Introduction
Renal cell carcinoma (RCC) accounts for ~85% of all
kidney tumors, and is one of the most common types of cancer of the
urinary system (1,2). RCC has several subtypes, including clear
cell RCC (ccRCC), papillary RCC, and chromophobe RCC. ccRCC, which
is associated with a high risk of metastasis, is the most common
subtype of RCC, accounting for 75–80% of all RCC cases (3). RCC is not sensitive to conventional
chemotherapy and radiotherapy, and its prognosis remains poor
(4–6).
Targeted therapy is a novel method for the treatment of RCC
(7). However, the detailed molecular
mechanism of RCC progression remains poorly understood; therefore,
the identification of novel therapeutic targets has an important
significance.
MicroRNAs (miRNAs) are a type of endogenous
non-coding RNA, which are able to effectively regulate the
expression of target genes by binding to the 3′-untranslated region
(3′-UTR) or by hybridizing in the coding sequence. They serve
important roles in the majority of biological processes, including
cell development, proliferation, differentiation and apoptosis
(8–10). An increasing volume of evidence has
demonstrated that the abnormal expression of miRNAs is a common
feature of cancer, and that they serve an important role in the
occurrence and development of tumors (11). The miRNA-183 (miR-183) cluster is
located on chromosome 7q32, and consists of miR-96, miR-182 and
miR-183 (12). Previous studies have
revealed that miR-183 may function as an oncogene in the majority
of cancer types, including, pancreatic, lung, gastric, breast and
colorectal cancer (CRC) (13–17). However, it may also function as a
tumor suppressor in certain types of cancer, including cervical
cancer (18). Therefore, the role of
miR-183 may depend on the type of cancer it is identified in.
The clinical significance of miR-183 has previously
been investigated in RCC. For example, Zhang et al (19) identified that the serum expression
level of miR-183 was significantly higher in patients with RCC than
that in healthy volunteers. Furthermore, its expression level was
significantly associated with sensitivity to natural killer cell
therapy (19). In order to further
investigate the role of miR-183 in the occurrence and development
of RCC, TargetScan analysis was conducted to predict the target
gene of miR-183, and results demonstrated that Dickkopf-related
protein-3 (DKK-3) was a target gene of miR-183. Previously, Ueno
et al (20) previously
identified that miR-183 was an oncogene targeting DKK-3 in prostate
cancer; however, whether there is an association between miR-183
and DKK-3 in RCC remains unknown. The present study focused on the
effects of miR-183 on RCC cells in addition to the identification
of its direct target gene, DKK-3, in order to illuminate the
molecular mechanisms of miR-183 in the initiation and progression
of RCC.
Materials and methods
Cell lines and cell culture
The normal human proximal tubule epithelial HK-2
cell line and four human RCC cell lines (ACHN, 786-O, Caki-1 and
Caki-2) were obtained from the Cell Bank of Type Culture Collection
of Chinese Academy of Sciences (Shanghai, China). The HK-2 cells
were cultured in keratinocyte serum-free medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The four RCC cell lines
were cultured in RPMI-1640 medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA), supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). The culture medium
was supplemented with 50 µg/ml streptomycin and 50 U/ml penicillin.
The cells were cultured at 37°C in a humidified atmosphere with 5%
CO2.
Cell transfection
The ACHN, 786-O, Caki-1 and Caki-2 cells were seeded
onto 24-well plates at a density of 5×104 cells per well
and were incubated at 37°C overnight prior to transfection. The
miR-183 inhibitor and the negative control (NC) were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The primer
sequences were as follows: miR-183 inhibitor forward,
5′-AGUGAAUUCUACCAGUGCCAUA-3′ and reverse,
5′-UAUGGCACUGGUAGAAUUCACU-3′; NC forward,
5′-CAGUACUUUUGUGUAGUACAA-3′ and reverse,
5′-UUGUACUACACAAAAGUACUG-3′. Transfection with the miR-183
inhibitor (50 nM) and the respective NC (50 nM) was performed using
Lipofectamine 2000® (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Following transfection, the cells were maintained at 37°C for 48 h
for additional experiments.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA, containing miRNA, was extracted from
harvested RCC cells using 1 ml TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. RNA was transcribed to cDNA using an EasyScript
First-Strand cDNA Synthesis SuperMix kit (TransGen Biotech, Inc.,
Slough, UK) according to the manufacturer's instructions. qPCR was
performed using the SYBR-Green PCR kit (Takara Bio, Inc., Otsu,
Japan) on the ABI 7500 Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. Reaction conditions included: 95°C for
10 min, followed by 40 cycles of 95°C for 10 sec, 57°C for 20 sec,
and 72°C for 15 sec. GAPDH or U6 were used as endogenous controls.
