Introduction
Endometrial cancer (EC) is the 5th most common
malignancy among females globally with a rising morbidity number,
and >90% of cases occur in females >50 years of age (1). The prognosis of patients with advanced
endometrial carcinoma and who are at least 60-years old is poor,
the mortality rate is high, and it was demonstrated that invasion
and metastasis are the primary factors leading to the mortality of
patients with EC (2). Therefore, the
key of the treatment and improvement of prognosis of patients with
EC is to study the mechanisms of invasion and metastasis, and
design appropriate intervention strategies.
Tumor tissue is composed of the tumor parenchyma
cells and stromal cells. Various studies have indicated that tumor
microenvironment serves an important role in the occurrence and
development of tumors (3,4), and stromal cell are the major component
of the tumor microenvironment. Cancer-associated fibroblasts
(CAFs), as the most abundant cellular components in the tumor
microenvironment, serve an important role in tumor growth,
angiogenesis, invasion, metastasis and clinical prognosis through
the remodeling of the extracellular matrix and secretion of growth
factors that regulate tumor cell proliferation, survival and
dissemination (5).
Epithelial-mesenchymal transition (EMT) is a
differentiation process that directs polarized epithelial cells to
differentiate into mesenchymal cells. EMT, as an important process
of invasion and metastasis of the malignant tumors, has been widely
studied (6,7). There is increasing evidence indicating
that EMT is stimulated by signals from the tumor microenvironment,
including a variety of growth factors and cytokines (8,9). These
studies have demonstrated that CAFs may regulate EMT; however, the
underlying mechanisms remain incompletely understood. In the
present study, CAFs were isolated and cultured, the association
between CAFs and invasion and metastasis was analyzed and the
possible association between the growth factors and EMT was
studied.
Materials and methods
Ethics statement
The present study was approved by the Ethical
Committee of the Second Affiliated Hospital of Zhejiang University
School of Medicine (Hangzhou, China). Written informed consent was
obtained from the participant prior to enrolment in the present
study. The use of endometrial adenocarcinoma tissue was also
approved by the aforementioned ethical committee.
Isolation and purification of CAFs and
normal fibroblasts (NFs)
EC tumor tissues and adjacent normal tissues (at
least 3 cm away from the EC tumor margin) were isolated during
surgical resection from one female patient (40 years-old), with
inclusion/exclusion criteria being that the sample be diagnosed
(January 2016) as EC prior to or during surgery, without the
presence of any other tumor type, and with cell isolation from the
tumor tissues or adjacent tissues being obtained 2 h after the
operation. The tumor or normal tissue from one patient was removed
and digested in 1 mg/ml collagenase I (cat. no. C0130;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) solution overnight
at 37°C. Following centrifugation (300 × g at room temperature for
10 min), cells (sourced from the aforementioned patient) were
passed through 100 and 40 mm filters in turn, and then the cell
suspension was cultured in Dulbecco's modified Eagle's medium
supplemented (DMEM; Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) with 10% fetal bovine serum (FBS; Hyclone; GE Healthcare
Life Sciences) for several days at 37°C. As CAFs or NFs that are
more sensitive to trypsin were detached from the flask, while
epithelial cells remained attached, differential trypsinization was
utilized to separate and purify the CAFs or NFs. Following several
rounds of differential trypsinization, the CAFs or NFs were
purified, and then cultured.
Immunofluorescence assay analysis of
cell purity and activated fibroblasts
The purity of the CAFs and NFs was determined by
analyzing the fibroblast-specific protein vimentin, and activated
fibroblast marker fibroblast activation protein (FAP) and α-smooth
muscle actin (α-SMA). Briefly, cells were fixed in 4%
paraformaldehyde in PBS solution at room temperature for 30 min.
Following washing with PBS three times, cells were treated in a
permeabilization (0.1% saponin in PBS) solution. Subsequently, the
cells were incubated with anti-vimentin (cat. no. ab92547;
dilution, 1:500), anti-FAP (cat. no. ab53066; dilution, 1:100) and
anti-α-SMA (cat. no. ab5694; dilution, 1:100; all from Abcam,
Cambridge, MA, USA) at 4°C overnight. Anti-Rabbit IgG-fluorescein
isothiocyanate (cat. no. ab97050; Abcam; dilution, 1:200) was used
and counterstaining was performed with DAPI (cat. no. C1006;
Beyotime Institute of Biotechnology, Shanghai, China) at room
temperature for 15 min. Visual analysis was performed with an
Olympus fluorescence microscope (CX71; Olympus Corporation, Tokyo,
Japan) at magnification, ×400.
Preparation of conditioned medium
When CAFs and NFs had reached 70–80% confluence, the
medium was replaced with fresh serum-free DMEM and cultured at 37°C
in a 5% CO2 atmosphere for 48 h. Then, the culture
medium was collected, centrifuged at 300 × g at room temperature
for 5 min and the cell debris was removed. The supernatant was
designated CAFs/NFs conditional medium (CM).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
HEC-1A and RL95-2 cells (Type Culture Collection of
the Chinese Academy of Sciences, Shanghai, China) were cultured in
the CM supplemented with 10% FBS at 37°C in a 5% CO2
humidified atmosphere for 48 h. Subsequent to culture, total RNA
was extracted using RNA Isolation kit (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Concentration of total RNA was measured
using light densitometry, and 1.5 µg of total RNA was reverse
transcribed to cDNA using the PrimeScript™ II 1st strand
cDNA synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China)
and subsequently diluted with nuclease-free water to 10 ng/µl cDNA.
qPCR was performed using the VeriQuest SYBR-Green qPCR Master Mix
kit (Thermo Fisher Scientific, Inc.) in a 25 µl volume. DNA was
amplified with an initial denaturation at 95°C for 3 min, followed
by 45 cycles of 95°C for 15 sec (denaturation), 60°C for 30 sec
(annealing) and 72°C for 3 mins (elongation), then 72°C for 10 mins
(final extension). The primers used in the present study are
summarized in Table I. Average
threshold cycle (Cq) values for the triplicate PCR reactions were
normalized against the average Cq values of β-actin from the same
cDNA sample (10).
 | Table I.Primers used in the reverse
transcription quantitative polymerase chain reaction. |
Table I.
Primers used in the reverse
transcription quantitative polymerase chain reaction.
| Genes | Sequence
(5′-3′) |
|---|
| Epithelial | F:
AAGCGTGAGTCGCAAGAATG |
| cadherin | R:
TCTCCAGGTTTTCGCCAGTG |
| Neural | F:
CAGAAAATAACGTTCTCCAGTTGCT |
| cadherin | R:
CCCCGTGTGTTAGTTCTGCT |
| Vimentin | F:
GACGCCATCAACACCGAGTT |
|
| R:
CTTTGTCGTTGGTTAGCTGGT |
| β-actin | F:
CCTGTACGCCAACACAGTGC |
|
| R:
ATACTCCTGCTTGCTGATCC |
Western blotting
HEC-1A and RL-952 cells were cultured in the CM at
37°C in a 5% CO2 humidified atmosphere for 48 h.
