Introduction
Lung cancer, the most common type of cancer, has the
highest morbidity rate among all types of malignancies worldwide
(1,2),
accounting for 30% of all incidences of cancer-associated mortality
(3). In clinical practice, the two
major types of lung cancer are small cell lung cancer (SCLC) and
non-SCLC (NSCLC) (4), of which NSCLC
accounts for ~80% of all lung cancer cases (5). Current therapies for NSCLC include
chemotherapy, radiotherapy, targeted therapy and surgery, which are
used alone or in combination (6).
Despite the use of these therapies, the overall 5-year survival
rate of NSCLC remains low, at 15% (7). Owing to the development of drug
resistance and the severe side effects experienced by patients,
effective and safe novel agents are required for the treatment of
NSCLC (1).
Angiotensin II (Ang II), a key biological peptide in
the renin-angiotensin system, is a vasoconstrictor that controls
cardiovascular function and renal homeostasis (8). Ang II receptor blockers (ARBs) are
extensively used as anti-hypertensive drugs (9). Studies have reported that angiogenesis
is essential for tumor progression and metastasis (10,11).
Furthermore, studies have demonstrated that ARBs have the potential
possibility to restrain the growth of several types of cancer cells
(12,13). Ang II binds two receptor subtypes, the
Ang II type 1 receptor (AT1R) and the Ang II type 2 receptor
(AT2R), to mediate a series of biological effects (14). AT1R mediates the main functions of Ang
II, including tumor growth and angiogenesis (15). Treatment with telmisartan, a specific
AT1R blocker, leads to apoptosis and the inhibition of cell cycle
progression in cancer cells (16,17). It
has been reported that telmisartan has anti-proliferative activity
in prostate and renal cell cancer (18,19);
however, there are few relevant reports on the anti-proliferative
activity of telmisartan in NSCLC cells (20).
In the present study, the Cell Counting Kit-8
(CCK-8), Transwell assay and western blot analysis was used to
determine whether telmisartan treatment could inhibit the
proliferation and invasion of A549 NSCLC cells, which may be
regulated via phosphoinositide 3-kinase (PI3K)/RAC
serine/threonine-protein kinase (AKT) inhibition-induced
apoptosis.
Materials and methods
Cell lines and cell culture
The NSCLC A549 cell line was obtained from the
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured in Dulbecco's Modified Eagle's Medium (DMEM; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml penicillin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 0.1 mg/ml streptomycin (Sigma-Aldrich;
Merck KGaA). All cell lines were incubated at 37°C in a humidified
atmosphere containing 5% CO2. When cells entered into
the logarithmic growth phase, they were washed three times with PBS
and digested with 0.25% trypsin (Beijing Solarbio Science &
Technology, Co., Ltd., Beijing, China). When the cells detached,
DMEM was re-added to terminate digestion and resuspended the cells
into single cell suspension, then the cells (100 µl,
1×105) were seeded into a 6-well plate for subsequent
experiments.
Cell Counting Kit-8 (CCK-8)
proliferation assay
A 100 µl suspension of A549 cells was seeded into
96-well plate with the standard 1,000 cells/well. In this assay, 5
groups were used, which were control (DMEM only), vehicle [0.1%
dimethyl sulfoxide (DMSO); Ameresco, Inc., Framingham, MA, USA)]
and 0.2, 2 and 20 µM telmisartan (MedChem Express, Princeton, NJ,
USA). Following this, cells were cultured in a CO2
incubator and the cell viability was detected at 24, 48, 72 h. A
total of 10 µl CCK-8 (Beijing Solarbio Science & Technology,
Co., Ltd.) reagent was added into each well and the plates were
incubated at 37°C for 1.5 h. The optical density value was measured
at a wavelength of 450 nm using a microplate reader and a
proliferation curve was plotted. The assay was performed in
triplicate. In the following experiments, the negative control (NC)
group was treated with 0.1% DMSO, and the treated group with 20 µM
telmisartan.
Cell invasion and migration assessed
via transwell assay
A total of 100 µl Matrigel (diluted 1:6 in
serum-free DMEM; BD Biosciences, Franklin Lakes, NJ, USA), was
melted overnight and then added to the upper chamber of a 24-well
transwell plate (EMD Millipore, Billerica, MA, USA). Following
shaking, the Matrigel was placed into 37°C CO2 incubator
cultivating for 4–6 h until the gel set, and then the culture
medium was dried. The cells, which had been treated with 20 µM
telmisartan for 24 h, were prepared using serum-free DMEM in a cell
suspension. A total of 100 µl (1×105) cell suspension
were added to the upper chamber of the transwell. Following this,
500 µl complete DMEM (containing 10% FBS) was mixed into the bottom
chamber of the transwell. Cells were incubated for 24 h, then the
transwell was taken out and the remaining cells in the upper
chamber were removed with a cotton swab. After washing with PBS,
the cells were fixed in 4% paraformaldehyde for 30 min at room
temperature, and stained with 0.1% crystal violet for 20 min at
room temperature. Following washing with PBS, five clear fields of
vision were selected random using a light microscope
(magnification, ×200), and images were captured to observe the cell
count.
The migration experiment procedure was similar to
the invasion assay; however, the Matrigel was not applied and the
5,000 cells were seeded in the upper chambers.