The relative expression levels were calculated using the
2−ΔΔCq method (21). All
RT-qPCR experiments were conducted in triplicate. PCR amplification
was performed using the following primers: miR-183 forward,
5′-CGCGGTATGGCACTGGTAGA-3′ and reverse,
5′-AGTGCAGGGTCCGAGGTATTC-3′; DKK-3 forward,
5′-AGGACACGCAGCACAAATTG-3′ and reverse,
5′-CCAGTCTGGTTGTTGGTTATCTT-3′; U6-forward, 5′-AGAGCCTGTGGTGTCCG-3′
and reverse, 5′-CATCTTCAAAGCACTTCCCT-3′; GAPDH-forward,
5′-CATCACCATCTTCCAGGAGCG-3′ and reverse,
5′-TGACCTTGCCCACAGCCTTG-3′.
MTT cell proliferation assay
The MTT assay was conducted to measure the
proliferation of RCC cells using MTT kit from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germamy), according to the manufacturer's
instructions. Following miR-183 inhibitor or mimic transfection,
RCC 786-O and ACHN cells were seeded onto 96-well plates
(4×103 cells/well) and were incubated at 37°C with 5%
CO2. Following incubation for 24, 48 and 72 h
post-transfection, cell proliferation was examined. A total of 20
µl MTT solution was added to each well and the cells were
subsequently incubated for 4 h prior to measurement. Following
removal of the medium, 150 µl dimethyl sulfoxide (DMSO) was added
to each well and the plates were agitated at a low speed for 10 min
to ensure the purple formazan was fully dissolved. The optical
densities of each well were measured at 490 nm using a microplate
reader. All experiments were performed in triplicate.
Cell invasion assay
The invasive potential of cancer cells was
determined using Transwell chambers and Transwell inserts. In
brief, 1×105 RCC 786-O and ACHN cells that had been
transfected with a miR-183 inhibitor or mimic were resuspended in
200 µl RPMI-1640 medium without serum and were seeded into the
upper chamber. RPMI-1640 medium containing 20% FBS was added to the
lower chamber. Following incubation for 48 h at 37°C with 5%
CO2, cells that had migrated to the lower surface of
filters were fixed with 4% paraformaldehyde at room temperature for
30 min, and then were stained with 0.5% crystal violet at room
temperature for 30 min. The number of migrating cells was counted
using a confocal microscope in five fields at a magnification of
×200. All experiments were performed in triplicate.
miRNA target prediction and luciferase
assay
miRNA targets were predicted using the TargetScan
algorithms (http://www.targetscan.org), and DKK-3
was predicted to be a target of miR-183. Luciferase reporter
plasmids [PmirGLO-DKK-3-3′UTR wild-type (WT) and
PmirGLO-DKK-3-3′UTR mutant (MUT)] were purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China). Transient transfection of
miR-183 mimics and/or plasmids were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
786-O cells were seeded onto 96-well plates (5×103/well)
and were co-transfected with 100 ng WT or MUT reporter with 50
nmol/l miR-183 or miR-negative control (NC), according to the
manufacturer's instructions. After 48 h, the luciferase activity
was measured using the Dual-Luciferase Reporter Assay system
(Promega Corporation, Madison, WI, USA). Firefly luciferase
activity was normalized to the corresponding Renilla
luciferase activity.
Western blot analysis
A total of 48 h after transfection with the miR-183
inhibitor or mimic, the cancer cells were collected and washed
three times with phosphate-buffered saline. Following the addition
of the cell lysis solution, the cancer cells were centrifuged at
14,000 × g for 15 min at 4°C. Total protein was extracted from RCC
cells using a protein extraction kit (Beyotime Institute of
Biotechnology, Jiangsu, China), according to the manufacturer's
instructions. Protein concentration was quantified using a Bradford
protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein (50 µg) was separated using 10% SDS-PAGE gels, and then
electrophoretically transferred onto nitrocellulose membranes
(Bio-Rad Laboratories, Inc.). The membranes were subsequently
blocked with 5% skimmed milk for 2 h at room temperature, the
primary antibodies were added and incubated overnight at 4°C. The
primary antibodies used were as follows: anti-DKK-3 antibody
(dilution 1:100; cat. no. 10365-1-AP; Proteintech Group, Inc.,
Chicago, IL, USA), and anti-GADPH antibody (dilution 1:1,000
dilution; cat. no. ab9484; both from Abcam, Cambridge, UK).
Subsequently, a horseradish peroxidase-conjugated secondary
antibody (dilution 1:5,000; cat. no. ab6789; Abcam) was added and
incubated for 2 h at room temperature. The blots were visualized
using an enhanced chemiluminescence kit (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA), and analyzed with Quantity One
software version 4.6.2 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are presented as mean ± standard deviation.
Statistically significant differences between two groups were
determined using the two-tailed Student's t-test. One-way analysis
of variance (ANOVA) was used for comparisons among multiple groups.
