Introduction
Osteosarcoma is one of the most common forms of
childhood cancer, and is characterized by its poor overall
prognosis and high mortality rate (1). Osteosarcoma is a highly aggressive
neoplasm typically composed of spindle cells and it metastasizes
predominantly to the lungs (2). Thus,
the development of novel curative strategies to prevent lung
metastasis is highly desirable. Accordingly, one of the key aims in
current osteosarcoma research is to further understand the
underlying molecular mechanisms of invasion and to provide an
experimental basis for the development of therapeutics for
osteosarcoma.
Ras-related proteins regulate various cellular
processes, including cell adhesion, differentiation, cell cycle
control, cytoskeletal organization and metabolic turnover (3,4).
Ras-related proteins transform between active GDP-bound and
inactive GTP-bound forms (4).
Ras-related proteins comprise a large family of small molecular
weight guanine nucleotide binding proteins that includes five
different members: Ras-related protein (Rap)-1a, Rap1b and Rap2a,
Rap2b, Rap2c (5,6). Previous studies have demonstrated that
Rap is able to enhance metastasis in breast and prostate cancer
cells (7,8), and it has been previously revealed that
the Rap2 family members' full-length sequence open reading frame
contains 561 bp, encoding 186 amino acids (9). Subsequent studies have demonstrated that
Rap2 is a regulator of LFA-1-mediated migration and is highly
expressed in human thyroid cancer cells (8,10). Thus,
decreasing Rap2 activity may indicate a novel therapeutic approach.
U2OS is one of the most commonly used types of osteosarcoma cell
and is representative of human osteosarcoma cells (11). Accordingly, U2OS cell lines in which
Rap2c expression was silenced or overexpressed were constructed to
investigate the effects of Rap2c on the invasion of osteosarcoma
cells. In the present study, the function of Rap2c in regulating
the proliferation and apoptosis of U2OS cells was analyzed.
Furthermore, the effect of Rap2c on the migration of U2OS and the
activity of matrix metalloproteinase-2 (MMP2) were detected.
Finally, in order to explore the underlying mechanisms by which
Rap2c is involved in osteosarcoma, the expression levels of B cell
lympphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), tissue
inhibitor of metalloproteinases 2 (Timp2), protein kinase B (Akt)
and phosphorylated (p)-Akt473 were examined in U2OS cells. The data
demonstrated that increasing Rap2c significantly promoted the
invasion and migration of U2OS cell in vitro, and increased
the expression level of p-Akt473. The data indicated that Rap2c may
serve as a novel therapeutic target for osteosarcoma.
Materials and methods
Cell line and culture conditions
U2OS (human osteosarcoma cell line) were purchased
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and cultured in Dulbecco's modified Eagle medium
(DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in an incubator
containing 5% CO2.
Plasmid construction
Total RNA was extracted from U2OS cells using the
Qiagen RNeasy kit (Qiagen Sciences, Inc., Gaithersburg, MD, USA),
and the first-strand cDNA was synthesized using the PrimeScript RT
reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). Then,
Rap2c cDNA was amplified using Taq polymerase (Tiangen Biotech Co.,
Ltd., Beijing, China). The primers sequences were as follows:
forward, 5′-AAGCTTATGAGGGAATACAAG-3′ and reverse,
5′-GAATTCTTACTGGACGACAC-3′. Consensus sequences for the restriction
enzymes EcoRI and HindIII (Takara Biotechnology Co.,
Ltd., Dalian, China) are underlined. cDNA was then subcloned into
pcDNA3.1 at the EcoRI and HindIII sites. The identity
of the clones were verified using sequencing.
Overexpression of Rap2c
Cells were transfected with pcDNA3.1-control or
pcDNA3.1-Rap2c expression plasmids using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and grown to 90%
confluence according to the manufacturer's protocol. The quantity
of plasmids used for transfection was 4 µg for each 35 mm culture
dish. The medium containing the transfection reagents was removed 4
h after transfection and replaced with fresh medium. Cells were
then collected 24 h after transfection and used in the following
experiments.
Knockdown of Rap2c
Rap2c-targeted small interfering RNA (siRNA) was
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China) and
transfected using siLentFect lipid reagent (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) according to the manufacturer's protocol.
Briefly, non-specific control siRNA, Rap2c#1, Rap2c#2 or Rap2c#3
siRNAa were transfected by siLentFect when cells reached ~50%
confluence. A total of 6 h after transfection, the medium
containing transfection reagents was removed and fresh medium
containing FBS was added. Cells were harvested for subsequent
experiments 48 h after transfection. The quantity of the siRNAs
used for transfection was 200 pmol for each 35 mm culture dish. The
three types of siRNA duplexes targeting human Rap2c that were used
are listed in Table I. A non-specific
siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′, 5′-ACGUGACACGUUCGGAGAATT-3′)
was transfected as a control.
 | Table I.siRNA duplexes used for the knockdown
of Rap2c. |
Table I.
siRNA duplexes used for the knockdown
of Rap2c.