Cell apoptosis assay
Apoptotic A549 cells were evaluated using an Annexin
V-fluorescein isothiocyanate (Annexin V-FITC)/propidium iodide (PI)
Apoptosis Detection kit (4A Biotech Co. Ltd., Beijing, China)
according to the manufacturer's protocol. The NC group was treated
with 0.1% DMSO. Following the treatment of A549 cells with 20 µM
telmisartan and digested with 0.25% trypsin (without EDTA),
collected in a centrifuge tube, centrifuged at 800 × g at 4°C for 5
min, resuspended in 4°C pre-cooled PBS and finally centrifuged once
again at 800 × g at 4°C for 5 min. The supernatant was aspirated
and the cells were resuspended in 1× Binding Buffer (Biomiga Inc.,
San Diego, CA, USA). Cell density was regulated to
~1×106/ml. A total of 100 µl cell suspension was added
into a 5-ml flow tube and stained with 5 µl Annexin V-FITC
(Apoptosis Detection kit) for 5 min at room temperature in the
dark. Next, cells were stained with 10 µl propidium iodide (PI) and
incubated at room temperature in the dark for 5 min, and 400 µl PBS
was added to detect cells. The cells were then assessed using a
flow cytometer and results were analyzed using FlowJo software
(version 7.6.3; FlowJo, LLC, Ashland, OR, USA). The assay was
performed in triplicate.
Western blot analysis
When the cell confluence was ~80%, the cells were
treated with 20 µM telmisartan or 0.1% DMSO. After 24 h, the 6-well
plate was placed on ice. Next, Radioimmunoprecipitation Assay lysis
buffer (including protease inhibitor; Beijing ComWin Biotech Co.,
Ltd., Beijing, China) was added to extract protein. The protein
concentration was measured using the BCA Protein Assay kit (Beijing
ComWin Biotech Co., Ltd.). Next, the protein was heated at 95°C for
5 min. A total of ~20 µg protein per lane was separated using 10%
SDS-PAGE and transferred onto a polyvinylidene fluoride membrane.
The membrane was blocked using 5% skimmed milk for 1 h at room
temperature. Next, the membrane was incubated with the primary
antibodies listed below at 4°C overnight. The membrane was then
washed three times for 5 min with 0.1% Tween 20-TBS, then incubated
with the appropriate secondary antibodies, listed below, for 1 h at
room temperature. Following washing of the membrane (as
aforementioned), bands were visualized using an enhanced
chemiluminescence reagent (PerkinElmer, Inc., Waltham, MA, USA).
Quantity One software (version 4.62; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to perform densitometry analysis.
The following antibodies were used. Primary
antibodies: AKT (dilution, 1:1,000; cat. no. 4691; Cell Signaling
Technology, Inc., Danvers, MA, USA), phosphorylated (p)-AKT
(dilution, 1:1,000; cat. no. 4060; Cell Signaling Technology,
Inc.), mechanistic target of rapamycin (mTOR) (dilution, 1:1,000;
cat. no. 2983; Cell Signaling Technology, Inc.), p-mTOR (dilution,
1:1,000; cat. no. 5536; Cell Signaling Technology, Inc.), Bcl-2
(dilution, 1:1,000; cat. no. 12789-1-AP; ProteinTech Group, Inc.,
Chicago, IL, USA), Bax (dilution 1:1,000; cat. no. 23931-1-AP;
ProteinTech Group), caspase-3 (dilution, 1:1,000; cat. no.
25546-1-AP; ProteinTech Group), cyclin D1 (dilution, 1:1,000; cat.
no. 60186-1-Ig; ProteinTech Group), p70 S6 kinase (p70S6K)
(dilution, 1:1,000; cat. no. 14485-1-AP; ProteinTech Group),
Tubulin (dilution, 1:5,000; cat. no. 10759-1-AP; ProteinTech
Group); Secondary antibodies: horseradish peroxidase-labeled goat
anti-rabbit (dilution 1:5,000; cat. no. SA00001-2; ProteinTech
Group). The relative expression of each protein was normalized to
tubulin levels in each sample.
Statistical analysis
Statistical analysis of experimental data was
performed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as mean ± standard deviation. Student's t-test was used
to analyze the difference between the telmisartan-treatment and NC
groups. Analysis of variance with post hoc Fisher's least
significant difference test was used for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Telmisartan inhibits A549 cell
proliferation
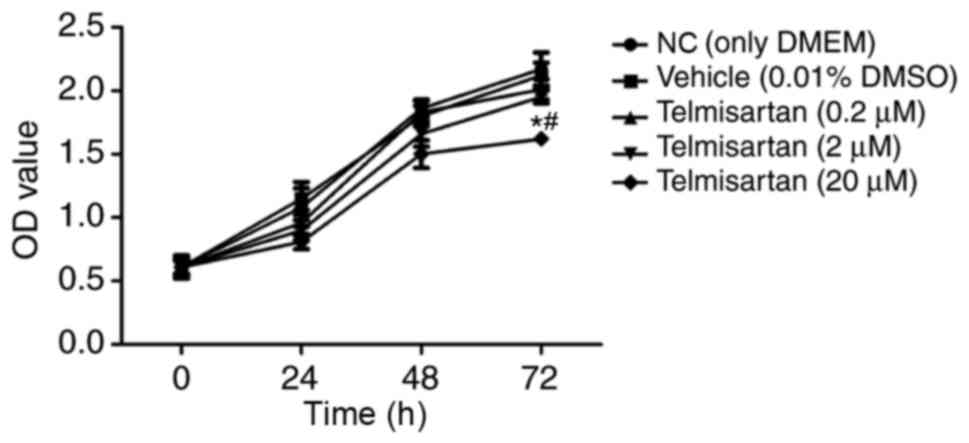
To evaluate the effect of telmisartan on NSCLC, the
NSCLC A549 cell line was treated with 0.2, 2 and 20 µM telmisartan
to detect cell proliferation rates. In this experiment, the cells
were measured at 24, 48, 72 h. The results of the CCK-8
proliferation assay indicated that only the 20 µM telmisartan
treatment group exhibited a significant reduction in the
proliferation of A549 cells following 72 h (P<0.05; Fig. 1). These results demonstrated that
telmisartan could effectively inhibit A549 proliferation in a dose-
and time-dependent manner; therefore, 20 µM telmisartan was used in
the following experiments.
