations between MMP-14 and
clinicopathological features have been investigated in various
human carcinomas, little is known about MMP-14 in breast cancer
patients. Perhaps due to the lack of samples, reports regarding the
relationship between MMP-14 and the clinical characteristics of the
patients have been scarce. In the present study, we initially
identified the expression of BCSC-1 and MMP-14 in breast cancer
using tissue microarrays. The immunohistochemical staining revealed
that BCSC-1 was expressed at a low level while MMP-14 was expressed
at a high level in breast cancer tissues compared with normal
breast tissues, suggesting that BCSC-1 and MMP-14 may be involved
in the pathogenesis of breast cancer. Subsequently, 127 cases of
breast cancer were collected for further confirmation, and the
immunohistochemical staining of these tissues was consistent with
that of the tissue microarrays, suggesting that BCSC-1 and MMP-14
are involved in the pathogenesis of breast cancer. The results of
RT-qPCR and western blot analysis also confirmed the
immunohistochemistry results. Additionally, the results indicated
that BCSC-1 and MMP-14 expression statuses are closely related to
tumor cellular differentiation, lymph node metastasis and distant
metastasis (P<0.05); however, they were not significantly
associated with patient age, histological type or clinical stage
(P>0.05). These findings indicate that BCSC-1 and MMP-14 may
participate in breast cancer metastasis. A correlational analysis
between BCSC-1 and MMP-14 was also conducted in the present study,
revealing a negative correlation between the two proteins
(P<0.05).
In summary, our study suggested that BCSC-1 is
expressed at a low level and MMP-14 is highly expressed in breast
cancer tissues compared with normal breast tissues, and that these
correlate with tumor cellular differentiation, lymph node
metastasis and distant metastasis. Therefore, these proteins may
play an important role in breast cancer progression and act as
predictive biomarkers.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (81373185); the
Natural Science Foundation of Shandong, China (ZR2009CM019,
ZR2014HL058); Shandong Province Health Department (2013WS0287,
2014WS0462); Student Innovation Program of Weifang Medical
University (KX2016020).
References
|
1
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hong W and Dong E: The past, present and
future of breast cancer research in China. Cancer Lett. 351:1–5.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin ES, Cesari R, Pentimalli F, Yoder
K, Fishel R, Himelstein AL, Martin SE, Godwin AK, Negrini M and
Croce CM: The BCSC-1 locus at chromosome 11q23-q24 is a candidate
tumor suppressor gene. Proc Natl Acad Sci USA. 100:pp. 11517–11522.
2003; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou YQ, Chen SL, Ju JY, Shen L, Liu Y,
Zhen S, Lv N, He ZG and Zhu LP: Tumor suppressor function of BCSC-1
in nasopharyngeal carcinoma. Cancer Sci. 100:1817–1822. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazumder Indra D, Mitra S, Singh RK, Dutta
S, Roy A, Mondal RK, Basu PS, Roychoudhury S and Panda CK:
Inactivation of CHEK1 and EI24 is associated with the development
of invasive cervical carcinoma: Clinical and prognostic
implications. Int J Cancer. 129:1859–1871. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anghel SI, Correa-Rocha R, Budinska E,
Boligan KF, Abraham S, Colombetti S, Fontao L, Mariotti A, Rimoldi
D, Ghanem GE, et al: Breast cancer suppressor candidate-1 (BCSC-1)
is a melanoma tumor suppressor that down regulates MITF. Pigment
Cell Melanoma Res. 25:482–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao CL, Yu WJ, Gao ZQ, Li WT, Gao W, Yang
WW, Feng WG and Ju JY: Association of BCSC-1 with human esophageal
squamous cell carcinoma. Neoplasma. 62:765–769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugiyama N, Gucciardo E, Tatti O,
Varjosalo M, Hyytiäinen M, Gstaiger M and Lehti K: EphA2 cleavage
by MT1-MMP triggers single cancer cell invasion via homotypic cell
repulsion. J Cell Biol. 201:467–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
von Nandelstadh P, Gucciardo E, Lohi J, Li
R, Sugiyama N, Carpen O and Lehti K: Actin-associated protein
palladin promotes tumor cell invasion by linking extracellular
matrix degradation to cell cytoskeleton. Mol Biol Cell.