Subsequent to culture, the cells were collected and lysed with
ice-cold radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology) with 1 mmol/l phenylmethanesulfonyl
fluoride (Beyotime Institute of Biotechnology), and the
concentrations were measured using a micro BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). A total of 50 µg per lane
of nucleoproteins or cytoplasmic proteins was resolved on 12% PAGE
(Invitrogen; Thermo Fisher Scientific, Inc.), transferred to
polyvinylidene fluoride membranes and visualized with enhanced
chemiluminescence western blot detection reagents (Pierce, USA).
Immunoblotting was performed using mouse anti-E-cadherin (cat. no.
ab1416; Abcam; dilution, 1:50), mouse anti-N-cadherin (cat. no.
ab98952; Abcam; dilution, 1:1,000) and mouse anti-vimentin
antibodies (cat. no. ab8978; Abcam; dilution, 1:500) at 37°C for 2
h, followed by incubation with the goat anti-mouse appropriate
horseradish-peroxidase-conjugated IgG secondary antibodies (cat.
no. ab97023; Abcam; dilution, 1:5,000) for 1 h at room temperature.
GAPDH (cat no. ab8245; Abcam; dilution, 1:1,000) levels were used
for normalization. Protein bands were scanned and quantified using
a ChemiDoc MP image analysis system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), and also analyzing using Image J2X software
(Rawak Software, Inc., Dresden, Germany).
Quantitative detection of various
growth factors by ELISA
The secretion of epidermal growth factor (EGF),
transforming growth factor-β, (TGF-β), hepatocyte growth factor
(HGF) and fibroblast growth factor-2 (FGF-2-2) was assessed by
ELISA. The Human EGF ELISA (cat. no. ab179888; Abcam), Human TGF-β
ELISA (cat. no. ab100647; Abcam), Human HGF ELISA (cat. no.
ab100534; Abcam) and Human FGF-2 basic Quantikine ELISA (cat. no.
DFB50; BD Biosciences, Franklin Lakes, NY, USA) kits were used to
determine the concentrations in the CM. The absorbance (OD) was
measured at 450 nm wavelength.
Matrigel® invasion
assay
Firstly, 40 µl Matrigel® (cat. no.
353097; BD Biosciences) was added into the polycarbonate membrane
filters and incubated for 24 h at 37°C. Following this incubation,
EC lines HEC1-A and RL-952 were trypsinized using a trypsin
solution (cat no. C0201; Beyotime Institute of Biotechnology) and
resuspended to a density of 5×105/ml in the CM. A total
of ~1×105 CAFs or NFs resuspended in CM were added into
the upper Transwell chamber. A total of 600 µl culture medium with
10% FBS was added into the lower well. Following incubation at 37°C
for 60 h, cells that remained on the upper surface of the filters
were removed using a cotton bud, and cells that migrated into the
lower surface of the filters were fixed at room temperature for 20
min in 4% paraformaldehyde followed by staining for 30 min with
0.1% crystal violet dye at room temperature. A total of 5 fields of
view were counted randomly to calculate the number of cells
invading through the polycarbonate membrane at magnification, ×100
using an inverted/light microscope (CX71; Olympus Corporation).
Each experiment was performed in triplicate.
Wound healing assay
HEC1-A and RL-952 cells were trypsinized using a
trypsin solution (cat no. C0201; Beyotime Institute of
Biotechnology), and 2×106 cells/well were seeded into
6-well plates cultured for 36 h until they reached 100% confluence.
Subsequently, each well was divided into 3–5 grids. An artificial
homogenous wound was created by scratching the cell monolayer with
the 200 µl pipette tip, and then the cells were washed 2 times with
free-serum medium. The cells were cultured in CM for 48 h.
Microscopic images of the same area were captured at 0, 24 and 48 h
time points using an inverted/light microscope (CX71; Olympus
Corporation) at magnification, ×100. The cell migration distance
was calculated using the equation: Initial distance-final
distance.
Effect of exogenous growth factors on
the migration and invasion of EC cells
Exogenous growth factors EGF (cat. no. E5036;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), TGF-β (cat. no.
155613; Abcam) HGF (cat. no. 105061; Abcam) and FGF-2-2 (cat. no.
61845; Abcam) were added into the conditional medium of NFs [as the
fortified CM (FCM) of NFs], and their concentrations were 40, 200,
50 and 95 pg/ml, respectively, so that the final concentrations of
these four growth factors was close to their concentration in the
conditional medium (CM) of CAFs. Then, Matrigel®
invasion and wound healing assays were performed using the FCM and
CM, according to the aforementioned protocols.
In vivo xenograft experiments
Female non-obese diabetes-severe combined immune
deficiency mice (n=20) at the age of 6–8 weeks (18–20 g) were
obtained from the Laboratory Animal Centre of Zhejiang University
(Hangzhou, China). The mice were freely fed with standard forage
and clean water, and maintained on a 12-h light/dark cycle under
room temperature (25±1°C) and humidity of 55±10%. They were
randomly divided into two groups (10 mice per group): The NFs group
(0.5×106 RL-952 + 0.5×106 NFs) and CAFs group
(0.5×106 RL-952 + 0.5×106 CAFs). Cell
suspensions (RL-952 cells) in 200 µl serum-free medium were
subcutaneously injected into the 2 flanks of each mouse. Following
4 weeks, the tumor and lung samples were carefully isolated, and
tumor weight and volume of each tumor samples were measured. Tumor
tissues were monitored by caliper measurements of the length and
width. Tumor volumes were calculated according to the formula:
Volume=width × length × (width + length)/2.
Immunohistochemical analysis
The lung tissue was fixed in 4% paraformaldehyde-PBS
solution at room temperature for 30 min, and sliced into 3–5 µm
sections. Following deparaffinization at room temperature (dipped
successively in xylene twice, 10 min/time) and hydration (100%
ethanol for 5 min, 95% ethanol for 5 min, 90% ethanol for 5 min,
85% ethanol for 5 min, 75% ethanol for 5 min, and then washed using
PBS twice, 5 min/time), the slides were treated with peroxide after
blocking with serum (Invitrogen, USA). The anti-rabbit primary
antibodies against vimentin (cat. no. ab92547; Abcam; dilution,
1:500), Zinc finger protein SNAI1 (Snail; cat. no. ab180714; Abcam;
dilution, 1:100), cluster of differentiation (CD)44 (cat. no.
ab157107; Abcam; dilution, 1:1,000) and CD24 (cat. no. ab214231;
Abcam; dilution, 1:200) were incubated at 4°C overnight. Subsequent
to washing with PBS three times and incubating with goat
anti-rabbit horseradish-peroxidase-conjugated IgG secondary
antibody (cat. no. ab6721; Abcam; dilution, 1:1,000) at 37°C for 30
min, the slides were treated with DAB kit (ZLI-9017; OriGene
Technologies, Inc., Rockville, MD, USA) at room temperature for 10
min. Finally, the slides were lightly counterstained at room
temperature with hematoxylin (cat no. C0107; Beyotime Institute of
Biotechnology) for 3 min, washed five times with PBS solution,
dehydrated (two times successively using 70% ethanol for 2 sec, 80%
ethanol for 2 sec, 90% ethanol for 5 sec, 95% ethanol for 10 sec,
100% ethanol for 30 sec, then washed in xylene twice until
transparent) and mounted at room temperature and finally subjected
to neutral resin sealing. Vimentin, Snail, CD44 and CD24 expression
in the lung tissues were determined by counting 5 random visual
fields with an inverted/light microscope (CX71; Olympus
Corporation) at magnification, ×400.