Telmisartan inhibits A549 cell
invasion and migration
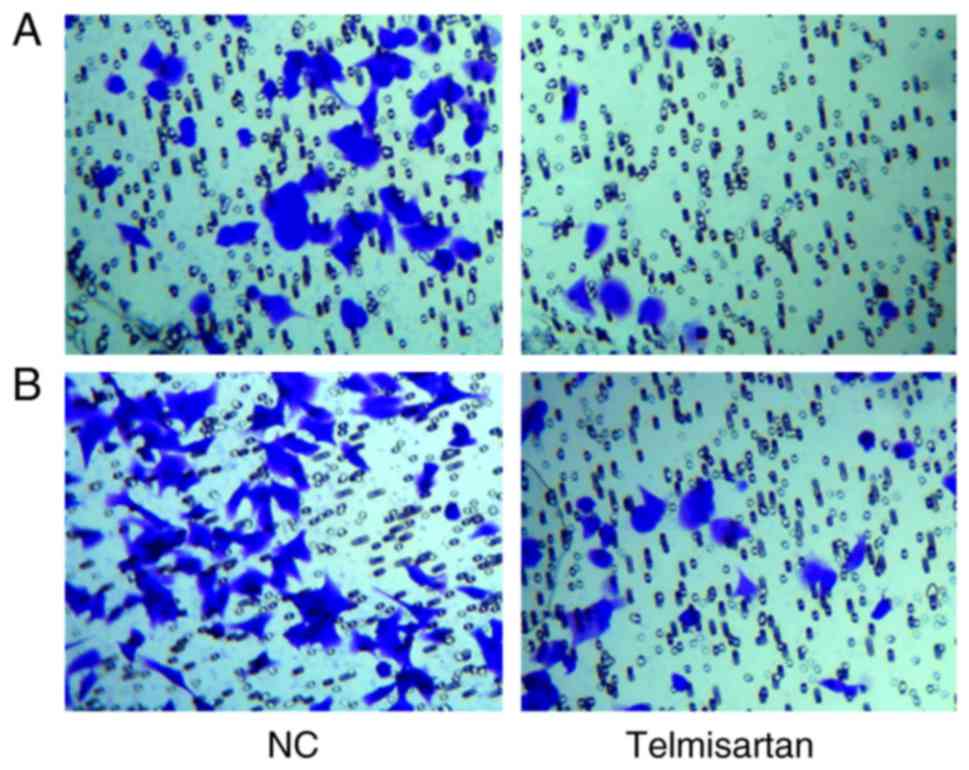
A transwell assay was used to analyze the effects of
telmisartan on the invasive and migratory ability of A549. After
crystal violet staining the A549 cells were reduced via the
telmisartan treatment in the invasion experiment. Additionally, the
cells were also reduced in the migration experiment. The number of
invaded cells in the telmisartan-expressing group was less than
control group (8±2 vs. 46±4 cells invaded, respectively) and
indicated a significant difference (P<0.05; Fig. 2A). The migratory capacity of cells was
also inhibited in the telmisartan-expressing group compared with
the control group (29±3 vs. 74±5 cells migrated, respectively),
indicating the negative effect of telmisartan on the migration
ability of A549 cells (P<0.05; Fig.
2B), compared with the control group. These results indicated
that telmisartan could significantly inhibit the invasion and
migration of A549.