25:2556–2570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue M, Fang Y, Sun G, Zhuo W, Zhong J,
Qian C, Wang L, Wang L, Si J and Chen S: IGFBP3, a transcriptional
target of homeobox D10, is correlated with the prognosis of gastric
cancer. PLoS One. 8:e814232013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ulasov I, Yi R, Guo D, Sarvaiya P and
Cobbs C: The emerging role of MMP14 in brain tumorigenesis and
future therapeutics. Biochim Biophys Acta. 1846:113–120.
2014.PubMed/NCBI
|
|
13
|
Di D, Chen L, Wang L, Sun P, Liu Y, Xu Z
and Ju J: Downregulation of human intercellular adhesion molecule-1
attenuates the metastatic ability in human breast cancer cell
lines. Oncol Rep. 35:1541–1548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Albrechtsen R, Kveiborg M, Stautz D,
Vikeså J, Noer JB, Kotzsh A, Nielsen FC, Wewer UM and Fröhlich C:
ADAM12 redistributes and activates MMP-14, resulting in gelatin
degradation, reduced apoptosis and increased tumor growth. J Cell
Sci. 126:4707–4720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taylor SH, Yeung CY, Kalson NS, Lu Y,
Zigrino P, Starborg T, Warwood S, Holmes DF, Canty-Laird EG, Mauch
C and Kadler KE: Matrix metalloproteinase 14 is required for
fibrous tissue expansion. Elife. 4:e093452015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong Y, Chen G, Gao M and Tian X:
Increased expression of MMP14 correlates with the poor prognosis of
Chinese patients with gastric cancer. Gene. 563:29–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang YZ, Wu KP, Wu AB, Yang ZC, Li JM, Mo
YL, Xu M, Wu B and Yang ZX: MMP-14 overexpression correlates with
poor prognosis in non-small cell lung cancer. Tumour Biol.
35:9815–9821. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nyalendo C, Sartelet H, Barrette S, Ohta
S, Gingras D and Béliveau R: Identification of membrane-type 1
matrix metalloproteinase tyrosine phosphorylation in association
with neuroblastoma progression. BMC Cancer. 9:4222009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu KP, Li Q, Lin FX, Li J, Wu LM, Li W and
Yang QZ: MT1-MMP is not a good prognosticator of cancer survival:
Evidence from 11 studies. Tumour Biol. 35:12489–12495. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jacob A and Prekeris R: The regulation of
MMP targeting to invadopodia during cancer metastasis. Front Cell
Dev Biol. 3:42015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williams KC, McNeilly RE and Coppolino MG:
SNAP23, Syntaxin4, and vesicle-associated membrane protein 7
(VAMP7) mediate trafficking of membrane type 1-matrix
metalloproteinase (MT1-MMP) during invadopodium formation and tumor
cell invasion. Mol Biol Cell. 25:2061–2070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyazawa Y, Uekita T, Ito Y, Seiki M,
Yamaguchi H and Sakai R: CDCP1 regulates the function of MT1-MMP
and invadopodia-mediated invasion of cancer cells. Mol Cancer Res.
11:628–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McLaughlin SL, Ice RJ, Rajulapati A,
Kozyulina PY, Livengood RH, Kozyreva VK, Loskutov YV, Culp MV, Weed
SA, Ivanov AV and Pugacheva EN: NEDD9 depletion leads to MMP14
inactivation by TIMP2 and prevents invasion and metastasis. Mol
Cancer Res. 12:69–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ampuja M, Jokimäki R, Juuti-Uusitalo K,
Rodriguez-Martinez A, Alarmo EL and Kallioniemi A: BMP4 inhibits
the proliferation of breast cancer cells and induces an
MMP-dependent migratory phenotype in MDA-MB-231 cells in 3D
environment. BMC Cancer. 13:4292013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ager EI, Kozin SV, Kirkpatrick ND, Seano
G, Kodack DP, Askoxylakis V, Huang Y, Goel S, Snuderl M, Muzikansky
A, et al: Blockade of MMP14 activity in murine breast carcinomas:
Implications for macrophages, vessels, and radiotherapy. J Natl
Cancer Inst. 107(pii): djv0172015.PubMed/NCBI
|
|
26
|
Zhang LL, Wu J, Liu Q, Zhang Y, Sun ZL and
Jing H: MiR-886-5p inhibition inhibits growth and induces apoptosis
of MCF7 cells. Asian Pac J Cancer Prev. 15:1511–1515. 2014.
View Article : Google Scholar : PubMed/NCBI
|