Introduction
Therapeutic regimens utilized for recurrent
craniopharyngioma (CP) primarily comprise repeat surgery,
radiotherapy and stereotactic intracavitary therapy (radiotherapy
or chemotherapy) (1). In 1985, Taasan
et al (2) reported on 6
patients (3 with CP and 3 with glioma) who were treated with
stereotactic intracavitary brachytherapy using phosphorus-32
(32P) colloid. Results demonstrated that all patients
experienced an improved clinical curative effect. Furthermore, the
standard clinical 32P colloid dosage (2) based on the size of the cystic cavity was
proposed. In 2004, Hasegawa et al (3) reported on 49 CP patients with a mean
cyst volume of 13 ml. All patients were treated with stereotactic
32P intracavitary irradiation. A radiation dose beween
189 and 250 Gy (mean, 224 Gy) was targeted at the cyst wall during
five half-lives of the isotope. The survival rates were 90% at 5
years and 80% at 10 years after initial diagnosis. The tumor cyst
control rates were 76% at 5 years and 70% at 10 years after initial
diagnosis. In 2012, Kickingereder et al (4) summarized the application of stereotactic
32P colloid irradiation in 53 patients with cystic CP:
Use of a capsule volume of 0.5–78.9 ml, assuming a capsule wall
dose of 200–250 Gy, a 32P colloid dose of 0.03–78.9 mCi
and pull-out fluid at 0–29.3 ml, and follow-up following
affirmation of the curative effect. The application of
32P colloid for radiotherapy on the treatment of cystic
CP using stereotactic surgery is a key topic of interest within the
Institute of Neurosurgery (Navy General Hospital, Beijing, China)
(5,6).
According to the current literature, studies on intracavitary drug
distribution, surgical leakage and 32P deposition in
treating patients with CP using intracavitary radiotherapy with
32P colloid are limited in number. Furthermore, to the
best of our knowledge, there are currently no studies on the
monitoring of cerebrospinal fluid (CSF) and blood parameters
following 32P colloid treatment for patients with CP
(2,3).
In 2012, Trippel and Nikkhah (7)
summarized previous literature on the use of stereotactic
intracavitary radionuclide therapy for patients with CP and
suggested that drug distribution prior to and following
radionuclide injection should be compared in order to avoid leakage
or connection to the ventricular system; otherwise fever, headache,
hypothalamic radiation injury and other effects may occur.
Therefore, a safe and convenient technique is required to monitor
the distribution of 32P injected into the CP cavity, and
to detect whether leakage is present. In 2013, Denis-Bacelar et
al (8) performed single-photon
emission computed tomography (CT) imaging pre- and post-surgery and
identified that the mean absorbed dose of 32P colloid
delivered to the cyst wall was <50% of the total dose and
outlined the requirement for further studies on the drug
metabolism, residual deposition and safety of 32P for
patients with CP.
Materials and methods
Clinical data
Primary drugs and equipment
In total, 300 mgI/ml 32P colloid (Atom
Hi-tech Co. Ltd., Beijing, China) and iopamidol-300 (Shanghai
Bracco Sine Pharmaceutical Corp., Ltd., Shanghai, China) were
injected into the CP cystic cavity of each patient (1:1 dilution)
one time. 32P is a sterile and green colloidal solution
with a nuclear purity >99.9%, (pH 7.0), a chemical concentration
of 2.32 mg/ml and a radioactive concentration >1,850 mBq/ml. A
CAPRAC well-type NaI γ counter (Beijing Huaruison Science and
Technology Development Co. Ltd., Beijing, China) was used to
quantitatively measure the 32P colloid per minute
radioactive count.
Subjects and grouping
Patients who were recently diagnosed with primary or
recurrent cystic CP were enrolled into the study between March 2012
and October 2015. Only patients who were not allergic to iodine and
required surgical intervention were enrolled as study subjects.
Written informed consent was obtained from either the patient or a
relative, and the present study was approved by the Ethics
Committee of the Navy General Hospital. In total, 40 patients (26
men and 14 women) aged between 10 and 65 years of age (mean age,
34.3) were selected. The equation for calculating the dose/volume
[previously described by Taasan et al (2)] was referred to for the tailored
calculation of therapeutic doses [in milliCurie (mCi)] based on
clinical and surgical requirements. For the analysis of drug
distribution, patients were divided into the following four groups
according to therapeutic dose: 0.5 mCi (0.2 ml; n=10), 1 mCi (0.4
ml; n=10), 1.5 mCi (0.6 ml; n=10) and 2 mCi (0.8 ml; n=10).