Statistical analyses
The data were analyzed by one-way analysis of
variance and an unpaired Student's t-test to determine statistical
significance using SPSS 16.0 statistical software (SPSS, Inc.,
Chicago, IL, USA). Furthermore, a least significant difference
post-hoc test was employed where equal variances were assumed,
while Dunnett's T3 test was used when equal variances were not
assumed. Each experiment was repeated at least three times. The
results are presented as mean ± standard error of the mean. A
two-tailed P<0.05 was considered to indicate a statistically
significant difference.
Results
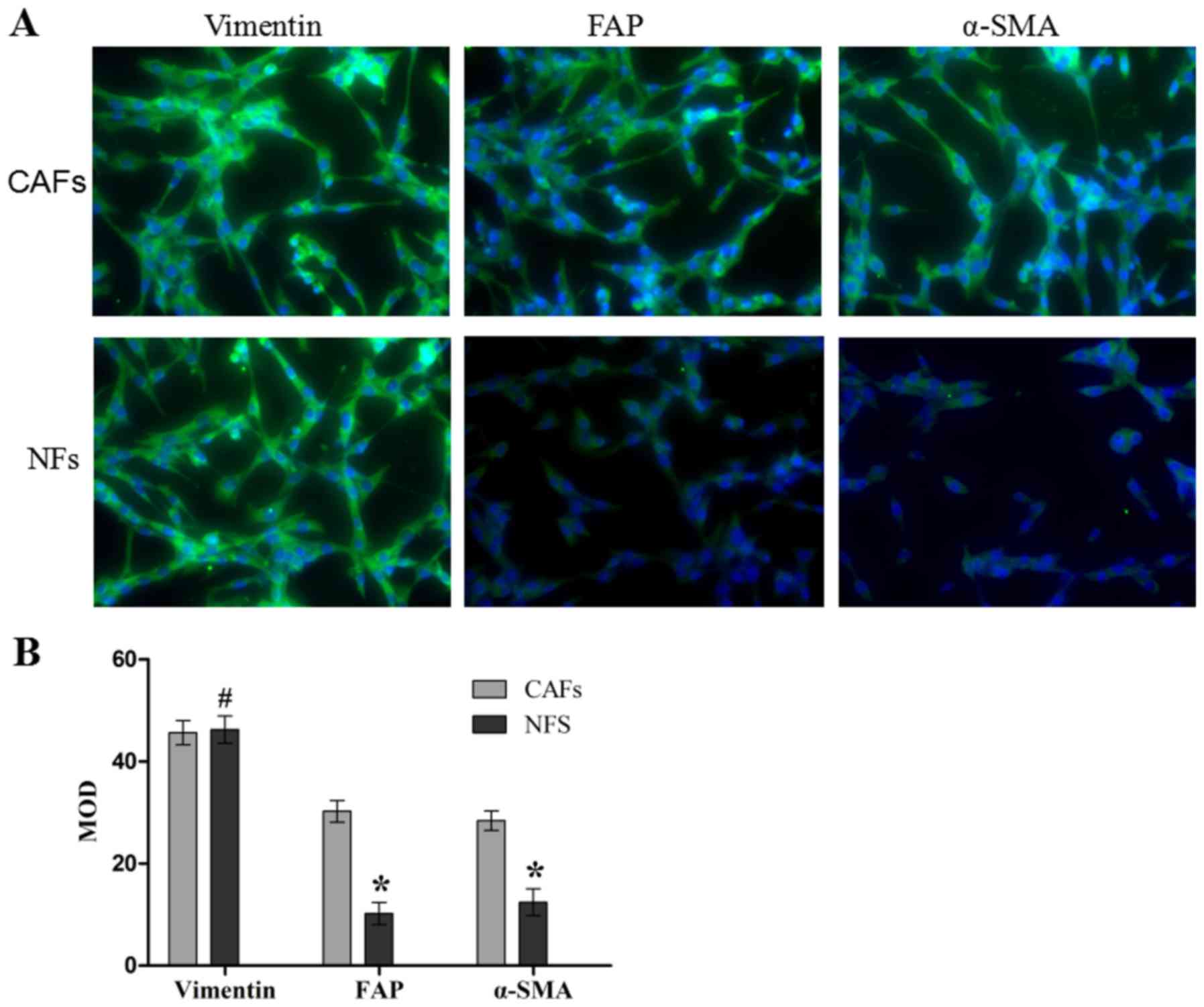
Isolation and level of activation of
isolated CAFs and NFs
The purity and activation degree of the isolated
cells was assessed by immunofluorescence (Fig. 1). The CAFs and NFs demonstrated a
positive expression of vimentin (a fibroblast-specific protein),
which meant that the CAFs and NFs were successfully isolated and
cultured. In addition, CAFs exhibited positive expression of FAP
and α-SMA through a marked green fluorescent signal, and the
expression of FAP and α-SMA in NFs was identified to be weakly
positive by a weak green fluorescent signal, and the differences
between these expression levels were significant compared with the
NFs group (both P<0.05). These results suggest that the CAFs and
NFs were successfully isolated and cultured, and that the
activation degree of CAFs was increased compared with that of
NFs.
CAFs induce the progress of EMT
RT-qPCR and western blotting were used to analyze
E-cadherin, N-cadherin and vimentin expression levels (Fig. 2). Compared with the NFs groups, the
expression levels of N-cadherin and vimentin in the CAFs group was
significantly upregulated (P<0.05), while the expression level
of E-cadherin was markedly downregulated (P<0.05).
