Introduction
One of the common hallmarks of cancer is abnormal
mitosis (1). Kinetochores are the key
complexes that regulate mitotic chromosome segregation by
generating physical connections between chromosomes and spindle
microtubule polymers (2,3). The Ndc80 complex, a hetero-tetramer
protein complex of Ndc80, Nuf2, Spc24 and Spc25, is at the core of
the kinetochore and is the key kinetochore coupler (4). Recent studies have demonstrated that
abnormal expression of the Ndc80 complex is involved in the
progression of human cancer (5–7). For
example, NDC80 (7) and NUF2 (6) were reported as oncogenes in colon cancer
and osteosarcoma. SPC25, together with SPC24, binds the kinetochore
at one end of the Ndc80 complex (8).
However, the functional roles of SPC25 in cancer remain
unknown.
In the past decade, prostate cancer (PCa) is the
most frequently diagnosed type of cancer in Chinese males (9). Over the past three decades, certain
genes, including androgen receptor (10), speckle-type POZ protein (11,12), motor
neuron and pancreas homeobox 1 (13,14), were
identified as key regulators in PCa. As a result, the survival rate
of patients with localized PCa has been improved owing to surgery
and radiotherapy (15). However, the
molecular mechanisms underlying PCa progression remain poorly
understood. Therefore, the identification of novel regulators as
diagnostic and therapeutic strategies is urgently required.
The present study investigated the expression of
SPC25 in PCa tissues using The Cancer Genome Atlas, and
investigated the potential roles of SPC25 in regulating cell
proliferation, cell cycle, cell migration and apoptosis. The
present study may provide useful information for the identification
of novel therapeutic and prognostic targets for PCa.
Materials and methods
Patients and clinicopathological
data
The detailed SPC25 expression data of 490 patients
with PCa were downloaded from The Cancer Genome Atlas (TCGA,
https://tcga-data.nci.nih.gov/tcga/)
database by using the Firebrowse dataset (http://firebrowse.org/) (16,17). The
RNA expression data (level 3) were generated from the HiSeq 2000
sequencing platform (Illumina Inc., San Diego, CA, USA) by RNASeqV2
post-processing pipelines and were presented as RNA-Seq by
Expectation-Maximization normalized count data. Patient clinical
features, including age at diagnosis, days to last follow-up,
pathological tumor (T) stage and node (N) stage, were
retrospectively obtained from patient records. All the patients
were staged using the 2009 Tumor-Node-Metastasis (TNM)
classification of the American Joint Committee on
Cancer/International Union Against Cancer (18). The Gleason grading system was also
used to evaluate the prognosis of men with prostate cancer using
samples from a prostate biopsy. The Gleason scores range from 2 to
10, with higher number indicating greater risks and higher
mortality (19). In order to further
investigate the prognostic value of SPC25 in PCa, the overall
survival rates of patients with high or low SPC25 expression were
assessed using the Kaplan-Meier method by using GSE21032 dataset,
which was reported by Taylor et al. The upper 75% SPC25 mRNA
expression in all PCa tissues was used as the cut-off point to
divide all cases into high (n=36, ≥75% SPC25 expression), and low
(n=105, <75% SPC25 expression), groups and clinicopathological
characteristics, including the Gleason score.
Lentiviral constructs and
transfections
A total of 6 µg SureSilencing short hairpin RNA
(shRNA) plasmids (Qiagen GmbH) were used against SPC25 to knockdown
SPC25 expression levels using standard molecular techniques. The
SPC25 shRNA sequence was as follows:
3′-CCGGCCATCAAAGCATTTGCAGAAACTCGAGTTTCTGCAAATGCTTTGATGGTTTTTG-5′,
which were purchased from Shanghai GenePharma Co., Ltd. (Shanghai,
China). 293T cells were infected with the recombinant lentiviral
vectors using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) to generate stably transfected
cells. A total of 4 h after transfection, the Opti-MEM was changed
to RPMI-1640 medium containing 10% fetal bovine serum (GE
Healthcare Life Sciences, Little Chalfont, UK), 100 U/ml penicillin
and 100 µg/ml streptomycin, and were cultured at 37°C in 5%
CO2. After 24 h, concentrated lentiviruses were
collected. Opti-modified Eagle's medium (Opti-MEM) which was ideal
for use during cationic lipid transfections especially
Lipofectamine 2000 transfection reagents was purchased from Thermo
Fisher Scientific, Inc (cat. no. 31985062). Concentrated
lentiviruses were transfected at a multiplicity of infection (MOI)
of 40 in serum-free RPMI-1640 medium. The supernatant was replaced
with complete culture medium (RPMI-1640 medium containing 10% fetal
bovine serum; GE Healthcare Life Sciences) after 24 h. The
expression of SPC25 shRNA in infected cells was determined by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). The SPC25 shRNA sequence was as follows:
3′-CCGGCCATCAAAGCATTTGCAGAAACTCGAGTTTCTGCAAATGCTTTGATGGTTTTTG-5′,
which were purchased from Shanghai GenePharma Co., Ltd. (Shanghai,
China). Knockdown effects of these shRNA plasmids on endogenous
SPC25 expression were validated 48 h following their transfection
using by RT-qPCR.
Cell culture
Prostate cancer PC-3 cells and 293T cells were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and confirmed by short tandem repeat analysis (20). Cells were cultured in RPMI-1640 medium
containing 10% fetal bovine serum (GE Healthcare Life Sciences),
100 U/ml penicillin and 100 µg/ml streptomycin, and were cultured
at 37°C in 5% CO2.