The Student-Newman-Keuls test was used as a post hoc test following
ANOVA. P<0.05 was considered to indicate a statistically
significant difference. All statistical analysis was conducted
using the SPSS 18.0 statistical software package (SPSS Inc.,
Chicago, IL, USA) and GraphPad Prism 6 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
Expression of miR-183 in RCC cell
lines
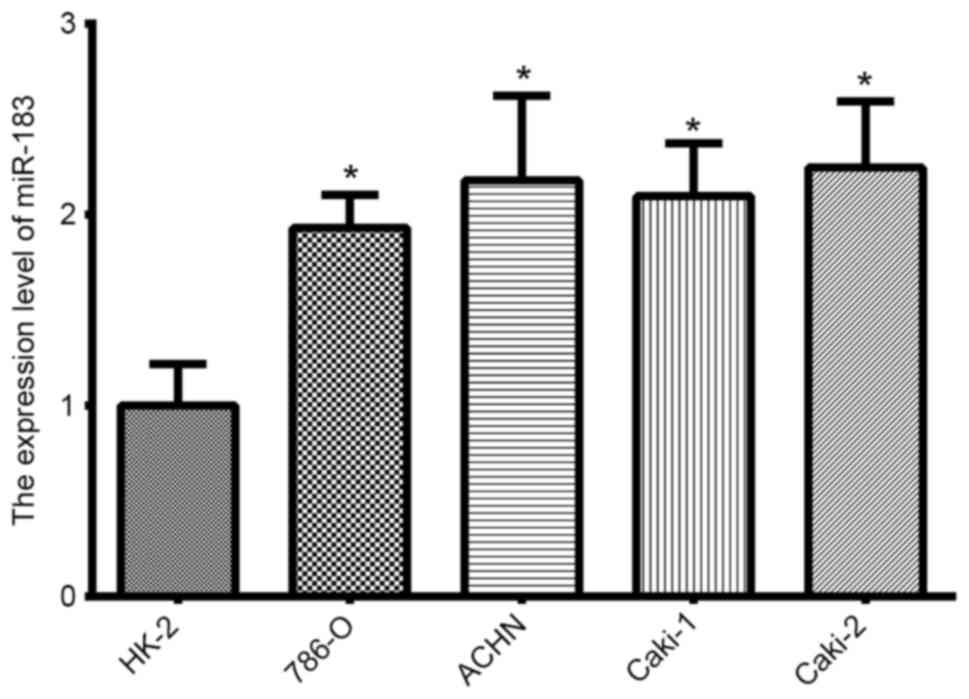
The expression of miR-183 in RCC cell lines was
analyzed using RT-qPCR. In comparison to the immortalized normal
proximal tubule epithelial HK-2 cell line, miR-183 expression was
significantly upregulated in all four RCC lines (Fig. 1; P<0.05).
Validation of cell transfection
efficiency
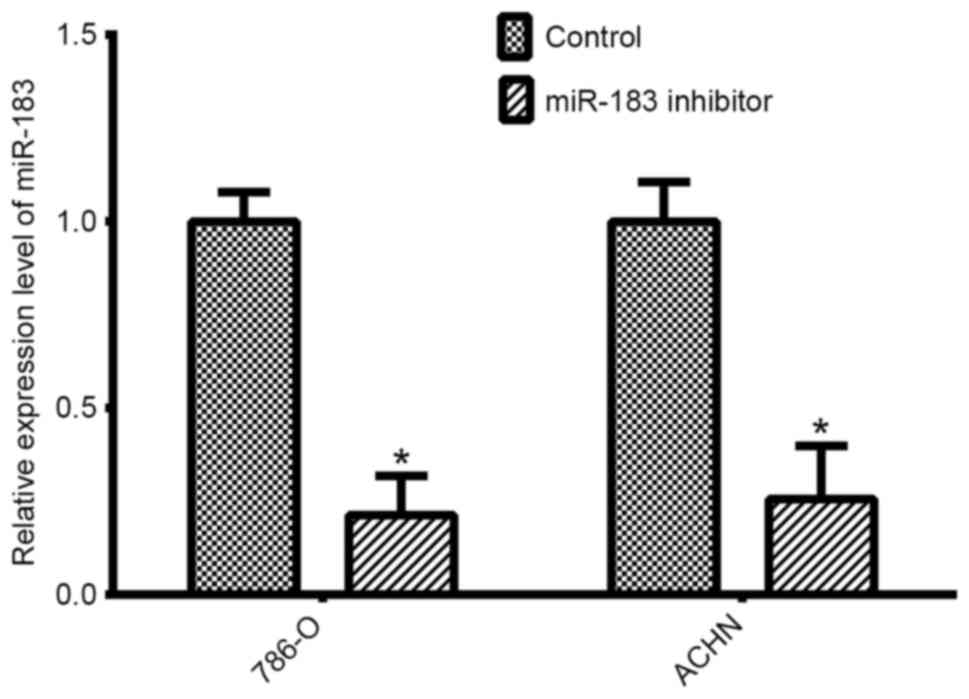
Silencing experiments were performed in two RCC
lines (786-O and ACHN). qPCR was performed to determine the
transfection efficiency of the miR-183 inhibitor compared with the
negative control. The expression levels of miR-183 were
significantly lower in 786-O cells (P<0.05) and ACHN cells
(P<0.05) transfected with the miR-183 inhibitor, compared with
expression in the respective negative control groups for each of
the cell lines (Fig. 2).