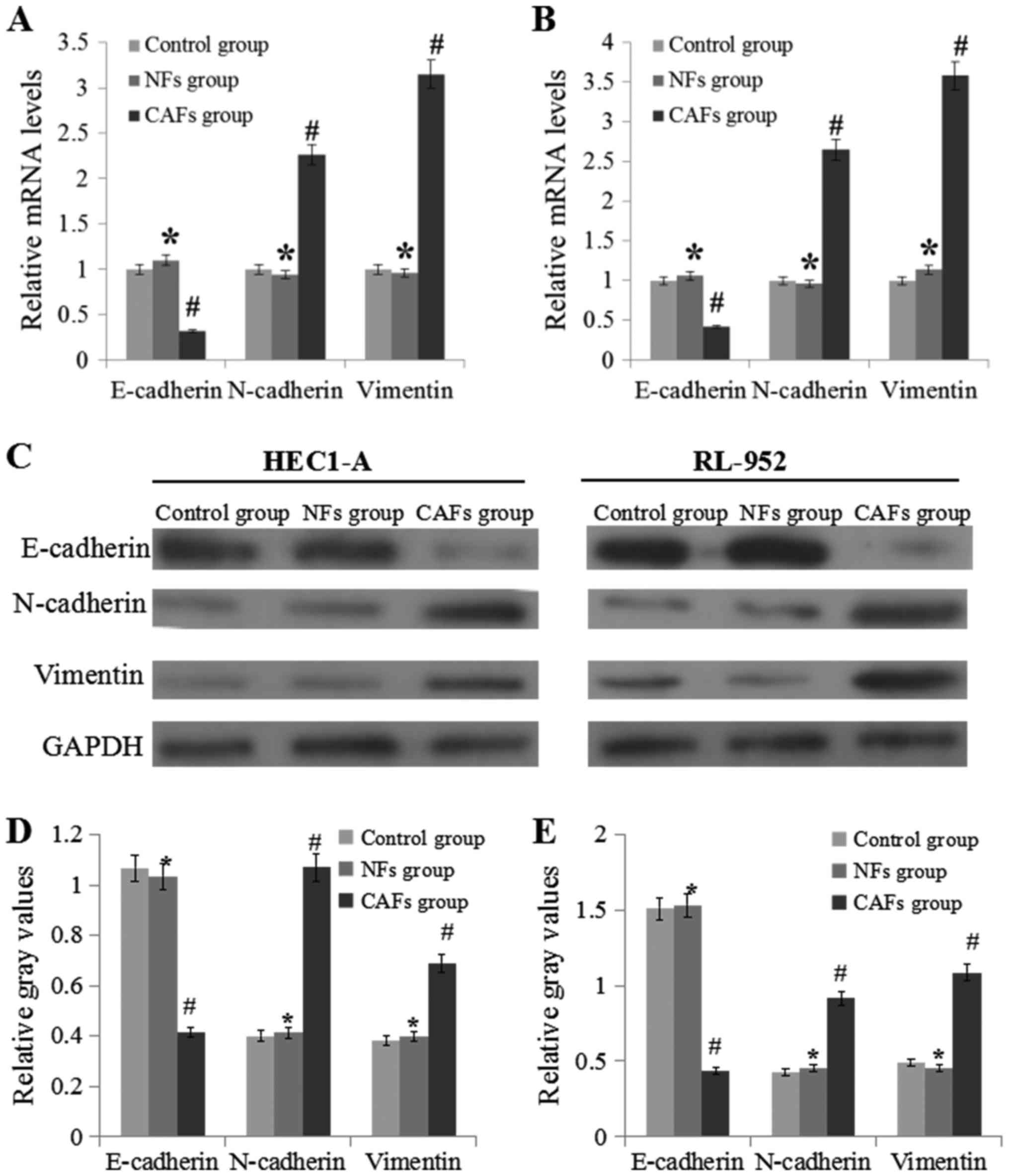 | Figure 2.CAF regulate the mRNA and protein
expression levels of E-cadherin, N-cadherin and vimentin. (A) mRNA
expression levels of E-cadherin, N-cadherin and vimentin in HEC1-A
cells. (B) mRNA expression levels of E-cadherin, N-cadherin and
vimentin in RL-952 cells. (C) Experimental schematic image of
western blotting experiments performed in the HEC1-A and RL-952
cells. (D) Relative levels of E-cadherin, N-cadherin and vimentin
to GAPDH in HEC1-A cell samples. (E) Relative levels of E-cadherin,
N-cadherin and vimentin to GAPDH in RL-952 cell samples. Data are
presented as the mean ± the standard error of the mean of three
independent experiments. *P>0.05 vs. control group.
#P<0.05 vs. NFs group. E-cadherin, epithelial
cadherin; N-cadherin, neural cadherin; CM, conditional medium;
CAFs, cancer-associated fibroblasts; NFs, normal fibroblasts. |