| siRNA | siRNA duplexes,
5′-3′ |
|---|
| Rap2c#1 |
CAGGAUAUCAAGCCAAUGATT |
|
|
UCAUUGGCUUGAUAUCCUGTT |
| Rap2c#2 |
GAAGCAAGAUCAGUGUUGUTT |
|
|
ACAACACUGAUCUUGCUUCTT |
| Rap2c#3 |
GAGAUCGUCAGGCAAAUGATT |
|
|
UCAUUUGCCUGACGAUCUCTT |
Western blotting
Following transfection with expression plasmids for
24 h or siRNA for 48 h, total proteins were extracted from U2OS
cells using lysis buffer (Beyotime Institute of Biotechnology,
Haimen, China) and the concentration of protein was determined
using enhanced BCA protein assay kit (Beyotime Institute of
Biotechnology). Total proteins were boiled with loading buffer
(Beyotime Institute of Biotechnology) and 100 µg protein per lane
were loaded and separated by 12.5% SDS-PAGE for electrophoresis and
then transferred onto nitrocellulose membrane. Following blocking
with 5% non-fat milk (BD Biosciences, Franklin Lakes, NJ, USA) at
room temperature in TBS containing 0.1% Tween-20 (TBST) for 2 h,
the membranes were incubated at 4°C overnight with primary
antibodies against the following proteins: Rap2c (anti-rabbit;
1:1,000; cat. no. ab138300; Abcam, Cambridge, UK), B cell
lymphoma-2 (Bcl-2; anti-rabbit; 1:500; cat. no. sc-492; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), Bcl-2-associated X protein
(Bax; anti-rabbit; 1:500; cat. no. sc-493; Santa Cruz
Biotechnology, Inc.), tissue inhibitor of metalloproteinases 2
(Timp2; anti-rabbit; 1:1,000; cat. no. 5738; Cell Signaling
Technology, Inc., Danvers, MA, USA), Akt (anti-rabbit; 1:2,000;
cat. no. 9272s; Cell Signaling Technology, Inc.), p-Akt473
(anti-rabbit; 1:1,000; cat. no. 4060s; Cell Signaling Technology,
Inc.) and β-actin (anti-mouse; 1:2,000; sc-58673; Santa Cruz
Biotechnology, Inc.). Following incubation with horseradish
peroxidase-conjugated anti-mouse IgG (1:10,000; cat. no.
P/N926-80010; LI-COR Biosciences, Lincoln, NE, USA) or anti-rabbit
IgG (1:10,000; cat. no. P/N926-80011; LI-COR Biosciences) at 37°C
for 2 h at room temperature, membranes were then washed and scanned
on the Odyssey Two-Color Infrared Imaging System (LI-COR
Biosciences). The densitometric analysis for the quantification of
the bands was performed using ImageJ software (version 1.46;
National Institutes of Health, Bethesda, MD, USA).
Cell proliferation assay
Following transfection, U2OS cells were seeded
(5×103/well) into 96-well culture plates. Cell
proliferation was detected using Cell Counting kit-8 (CCK-8; Vicmed
Biotech Co., Ltd., Xuzhou, China; http://vicmed2013.bioon.com.cn/) every 24 h for up to
96 h. In total, 100 µl serum-free culture medium and 10 µl CCK-8
solutions were added into each well and cells were incubated at
37°C for 2 h. The absorbance was measured at 490 nm using an
ELX-800 spectrometer reader (BioTek Instruments, Inc., Winooski,
VT, USA). Each experiment was performed in triplicate.
Detection of apoptosis using flow
cytometry
U2OS cells were plated in six-well plates and
transfected with expression plasmids for 24 h, or siRNA for 48 h.
Cells were collected and labeled using the Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Detection kit
(BD Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol, and the apoptosis fraction was analyzed
using a FACScan cytometer (BD Biosciences). Data was analyzed using
FlowJo software (FlowJo LLC, Ashland, OR, USA).
Wound-healing assay
For the wound-healing assay, U2OS cells were seeded
into 6-well plates in culture medium and were grown to 90%
confluence. Following transfection with Rap2c plasmid or Rap2c
siRNAs, a wound was made by pulling a 200 µl pipette tip along the
center of the plate; the confluent monolayer was washed three times
with PBS to remove cell debris. The wound monolayer was captured at
0 and 24 h using a light microscope (Nikon Corporation, Tokyo,
Japan) at ×100 magnification. The rate of wound healing was
determined by comparing the wound width at 24 h to the wound width
at 0 h.
Migration and invasion assay
Cell migration and invasion assays were performed
using Transwell units with 8.0 µm-pore polycarbonate filters
(Corning Incorporated, Corning, NY, USA). For the migration assay,
the underside of a Transwell filter was coated with 10 µg/ml
fibronectin from human plasma (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C overnight. In brief, a total of
1×105 U2OS cells were seeded in the upper chamber, and
600 µl DMEM supplemented with 10% FBS was placed in the lower
compartment. After being cultured at 37°C in a CO2
incubator for 24 h, the cells in the upper chamber were fixed in
methanol for 30 min and stained with leucocrystal violet (Beyotime
Institute of Biotechnology) for 15 min at room temperature. Cells
in the upper chamber were removed with a cotton swab and the number
of cells that migrated across the membrane was counted in five
randomly selected microscopic fields in at a minimum of three
independent experiments. For the invasion assay, a similar protocol
was performed using Matrigel-coated (BD Biosciences) instead of
Transwell chambers. The cells that suspended into the well bottom
were quantified after 48 h incubation at 37°C. Cells in the upper
chamber were subjected to microscopic inspection and then
photographed randomly at ×100 magnification.
Gelatin zymography
It has been reported that matrix metalloproteinases
(MMPs) serve a pivotal role in cellular invasion and metastasis
(12). Therefore, the activity of
matrix metalloproteinase MMP2 secreted by U2OS cells was evaluated
using gelatin zymography. Briefly, 40 µl of serum-free DMEM was
denatured in SDS buffer under non-reducing conditions (63 mM
TrisHCl, 10% glycerol, 2% SDS and 0.0025% bromophenol blue;
Beyotime Institute of Biotechnology) without heating and
electrophoresed for 3 h in 10% SDS-PAGE with 0.1% gelatin
(Sigma-Aldrich; Merck KGaA). The SDS-PAGE gels were renatured by
incubating the gel in renaturing buffer (2.5% Triton X-100) for 30
min twice at room temperature and equilibration in developing
buffer (50 mM Tris base, 40 mM HCl, 200 mM NaCl, 5 mM
CaCl2, and 0.02% Brij) for 48 h at 37°C. The gels were
rinsed in staining buffer (0.1% Coomassie Brilliant Blue R-250, 40%
ethanol, and 10% acetic acid; Sigma-Aldrich; Merck KGaA) and then
in de-staining buffer (10% ethanol and 7.5% acetic acid). Regions
of protease activity appeared as bands against a dark blue
background.