Additionally, 8 patients from all groups, with the exception of the
1.5 mCi group (as a result of the little effect of the drug dose
between 1.0–2.0 mCi on the experimental results of drug safety
study) were randomly selected for an evaluation of drug safety
through comparison of hematological parameters prior to and
following surgery among different groups and comparison of residue
32P depositions in venous blood and urine among
different groups. Due to the large number of specimens (13 ml
preoperative blood samples, 13 ml blood samples from 7 days after
surgery, 2 ml blood samples from 1, 3 and 7 days after surgery, 2
ml urine samples from 1, 3 and 7 days after surgery) required from
each patient in this part of the study, in order to ensure the
smooth progress of the experiment (a smaller number of patients
would be easier to monitor because the drug safety analysis
required 7 days in hospital following surgery), the number of cases
included in the drug safety analysis was reduced.
Therapeutic methods
Surgery
Local anesthesia was adopted for surgery. The
Leksell head frame was used for all patients preoperatively. The
positioning magnetic resonance imaging (MRI) scan was performed,
and the acquired images were transferred to the stereotactic
imaging workshop for artificial puncture route planning and the
calculation of cystic cavity volume and drug dose. The Aero Tech
stereotactic surgery planning system (supplied by the Image Center
of Beihang University, Beijing, China) was used to determine the
surgical target and calculate coordinates, whereas the
three-dimensional simulation was adopted to determine the optimal
surgical approach that should avoid important structures, including
blood vessels and optic nerves. During the surgery, 32P
colloid was administered for the stereotactic intracavitary therapy
in a 1:1 dilution with iopamidol 300 (300 mgI/ml). Drug
distribution was assessed using head CT scans within 2 h after
surgery.
Physical examination and imaging
examination
Ophthalmic examination (visual acuity, visual field
and fundus) and head MRI or CT examinations were performed prior to
and following surgery.
Hematological examination
Prior to and 7 days after surgery, the following
haematological parameters were assessed for each patient: Blood
routine, prothrombin time, activated partial thromboplastin time,
thrombin time, fibrinogen, serum total protein, serum albumin,
alanine aminotransferase (ALT), aspartate aminotransferase,
alkaline phosphatase, creatinine, blood urea nitrogen, potassium
ion (K+) levels, sodium ion (Na+) levels,
chloride ion (Cl−) levels and pituitary hormones
[including thyroid-stimulating hormone (TSH), adrenocorticotropic
hormone (ACTH), luteinizing hormone (LH), follicle-stimulating
hormone, prolactin (PRL) and growth hormone (GH)]. Patients were
require to fast for 10 h prior to blood sampling (13 ml). Patients
who demonstrated a decrease in hormone levels were administered
hormone supplements (including thyroxine tablets, dexamethasone
tablets and ambroxol tablets) and regular follow-ups (the first
week, the first month, the third month and the half year after
discharge) were performed the major hematological parameters (ALT,
AST, K+, Na+, Cl−, TSH, ACTH, PRL
and GH) were compared between the groups with the difference value
(D-value) of the blood index prior to and following the
operation.
Detection of residual 32P
deposition
Venous blood (2 ml) and urine (2 ml) were obtained
from all patients at 1, 3 and 7 days post-surgery. Radioactive
counts per minute (CPM) within each sample were quantitatively
measured using the CAPRAC well-type NaI γ counter to evaluate
32P deposition.
Statistical analysis
SPSS software (version 17; SPSS, Inc., Chicago, IL,
USA) was used for data analysis. Results are presented as mean ±
standard deviation. Data were analyzed for normality, and an
analysis of variance for randomized block design was performed. The
Student-Newman-Keuls test was then utilized for group comparisons
and Dunnett's test was used to analyze blood and urine CPM values
between each study group and the negative control group (normal
blood and urine specimens). An analysis of variance and
Bonferroni's correction were used to analyze leakage rate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of general data between
study groups
No significant differences were identified between
patients (n=40) with regard to the general condition (age, weight
and surgical duration; P>0.05; Table
I).
 | Table I.Comparison between groups with regard
to the general condition of each patient (mean ± standard
deviation). |
Table I.