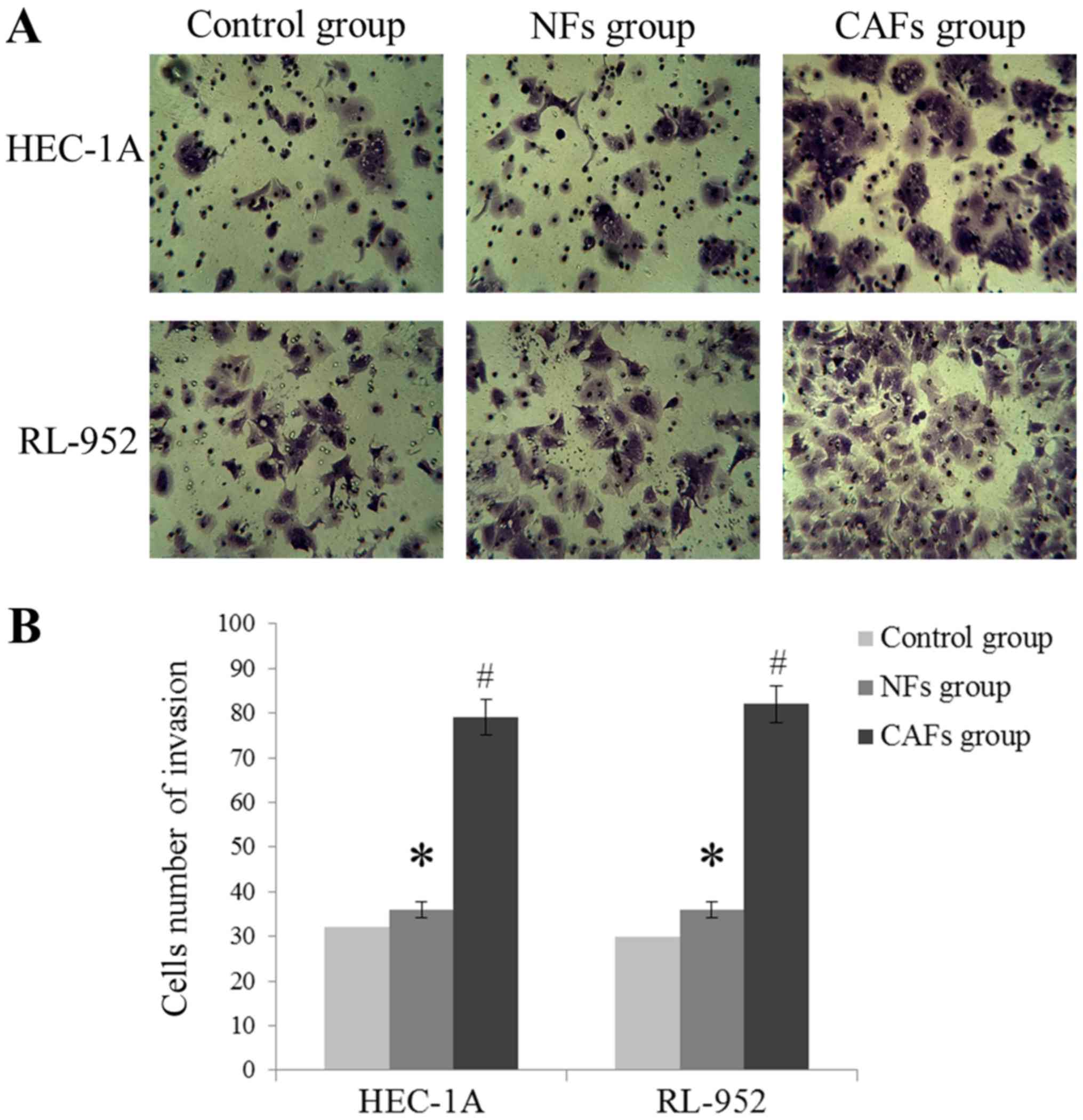
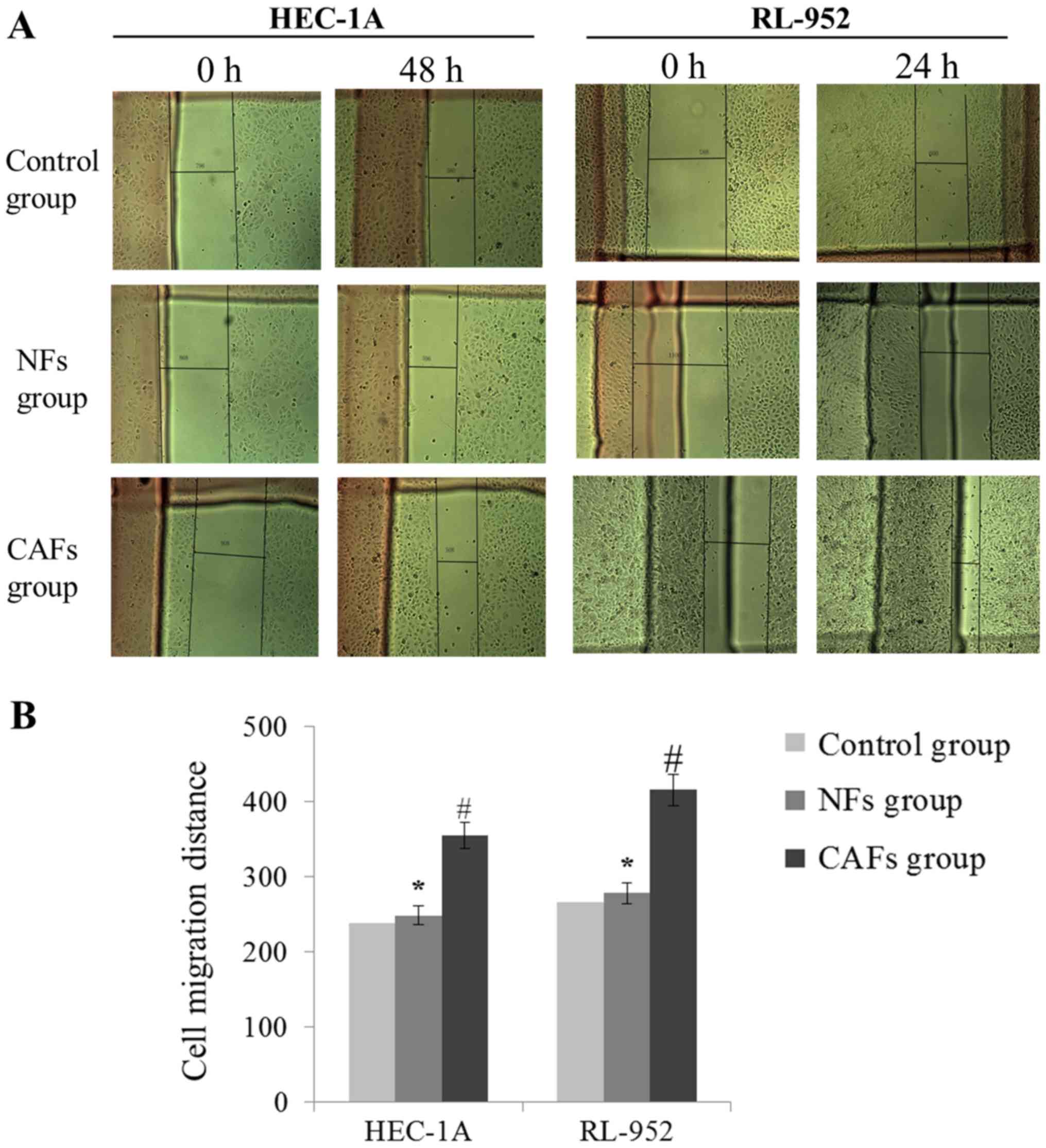
CAFs increase EC cell invasion and
migration
As demonstrated in Fig.
3, HEC-1A and RL-952 cell invasion levels were markedly
increased in the CAFs group, compared with the NFs group
(P<0.05), exhibited by the increase of the number of invading
cells. In addition, compared with the NFs group, the wound healing
assay indicated that the migration distance was significantly
increased in the CAFs group (P<0.05; Fig. 4). These results suggest that the CM of
CAFs may induce migration and invasion of EC cells.
CAFs induce the secretion of EGF,
TGF-β, HGF and FGF-2
In comparison between the CAFs and NFs, the
concentration of EGF, TGF-β, HGF and FGF-2 were increased in CM of
CAFs, and the difference was significant (P<0.05). The results
are summarized in Table II.
 | Table II.Various cytokine levels in the
conditional medium by ELISA. |
Table II.