Downregulation of miR-183 inhibits
786-O and ACHN cell proliferation
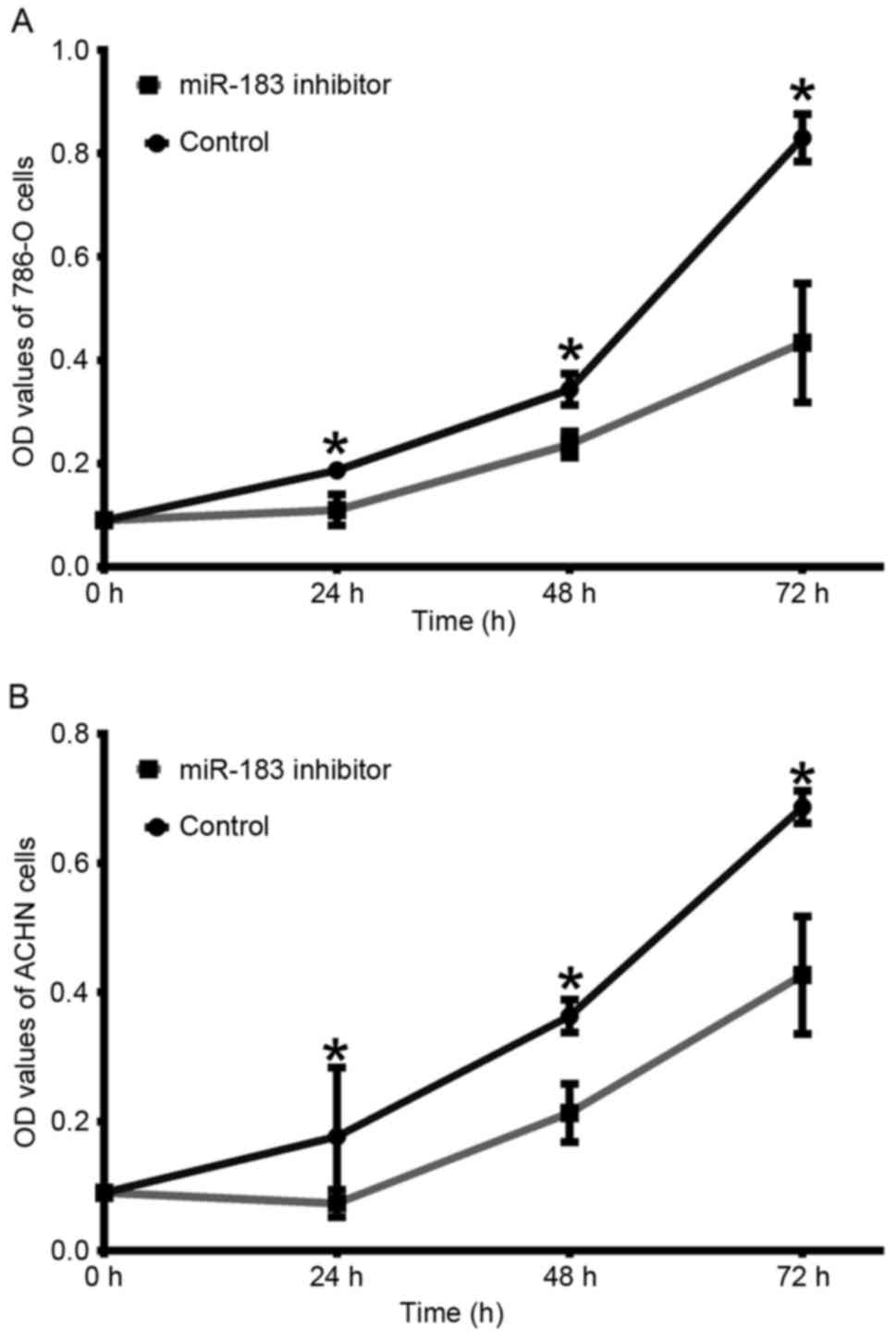
In order to determine whether miR-183 was able to
affect RCC cell proliferation, an MTT assay was performed.
Following transfection with the miR-183 inhibitor, the
proliferation of 786-O cells was decreased by 7.67% (24 h;
P<0.05), 10.67% (48 h; P<0.05) and 39.67% (72 h; P<0.05)
compared with the negative control group (Fig. 3A). In the ACHN cells, cell
proliferation was decreased by 11.33% (24 h; P<0.05), 15.00% (48
h; P<0.05) and 26.00% (72 h; P<0.05) compared with the
negative control group (Fig. 3B).