Comparison between groups with regard
to the general condition of each patient (mean ± standard
deviation).
| Group | n | Age, years | Weight, kg | Surgical duration,
h |
|---|
| 0.5 mCi | 10 | 31.10±18.41 | 60.50±16.43 | 1.59±0.50 |
| 1.0 mCi | 10 | 30.10±18.07 | 58.80±17.50 | 1.64±0.37 |
| 1.5 mCi | 10 | 32.20±20.23 | 61.50±19.86 | 1.62±0.40 |
| 2.0 mCi | 10 | 29.60±19.25 | 62.90±17.04 | 1.57±0.45 |
Medical history
In total, 24 patients had a history of previous CP
resection, 29 underwent internal radiotherapy with 32P
colloid for CP following stereotactic aspirations of cystic fluid,
11 underwent stereotactic aspiration alone, 7 accepted γ-knife
treatment, 4 were treated with a ventriculoperitoneal shunt and 2
were implanted with Ommaya reservoirs.
Preoperative signs, symptoms and
laboratory results
The primary clinical symptoms regarding intracranial
hypertension included headache, nausea and vomiting in 18 patients,
and polydipsia and polyuria in 12 patients. Vision loss was
identified in 34 patients (4 with monocular blindness), with
varying degrees of visual field defects. Developmental disorders,
which were short and without the development of secondary sexual
characteristics, were observed in 4 patients. Abnormal pre-surgery
levels were observed for the following hormones: TSH (n=10; the
normal range values, 0.49–4.91 mIU/l), ACTH (n=22; the normal range
values, 7.2–63.3 pg/ml), LH [n=1; the normal range values, period
of follicular (2.22–10.9 IU/l), period of ovulation (19.1–103
IU/l), period of luteal (1.2–12.9 IU/l), period of the menopause
(10.9–58.6 IU/l)], PRL (n=5; the normal range values, 70.8–566.5
mIU/l) and GH (n=4; the normal range values, 0–5 ng/ml).
Clinical efficacy
Surgery was successful for all patients; the volume
of the capsule was reduced, the injection was successful, and the
postoperative symptoms were relieved without complications.
Overall, 1 patient formed a small epidural hematoma, which was
treated using the conservative regimen (hemostasis, dehydrated
drugs and symptomatic treatment); 1 patient suffered from a small
amount of bleeding from the frontal lobe puncture path, which was
improved following the conservative regimen; 19 patients exhibited
small amounts of intracranial gas accumulation, which were
self-absorbed; the cystic volume was significantly decreased in 37
patients post-surgery; hormone levels returned to the normal
reference range post-surgery in 5 patients (TSH, n=2; PRL, n=2 and
GH, n=1), whereas 3 patients (TSH, n=2 and ACTH, n=1) exhibited
abnormal hormone levels, and no other marked alterations were
observed. Improvement in vision was observed in 21 patients
following surgery and no other marked changes were observed during
ophthalmic examination. No wound or intracranial infection was
observed following surgery. Results demonstrated significant
differences between the predicted cystic cavity volume prior to
surgery and the cystic fluid volume aspirated during surgery among
different dose groups (P<0.05; Table
II).
 | Table II.Comparison of each patient's cystic
cavity volume, extraction fluid volume and leakage rate between
groups prior to surgery (mean ± standard deviation). |
Table II.
Comparison of each patient's cystic
cavity volume, extraction fluid volume and leakage rate between
groups prior to surgery (mean ± standard deviation).