Various cytokine levels in the
conditional medium by ELISA.
| Cytokines,
pg/ml | Control medium | NFs | CAFs |
|---|
| EGF | 0.26±0.12 | 1.23±0.85 |
43.52±4.26a |
| TGF-β | 0.34±0.11 | 2.12±1.02 |
212.58±10.24a |
| HGF | 0.12±0.09 | 1.45±0.75 |
52.37±3.26a |
| FGF-2 | 0.97±0.55 | 3.12±1.46 |
95.64±8.97a |
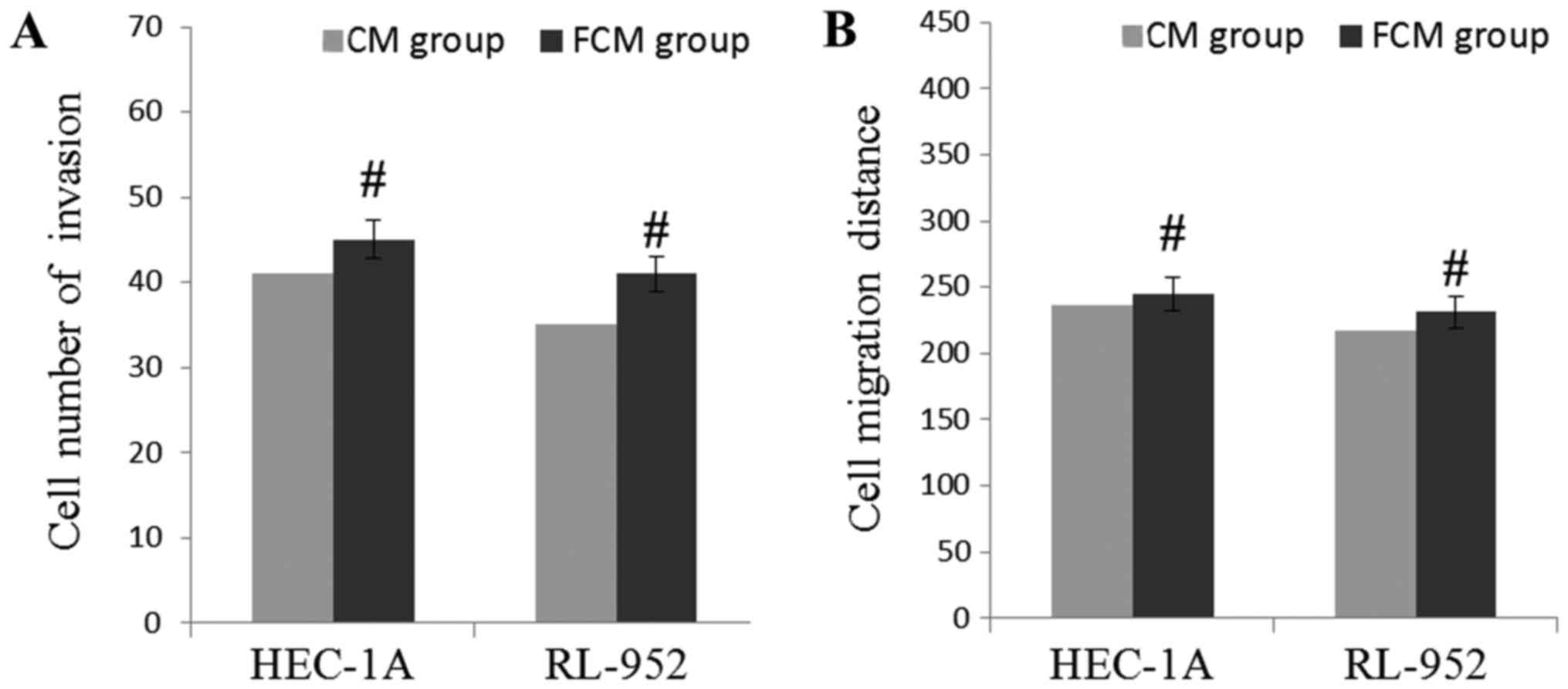
Exogenous growth factors induce
invasion and migration of EC cells
In order to confirm whether these growth factors
affected the levels of invasion and migration, EGF, TGF-β, HGF and
FGF-2 were artificially added into CM of NFs, and the levels of
cell invasion and migration were observed. The results are
demonstrated in Fig. 5. Compared with
the CM of CAFs, the number of invading HEC-1A and RL-952 cells were
markedly increased in the FCM of the NFs group, but the difference
was not significant (P>0.05). In addition, compared with the CM
of CAFs, the wound healing assay indicated that the migration
distance was significantly increased in the FCM of NFs group, but
the difference between the CAFs and NFs group was not significant
(P>0.05). Compared with Figs. 3
and 4, these results suggest that
these growth factors may induce the migratory and invasive
capabilities of EC cells.
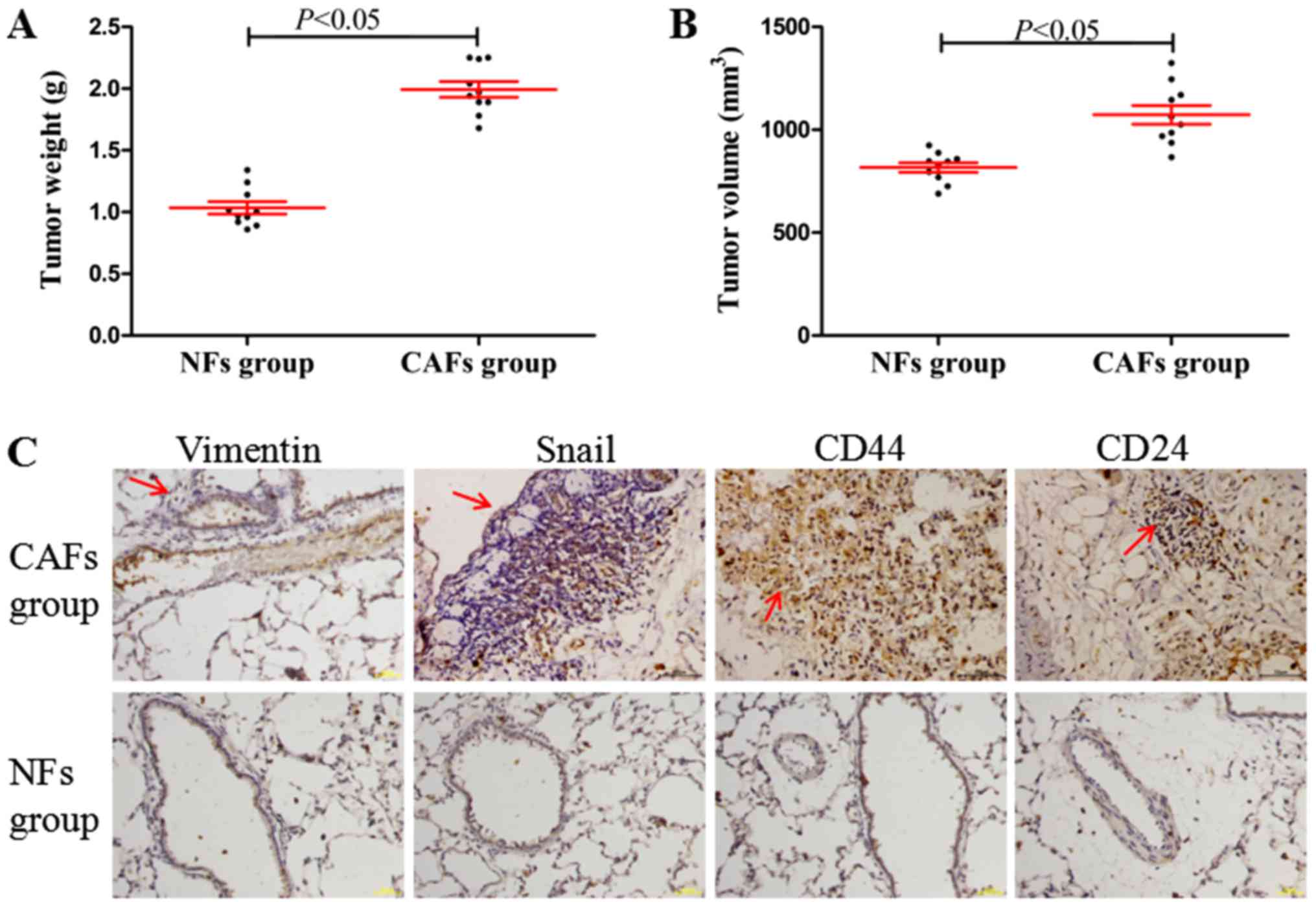
CAFs induce lung metastasis and the
EMT process in vivo
In the in vivo xenograft experiments, it was
identified that the tumor weights and volumes (maximum tumor volume
obtained: ~9.2×7.9 mm) in CAFs group were significantly increased
compared with those in the NFs group (Fig. 6A and B; P<0.05). Furthermore,
compared with the NFs group (Fig. 6C;
no marked lung metastasis, observed under a microscope, and
positive expression determined using immunohistochemistry), the
CAFs group demonstrated significant lung metastasis, while the
EMT-associated proteins (vimentin and Snail) and the cancer stem
cell molecular markers CD44 and CD24 demonstrated marked positive
staining in areas of lung metastasis (Fig. 6C). These results indicated that CAFs
may induce tumorigenesis, tumor metastasis and the EMT process
in vivo.