These results suggested that downregulation of miR-183 may inhibit
RCC cell viability.
Downregulation of miR-183 inhibits the
invasion of 786-O and ACHN cells
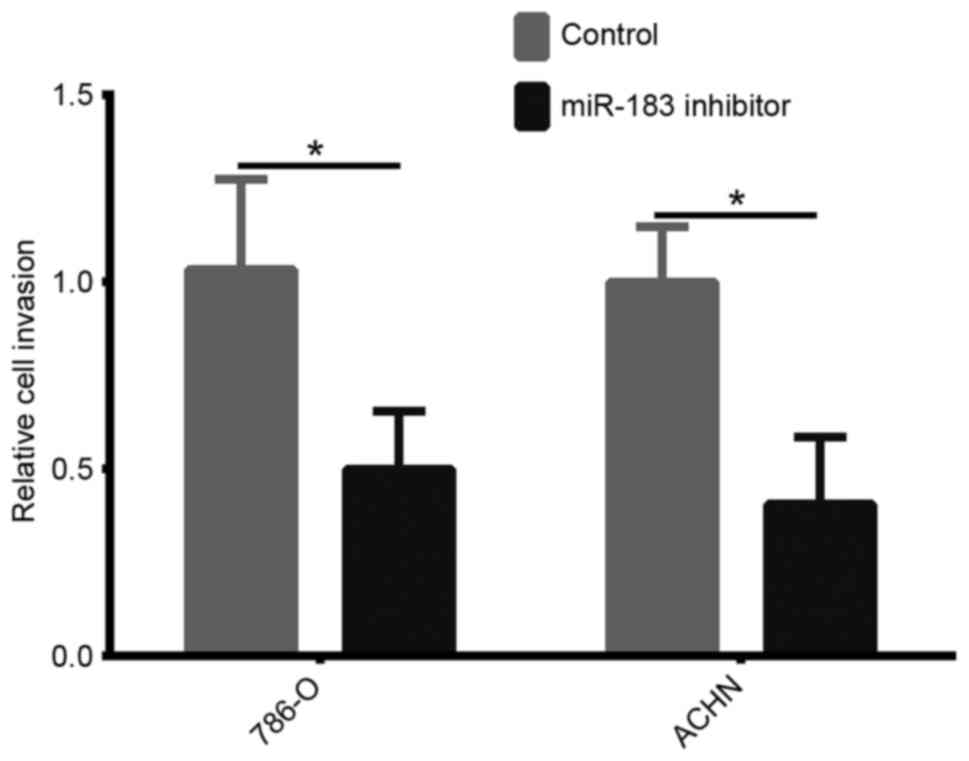
In order to investigate the role of miR-183 on cell
invasion, an invasion assay was performed. As demonstrated in
Fig. 4, the number of invasive 786-O
and ACHN cells transfected with the miR-183 inhibitor was
significantly reduced compared with that in the control groups
(both P<0.05). These results indicated that downregulation of
miR-183 may decrease the invasion of 786-O and ACHN cells.
DKK-3 is a direct target gene of
miR-183
DKK-3 was predicted as a target gene of miR-183
using TargetScan. The 3′-UTR and a mutant 3′-UTR of DKK-3 were used
to verify DKK-3 as a target gene of miR-183 (Fig. 5A). The target sequences in the WT
DKK-3 3′-UTR and a MUT sequence were cloned into the luciferase
reporter. Following co-transfection of the reporters with a miR-183
mimic or mimic-NC into 786-O cells, the luciferase activity was
recorded. The data demonstrated that miR-183 induced an evident
decrease in the firefly luciferase activity when compared with the
mimic-NC group in the WT reporter-transfected cells. However, the
same effect was not observed in the MUT group (Fig. 5B; P<0.05). Additionally, the
regulatory effect of the miR-183 inhibitor on DKK-3 was
investigated by RT-qPCR and western blot analysis in 786-O cells,
identifying that the miR-183 inhibitor increased the DKK-3 mRNA and
protein levels in 786-O cells (Fig. 5C
and D). Taken together, these results indicated that miR-183
directly targets DKK-3 in RCC cells.