Statistical analysis
Statistical analyses were conducted using SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). All
quantitative data are presented as mean ± standard deviation (SD).
The differences between groups were analyzed using unpaired
Student's t-test when only 2 groups were compared, or one-way ANOVA
analysis of variance when >2 groups were compared. The
Student-Newman-Keuls test was used as a post hoc test following
ANOVA. All experiments were performed at least three times, unless
otherwise indicated. P<0.05 was considered to indicate a
statistically significant difference.
Results
Rap2c has no effect on cell
proliferation
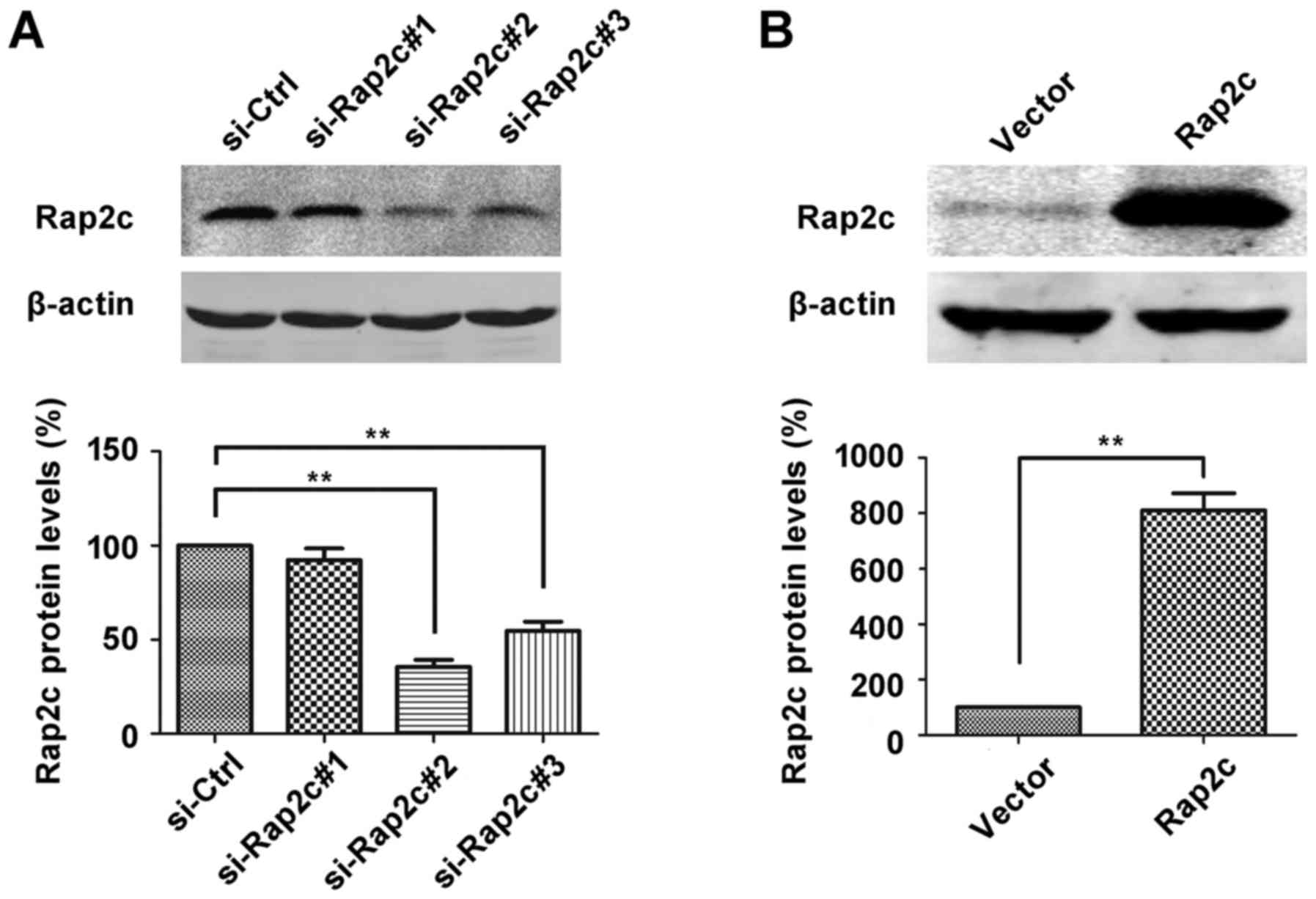
To investigate the oncogenic role of Rap2c, siRNA
targeting Rap2c or pcDNA3.1-Rap2c expression plasmid was
transiently transfected into U2OS cells and Rap2c protein
expression was evaluated using western blot analysis. As
illustrated in Fig. 1A, the
expression level of Rap2c protein was significantly downregulated
in si-Rap2c#2 and si-Rap2c#3 groups compared with the si-control
group. However, si-Rap2c#2 exhibited the most marked effect in
decreasing the expression levels of Rap2c and was therefore used in
subsequent experiments. Furthermore, the expression of Rap2c was
significantly upregulated following transfection of U2OS cells with
the Rap2c expression plasmid (Fig.