Telmisartan promotes A549 cell
apoptosis
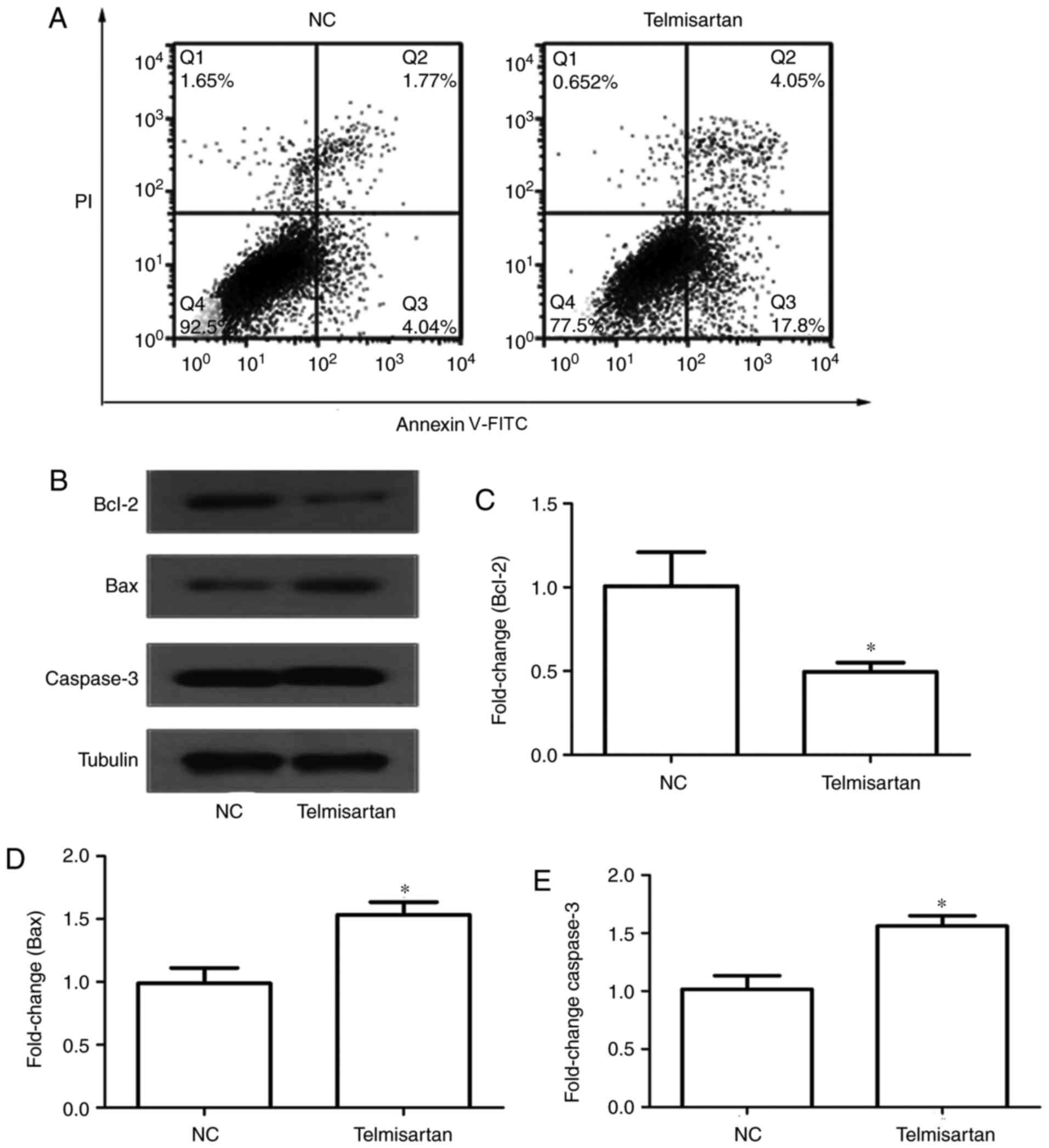
An annexin V-FITC/PI double-staining assay was used
to determine the effect of telmisartan on A549 cell apoptosis.
Following the induction of serum-free apoptosis, the apoptosis rate
in the telmisartan-treated group was significantly higher than that
in the control group (21.85 vs. 5.81%, respectively; P<0.05). In
addition, it was determined that telmisartan had a greater effect
on early apoptosis (17.8%) compared with late apoptosis (4.05%)
(Fig. 3A). Furthermore, western blot
analysis was used to analyze the protein expression of apoptosis
regulators, including the anti-apoptotic protein Bcl-2, and the
pro-apoptotic proteins caspase-3 and Bax (Fig. 3B). The expression of the pro-apoptotic
proteins caspase-3 and Bax increased in the telmisartan-treated
group compared with the control group, whereas the expression of
the anti-apoptotic protein Bcl-2 decreased (P<0.05; Fig. 3C-E). These results indicated that
telmisartan could promote A549 cell apoptosis.
Telmisartan suppresses the PI3K
pathway in A549 cells
In tumors the PI3K/AKT pathway is extremely
important. Following telmisartan treatment, mTOR, p70S6K and cyclin
D1 proteins were selected as indicators for the evaluation of the
activity of the PI3K/AKT signaling pathway. The results of western
blot analysis indicated that the phosphorylation level of mTOR
decreased significantly in telmisartan-treated A549 cells
(P<0.05). Similarly, the expression levels of p70S6K and cyclin
D1 were decreased following telmisartan treatment (P<0.05;
Fig. 4). These results indicated that
telmisartan-induced inhibition of A549 cell growth might be
regulated via the PI3/AKT pathway.