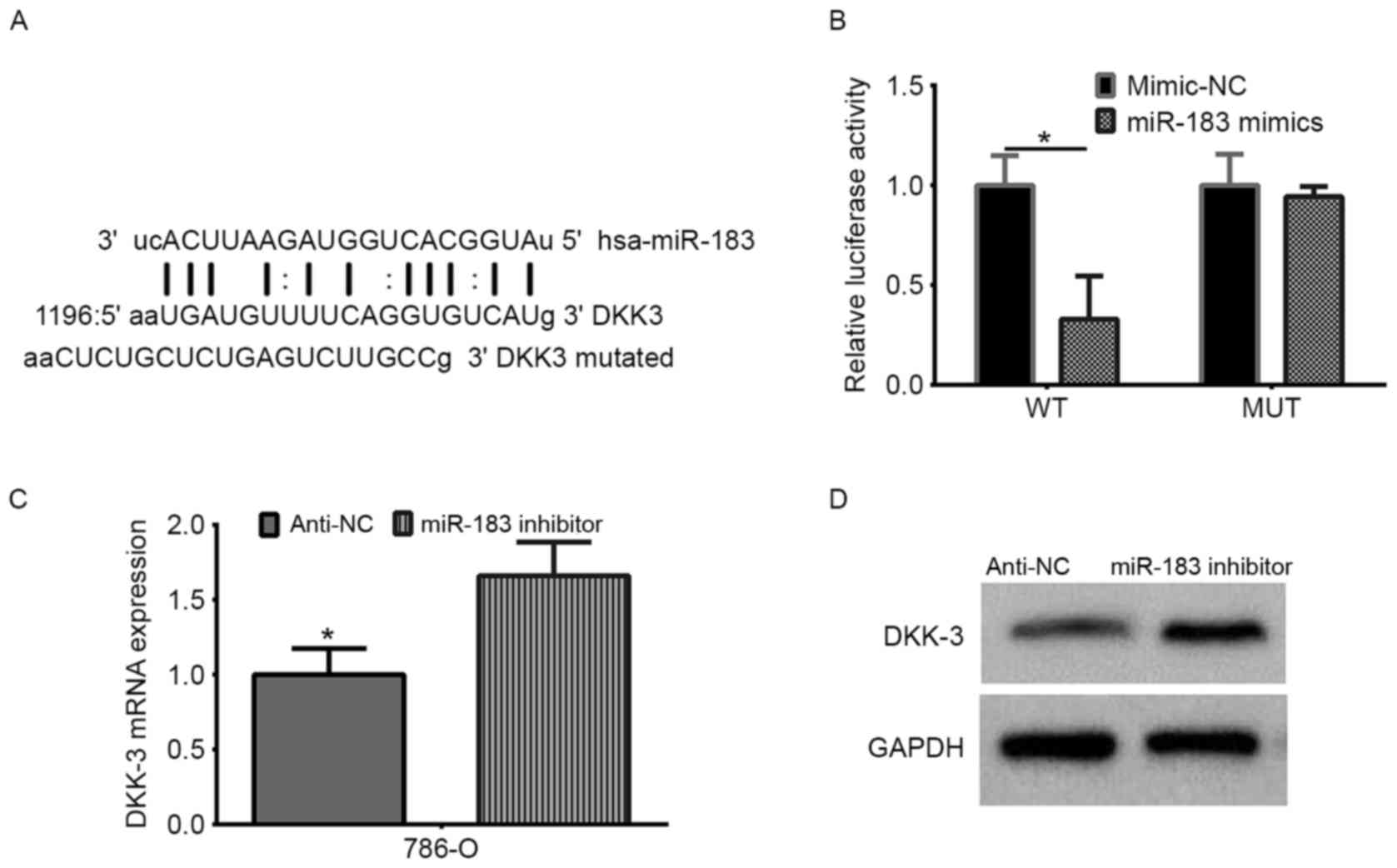 | Figure 5.DKK-3 is the direct target of miR-183
in renal cell carcinoma. (A) The miR-183 binding site in the DKK-3
3′-UTR, as predicted by TargetScan. The upper sequence is miR-183,
the middle sequence is the target WT, and the bottom sequence is
the target-mutated sequence. (B) The firefly luciferase activity in
the 786-O cell line was detected following co-transfection of the
WT or MUT 3′-UTR (100 ng) with the miR-183 mimic or mimic-NC (50
nmol/l). The firefly luciferase activity of each sample was
normalized to Renilla luciferase activity. *P<0.05 vs.
mimic NC. (C) Reverse transcription-quantitative polymerase chain
reaction analysis of the relative mRNA levels of DKK-3 following
miR-183 inhibitor transfection. (*P<0.05 vs. anti-miR-183, as
determined by Student's t-test). (D) Western blot analysis of DKK-3
protein expression levels following transfection with a miR-183
inhibitor. DKK-3, Dickkopf-related protein 3; miR, microRNA; WT,
wild-type; MUT, mutant; UTR, untranslated region; NC, negative
control. |
Discussion
As RCC is not sensitive to traditional radiotherapy
or chemotherapy, there is a pressing demand to identify more
efficient novel treatment strategies, such as targeted therapies.
miRNAs are able to regulate gene expression by binding to the
3′-UTR of target genes or by hybridizing in the coding sequence
(8). An increasing volume of evidence
has demonstrated that miRNAs are abnormally expressed in the
majority of cancer types. Furthermore, miRNAs may regulate cancer
cell proliferation, invasion and migration, suggesting that they
serve an important role in cancer occurrence and progression
(9). Therefore, miRNAs have the
potential to be used as cancer diagnostic and prognostic
biomarkers, as well as target points for cancer therapeutics
(8,11).