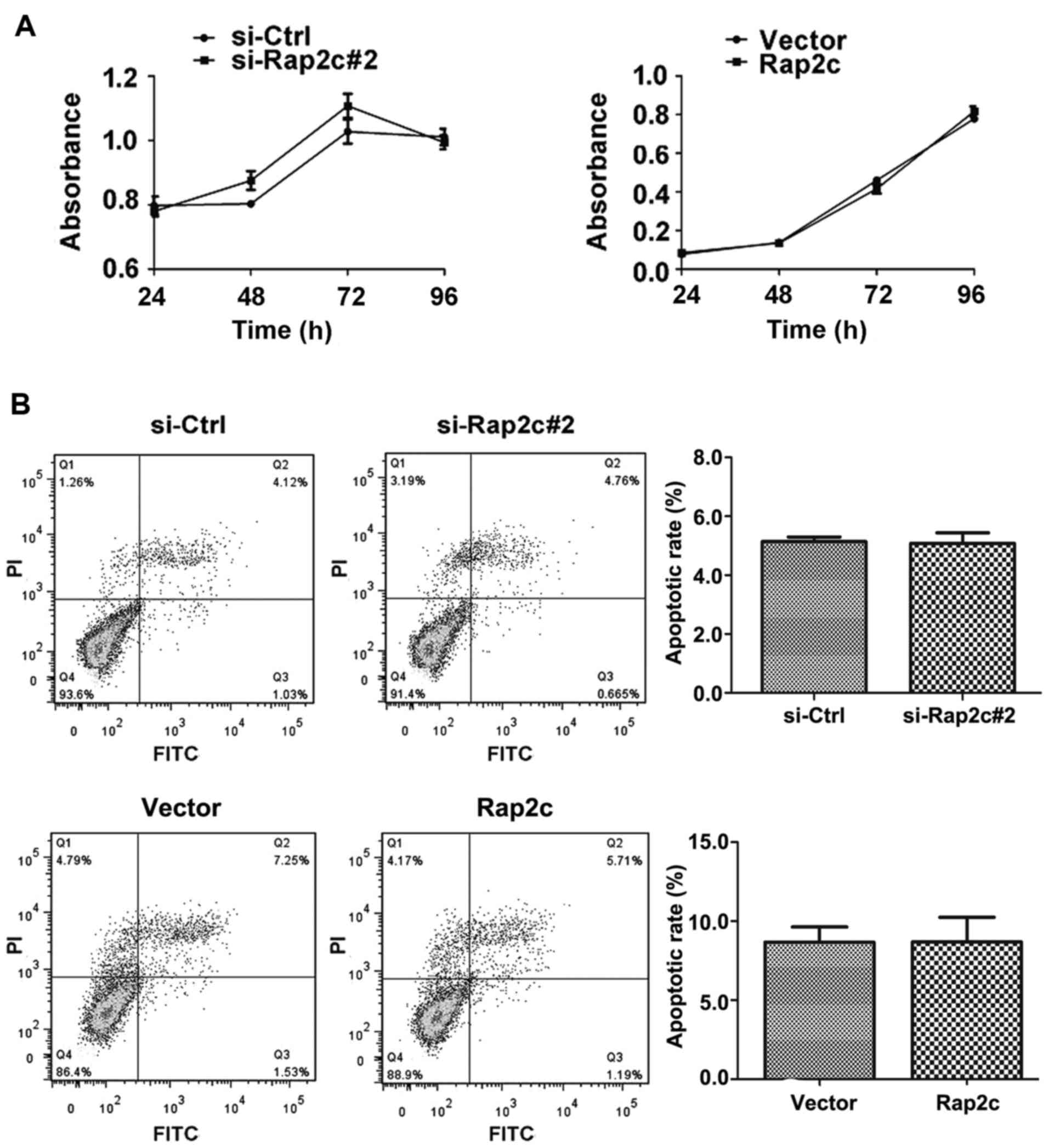
1B). A CCK-8 assay was performed following the transfection of
U2OS cells with Rap2c siRNA or a Rap2c expression plasmid. The
results demonstrated that there was no significant difference in
the rate of proliferation between the Rap2c-knockdown cells and
those treated with the negative control siRNA (Fig. 2A). Similarly, the overexpression of
Rap2c had no effect on cell proliferation, when compared with the
rate of proliferation in cells transfected with the empty control
vector (Fig. 2A).
Rap2c has no effect on the apoptosis
of cancer cells
To investigate whether Rap2c influences carcinoma
cell survival, cell apoptosis was analyzed using flow cytometry
with Annexin V-FITC/PI. Following the transfection of U2OS cells
with Rap2c siRNA or a Rap2c expression plasmid, no significant
difference in the proportion of cells undergoing apoptosis was
observed between either of the Rap2c-treatment groups and the
control groups (Fig. 2B).