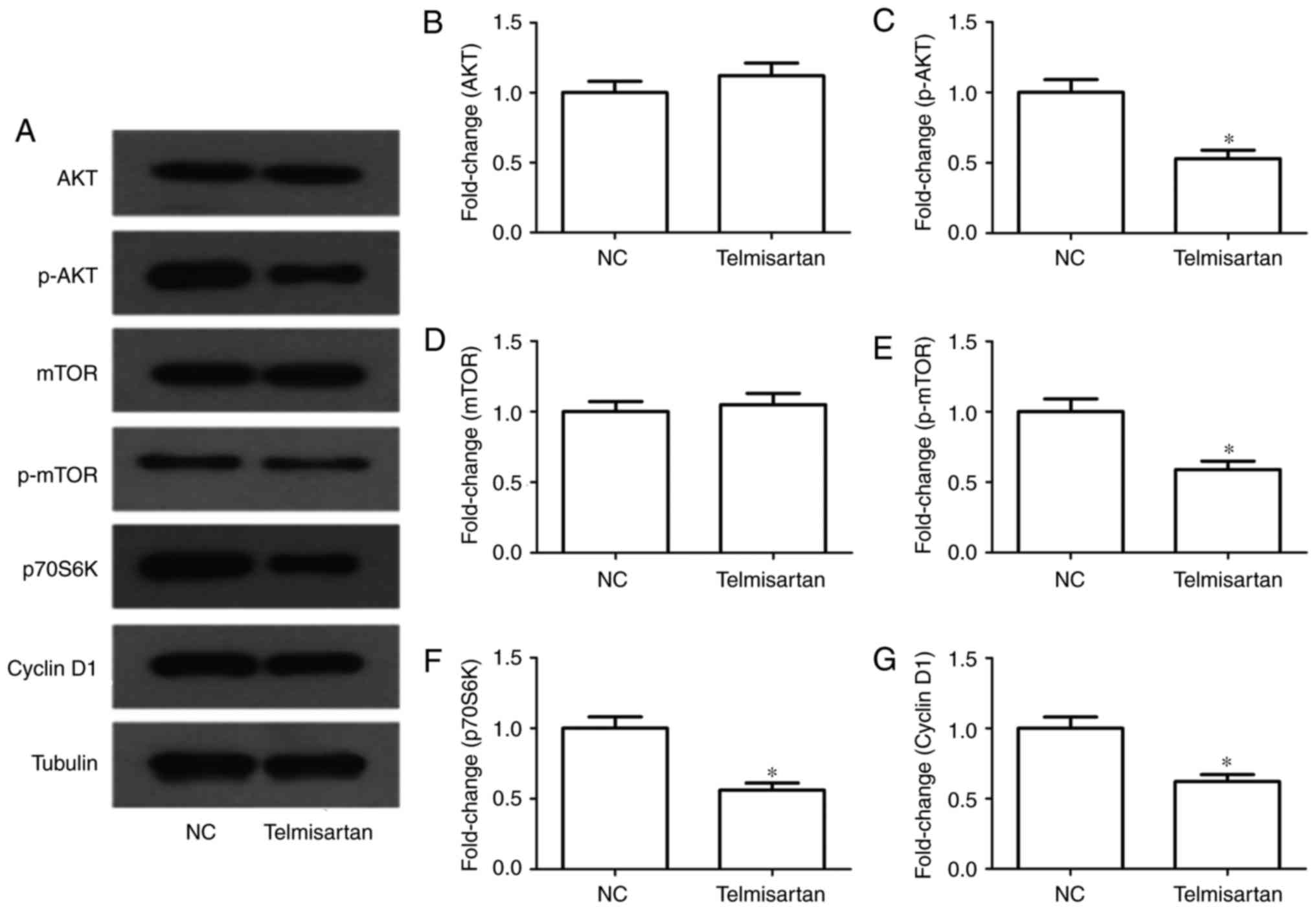 | Figure 4.Effects of telmisartan on the PI3K
signaling pathway in A549 cells. (A) Expression levels of AKT,
p-AKT, mTOR, p-mTOR, p70S6K and cyclin D1 were measured in A549
cells treated with 20 µM telmisartan by western blot analysis.
(B-G) The relative protein levels of AKT, p-AKT, mTOR, p-mTOR,
p70S6K and cyclin D1 compared with the control group. *P<0.05,
compared with NC group. PI3K, phosphoinositide 3-kinase; p-AKT,
phosphorylated RAC serine/threonine-protein kinase; mTOR,
mechanistic target of rapamycin; p70S6K, p70-S6 kinase; NC,
negative control. |
Discussion
In the present study, a preliminary investigation
into the effects and potential underlying molecular mechanisms of
telmisartan was conducted. Telmisartan inhibited the proliferation,
invasion and migration of A549 cells. Furthermore, it was
demonstrated that cell apoptosis was significantly promoted via
telmisartan. These effects mediated by telmisartan on A549 cells
may be associated with the inhibition of the PI3K/AKT signaling
pathway.
Ang II, as a vasoconstrictor, is a multifunctional
and bioactive octapeptide of the renin-angiotensin system, which
controls cardiovascular function and kidney homeostasis (21). The prior published data have
demonstrated an association between Ang II and cancer development
(22,23). Penafuerte et al (24) revealed that Ang II is a major upstream
regulator of cancer cachexia. Recently, studies have reported that
Ang II stimulates angiogenesis and tumor growth, particularly in
breast and pancreatic cancer (25,26). The
local production of Ang II in gastric cancer has been indicated to
promote lymph node metastasis and cancer progression (27). By contrast, ARBs have been
demonstrated to be effective in reducing tumor growth, angiogenesis
and metastasis in mouse models in vivo (28,29). Ang
II binds two receptor subtypes to mediate its biological effects,
AT1R and AT2R (30). Studies have
demonstrated that the main functions of Ang II in tumor growth are
mediated by the AT1R (31). ARBs
block the activation of AT1R, thus inhibiting the renin-angiotensin
system (32).
In the present study, NSCLC A549 cells were treated
with telmisartan. The results of the CCK-8 proliferation assay
indicated that telmisartan could inhibit A549 proliferation in a
dose- and time-dependent manner. In transwell assays, telmisartan
significantly inhibited the invasion and migration of A549.
Telmisartan is a specific AT1R blocker, which has the strongest
binding affinity for AT1R in ARBs (16,33). It
has previously been reported that Ang II can potentiate breast
cancer cell migration (25). Godugu
et al (7) revealed that
telmisartan could enhance the anticancer effect on A549 cells;
however, whether telmisartan can inhibit proliferation, promote
migration, invasion and apoptosis in lung cancer cells has not, to
the best of our knowledge, been studied. The results of the present
study demonstrated that telmisartan could inhibit A549 cell
proliferation and migration.