RT-qPCR
Total RNA was extracted from PC-3 cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was
synthesized using a PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). qPCR was performed with
cDNA samples using the iQSYBRGreenSupermix and ABI Prism 7900
platform (both Bio-Rad Laboratories, Inc., Hercules, CA, USA),
according to the manufacturer's protocol. PCR cycling conditions
were 50°C for 2 min, followed by 95°C for 10 min, 40 cycles of 95°C
for 15 sec and 60°C for 1 min. The 2−ΔΔCq method was
used to calculate the relative expression level by normalizing to
GAPDH levels. The following primer sequences were used: SPC25
forward, 5′-AGAAGAACGAATGGTTGAGAT-3′ and reverse,
5′-TCCTGGATATTTGCAGTCAGT-3′; and GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. Each sample was run in triplicate to
ensure quantitative accuracy.
Plate analysis with the adherent cell
cytometry system Celigo®
PC-3 cells were stained with fluorescence nuclear
staining (Hoechst nuclei stain; 2.6 µg/ml; Invitrogen; Thermo
Fisher Scientific, Inc.) for 10 min at 37°C. The adherent cell
cytometry system, Celigo® (analyzed by Application
Programing Interface, version 1.0, software), allowed rapid
quantification of cellular fluorescence expression as previously
described (21). siGLO Green
Transfection Indicator (50 nM, Dharmacon Inc., Lafayette, CO, USA)
was transfected into PC-3cells with DharmaFECT 1 (0.15 µg/100 µl
well, Dharmacon Inc.). After 24 h, cells were washed with 1X PBS
and stained with Hoechst nuclei stain (2.6 µg/ml; Invitrogen;
Thermo Fisher Scientific, Inc.). Plates were analyzed using the
adherent cell cytometer equipped with bright field and fluorescent
channels: A blue 4′,6-diamidino-2-phenylindole filter for the
Hoechst nuclei stain and a green filter for the siGLOGreen
(Dharmacon Inc.). Gating parameters were adjusted for each
fluorescence channel to exclude background and other non-specific
signals. The Celigo® system provided a gross
quantitative analysis for each fluorescence channel and individual
well, including total count and average integrated red fluorescence
intensity of gated events.
Cell proliferation assay
An MTT assay was performed in order to evaluate
changes in cell proliferation. A total of 5,000 transfected PC-3
cells were seeded onto 96-well plates at a final volume of 100 µl
medium/well (RPMI-1640 medium containing 10% fetal bovine serum; GE
Healthcare Life Sciences). Proliferation was assessed at 0, 24, 48,
72 and 96 h. Cell proliferation was quantified by adding 20 µl MTT
(0.5 mg/ml; Sigma-Aldrich; Merck KGaA, dissolved in dimethyl
sulfoxide; Sigma-Aldrich; Merck KGaA), according to the
manufacturer's protocol. Following a 4 h incubation, the plates
were monitored using a PowerWave XS Microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA), which measured absorbance at
490 nm. The absorbance at 570 nm was used as a reference. Each
experiment was performed at least in triplicate.
Cell cycle and apoptosis assay
Following transfection for 48 h, cells were
harvested and washed with phosphate-buffered saline (PBS) three
times. Cells were incubated with PBS containing 0.03% Triton X-100,
100 ng/ml RNase A and 50 ng/ml propidium iodide (PI) for 15 min at
37°C. The distribution of cells in the different phases of the cell
cycle was analyzed using a FACSanto flow cytometer (BD Biosciences,
San Jose, CA, USA). Data on cell cycle distribution were analyzed
using ModFit LT 3.0 software (BD Biosciences). For the apoptosis
assay, cells were assayed with an Annexin V-APC Apoptosis Detection
kit (eBioscience; Thermo Fisher Scientific, Inc.) and were analyzed
using a flow cytometer (BD Biosciences).
Microarray and expression
datasets
Total RNA was extracted from shSPC25 and shRNA
control samples using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and the RNeasy mini kit (Qiagen GmbH, Hilden,
Germany). Total RNA was quantified by the NanoDrop ND-2000 (Thermo
Fisher Scientific, Inc.) and the RNA integrity was assessed using
Agilent Bioanalyzer 2100 (Agilent Technologies, Inc., Santa Clara,
CA, USA).
Global expressions of mRNAs in 3 SPC25 shCtrl
samples and 3 shSPC25 were examined using the Gene Chip Prime View
Human Gene Expression Array (Thermo Fisher Scientific, Inc.). The
sample labeling, microarray hybridization and washing were
performed according to the manufacturer's protocols. The raw
microarray data were uploaded to the Gene Expression Omnibus (GEO)
public repository (http://www.ncbi.nlm.nih.gov/geo/; GEO series no.
GSE73397). Raw data were normalized using the log2 scale. Two-class
unpaired significance analysis of microarray (22) was employed to filter significantly
differentially expressed mRNAs between shCtrl and shSPC25. To begin
with, the raw data were normalized using the quantile algorithm.
The probes for which ≥1 out of 2 conditions have flags in ‘P’ were
selected for further data analysis. Differentially expressed mRNAs
were subsequently identified through fold change. The threshold set
for upregulated and downregulated genes was a fold change ≥2.0.
Subsequently, Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway and Gene Ontology (GO) analyses of differently expressed
downstream genes of SPC25 were performed using an Ingenuity Pathway
Analysis system (https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis/).
Statistical analysis
The numerical data are presented as the mean ±
standard deviation of ≥3 repeats. To determine the associations
between SPC25 expression and the clinical characteristics of the
tumors, Student's t-tests or Mann-Whitney U-tests were used as
appropriate. For variables ≥3 groups, the Kruskal-Wallis H test was
used for non-parametric analysis. Tukey HSD was used as the post
hoc test. Kaplan Meier analysis, followed by the log-rank test, and
Cox regression analyses were utilized to assess the association
between SPC25 and overall survival as well as the prognosis of PCa.
All tests were two-sided and P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using the SPSS software package, version 15.0 (SPSS,
Inc., Chicago, IL, USA).