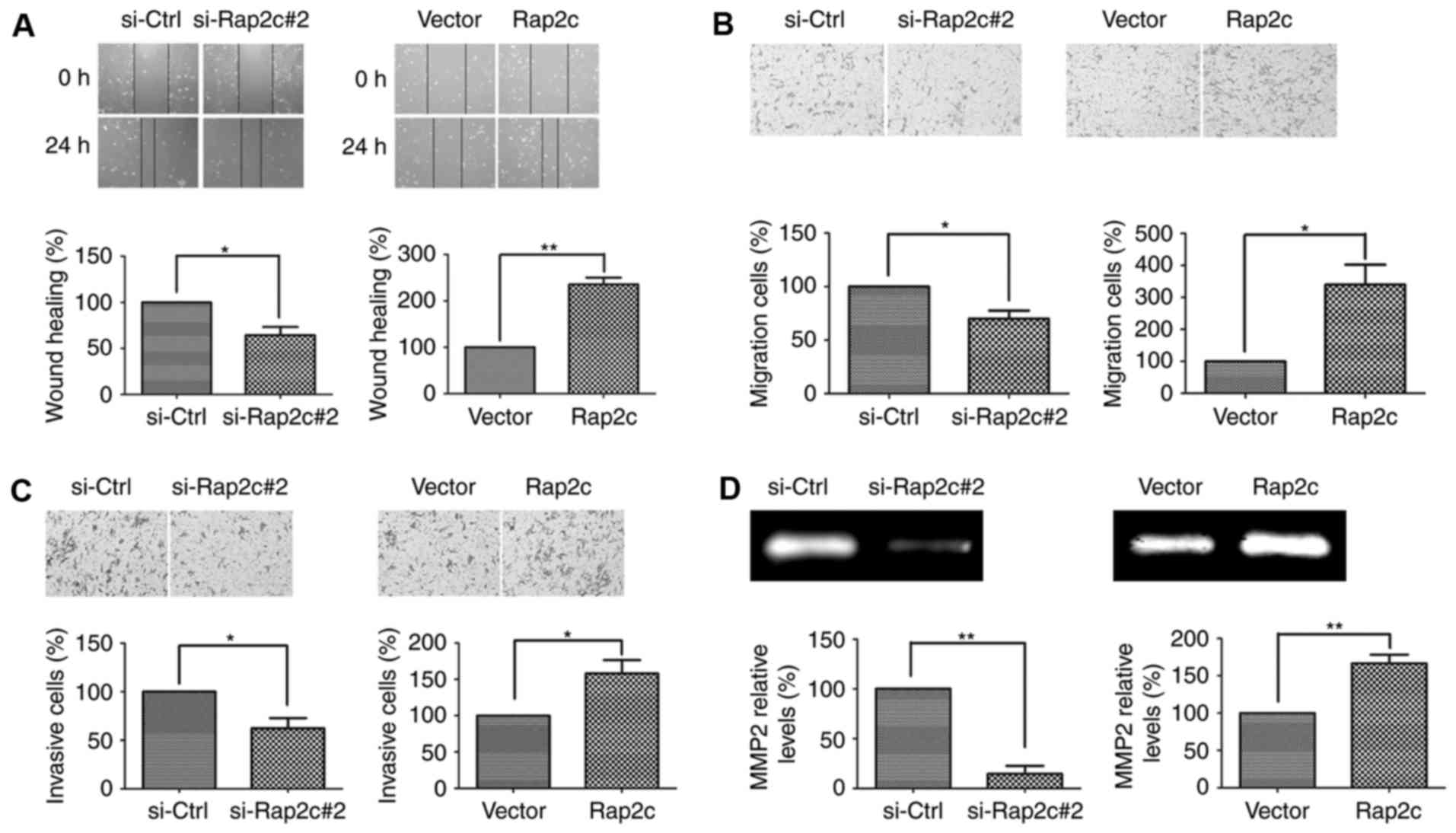
Rap2c promotes invasion and migration
of osteosarcoma cell
The effects of Rap2c on the migration of U2OS cells
was measured using wound-healing assays. The results of these
assays demonstrated that decreased wound closure was observed in
Rap2c-knockdown U2OS cells. By contrast, overexpression of Rap2c
increased the degree of wound closure in U2OS cells (Fig. 3A). Moreover, Transwell cell migration
assays demonstrated that knockdown of Rap2c markedly decreased the
migratory ability of U2OS cells. Conversely, overexpression of
Rap2c promoted the migratory ability of U2OS cells (Fig. 3B). Furthermore, the invasive ability
of Rap2c cells was measured using transwell cell invasion assays,
the results of which demonstrating that Rap2c promoted U2OS cells
invasion (Fig. 3C). These results
indicated that Rap2c may have a significant effect on cell
migration and invasion in vitro.
Rap2c upregulates the MMP2 activity
and downregulates the protein level of Timp2
The present study revealed that MMP2 secretion was
increased in Rap2c-overexpressing U2OS cells and decreased in
Rap2c-knockdown U2OS cells (Fig. 3D).