| Group | n | Leakage rate, % | Predicted cystic
cavity volume, ml | Extracted cystic
fluid volume, ml |
|---|
| 0.5 mCi | 10 | 0 | 3.54±0.66 | 3.46±0.64 |
| 1.0 mCi | 10 | 10 |
7.44±1.08a |
7.12±1.35a |
| 1.5 mCi | 10 | 20 |
12.29±1.84a,b |
12.79±1.47a,b |
| 2.0 mCi | 10 | 60a,b |
16.84±1.01a,b,c |
16.95±1.51a,b,c |
Drug distribution and leakage
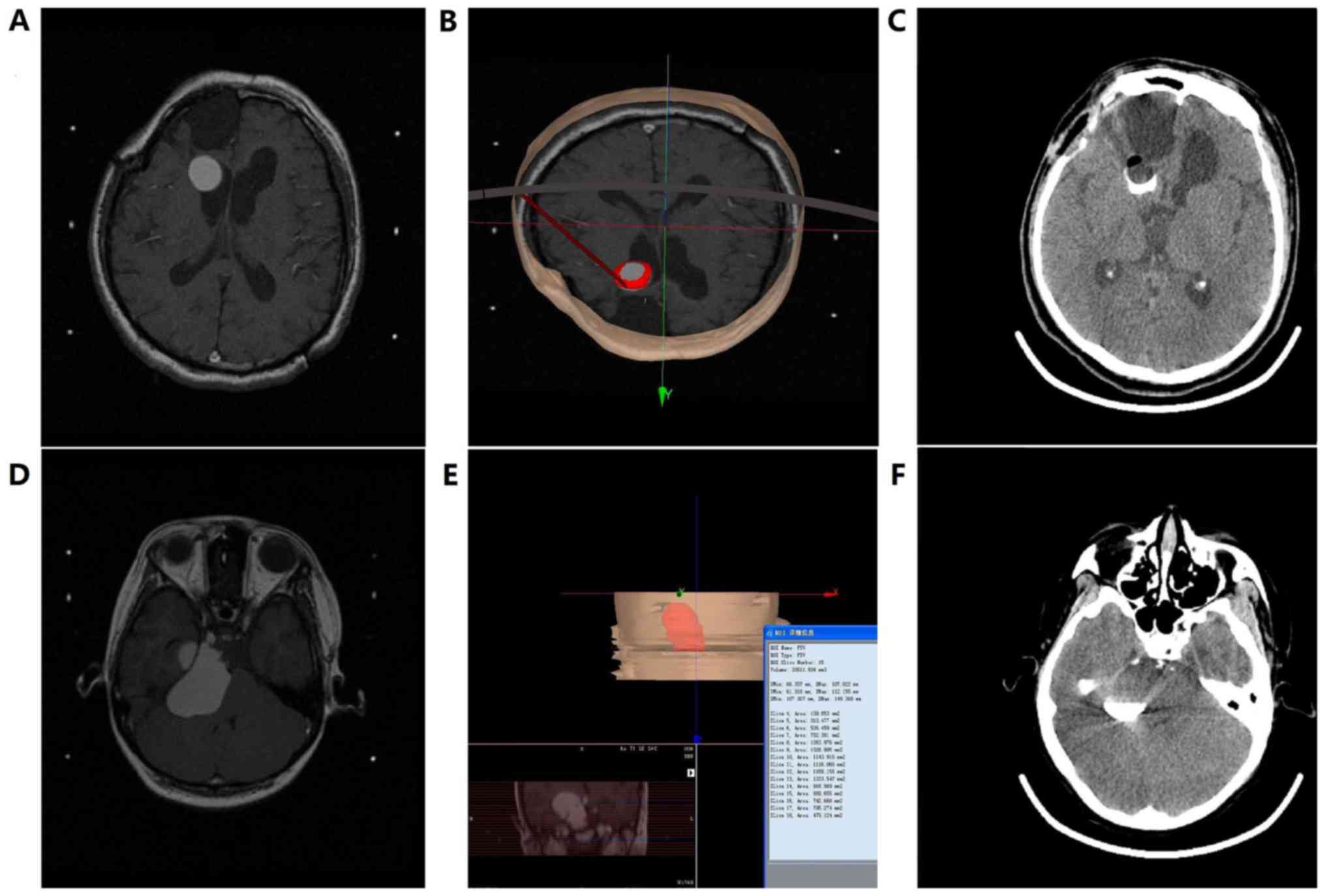
Head CT results obtained from all patients 2 h after
surgery demonstrated that heterogeneous drug and contrast agent
distributions were present in 6 patients within the 2.0 mCi group,
2 patients within the 1.5 mCi group and 2 patients within the 1.0
mCi group. This was due to the fact that the ventricles were
inevitably involved in the puncture routes, resulting in shrinkage
of the cavity volume following the intracavitary CSF leakage
(Fig. 1A-C). However, the remaining
patients demonstrated that the drug and the contrast agent were
heterogeneously distributed and no leakage was identified.
Therefore, cavity volumes were significantly decreased (Fig. 1D-F) and the leakage rate was
significantly increased between the 2.0 mCi group and the 1.0 and
0.5 mCi groups (both P<0.05; Table
II).