Results
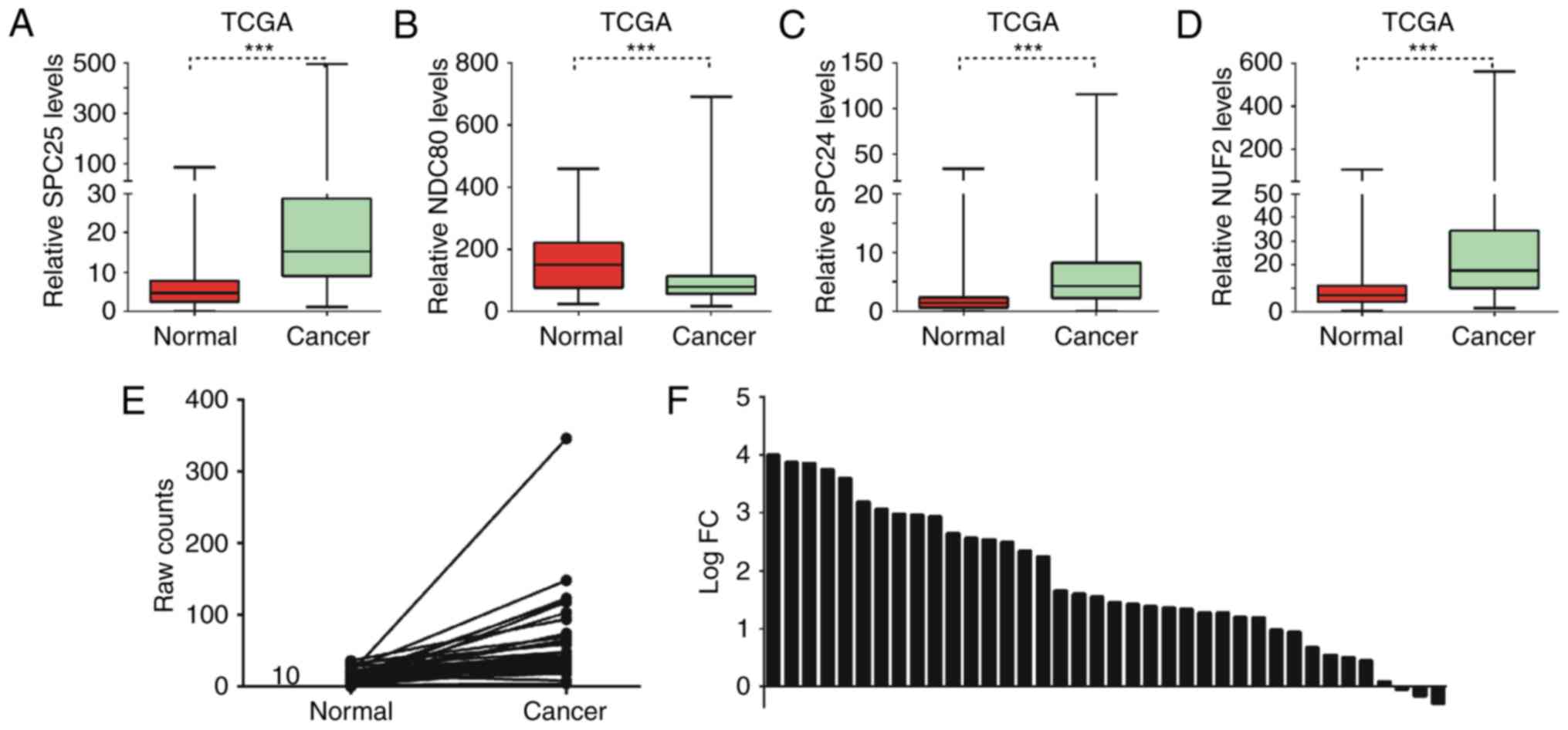
SPC25 was upregulated in PCa
The present study used the TCGA database to
investigate the expression pattern of the Ndc80 complex in PCa. It
was revealed that SPC25, SPC24 and NUF2 were significantly
upregulated and NDC80 was downregulated in PCa samples compared
with matched normal tissues (Fig.
1A-D). To the best of our knowledge, the functional roles of
SPC25 in cancer had not previously been reported. Therefore, SPC25
was selected for further study. The results of the present study
demonstrated that SPC25 was significantly upregulated in PCa
samples compared with matched normal tissues (Fig. 1E). Additionally, >90% of PCa
tissues expressed high levels of SPC25, while only ~10% (3/38) of
matched adjacent normal tissues expressed high levels of SPC25
(Fig. 1F).