Thus, Rap2c may promote migration and invasion of osteosarcoma
cells via the upregulation of MMPs activities. To further validate
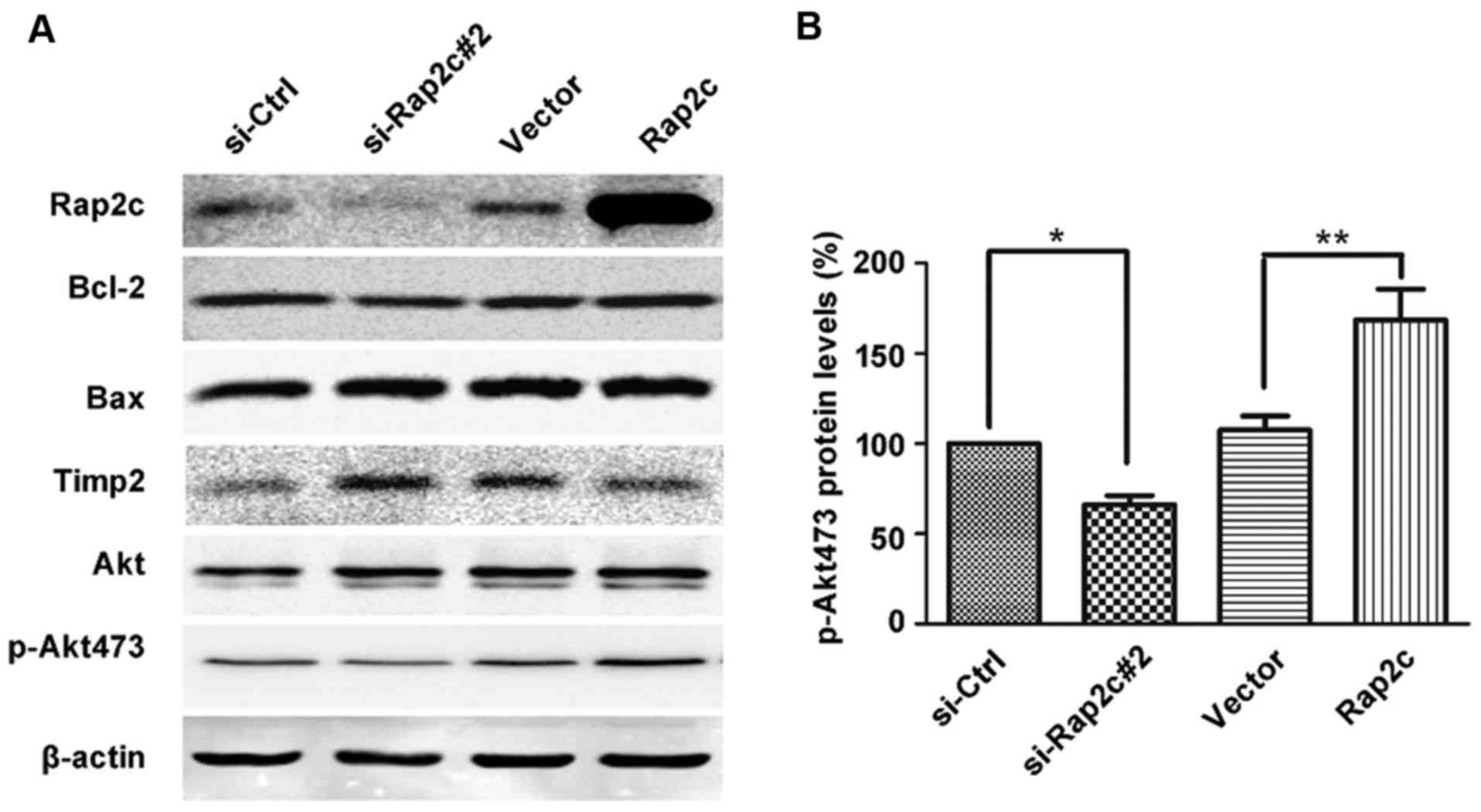
this conclusion, the protein level of Timp2 was analyzed. As
expected, the results of the present study demonstrated that the
level of Timp2 protein was markedly increased in U2OS cells
following the knockdown of Rap2c (Fig.
4A). These results demonstrated that Rap2c upregulated MMP2
activity and downregulated levels of Timp2 protein, which may
subsequently have led to an increase in migration and invasion in
U2OS cells. In addition, the expression levels of Bcl-2 and Bax
were not altered in Rap2c-transfected cells (Fig. 4A); this result was consistent with the
previous proliferation and apoptosis data.
Akt phosphorylation is involved in
Rap2c-mediated migration and invasion of osteosarcoma cells
Tumor growth and metastasis are caused by highly
integrated multistep cellular events regulated by various signaling
molecules, including PI3K/Akt (13).
Emerging evidence reveals that MMP2 secretion and the invasion of
tumor cells are associated with PI3K/Akt signaling pathway
(13–15). Tumor angiogenesis and invasion are
triggered via the abundant secretion of MMPs by tumor cells; Akt is
a key regulator of the intracellular processes promoting cell
growth, migration and survival. It has been identified that the
activation of Akt pathway stimulates MMP production by cancer
cells, and that pharmacological inhibition of Akt can suppress the
expression of MMP2 (16,17). To understand whether Rap2c regulates
U2OS cell migration and invasion via Akt, the expression levels of
Akt proteins were detected in the present study. Results revealed
that Rap2c overexpression increased the phosphorylation of Akt,
which has a single residue at serine 473 (p-Akt473).
Correspondingly, the downregulation of Rap2c decreased p-Akt473
expression (Fig. 4A and B).
Discussion
Rap2c is a member of the Ras superfamily of small
GTPases (7). Ras gene mutations are
involved in a variety of human tumors and are poor prognostic
indicators for patient survival (18,19). Ras
gene proteins can present in two states: The inactive state, in
which the GTP has been hydrolyzed to GDP, and an active state, in
which GTP is bound to Ras. In physiological conditions, the active
isoform of Ras gene induces cell proliferation through the
Ras-dependent kinase cascade (20).
Aberrant Rap activation leads to cancer cell proliferation and
tumorigenesis, thereby contributing to several types of malignancy,
including prostate and thyroid cancer (21,22). The
Rap small GTPases are characterized important regulators of cell
adhesion and serve a key function in cell adhesion (23,24). It
has been previously reported that Rap2a and Rap2b are involved in
tumor progression and act as an oncogene in human cancer (25–27).
However, until recently, little was known about the function of
Rap2c protein in mammalian cells.
In the present study the following experiments were
used: Cell proliferation assay, wound-healing assay, migration
assay, invasion assay and western blotting, which were used to
investigate the function of Rap2c on U2OS cells. Results from the
present study demonstrated that upregulation of Rap2c promotes the
invasive and migratory capacities of cancer cells. However, CCK-8
and Annexin V-FITC/PI flow cytometry assays revealed that Rap2c has
no effect on the proliferation or rate of apoptosis in U2OS cells.