Drug safety
Comparison of hematological parameters
prior to and following surgery among different groups
No significant differences were identified in group
comparisons between blood routine results, blood biological
parameters and pituitary hormone levels among 24 patients
pre-surgery and at 7 days post-surgery (P>0.05; Table III). Prior to surgery, blood routine
results and blood biological parameters from all the patients were
within the normal reference ranges. However, within the 2.0 mCi
group, two patients presented with increased ALT (the normal range
values, 5–35 U/l) levels, one exhibited decreased levels of
K+ (the normal range values, 3.5–5.3 mmol/l),
Na+ (the normal range values, 136–145 mmol/l), and
Cl− (the normal range values, 96–108 mmol/l) and one
demonstrated a mildly prolonged coagulation time following surgery,
while in the 1.0 mCi group, one patient demonstrated decreased
levels of K+, Na+ and Cl−
following surgery. Abnormal pituitary hormone levels were
identified in patients in different groups prior to surgery: 3
patients demonstrated decreased TSH levels, 2 demonstrated
abnormally decreased ACTH levels, 1 demonstrated slightly increased
PRL levels and 1 demonstrated decreased GH levels in the 2.0 mCi
group, whereas 1 patient demonstrated decreased TSH levels, 1
demonstrated decreased ACTH levels and 1 demonstrated decreased GH
levels in the 1.0 mCi group. Abnormal postoperative pituitary
hormone levels were also identified in patients in different
groups: In the 2.0 mCi group, 2 patients exhibited decreased TSH
levels, 2 exhibited decreased ACTH levels and 1 exhibited decreased
GH levels, whereas in the 1.0 mCi group, 1 patient exhibited
decreased TSH levels and 1 patient exhibited decreased ACTH levels.
In the 0.5 mCi group, 1 patient demonstrated decreased ACTH levels
following surgery. Although abnormal variations were observed prior
to and following surgery in certain individual surgical indices, no
significant differences were identified when compared with major
hematological parameters (ALT, AST, K+, Na+,
Cl−, TSH, ACTH, PRL and GH) from each group prior to and
7 days after surgery (P>0.05).
 | Table III.D-value results of main blood test
indices for differences prior to and following surgery for each
group (mean ± standard deviation). |
Table III.
D-value results of main blood test
indices for differences prior to and following surgery for each
group (mean ± standard deviation).
| Group | n | ALT, U/l | AST, U/l | K+,
mmol/l | Na+,
mmol/l | Cl−,
mmol/l | TSH, mIU/l | ACTH, pg/ml | PRL, IU/l | GH, ng/ml |
|---|
| 0.5 mCi | 8 | −0.74±1.77 | −0.78±1.65 | 0.61±0.32 | 6.13±4.04 | 5.22±3.82 | 0.08±0.05 | 2.35±0.99 | 2.79±1.64 | 0.01±0.01 |