The results of the apoptosis assay indicated that
telmisartan had a greater effect in early apoptosis, compared with
late apoptosis, indicating that the Bcl-2 intrinsic mitochondrial
apoptosis pathway was activated. The results of western blot
analysis demonstrated that the expression of the pro-apoptotic
proteins caspase-3 and Bax increased in the telmisartan-treated
groups whereas that of the anti-apoptotic protein Bcl-2 decreased.
Bcl-2 is a negative regulator of apoptosis, whereas Bax and
caspase-3 are positive regulators of apoptosis (34). Apoptosis is an important antitumor
target, and a number of antitumor drugs function by inducing
apoptosis (35). The activation of
apoptosis is regulated via multiple signaling pathways, of which
PI3K/AKT is one of the more important (36). The results of western blot analysis
indicated that levels of p-AKT, p-mTOR, p70S6k and cyclin D1 were
significantly decreased in the telmisartan-treated group, which
indicated that telmisartan may exert an antitumor effect through
the regulation of apoptosis via the PI3K/AKT pathway. The PI3K/AKT
pathway can mediate cell survival signals via the Bcl-2 family
(37). AKT has direct effects on the
effectors of the apoptotic pathway, including Bcl-2 (38). Bcl-2 activity is regulated via p-AKT
in various cell types (39). AKT
phosphorylates Bax, disrupting its interaction with Bcl-XL to
protect cells from apoptosis (40).
In addition, AKT can phosphorylate Bcl-2, making it resistant to
protein degradation and ultimately protecting the cell from
apoptosis (41). The effect of mTOR
on Bcl-2 is similar to that of AKT (42). Additionally, p70S6K and cyclin D1 may
also be involved in pathways that telmisartan effects in cancer
cells. Cyclin D1 and p70S6K are located downstream of the PI3K/AKT
pathway, which is closely associated with cell apoptosis (43,44).
Cyclin D1 is a member of the cyclin D family of proteins that serve
a pivotal role in facilitating the cell cycle (45). The expression of cyclin D1 is
increased in different types of tumor tissues (46). The present results were consistent
with these studies (41–46), which indicated that telmisartan
inhibited signaling via the PI3K/AKT pathway in A549 cells, and
reduced the phosphorylation of mTOR, p70S6K and cyclin D1. In
summary, the downregulation of the PI3K/AKT pathway may have
contributed to the inhibitory effect of telmisartan on A549
cells.
In conclusion, the present study demonstrated that
telmisartan could inhibit the proliferation, invasion and migration
of lung cancer cells and promote their apoptosis via the PI3K/AKT
signaling pathway. The present study provides a prospect for
further clinical research of telmisartan in lung cancer
treatment.
Glossary
Abbreviations
Abbreviations:
|
Ang II
|
angiotensin II
|
|
NSCLC
|
non-small cell lung cancer
|
|
AT1R
|
angiotensin II type 1 receptor
|
|
AT2R
|
angiotensin II type 2 receptor
|
|
ARBs
|
angiotensin II receptor blockers
|
References
|
1
|
Pan ST, Zhou ZW, He ZX, Zhang X, Yang T,
Yang YX, Wang D, Qiu JX and Zhou SF: Proteomic response to
5,6-dimethylxanthenone 4-acetic acid (DMXAA, vadimezan) in human
non-small cell lung cancer A549 cells determined by the
stable-isotope labeling by amino acids in cell culture (SILAC)
approach. Drug Des Devel Ther. 9:937–968. 2015.PubMed/NCBI
|
|
2
|
Han ML, Zhao YF, Tan CH, Xiong YJ, Wang
WJ, Wu F, Fei Y, Wang L and Liang ZQ: Cathepsin L
upregulation-induced EMT phenotype is associated with the
acquisition of cisplatin or paclitaxel resistance in A549 cells.
Acta Pharmacol Sin. 37:1606–1622. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kallianos A, Tsimpoukis S, Zarogoulidis P,
Darwiche K, Charpidou A, Tsioulis I, Trakada G, Porpodis K,
Spyratos D, Panoutsopoulos A, et al: Measurement of exhaled
alveolar nitrogen oxide in patients with lung cancer: A friend from
the past still precious today. Onco Targets Ther. 6:609–613.
2013.PubMed/NCBI
|
|
4
|
Jiang M, Zhong T, Zhang W, Xiao Z, Hu G,
Zhou H and Kuang H: Reduced expression of miR-205-5p promotes
apoptosis and inhibits proliferation and invasion in lung cancer
A549 cells by upregulation of ZEB2 and downregulation of erbB3. Mol
Med Rep. 15:3231–3238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan Y, Zheng S, Li Q, Xiang X, Gao T, Ran
P, Sun L, Huang Q, Xie F, Du J and Xiao C: Overexpression of
miR-30a in lung adenocarcinoma A549 cell line inhibits migration
and invasion via targeting EYA2. Acta Biochim Biophys Sin
(Shanghai). 48:220–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keith RL and Miller YE: Lung cancer
chemoprevention: Current status and future prospects. Nat Rev Clin
Oncol. 10:334–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Godugu C, Patel AR, Doddapaneni R,
Marepally S, Jackson T and Singh M: Inhalation delivery of
Telmisartan enhances intratumoral distribution of nanoparticles in
lung cancer models. J Control Release. 172:86–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuyama M, Funao K, Kuratsukuri K,
Tanaka T, Kawahito Y, Sano H, Chargui J, Touraine JL, Yoshimura N
and Yoshimura R: Telmisartan inhibits human urological cancer cell
growth through early apoptosis. Exp Ther Med. 1:301–306. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li P, Koike T, Jiang HY, Wang ZH, Kawata Y
and Oshida Y: Acute treatment with candesartan cilexetil, an
angiotensin II type 1 receptor blocker, improves insulin
sensitivity in high-fructose-diet-fed rats. Horm Metab Res.