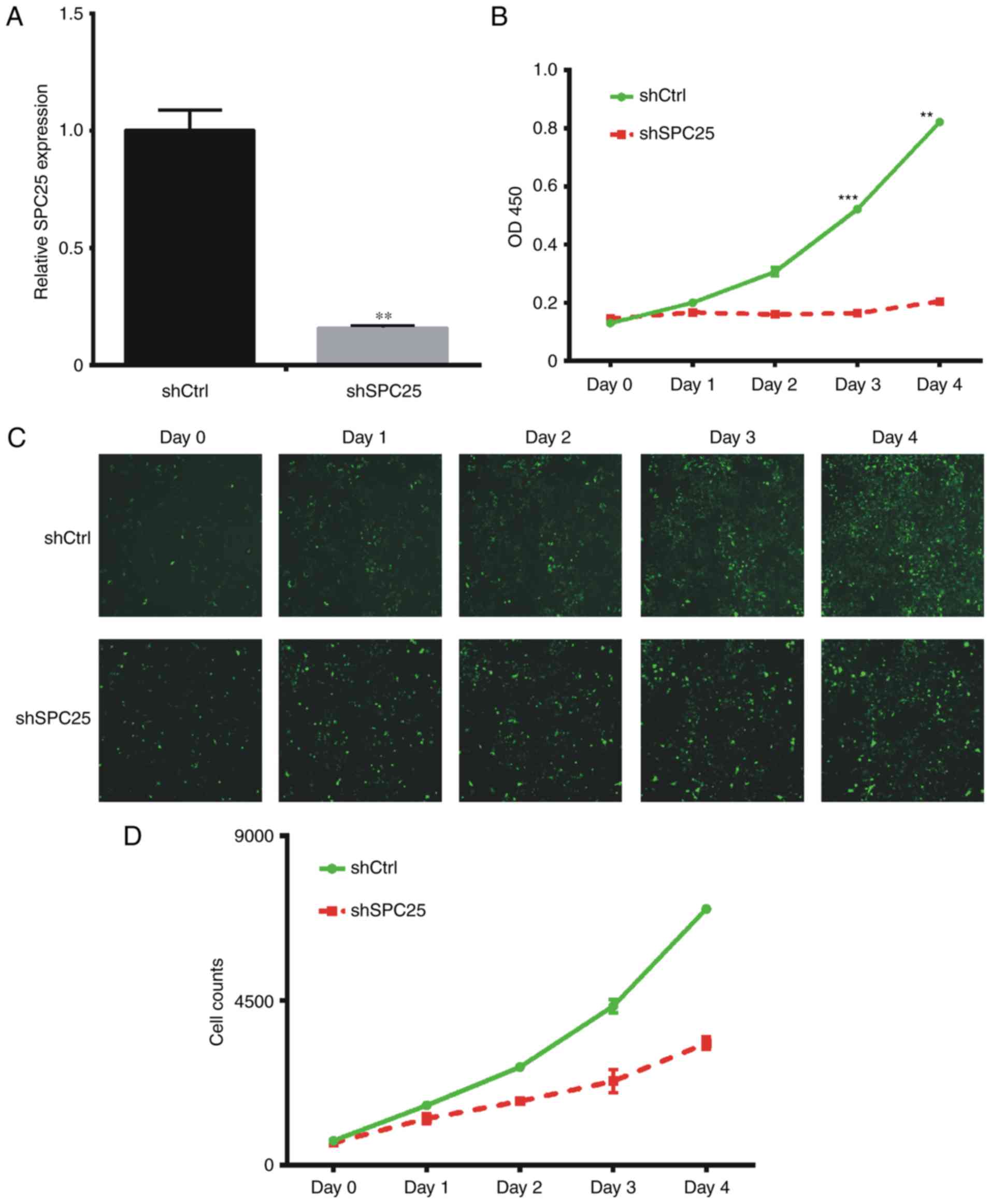
SPC25 knockdown inhibits cell
proliferation in PC-3 cells
In order to characterize the role of SPC25 in the
PC-3 cell line, SPC25 shRNA was used to knockdown its expression.
The results of the present study demonstrated that shSPC25
significantly reduced SPC25 mRNA expression compared with
expression in cells transfected with the negative control (Fig. 2A). When PC-3cells were transfected
with shSPC25, cell proliferation was decreased (Fig. 2B-D).
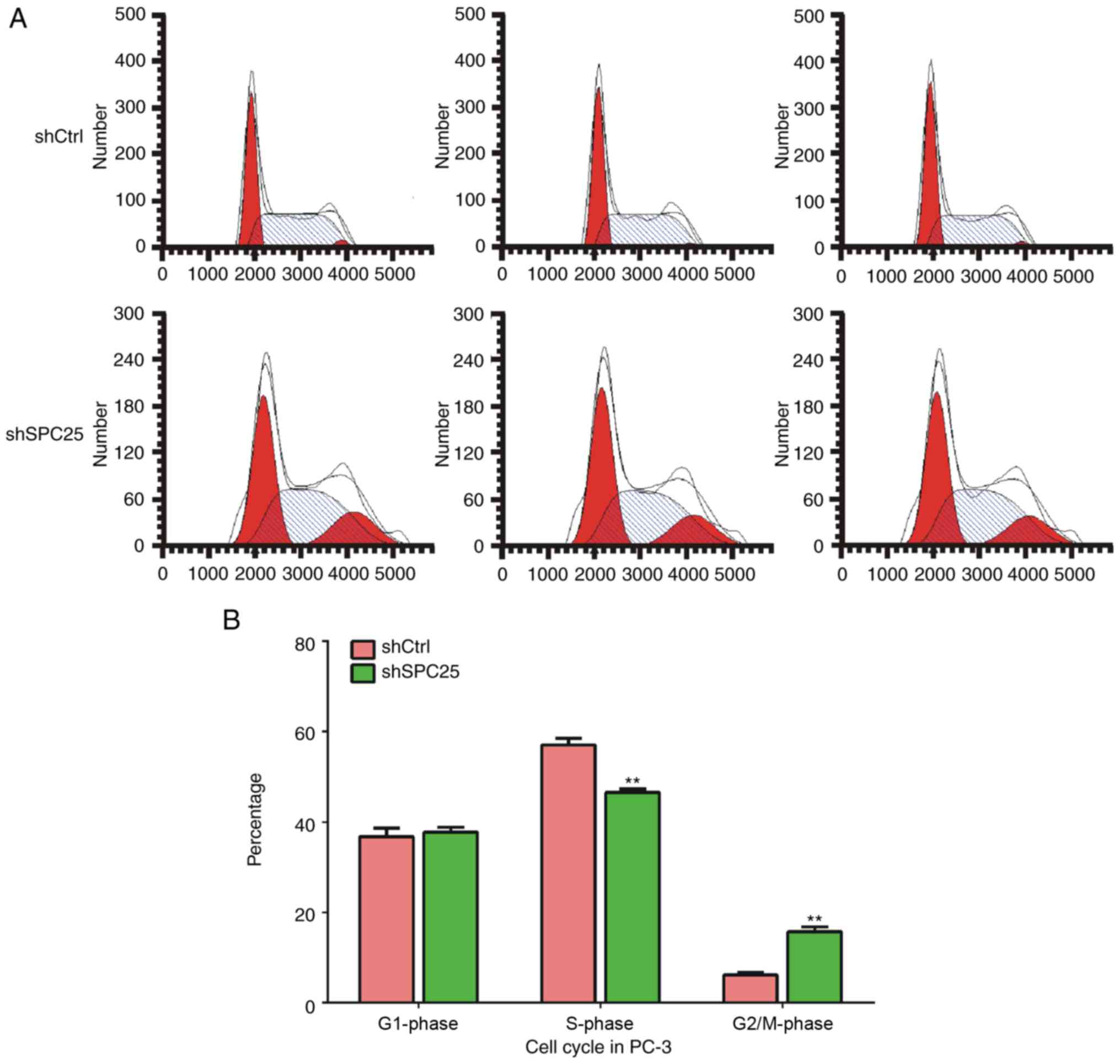
SPC25 knockdown decreased the number
of PCa cells in the S phase and increased the number in the G2/M
phase
Subsequently, the effect of SPC25 knockdown on cell
cycle progression in PC-3 cells was determined using a flow
cytometer. Knockdown of SPC25 in PC-3 cells increased the
percentage of cells in the G2/M phase and decreased the percentage
of cells in the S phase, but did not affect the percentage of cells
in the G1 phase compared with cells treated with the negative
control (Fig. 3A and B). These
results indicated that SPC25 was involved in regulating the PCa
cell cycle.