Discussion
It has been demonstrated that the progress of EMT
was associated with a downregulation of the apical and basolateral
epithelial cells specific tight and adherens junction proteins such
as E-cadherin, and increased expression of mesenchymal molecules
such as vimentin and N-cadherin (8,9).
E-cadherin and N-cadherin, functioning as adhesion molecules, and
vimentin, are considered the markers of EMT (11). In pancreatic cancer and human squamous
carcinoma cells, overexpression of N-cadherin is involved in EMT,
and is associated with a reduction in E-cadherin level (12,13).
Various studied have suggested that CAFs may reduce EMT (14). Downregulation of E-cadherin expression
and upregulation of N-cadherin reduces the adhesion ability of
cells and simultaneously enhances cell mobility, so that the cells
may migrate and invade, which is a typical feature of EMT (15). Kim et al (16) indicated that CAFs affected the
motility of cancer cells through inducing EMT. In the present
study, CM induced N-cadherin and vimentin expression, and inhibited
the expression of E-cadherin, indicating that CM of CAFs may induce
the EMT progress.
Although CAFs are key determinants in the malignant
progression of cancer, their functional contributions to this
process remain unclear (17).
Previous data demonstrated that CAFs may secrete a variety of
growth factors through autocrine and paracrine pathways (18). Under certain conditions, fibroblasts
changed the tumor progression and fibroblasts contribute to tumor
development through secreting certain cytokine factors (19–21). In a
mouse model, Tyan et al (22)
identified that the HGF level in CAFs was positively correlated
with their ability to enhance breast tumorigenesis. TGF-β is a
multifunctional cytokine that serves important roles in tumor
formation, progression and metastasis (23). In the CM of CAFs in the present study,
the concentration of EGF, TGF-β, HGF and FGF-2 were increased
compared with the CM of NFs, which indicated that CAFs may secrete
these various cytokines.
Based on the effect of cytokines on the tumor
progression, artificial adjustment the concentration of cytokines
may affect the progression of the tumor (24). In breast cancer cells, the reduction
of HGF concentration using a neutralizing antibody reduced
CAF-mediated colony formation (22).
Using comparisons of the gene expression profiles between 6 pairs
of tumor fibroblasts (TFs) and NFs from esophageal squamous cell
carcinoma (ESCC) using Affymetrix expression microarray, Zhang
et al (25) indicated that the
CM from TFs (of which the most significant result was the
upregulation of FGF receptor 2) was identified to be able to
promote ESCC tumor cell growth, migration and invasion in
vitro. In gastrointestinal cancer, increased TGF-β regulated
the tumor microenvironment and metastasis (26). In the present study, it was first
identified that the levels of EGF, TGF-β, HGF and FGF-2 in the CAFs
CM were increased compared with that in the NFs CM. In addition,
the CM of CAFs exhibited an increased ability to promote migration
and invasion compared with the conditioned medium of NFs. Notably,
in comparison with the regular CM of NFs, the addition of exogenous
growth factors to the CM of NFs increased EC cells migratory and
invasive capabilities. From these results, it was inferred that
EGF, TGF-β, HGF and FGF-2 were important for inducing migration and
invasion in EC cells. However, the migratory and invasive
capabilities in the FCM and CM groups were not completely similar.
This suggests that there are a number of additional cytokines, not
assessed within the present study, which may affect EC cell
migration and invasion, for example interleukin (IL)-6 receptor and
glycoprotein 130 (27). Treatment
with Janus kinase- and Signal transducer and activator of
transcription 3-specific inhibitors, AD412 and STATTIC,
respectively, significantly abrogated CAF-mediated cell
proliferation, indicating the role of IL-6 activation in EC cell
proliferation (27); also, aberrant
S100A4 expression may predict EC progression and serve an important
role in regulating EC cell invasion through EMT regulation.
Therefore, S100A4 is a promising therapeutic target (28). In the present study, only four growth
factors were studied; a more comprehensive analysis will be
included in our future studies.
Tumor growth depends on interactions between
multiple inter-dependent cell types, among these different cell
types, CAFs are becoming a topic of study as recipients and as
producers of pro-tumorigenic signals (29). There are a number of previous studies
concerning the role of CAFs and their mechanisms of action in
various tumors (27,28,30,31). Hwang
et al (32) examined
pancreatic cancer, and identified that cancer-associated stromal
fibroblasts exhibited an important role in supporting and promoting
growth and metastasis in pancreatic cancer. In human prostatic
cancer, Olumi et al (33)
suggested that human prostatic CAFs grown with initiated human
prostatic epithelial cells markedly stimulated growth and altered
the histology of the epithelial cell population. In the present
study, it was only identified that CAFs had a significant effect on
lung metastasis. Immunohistochemistry was utilized to detect the
markedly positive expressions of vimentin, Snail, CD44 and CD24 in
areas of lung metastasis; in the NFs group there was no marked
protein (vimentin, snail, CD44 and CD24) expression identified. The
number of metastasized tumors cannot be calculated using the naked
eye or immunohistochemistry assays; computed tomography (CT) and
magnetic resonance imaging (MRI) technology is required. As CT and
MRI were not used during the xenograft experiments; the number of
metastasized tumors was not calculated. However, by comparing the
results visually and with immunohistochemistry, it was concluded
that CAFs induced lung metastasis and the EMT process in
vivo. The present study again demonstrated the role of CAFs in
promoting tumorigenesis.