44:286–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marquardt G, Schulz TF and Schweighofer B:
Angiogenesis in cancer. Nature. 407:249–257. 2012.
|
|
11
|
Celus W, Di Conza G, Oliveira AI, Ehling
M, Costa BM, Wenes M and Mazzone M: Loss of caveolin-1 in
metastasis-associated macrophages drives lung metastatic growth
through increased angiogenesis. Cell Rep. 21:2842–2854. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imai N, Hashimoto T, Kihara M, Yoshida S,
Kawana I, Yazawa T, Kitamura H and Umemura S: Roles for host and
tumor angiotensin II type 1 receptor in tumor growth and
tumor-associated angiogenesis. Lab Invest. 87:189–198. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hinsley EE, de Oliveira CE, Hunt S,
Coletta RD and Lambert DW: Angiotensin 1–7 inhibits angiotensin
II-stimulated head and neck cancer progression. Eur J Oral Sci.
125:247–257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan SMH, Lau YS, Miller AA, Ku JM,
Potocnik S, Ye JM, Woodman OL and Herbert TP: Angiotensin II causes
β-cell dysfunction through an ER stress-induced proinflammatory
response. Endocrinology. 158:3162–3173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Araújo Júnior RF, Leitão Oliveira AL,
de Melo Silveira RF, de Oliveira Rocha HA, de França Cavalcanti P
and de Araújo AA: Telmisartan induces apoptosis and regulates Bcl-2
in human renal cancer cells. Exp Biol Med (Maywood). 240:34–44.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sukumaran S, Patel HJ and Patel BM:
Evaluation of role of telmisartan in combination with
5-fluorouracil in gastric cancer cachexia. Life Sci. 154:15–23.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee LD, Mafura B, Lauscher JC, Seeliger H,
Kreis ME and Gröne J: Antiproliferative and apoptotic effects of
telmisartan in human colon cancer cells. Oncol Lett. 8:2681–2686.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Funao K, Matsuyama M, Kawahito Y, Sano H,
Chargui J, Touraine JL, Nakatani T and Yoshimura R: Telmisartan is
a potent target for prevention and treatment in human prostate
cancer. Oncol Rep. 20:295–300. 2008.PubMed/NCBI
|
|
19
|
Funao K, Matsuyama M, Kawahito Y, Sano H,
Chargui J, Touraine JL, Nakatani T and Yoshimura R: Telmisartan as
a peroxisome proliferator-activated receptor-γ ligand is a new
target in the treatment of human renal cell carcinoma. Mol Med Rep.
2:193–198. 2009.PubMed/NCBI
|
|
20
|
Li J, Chen L, Yu P, Liu B, Zhu J and Yang
Y: Telmisartan exerts anti-tumor effects by activating peroxisome
proliferator-activated receptor-γ in human lung adenocarcinoma A549
cells. Molecules. 19:2862–2876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu TT, Niu HS, Chen LJ, Cheng JT and Tong
YC: Increase of human prostate cancer cell (DU145) apoptosis by
telmisartan through PPAR-delta pathway. Eur J Pharmacol. 775:35–42.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cambados N, Walther T, Nahmod K, Tocci JM,
Rubinstein N, Böhme I, Simian M, Sampayo R, Del Valle Suberbordes
M, Kordon EC and Schere-Levy C: Angiotensin-(1–7) counteracts the
transforming effects triggered by angiotensin II in breast cancer
cells. Oncotarget. 8:88475–88487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sobczuk P, Szczylik C, Porta C and
Czarnecka AM: Renin angiotensin system deregulation as renal cancer
risk factor. Oncol Lett. 14:5059–5068. 2017.PubMed/NCBI
|
|
24
|
Penafuerte CA, Gagnon B, Sirois J, Murphy
J, MacDonald N and Tremblay ML: Identification of
neutrophil-derived proteases and angiotensin II as biomarkers of
cancer cachexia. Br J Cancer. 114:680–687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodrigues-Ferreira S, Abdelkarim M,
Dillenburg-Pilla P, Luissint AC, di-Tommaso A, Deshayes F, Pontes
CL, Molina A, Cagnard N and Letourneur F: Angiotensin II
facilitates breast cancer cell migration and metastasis. PLos One.
7:e356672012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masamune A, Hamada S, Kikuta K, Takikawa
T, Miura S, Nakano E and Shimosegawa T: The angiotensin II type I
receptor blocker olmesartan inhibits the growth of pancreatic
cancer by targeting stellate cell activities in mice. Scand J
Gastroenterol. 48:602–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kinoshita J, Fushida S, Harada S, Yagi Y,
Fujita H, Kinami S, Ninomiya I, Fujimura T, Kayahara M, Yashiro M,
et al: Local angiotensin II-generation in human gastric cancer:
Correlation with tumor progression through the activation of
ERK1/2, NF-kappaB and survivin. Int J Oncol. 34:1573–1582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arafat HA, Gong Q, Chipitsyna G, Rizvi A,
Saa CT and Yeo CJ: Antihypertensives as novel antineoplastics:
Angiotensin-I-converting enzyme inhibitors and angiotensin II type
1 receptor blockers in pancreatic ductal adenocarcinoma. J Am Coll
Surg. 204:996–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
George AJ, Thomas WG and Hannan RD: The
renin-angiotensin system and cancer: Old dog, new tricks. Nat Rev
Cancer. 10:745–759. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liles C, Li H, Veitla V, Liles JT, Murphy
TA, Cunningham MW, Yu X and Kem DC: AT2R autoantibodies block
angiotensin II and AT1R autoantibody-induced vasoconstriction.