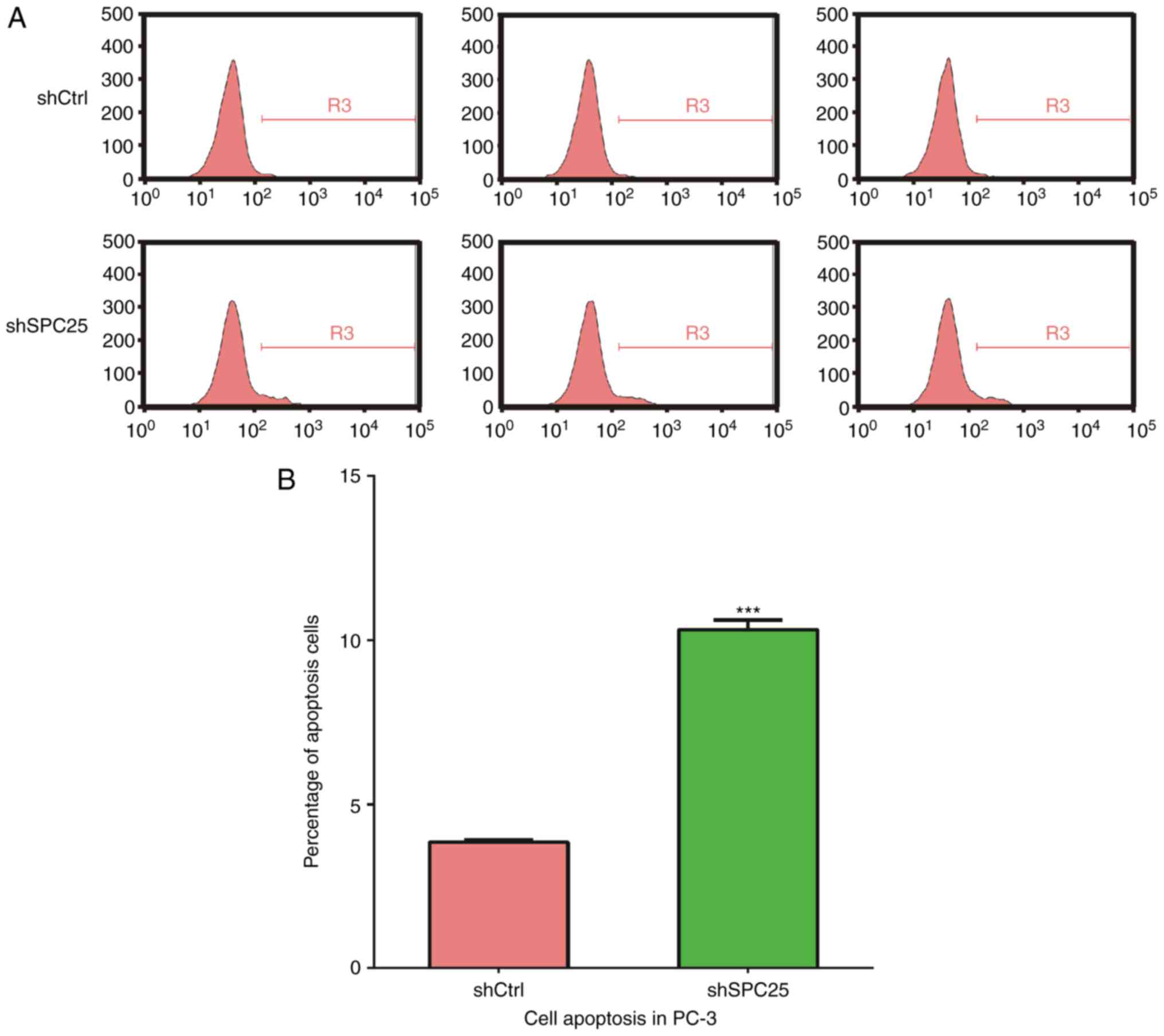
SPC25 knockdown promotes the apoptosis
of PCa cells
Subsequently, the role of SPC25 in the apoptosis of
PCa cells was investigated. PC-3 cells stably expressing shSPC25
and stained with Annexin V-APC were analyzed by flow cytometry. As
demonstrated in Fig. 4, SPC25
knockdown promoted the apoptosis of PC-3 cells.