Previous investigations revealed that miR-183 may
function as an oncogene in various types of cancer, including
pancreatic, lung, gastric, breast and colorectal cancer (13–17).
However, it may also function as a tumor suppressor in certain
cancer types, including cervical cancer (18). Therefore, the role of miR-183 may
depend on the type of cancer that it is expressed in. A previous
study by Huangfu et al (13)
identified that miR-183 was able to promote CRC occurrence and
progression through regulating the apoptosis and autophagy of CRC
cells by targeting the ultraviolet radiation resistance-associated
gene. Bi et al (22) also
investigated the detailed functional role of miR-183 in CRC and
identified that the ATP-binding cassette transporter A1/cholesterol
exporter may be one of the downstream targets of miR-183. Zhu et
al (15) demonstrated that
miR-183 may function as an oncogene in non-small cell lung
carcinoma by promoting proliferation, invasion and migration
through targeting protein tyrosine phosphatase non-receptor type 4.
Additionally, a study undertaken by Miao et al (14) identified that miR-183 may have the
potential to promote pancreatic cancer cell proliferation, invasion
and metastasis by targeting suppressor of cytokine signaling 6. Li
et al (16) demonstrated that
miR-183 may have the potential to regulate the proliferation,
invasion and apoptosis of gastric cancer cells by regulating
programmed cell death 4 (PDCD4), indicating that it may function as
an oncogene in gastric cancer. Similarly, Cheng et al
(17) identified that miR-183 may
function as an oncogene in breast cancer by promoting the
proliferation and invasion of breast cancer cells, and that PDCD4
is a target of miR-183.
In contrast to the aforementioned studies, a study
undertaken by Fan et al (18)
demonstrated that the expression level of miR-183 was significantly
lower in cervical tissues than in adjacent normal cervical tissues.
Furthermore, they revealed that miR-183 may function as a tumor
suppressor by regulating the proliferation, invasion and metastasis
of cervical cancer cells, and that matrix metallopeptidase-9 may be
one of the downstream targets of miR-183.
The Wnt signaling pathway has been investigated at
several molecular levels, as its involvement has been implicated in
tumorigenesis and disease progression (23). The DKK family functions as a negative
regulator of the Wnt signaling pathway, and therefore serves a
tumor-suppressive function (24).
Ueno et al have reported that DKK-3 expression was regulated
by histone modifications in the DKK-3 promoter region in RCC cells
(25). Furthermore, in our previous
study, DKK-3 levels were identified to be lower in the tumor
tissues compared with the adjacent normal tissues using
immunohistochemistry, western blot analysis and qPCR, indicating
that DKK-3 may have an associated mechanism in the development of
RCC (26). TargetScan identified
DKK-3 as a target gene of miR-183, in line with the results of a
study undertaken by Ueno et al (20), who had previously identified that
miR-183 was an oncogene targeting DKK-3 in prostate cancer
(20). However, whether there is an
association between miR-183 and DKK-3 in RCC remains to be
elucidated.
The clinical significance of miR-183 has previously
been investigated in RCC. For example, Zhang et al (19) demonstrated that the serum expression
level of miR-183 was significantly higher in patients with RCC than
in healthy volunteers. Furthermore, its expression level was
significantly associated with sensitivity to natural killer cell
therapy (19). The present study
expanded the knowledge presently available regarding the expression
and function of miR-183 in RCC. Firstly, in comparison to the
immortalized normal proximal tubule epithelial HK-2 cell line,
miR-183 expression was upregulated in all four RCC cell lines
(ACHN, 786-O, Caki-1 and Caki-2). Furthermore, downregulation of
miR-183 inhibited RCC cell proliferation and invasion, indicating
that miR-183 may function as an oncogene in RCC. Additionally, an
important molecular link was identified between miR-183 and DKK-3
in the present study through the prediction of TargetScan that
DKK-3 was a direct target gene of miR-183. Furthermore, the results
of the luciferase activity assay indicated that miR-183 directly
targeted the DKK-3 3′-UTR, as predicted by bioinformatics analysis.
Additionally, the regulatory effect of miR-183 on DKK-3 was
investigated by RT-qPCR and western blotting in 786-O cells. The
data demonstrated that downregulation of miR-183 increased DKK-3
expression at the mRNA and protein levels. These findings revealed
that miR-183 may have the potential to regulate DKK-3 expression
in vitro, and may have a tumor promoting role in RCC
development and progression.
In conclusion, the results of the present study
demonstrate that miR-183 functions as an oncogene by downregulating
DKK-3 expression, providing a potential diagnostic, prognostic and
therapeutic target for RCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Zhejiang Medical, Health, and Science and Technology Project (grant
no. 2016RCA028).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZXL designed the study; ZXL, XG, ZY, and YJJ carried
out the experiments and analyzed the data; ZXL, and XG drafted the
study. All authors read and approved the final study.