Consistent with this result, Rap2c did not alter the expression
levels of Bax or Bcl-2. These results indicated that Rap2c might
promote cancer cell motility. Furthermore, results demonstrated
that Rap2c regulated the expression of Timp2, MMP2 and p-Akt.
MMPs, secreted by tumor cells, can degrade the
extracellular matrix and serve an important function in tumor
progression (12,28). MMP-2 and MMP-9, commonly associated
with tumor metastasis and invasion, have been regarded as
biomarkers for various types of cancer (29). The present results demonstrated that
the siRNA-mediated downregulation of Rap2c significantly decreased
the expression of MMP-2, whereas Rap2c overexpression increased it.
Increased MMP2 secretion may be involved in mediating the effect of
Rap2c-enhanced migration and invasion of cancer cells. Timps have
the ability to inhibit the catalytic activity of MMPs and Timp2 is
a specific inhibitor of MMP2 (30).
MMP2 and Timp2 serve important functions in mediating tumor cell
metastasis (31,32). The present study evaluated the
association between Rap2c and Timp2 expression. The data
demonstrated that knockdown of Rap2c significantly increased Timp2
protein expression. By contrast, the expression of Timp2 was
markedly downregulated in U2OS cells following Rap2c
overexpression, which was consistent with the upregulation of MMP2
induced by Rap2c.
To fully elucidate the mechanisms involved in the
upregulation of MMP2 by Rap2c, the signaling pathways of MMPs
associated with cell invasion were investigated. Previous studies
revealed that the Akt signaling pathway may be involved in
metastatic potential and mediated the activity of MMPs (33,34). Thus,
the present study investigated whether Rap2c expression could
modulate the protein level of Akt in order to contribute to
evidence regarding the molecular pathways leading to Rap2c-mediated
upregulation of MMP2. Results demonstrated that overexpression of
Rap2c enhanced the phosphorylation of Akt, whereas and the
knockdown of Rap2c expression contributed to a reduction of p-Akt
protein levels. These results indicate that the Akt signaling
pathway may be involved in Rap2c-induced MMP2 expression.
Therefore, it is likely that this signaling molecule is not the
only one responsible for Rap2c-induced MMP2 expression and the
invasion of tumor cells. Further studies and the use of other
osteosarcoma cells are required to uncover other critical signaling
molecules involved in these events.
Collectively, this study provided evidence that
Rap2c may influence cancer cell invasion and migration.
Overexpression of Rap2c promoted the migration and invasion of U2OS
cells, and decreased Rap2c expression may contribute to the
inhibition of cancer cell invasion and migration. In addition, MMP2
activity and the Akt signaling pathway may be involved in
Rap2c-mediated tumor metastasis. Future work will be required to
elucidate whether this pathway occurs in vivo, and whether
there are other signaling pathways involved. To conclude, the
results of the present study provide information on a potential
therapeutic target that mediates the invasiveness of
osteosarcoma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572349), Jiangsu
Provincial Medical Talent, the Science and Technology Department of
Jiangsu Province (grant nos. BK20141149 and BK20150217) and
Education Departmental Nature Science Foundation of Jiangsu
Province (grant no. 15KJB320018).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arndt CA, Rose PS, Folpe AL and Laack NN:
Common musculoskeletal tumors of childhood and adolescence. Mayo
Clin Proc. 87:pp. 475–487. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang S, Ren Z, Han X, Yang J, Shan L, Li
L, Wang B, Zhang Q, Mu T, Chen K, et al: PLA2G16 expression in
human osteosarcoma is associated with pulmonary metastasis and poor
prognosis. PLoS One. 10:e01272362015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mittal V and Linder ME: Biochemical
characterization of RGS14: RGS14 activity towards G-protein alpha
subunits is independent of its binding to Rap2A. Biochem J.
394:309–315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albright CF, Giddings BW, Liu J, Vito M
and Weinberg RA: Characterization of a guanine nucleotide
dissociation stimulator for a ras-related GTPase. EMBO J.
12:339–347. 1993.PubMed/NCBI
|
|
5
|
Bokoch GM: Biology of the Rap proteins,
members of the ras superfamily of GTP-binding proteins. Biochemical
J. 289:17–24. 1993. View Article : Google Scholar
|
|
6
|
Pasheva E, Janoueix-Lerosey I, Tavitian A
and de Gunzburg J: Characterization of the Ras-related RAP2A
protein expressed in the baculovirus-insect cell system: Processing
of the protein in insect cells and comparison with the bacterially
produced unprocessed form. Biochem Biophys Res Commun. 198:973–982.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itoh M, Nelson CM, Myers CA and Bissell
MJ: Rap1 integrates tissue polarity, lumen formation, and
tumorigenic potential in human breast epithelial cells. Cancer Res.
67:4759–4766. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong X, Korch C and Meinkoth JL: Histone
deacetylase inhibitors upregulate Rap1GAP and inhibit Rap activity
in thyroid tumor cells. Endocr Relat Cancer. 18:301–310. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen W, Wang X, Deng C, Lv X, Fan Y, Men
J, Liang C and Yu X: Molecular cloning and characterization of a
novel ras-related protein (rap2) from Clonorchis sinensis.
Parasitol Res. 108:1021–1026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stanley P, Tooze S and Hogg N: A role for
Rap2 in recycling the extended conformation of LFA-1 during T cell
migration. Biol Open. 1:1161–1168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou H, Cui X, Yuan H, Zhang B, Meng C and
Zhao D: Effects of distinct drugs on gene transcription in an
osteosarcoma cell line. Oncol Lett. 14:4694–4700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
13
|
Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS,
Lee SH, Park IC, Rhee CH and Hong SI: Ionizing radiation enhances
matrix metalloproteinase-2 secretion and invasion of glioma cells
through Src/epidermal growth factor receptor-mediated p38/Akt and
phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res.