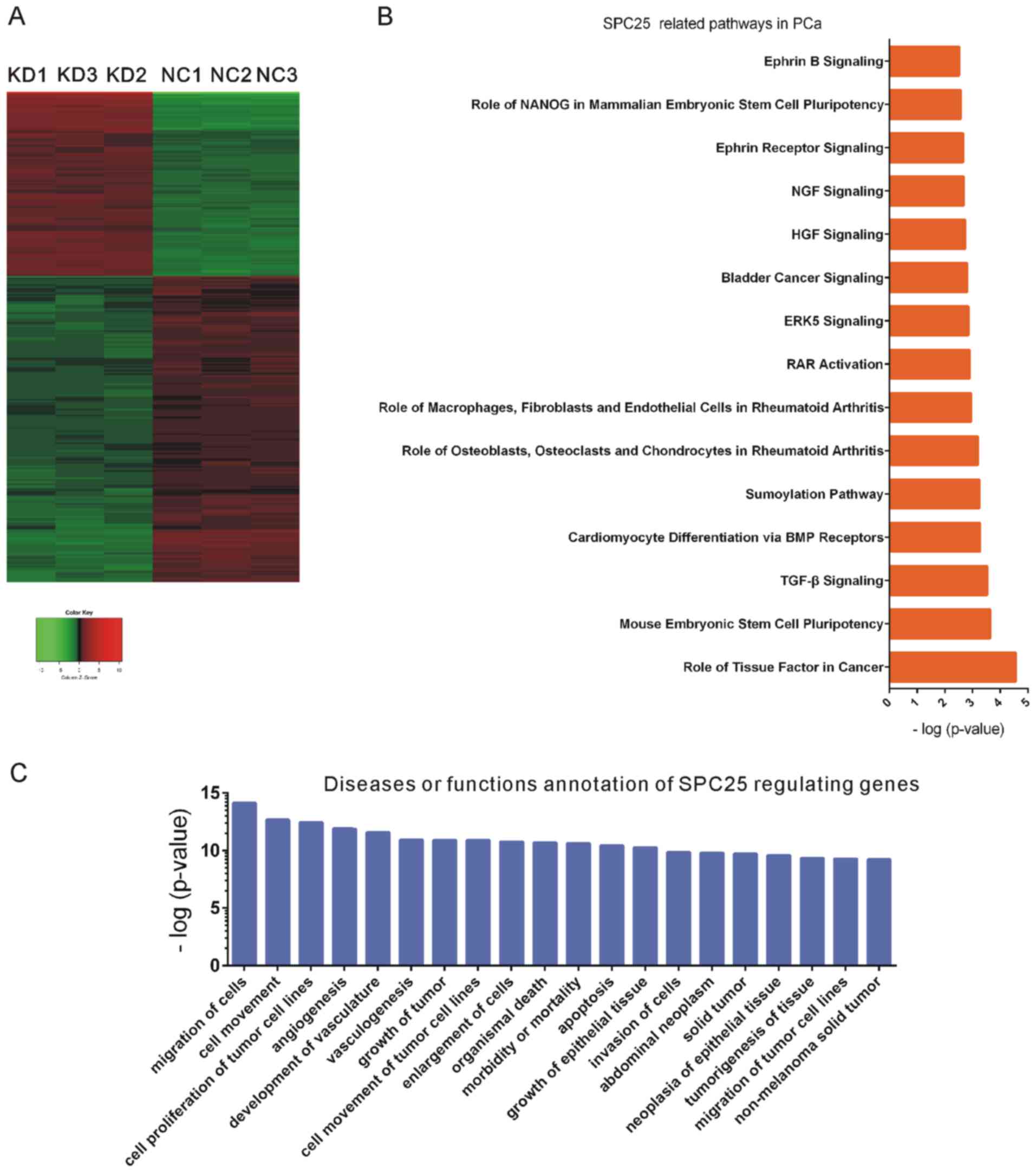
Bioinformatics analysis reveals
multiple functional roles of SPC25 in PCa
To further investigate the functional roles of SPC25
in PCa, mRNA expression profiling was used to detect global gene
expression levels following SPC25 knockdown. Analysis of the
microarray data revealed 193 upregulated and 297 downregulated
genes following SPC25 knockdown, with an average expression level
>1.5-fold (P<0.05; Fig. 5A).
Subsequently, Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway and Gene Ontology (GO) analyses of differently expressed
downstream genes of SPC25 were performed using an Ingenuity Pathway
Analysis system (www.ingenuity.com). SPC25 was significantly involved
in regulating role of tissue factor in cancer, mouse embryonic stem
cell pluripotency pathway, transforming growth factor-β signaling,
SUMOylation pathway, retinoic acid receptor activation,
extracellular-signal-regulated kinase 5 signaling, bladder cancer
signaling, hepatocyte growth factor signaling and nerve growth
factor signaling (Fig. 5B). GO
analysis demonstrated that SPC25 widely participated in the
regulation of the migration of cells, cell movement, cell
proliferation of tumor cell lines, angiogenesis, development of
vasculature, vasculogenesis, growth of tumor, cell movement of
tumor cells, enlargement of cells, organismal death, morbidity or
mortality, apoptosis, growth of epithelial tissue and invasion of
cells (Fig. 5C).