Hypertension. 66:830–835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kosugi M, Miyajima A, Kikuchi E, Kosaka T,
Horiguchi Y and Murai M: Effect of angiotensin II type 1 receptor
antagonist on tumor growth and angiogenesis in a xenograft model of
human bladder cancer. Hum Cell. 20:1–9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Liu J, Chen J, Li X, Wu Y, Chen
H, Wu W, Zhang K and Gu L: Angiotensin receptor blockers (ARBs)
reduce the risk of lung cancer: A systematic review and
meta-analysis. Int J Clin Exp Med. 8:12656–12660. 2015.PubMed/NCBI
|
|
33
|
Kakuta H, Sudoh K, Sasamata M and
Yamagishi S: Telmisartan has the strongest binding affinity to
angiotensin II type 1 receptor: Comparison with other angiotensin
II type 1 receptor blockers. Int J Clin Pharmacol Res. 25:41–46.
2005.PubMed/NCBI
|
|
34
|
Wei JC, Zhang T and Yang P: Effect of
gambogic acid on cell apoptosis and expressions of Bax, Bcl-2 and
Caspase-3 in colorectal cancer cells with. J Pract Med. 2016.
|
|
35
|
Mao YQ, Li XR and Lei S: Effect of several
anti-tumor drugs on apoptosis induction in jurkat cell line.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 14:681–685. 2006.(In Chinese).
PubMed/NCBI
|
|
36
|
González-Pérez PP and Cárdenas-García M:
Modeling and simulation of molecular mechanism of action of dietary
polyphenols on the inhibition of anti-apoptotic PI3K/AKT pathway.
Comput Mol Biosci. 3:39–52. 2013. View Article : Google Scholar
|
|
37
|
Guo H, Cui H, Peng X, Fang J, Zuo Z and
Deng J, Wang X, Wu B, Chen K and Deng J: Modulation of the PI3K/Akt
pathway and Bcl-2 family proteins involved in chicken's tubular
apoptosis induced by nickel chloride (NiCl2). Int J Mol
Sci. 16:22989–23011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Phatak NR, Stankowska DL and
Krishnamoorthy RR: Bcl-2, Bcl-xL and p-AKT are involved in
neuroprotective effects of transcription factor Brn3b in an ocular
hypertension rat model of glaucoma. Mol Vis. 22:1048–1061.
2016.PubMed/NCBI
|
|
39
|
Song T, Wang L, Mo Z, Mao L, Ma X, Niu R,
Gu K, Yan R, Ma P, Qi Y and Jiao Q: Expression of p-Akt in ovarian
serous carcinoma and its association with proliferation and
apoptosis. Oncol Lett. 7:59–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Premkumar DR, Jane EP, DiDomenico JD,
Vukmer NA, Agostino NR and Pollack IF: ABT-737 synergizes with
bortezomib to induce apoptosis, mediated by bid cleavage, bax
activation, and mitochondrial dysfunction in an Akt-dependent
context in malignant human glioma cell lines. J Pharmacol Exp Ther.
341:859–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bratton MR, Duong BN, Elliott S, Weldon
CB, Beckman BS, McLachlan JA and Burow ME: Regulation of
ERalpha-mediated transcription of Bcl-2 by PI3K-AKT crosstalk:
Implications for breast cancer cell survival. Int J Oncol.
37:541–550. 2010.PubMed/NCBI
|
|
42
|
Hamunyela RH, Serafin AM and Akudugu JM:
Strong synergism between small molecule inhibitors of HER2, PI3K,
mTOR and Bcl-2 in human breast cancer cells. Toxicol In Vitro.
38:117–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Halacli SO and Dogan AL: FOXP1 regulation
via the PI3K/AKT/p70S6K signaling pathway in breast cancer cells.
Oncol Lett. 9:1482–1488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu W, Ren H, Ren J, Yin T, Hu B, Xie S,
Dai Y, Wu W, Xiao Z, Yang X and Xie D: The role of
EGFR/PI3K/AKT/cyclinD1 signaling pathway in acquired middle ear
cholesteatoma. Mediators Inflamm. 2013:6512072013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao S, Yi M, Yuan Y, Zhuang W, Zhang D,
Yu X, Chen X, Teng B, Guan Z and Zhang Y: Expression of AKAP95,
Cx43, CyclinE1 and CyclinD1 in esophageal cancer and their
association with the clinical and pathological parameters. Int J
Clin Exp Med. 8:7324–7332. 2015.PubMed/NCBI
|
|
46
|
Cheng R, Liu YJ, Cui JW, Yang M, Liu XL,
Li P, Wang Z, Zhu LZ, Lu SY, Zou L, et al: Aspirin regulation of
c-myc and cyclinD1 proteins to overcome tamoxifen resistance in
estrogen receptor-positive breast cancer cells. Oncotarget.
8:30252–30264. 2017.PubMed/NCBI
|