Ethical approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Znaor A, Lortet-Tieulent J, Laversanne M,
Jemal A and Bray F: International variations and trends in renal
cell carcinoma incidence and mortality. Eur Urol. 67:519–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patard JJ, Leray E, Rioux-Leclercq N,
Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani
W, Abbou CC, et al: Prognostic value of histologic subtypes in
renal cell carcinoma: A multicenter experience. J Clin Oncol.
23:2763–2771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lardas M, Stewart F, Scrimgeour D, Hofmann
F, Marconi L, Dabestani S, Bex A, Volpe A, Canfield SE, Staehler M,
et al: Systematic review of surgical management of nonmetastatic
renal cell carcinoma with vena caval thrombus. Eur Urol.
70:265–280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part a: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davis ID, Xie W, Pezaro C, Donskov F,
Wells JC, Agarwal N, Srinivas S, Yuasa T, Beuselinck B, Wood LA, et
al: Efficacy of second-line targeted therapy for renal cell
carcinoma according to change from baseline in international
metastatic renal cell carcinoma database consortium prognostic
category. Eur Urol. 71:970–978. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cloney R: Non-coding RNA: Deciphering the
rules of microRNA targeting. Nat Rev Genet. 17:7182016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsui M and Corey DR: Non-coding RNAs as
drug targets. Nat Rev Drug Discov. 16:167–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lumayag S, Haldin CE, Corbett NJ, Wahlin
KJ, Cowan C, Turturro S, Larsen PE, Kovacs B, Witmer PD, Valle D,
et al: Inactivation of the microRNA-183/96/182 cluster results in
syndromic retinal degeneration. Proc Natl Acad Sci USA. 110:pp.
E507–E516. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huangfu L, Liang H, Wang G, Su X, Li L, Du
Z, Hu M, Dong Y, Bai X, Liu T, et al: miR-183 regulates autophagy
and apoptosis in colorectal cancer through targeting of UVRAG.
Oncotarget. 7:4735–4745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miao F, Zhu J, Chen Y, Tang N, Wang X and
Li X: MicroRNA-183-5p promotes the proliferation, invasion and
metastasis of human pancreatic adenocarcinoma cells. Oncol Lett.
11:134–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu C, Deng X, Wu J, Zhang J, Yang H, Fu
S, Zhang Y, Han Y, Zou Y, Chen Z and Lin S: MicroRNA-183 promotes
migration and invasion of CD133(+)/CD326(+) lung adenocarcinoma
initiating cells via PTPN4 inhibition. Tumour Biol. 37:11289–11297.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Deng L, Zhi Q, Meng Q, Qian A, Sang
H, Li X and Xia J: MicroRNA-183 functions as an oncogene by
regulating PDCD4 in gastric cancer. Anticancer Agents Med Chem.
16:447–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng Y, Xiang G, Meng Y and Dong R:
MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in
human breast cancer by targeting the PDCD4. Reprod Biol.
16:225–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan D, Wang Y, Qi P, Chen Y, Xu P, Yang X,
Jin X and Tian X: MicroRNA-183 functions as the tumor suppressor
via inhibiting cellular invasion and metastasis by targeting MMP-9
in cervical cancer. Gynecol Oncol. 141:166–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, Di W, Dong Y, Lu G, Yu J, Li J
and Li P: High serum miR-183 level is associated with poor
responsiveness of renal cancer to natural killer cells. Tumour
Biol. 36:9245–9249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ueno K, Hirata H, Shahryari V, Deng G,
Tanaka Y, Tabatabai ZL, Hinoda Y and Dahiya R: microRNA-183 is an
oncogene targeting Dkk-3 and SMAD4 in prostate cancer. Br J Cancer.
108:1659–1667. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bi DP, Yin CH, Zhang XY, Yang NN and Xu
JY: MiR-183 functions as an oncogene by targeting ABCA1 in colon
cancer. Oncol Rep. 35:2873–2879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Veeck J and Dahl E: Targeting the Wnt
pathway in cancer: The emerging role of Dickkopf-3. Biochim Biophys
Acta. 1825:18–28. 2012.PubMed/NCBI
|
|
25
|
Ueno K, Hirata H, Majid S, Chen Y, Zaman
MS, Tabatabai ZL, Hinoda Y and Dahiya R: Wnt antagonist DICKKOPF-3
(Dkk-3) induces apoptosis in human renal cell carcinoma. Mol
Carcinog. 50:449–457. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo CC, Zhang XL, Yang B, Geng J, Peng B
and Zheng JH: Decreased expression of Dkk1 and Dkk3 in human clear
cell renal cell carcinoma. Mol Med Rep. 9:2367–2373. 2014.
View Article : Google Scholar : PubMed/NCBI
|