| 1.0 mCi | 8 | −0.96±1.78 | −0.97±1.67 | 0.69±0.47 | 5.79±4.90 | 5.37±3.71 | 0.09±0.05 | 2.12±1.30 | 2.69±2.04 | 0.01±0.01 |
| 2.0 mCi | 8 | 0.08±4.68 | 0.11±4.44 | 0.69±0.80 | 6.90±4.83 | 5.41±3.94 | 0.09±0.07 | 2.32±1.81 | 2.32±2.96 | 0.01±0.01 |
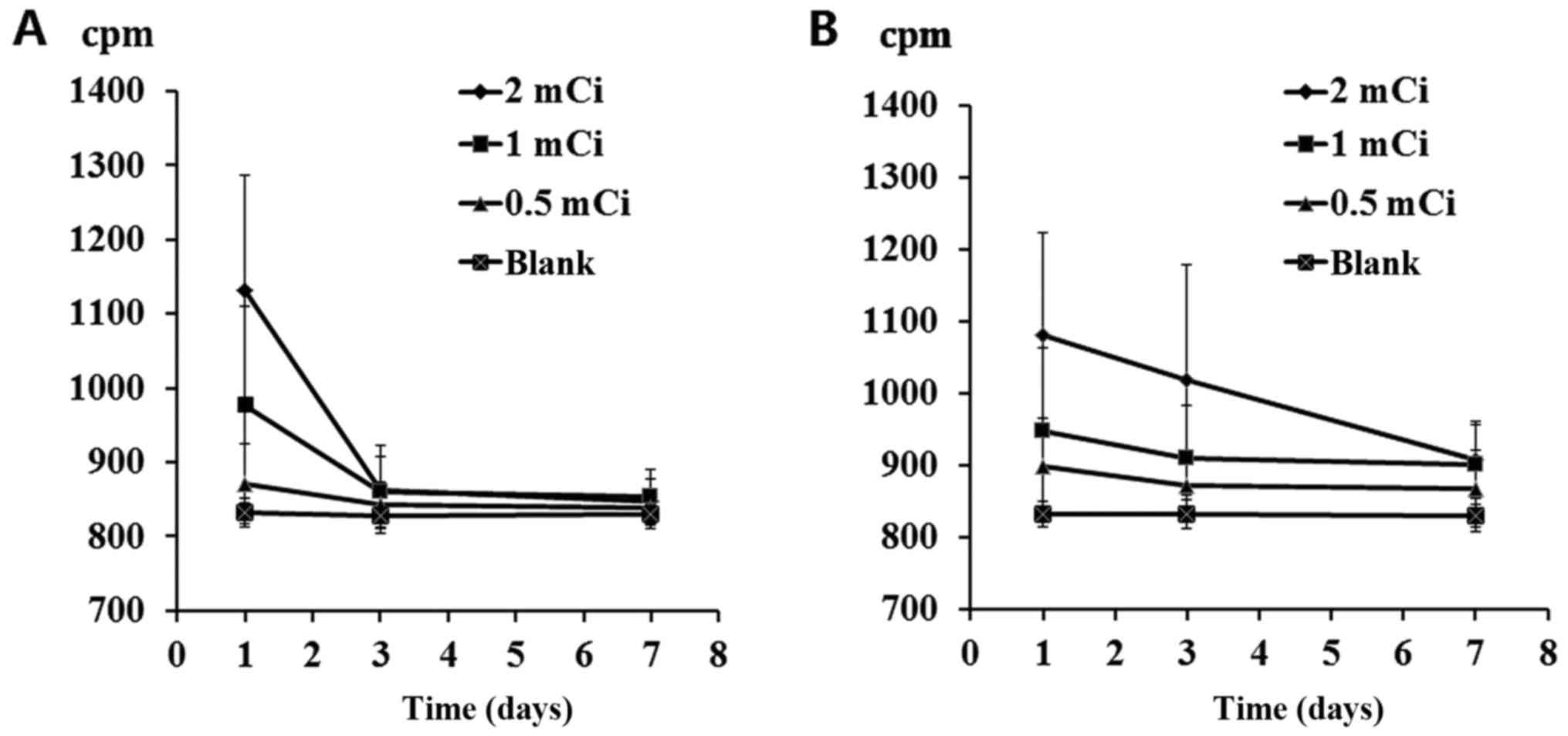
Residue 32P depositions
among different groups
Venous blood (2 ml) and urine (2 ml) were obtained
from 24 patients at 1, 3 and 7 days post-surgery. The CAPRAC
well-type NaI γ counter was used to measure the CPM and blank
controls were set accordingly. Results indicated a significant
difference in blood CPM values 1 day after surgery and in urine CPM
values 1, 3 and 7 days after surgery between the 2.0 and 1.0 mCi
groups and the control group (P<0.05). Blood deposition returned
to normal within 3 days, whereas urine deposition returned to
normal in ~7 days (Fig. 2).
Discussion
The present study successfully investigated the
distribution and leakage of 32P injected into the CP
cystic cavity using the contrast agent developing technology (a
head CT scan to check the iopamidol-300 has been injected into the
CP cystic cavity). This method, compared with the single-photon
emission computed tomography (CT) imaging (8), is advantageous with regard to
convenience, immediacy and low cost. Head CT scans, which were
performed following surgery, indicated the accumulation of a small
amount of intracavitary gas in the majority of patients. Negative
pressure caused by cyst fluid aspiration may have contributed to
the accumulation and these small amounts of gas may have entered
the cyst at the time of syringe replacement and/or drug injection.
Despite gas being self-absorbed in the long term, the intracranial
gas accumulation can still be avoided using a T-tube to improve the
injection process and withdrawing the puncture needle directly
following the completion of the injection. The primary reason for
the heterogeneous CT images was due to negative pressure being
created by the connection between the puncture route and the
ventricle, this caused the CSF to pass through the puncture hole in
the CP wall and enter into the cystic cavity, which diluted the
contrast agent and the 32P colloid. As the target is
typically set at the center of the cystic cavity, imaging results
demonstrated a CSF flushing dilution zone within the cavity center.
In such cases, when the cystic fluid is aspirated during the
surgery, the fluid color becomes gradually clear and the negative
pressure is removed. When the aspirated fluid volume is
significantly increased compared with the pre-surgical estimate,
CSF leakage occurs and the aspiration is terminated immediately.