Upregulation of SPC25 predicts a poor
prognosis in PCa
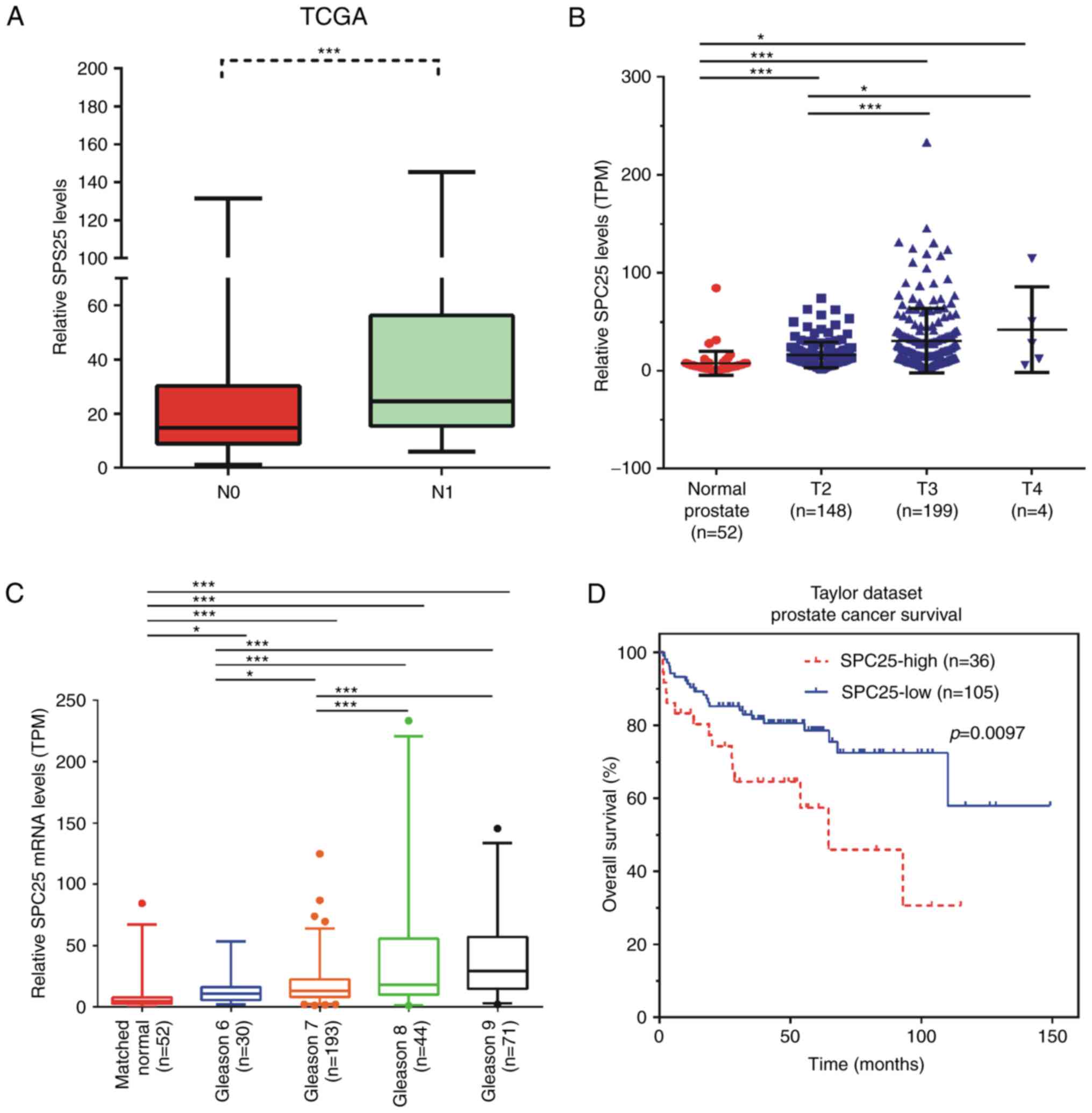
Furthermore, the present study evaluated the
possible prognostic value of SPC25 using TCGA RNA-seq data. The
results demonstrated that the expression level of SPC25 was
significantly associated with the pathological T stage and the N
stage of PCa. The results of the present study revealed that SPC25
levels were higher in N1 stage PCa samples than inN0 stage PCa
samples (Fig. 6A). It was also
revealed that SPC25 expression was significantly upregulated in
T3/T4 PCa samples compared with expression in T2 PCa samples
(Fig. 6B). These results suggested
that SPC25 may be involved in PCa metastasis.
Analysis of the TCGA database demonstrated that a
significantly higher expression of SPC25 was observed in Gleason 8
(P<0.001) and Gleason 9 (P<0.001) patients compared with
Gleason 6 and Gleason 7 patients (Fig.
6C) (19).
In order to further investigate the prognostic value
of SPC25 in PCa, the overall survival rates of patients with high
or low SPC25 expression were assessed using the Kaplan-Meier
method. Using the Taylor dataset (GSE21032) (23), 75% SPC25 mRNA expression in all PCa
tissues was used as the cut-off point to divide all cases into high
(n=36) and low (n=105) SPC25 expression groups. As demonstrated in
Fig. 6D, compared with patients with
high SPC25 expression, the 5-year BCR-free survival rates were
higher in patients with low SPC25 expression in this dataset
(Fig. 6D), indicating that the low
level of SPC25 was associated with a longer BCR-free survival
time.
Discussion
SPC25 is a member of the Ndc80 complex that serves
an important role in regulating mitotic chromosome segregation
(4). Abnormal expression of the Ndc80
complex was observed in different types of human cancer (5–7). For
example, SPC24, a co-factor of SPC25, was revealed to be critical
for the progression of anaplastic thyroid cancer (24). The present study focused on
investigating the roles of SPC25 in PCa. In a previous study, SPC25
was significantly overexpressed in human breast tumor tissues and
was associated with reduced overall survival (25). However, the functional roles of SPC25
in cancer remain unknown.
The present study analyzed the TCGA database in
order to investigate the expression pattern of the Ndc80 complex in
PCa. The results demonstrated that SPC25 was significantly
upregulated in PCa samples compared with expression in matched
normal tissues. These results suggested that SPC25 may act as an
oncogene in PCa. In order to characterize the role of SPC25 in PCa
cells, a loss of function assay was performed in the present study.