66:8511–8519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang JS, Lin CW, Hsieh YS, Cheng HL, Lue
KH, Yang SF and Lu KH: Selaginella tamariscina (Beauv.) possesses
antimetastatic effects on human osteosarcoma cells by decreasing
MMP-2 and MMP-9 secretions via p38 and Akt signaling pathways. Food
Chem Toxicol. 59:801–807. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Chen T, Zhang J, Mao Q, Li S,
Xiong W, Qiu Y, Xie Q and Ge J: Notch1 promotes glioma cell
migration and invasion by stimulating β-catenin and NF-κB signaling
via AKT activation. Cancer Sci. 103:181–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Groot JF, Fuller G, Kumar AJ, Piao Y,
Eterovic K, Ji Y and Conrad CA: Tumor invasion after treatment of
glioblastoma with bevacizumab: Radiographic and pathologic
correlation in humans and mice. Neuro Oncol. 12:233–242. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Folkins C, Shaked Y, Man S, Tang T, Lee
CR, Zhu Z, Hoffman RM and Kerbel RS: Glioma tumor stem-like cells
promote tumor angiogenesis and vasculogenesis via vascular
endothelial growth factor and stromal-derived factor-1. Cancer Res.
69:7243–7251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szpon L, Stal A, Zawadzki M, Lis-Nawara A,
Kielan W and Grzebieniak Z: K-ras gene mutation as an early
prognostic marker of colon cancer. Pol Przegl Chir. 88:15–19.
2016.PubMed/NCBI
|
|
19
|
Tao LY, Zhang LF, Xiu DR, Yuan CH, Ma ZL
and Jiang B: Prognostic significance of K-ras mutations in
pancreatic cancer: A meta-analysis. World J Surg Oncol. 14:1462016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mascaux C, Iannino N, Martin B, Paesmans
M, Berghmans T, Dusart M, Haller A, Lothaire P, Meert AP, Noel S,
et al: The role of RAS oncogene in survival of patients with lung
cancer: A systematic review of the literature with meta-analysis.
Br J Cancer. 92:131–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bailey CL, Kelly P and Casey PJ:
Activation of Rap1 promotes prostate cancer metastasis. Cancer Res.
69:4962–4968. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Altschuler DL and Ribeiro-Neto F:
Mitogenic and oncogenic properties of the small G protein Rap1b.
Proc Natl Acad Sci USA. 95:pp. 7475–7479. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McLeod SJ, Shum AJ, Lee RL, Takei F and
Gold MR: The Rap GTPases regulate integrin-mediated adhesion, cell
spreading, actin polymerization, and Pyk2 tyrosine phosphorylation
in B lymphocytes. J Biol Chem. 279:12009–12019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Price LS, Hajdo-Milasinovic A, Zhao J,
Zwartkruis FJ, Collard JG and Bos JL: Rap1 regulates
E-cadherin-mediated cell-cell adhesion. J Biol Chem.
279:35127–35132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bigler D, Gioeli D, Conaway MR, Weber MJ
and Theodorescu D: Rap2 regulates androgen sensitivity in human
prostate cancer cells. Prostate. 67:1590–1599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prabakaran I, Grau JR, Lewis R, Fraker DL
and Guvakova MA: Rap2A is upregulated in invasive cells dissected
from follicular thyroid cancer. J Thyroid Res. 2011:9798402011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie X, Liu H, Wang M, Ding F, Xiao H, Hu
F, Hu R and Mei J: miR-342-3p targets RAP2B to suppress
proliferation and invasion of non-small cell lung cancer cells.
Tumour Biol. 36:5031–5038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Wang X, Zhong W, Ren X, Sha X and
Fang X: Matrix metalloproteinases-2/9-sensitive peptide-conjugated
polymer micelles for site-specific release of drugs and enhancing
tumor accumulation: Preparation and in vitro and in vivo
evaluation. Int J Nanomedicine. 11:1643–1661. 2016.PubMed/NCBI
|
|
30
|
Pulukuri SM, Patibandla S, Patel J, Estes
N and Rao JS: Epigenetic inactivation of the tissue inhibitor of
metalloproteinase-2 (TIMP-2) gene in human prostate tumors.
Oncogene. 26:5229–5237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giannelli G, Erriquez R, Fransvea E,
Daniele A, Trerotoli P, Schittulli F, Grano M, Quaranta M and
Antonaci S: Proteolytic imbalance is reversed after therapeutic
surgery in breast cancer patients. Int J Cancer. 109:782–785. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Mo N, Li L, Cao Y, Wang W, Liang Y,
Deng H, Xing R, Yang L, Ni C, et al: Surgery-induced hippocampal
angiotensin II elevation causes Blood-brain barrier disruption via
MMP/TIMP in aged rats. Front Cell Neurosci. 10:1052016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hao J, Du H, Li W, Liu F, Lu J, Yang X and
Cui W: Anthocyanins protected hearts against ischemic injury by
reducing MMP-2 activity via Akt/P38 pathways. Am J Transl Res.
8:1100–1107. 2016.PubMed/NCBI
|
|
34
|
Wang X, Li X, Li C, He C, Ren B, Deng Q,
Gao W and Wang B: Aurora-A modulates MMP-2 expression via AKT/NF-κB
pathway in esophageal squamous cell carcinoma cells. Acta Biochim
Biophys Sin (Shanghai). 48:520–527. 2016. View Article : Google Scholar : PubMed/NCBI
|