Therefore, with the premise of ensuring a safe intracranial
puncture route, the ventricle system should be maximally avoided
during the pre-surgical route planning. Usually, when the route is
not connected to the ventricular system, negative pressure
increases as the aspiration continues. Negative pressure appears
when the fluid is completely aspirated, and therefore, further
aspiration should be avoided. In these cases, a significantly
decreased cavity size with homogeneous enhancement of contrast
agent is observed in the head CT scans following surgery. A
difference between the preoperative estimated cystic fluid volume
and the actual aspirated cystic fluid volume was investigated in
the present study. The differences observed may be associated with
the tracing accuracy of the surgery planning system for each cyst
cross-section presented on the MRI prior to surgery, the degree of
aspiration, the amount of CSF leakage and the presence of multiple
CP cysts, as well as whether they were interconnected. Furthermore,
the degree of cyst wall calcification also affects the planning for
the puncture route, whereas the elastic retraction degree of the
cyst wall also determines the amount of fluid aspiration and the
degree of cystic cavity shrinkage. Overall, as patient CP cystic
cavity volume increased, the volume of required 32P
colloid also increased [calculated based on the equation (2)]. Patients who required an increased
volume of 32P colloid also required a more complex
intracranial puncture route in order to avoid the ventricle
containing part of the protruding cyst wall(s), therefore, this
increased the risk of heterogeneous drug and contrast agent
distribution due to CSF leakage. However, it is important to note
that even if the imaging route plan avoids the ventricle system,
CSF leakage may still occur during surgery.
The present study also investigated the deposition,
excretion, toxicity and side effects of the 32P colloid
following surgery. Surgery was successful in all patients, and
according to the comparison of clinical and imaging results, the
effectiveness (classified as the volume of the capsule being
reduced, the injection was successful, and the postoperative
symptoms were relieved without complications) was 100%; however,
certain patients still suffered from poorly improved vision, visual
field and hormone levels, whereas other individuals exhibited
mildly decreased hormone levels. Patients who demonstrated a
decrease in hormone levels were administered hormone supplements
(including thyroxine tablets, dexamethasone tablets and ambroxol
tablets) and regular follow-ups were performed. All patient hormone
levels returned to their respective normal reference range and the
side effects caused by hormone supplements were effectively
managed. Results obtained from imaging analysis demonstrated a
slight decrease in CP cystic volume in certain individuals,
indicating the presence of CSF leakage from the intracranial
puncture route. Therefore, disease observation, blood tests and
imaging examination during the follow-up should be provided to
these patients. Intraventricular leakage of 32P colloid
is occasionally inevitable and the drug spreads along the CSF
circulation pathway following entrance into the ventricular system,
causing further radioactive damage to several types of neurological
system, including the hypothalamus. Despite comparison of
hematological parameters prior to and following surgery, results
demonstrated no significant different in any group; however
slightly elevated transaminase levels, mild coagulation
abnormalities and electrolyte imbalance occurred in certain
patients. A previous study (9)
demonstrated that 32P colloid caused coagulation and
liver dysfunction due to a decrease in antiplatelets; however,
these dysfunctions were completely restored on their own accord
following 8 weeks. Therefore, monitoring hematological parameters
within 1 month after surgery is of high importance. As the duration
of hospitalization is usually <7 days, it is essential to
increase the monitoring of each hematological parameter and symptom
following patient discharge. In the present study, time points for
measuring CPM following surgery in blood and urine samples were
rather limited and also all within 1 week, whereas the half-life of
32P is ~2 weeks and the excretion cycle is >2 weeks.
Therefore, the follow-up duration following discharge should be
prolonged, or preferably be replaced with continuous monitoring
until the CPM value has returned to normal. Due to the limited
research in this area, the complete metabolic pathway of
32P colloid remains unclear. The absorbance of
32P colloid into the blood through the cyst wall prior
to being decomposed by the liver requires investigation. However,
it is important to note that discharged 32P colloid only
accounts for a small proportion of the total injected dose and that
the majority of the drug is still distributed and deposited into
the CP cystic cavity until it loses its radioactivity; however,
further investigation into the presence of residue in feces and
sweat excretion are required.