In conclusion, the present study focused on
identifying the role of CAFs on the EMT process from the
perspective of the cytokines secreted. The results provide a novel
perspective for the treatment of endometrial cancer through
cytokines. Previous articles (28–30) have
not examined CAFs by describing and analyzing their cytokine
expression profiles, which is the primary focus of the present
study. The data suggest that CAFs may induce EMT through secreted
cytokines in endometrial cancer cells. Therapeutic drugs could be
designed to perform as regulators of these cytokines, and may be
useful for EC therapeutic intervention.
Acknowledgements
The present study was supported by the National
Natural Science Fund of China (grant no. 81301808).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aune D, Navarro Rosenblatt DA, Chan DS,
Vingeliene S, Abar L, Vieira AR, Greenwood DC, Bandera EV and Norat
T: Anthropometric factors and endometrial cancer risk: A systematic
review and dose-response meta-analysis of prospective studies. Ann
Oncol. 26:1635–1648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marnitz S and Köhler C: Current therapy of
patients with endometrial carcinoma. A critical review.
Strahlenther Onkol. 188:12–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trimboli AJ, Cantemir-Stone CZ, Li F,
Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H,
Chong JL, et al: Pten in stromal fibroblasts suppresses mammary
epithelial tumors. Nature. 461:1084–1091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cirri P and Chiarugi P:
Cancer-associated-fibroblasts and tumour cells: A diabolic liaison
driving cancer progression. Cancer Metastasis Rev. 31:195–208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaw SY, Majeed AA, Dalley AJ, Chan A,
Stein S and Farah CS: Epithelial to mesenchymal transition (EMT)
biomarkers-E-cadherin, beta-catenin, APC and Vimentin-in oral
squamous cell carcinogenesis and transformation. Oral Oncol.
48:997–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada
M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, et al: N-cadherin
expression and epithelial-mesenchymal transition in pancreatic
carcinoma. Clin Cancer Res. 10:4125–4133. 2014. View Article : Google Scholar
|
|
13
|
Islam S, Carey TE, Wolf GT, Wheelock MJ
and Johnson KR: Expression of N-cadherin by human squamous
carcinoma cells induces a scattered fibroblastic phenotype with
disrupted cell-cell adhesion. J Cell Biol. 135:1643–1654. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giannoni E, Bianchini F, Masieri L, Serni
S, Torre E, Calorini L and Chiarugi P: Reciprocal activation of
prostate cancer cells and cancer associated fibroblasts stimulates
epithelial-mesenchymal transition and cancer stemness. Cancer Res.
70:6945–6956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SH, Choe C, Shin YS, Jeon MJ, Choi SJ,
Lee J, Bae GY, Cha HJ and Kim J: Human lung cancer-associated
fibroblasts enhance motility of non-small cell lung cancer cells in
co-culture. Anticancer Res. 33:2001–2009. 2013.PubMed/NCBI
|
|
17
|
Du Y, Long Q, Zhang L, Shi Y, Liu X, Li X,
Guan B, Tian Y, Wang X, Li L and He D: Curcumin inhibits
cancer-associated fibroblast-driven prostate cancer invasion
through MAOA/mTOR/HIF-1α signaling. Int J Oncol. 47:2064–2072.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Räsänen K and Vaheri A: Activation of
fibroblasts in cancer stroma. Exp Cell Res. 316:2713–2722. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tlsty TD and Coussens LM: Tumor stroma and
regulation of cancer development. Annu Rev Pathol. 1:119–150. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumoto K and Nakamura T: Hepatocyte
growth factor and the Met system as a mediator of tumor-stromal
interactions. Int J Cancer. 119:477–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Birchmeier C, Birchmeier W, Gherardi E and
Vande Woude GF: Met, metastasis, motility and more. Nat Rev Mol
Cell Biol. 4:915–925. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tyan SW, Kuo WH, Huang CK, Pan CC, Shew
JY, Chang KJ, Lee EY and Lee WH: Breast cancer cells induce
cancer-associated fibroblasts to secrete hepatocyte growth factor
to enhance breast Tumorigenesis. PLoS One. 6:e153132011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Padua D, Zhang XH, Wang Q, Nadal C, Gerald
WL, Gomis RR and Massagué J: TGFbeta primes breast tumors for lung
metastasis seeding through angiopoietin-like 4. Cell. 133:66–77.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang W, Hiscox S, Matsumoto K and
Nakamura T: Hepatocyte growth factor/scatter factor, its molecular,
cellular and clinical implications in cancer. Crit Rev Oncol
Hematol. 29:209–248. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Fu L, Fu J, Hu L, Yang H, Rong
TH, Li Y, Liu H, Fu SB, Zeng YX and Guan XY: Fibroblast growth
factor receptor 2-positive fibroblasts provide a suitable
microenvironment for tumor development and progression in
esophageal carcinoma. Clin Cancer Res. 15:4017–4027. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Achyut BR and Yang L: Transforming growth
factor-β in the gastrointestinal and hepatic tumor
microenvironment. Gastroenterology. 141:1167–1178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Subramaniam KS, Omar IS, Kwong SC, Mohamed
Z, Woo YL, Mat Adenan NA and Chung I: Cancer-associated fibroblasts
promote endometrial cancer growth via activation of
interleukin-6/STAT-3/c-Myc pathway. Am J Cancer Res. 6:200–213.
2016.PubMed/NCBI
|
|
28
|
Hua T, Liu S, Xin X, Cai L, Shi R, Chi S,
Feng D and Wang H: S100A4 promotes endometrial cancer progress
through epithelial-mesenchymal transition regulation. Oncol Rep.
35:3419–3426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ostman A and Augsten M: Cancer-associated
fibroblasts and tumor growth-bystanders turning into key players.
Curr Opin Genet Dev. 19:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Teng F, Tian WY, Wang YM, Zhang YF, Guo F,
Zhao J, Gao C and Xue FX: Cancer-associated fibroblasts promote the
progression of endometrial cancer via the SDF-1/CXCR4 axis. J
Hematol Oncol. 9:8–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Subramaniam KS, Tham ST, Mohamed Z, Woo
YL, Mat Adenan NA and Chung I: Cancer-associated fibroblasts
promote proliferation of endometrial cancer cells. PLoS One.
8:e689232013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwang RF, Moore T, Arumugam T,
Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB and Logsdon CD:
Cancer-associated stromal fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Olumi AF, Grossfeld GD, Hayward SW,
Carroll PR, Tlsty TD and Cunha GR: Carcinoma-associated fibroblasts
direct tumor progression of initiated human prostatic epithelium.
Cancer Res. 59:5002–5011. 1999.PubMed/NCBI
|