It important to recognize the limitations of the
present study. The long-term distribution and leakage of
32P colloid could not be monitored continuously and
further measurements of 32P colloid radioactivity inside
the cyst and/or CSF fluid were not provided. Additionally, the
enrolled patients should have been followed up in order to
determine long-term efficacy. Furthermore, surgical manipulations
and route planning affected the present results and surgical
efficacy to varying degrees. Associated factors which influenced
results included the surgeon's surgical habits (artificial
differences in surgical software planning, artificial control
differences in cystic fluid aspiration level, artificial selection
of puncture needle, surgical technique difference, etc.),
estimations of the cystic volume and drug doses, accuracy of the
evaluation for visual recovery and symptom improvement,
intraoperative judgment for the amount and degree of cyst fluid
aspiration, and the patient's tolerance to the local anesthesia and
surgery. The CAPRAC well-type NaI γ counter was used to
quantitatively measure the 32P colloid radioactive CPM
in the patients' blood and urine, and to evaluate the presence of
residual 32P colloid deposition. This method, compared
with the previous liquid scintillation counter (10), is advantageous with regard to
convenience, immediacy and low cost during radioactive counting,
therefore, it is suitable for the continuous monitoring of a
patient's residual 32P colloid deposition. The present
study provides a method for evaluating the distribution of
32P colloid radiotherapy for craniopharyngioma and
proves its safety and efficacy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Innovation
and Development Foundation of the Navy General Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC and JZ designed the experiment, HC, WC, YW, HZ
and RL performed the experiment, HC and SG processed the data, HC
wrote and edited the paper.
Ethics approval and consent to
participate
Written informed consent was obtained from either
the patient or a relative, and the present study was approved by
the Ethics Committee of the Navy General Hospital.
Consent for publication
Written informed consent was obtained from either
the patient or a relative.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liubinas SV, Munshey AS and Kaye AH:
Management of recurrent craniopharyngioma. J Clin Neurosci.
18:451–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taasan V, Shapiro B, Taren JA, Beierwaltes
WH, McKeever P, Wahl RL, Carey JE, Petry N and Mallette S:
Phosphorus-32 therapy of cystic Grade IV astrocytomas: Technique
and preliminary application. J Nucl Med. 26:1335–1338.
1985.PubMed/NCBI
|
|
3
|
Hasegawa T, Kondziolka D, Hadjipanayis CG
and Lunsford LD: Management of cystic craniopharyngiomas with
phosphorus-32 intracavitary irradiation. Neurosurgery. 54:813–820.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kickingereder P, Maarouf M, El Majdoub F,
Fuetsch M, Lehrke R, Wirths J, Luyken K, Schomaecker K, Treuer H,
Voges J and Sturm V: Intracavitary brachytherapy using
stereotactically applied phosphorus-32 colloid for treatment of
cystic craniopharyngiomas in 53 patients. J Neurooncol.
109:365–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian Z, Liu Z and Wang Y: Stereotactic
intratumoral irradiation of huge craniopharyngioma. Zhonghua Zhong
Liu Za Zhi. 18:234–236. 1996.(In Chinese). PubMed/NCBI
|
|
6
|
Yu X, Zhang JN, Liu R, Wang YM, Sun JZ, Qi
SB, DU YN, Wang HW, Zhao HL and Liu ZH: Mixed craniopharyngioma:
Long-term results after gamma knife combined with stereotactic
brachytherapy. Zhonghua Wai Ke Za Zhi. 51:631–635. 2013.(In
Chinese). PubMed/NCBI
|
|
7
|
Trippel M and Nikkhah G: Stereotactic
neurosurgical treatment options for craniopharyngioma. Front
Endocrinol (Lausanne). 3:632012.PubMed/NCBI
|
|
8
|
Denis-Bacelar AM, Romanchikova M,
Chittenden S, Saran FH, Mandeville H, Du Y and Flux GD:
Patient-specific dosimetry for intracavitary 32P-chromic
phosphate colloid therapy of cystic brain tumours. Eur J Nucl Med
Mol Imaging. 40:1532–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Teng G, Zhang D, Song J, He S, Guo
J and Fang W: Toxicology of intrahepatic arterial administration of
interventional phosphorus-32 glass microspheres to domestic pigs.
Chin Med J (Engl). 112:632–636. 1999.PubMed/NCBI
|
|
10
|
Gaca P, Warwick PE and Croudace IW: Liquid
scintillation counters calibration stability over long timescales.
J Radioanal Nucl Chem. 314:753–760. 2017. View Article : Google Scholar : PubMed/NCBI
|