The results demonstrated that SPC25 knockdown may significantly
reduce PCa cell proliferation. Given that SPC25 is an important
component of the mitotic checkpoint machinery, the roles of SPC25
in regulating the PCa cell cycle were also investigated. It was
revealed that SPC25 knockdown induced a decrease in the number of
PCa cells in the S phase and an increase in the number of PCa cells
in the G2/M phase. Furthermore, SPC25 knockdown promoted the
apoptosis of PCa cells. Taken together, these results demonstrated
that SPC25 serves an oncogenic role in PCa by regulating the cell
cycle and apoptosis.
The present study demonstrated the effect of SPC25
on cell cycle regulation. In order to further investigate the
functional roles of SPC25 in PCa, bioinformatics analysis was
performed, in combination with a high-throughput array. A total of
193 genes were identified to be upregulated and 297 genes were
identified to be downregulated following SPC25 knockdown. In line
with the experimental results of the present study, GO analysis
revealed that SPC25 is involved in regulating cell proliferation
and apoptosis. Notably, SPC25 downstream genes were revealed to be
significantly enriched in cell invasion pathways. Furthermore, KEGG
pathway analysis demonstrated that SPC25 was involved in regulating
role of tissue factor in cancer, mouse embryonic stem cell
pluripotency, transforming growth factor-β signaling and
SUMOylation pathway. Although further validation is required, the
results of the present study provided novel information regarding
the role of SPC25 in regulating PCa progression.
PCa is one of the most frequently diagnosed types of
cancer worldwide (1). In the last
three decades, a series of genes, including prostate-specific
antigen (PSA) (26,27), prostate cancer associated 3 (26), and ubiquitin-like with PHD and RING
finger domains (28) were revealed to
be dysregulated in PCa and thus, may act as biomarkers. Notably,
PSA testing was the most widely used biomarker of PCa (27). However, there are limitations
regarding the accuracy of these tests (29). Therefore, there remains an urgent
requirement to identify novel biomarkers for PCa. In the present
study, the possible prognostic value of SPC25 was evaluated using
TCGA RNA-seq data. According to this analysis, the expression level
of SPC25 was significantly upregulated in T3/T4 PCa samples
compared with T2 PCa samples. These results also revealed that
SPC25 levels were higher in N1 stage PCa samples than in N0 stage
PCa samples. Analysis of the TCGA database also demonstrated that a
significantly higher expression of SPC25 was observed in patients
with high Gleason scores (Gleason 8 and Gleason 9) than in patients
with low Gleason scores (Gleason 6 and Gleason 7). Kaplan-Meier
analysis revealed that patients with PCa with a low expression of
SPC25 had a longer BCR-free survival time than those with a high
expression of SPC25. To the best of our knowledge, the present
study was the first to report that SPC25 was involved in the
prognosis of PCa.
To the best of our knowledge, the present study was
the first to demonstrate that SPC25 was significantly upregulated
in PCa. In order to investigate the molecular functional roles of
SPC25, a loss of function assay was performed and it was revealed
that SPC25 knockdown inhibited cell proliferation and induced a
decrease in the number of PCa cells in the S phase and an increase
in the number of cells in the G2/M phase. Further more, SPC25
knockdown promoted the apoptosis of PCa. Notably, bioinformatics
analysis revealed multiple functional roles of SPC25 in regulating
cell proliferation, apoptosis, invasion, role of tissue factor in
cancer, transforming growth factor-β signaling, and SUMOylation
pathway in PCa. Furthermore, the present study evaluated the
possible prognostic value of SPC25 using TCGA RNA-seq data and
revealed that SPC25 was upregulated in late pathological stages of
PCa. Kaplan-Meier analysis demonstrated that lower SPC25 expression
levels were associated with better survival of patients with PCa.
Taken together, the results of the present study suggested that
SPC25 serves an oncogenic role in PCa and may act as a novel
diagnostic and therapeutic target for PCa.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Social
Development Plan of Jiangsu Province-Standardization of Key Disease
Diagnosis and Treatment Projects (grant no. BE2016715) from Jiangsu
Province Science and Technology Commission.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
FC and HT were responsible for the study conception
and design. FC, JH, YF and HT developed the methodology. FC, YF and
JT analysed and interpreted the data. FC and HT wrote, reviewed and
revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dominguez-Brauer C, Thu KL, Mason JM,
Blaser H, Bray MR and Mak TW: Targeting mitosis in cancer: Emerging
strategies. Mol Cell. 60:524–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeLuca JG, Gall WE, Ciferri C, Cimini D,
Musacchio A and Salmon ED: Kinetochore microtubule dynamics and
attachment stability are regulated by Hec1. Cell. 127:969–982.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ciferri C, Pasqualato S, Screpanti E,
Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca
JG, De Wulf P, et al: Implications for kinetochore-microtubule
attachment from the structure of an engineered Ndc80 complex. Cell.
133:427–439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tooley J and Stukenberg PT: The Ndc80
complex: Integrating the kinetochore's many movements. Chromosome
Res. 19:377–391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu P, Chen X, Sun J, Bie P and Zhang LD:
siRNA-mediated knockdown against NUF2 suppresses pancreatic cancer
proliferation in vitro and in vivo. Biosci Rep. 35(pii):
e001702015.PubMed/NCBI
|
|
6
|
Fu HL and Shao L: Silencing of NUF2
inhibits proliferation of human osteosarcoma Saos-2 cells. Eur Rev
Med Pharmacol Sci. 20:1071–1079. 2016.PubMed/NCBI
|
|
7
|
Xing XK, Wu HY, Chen HL and Feng HG: NDC80
promotes proliferation and metastasis of colon cancer cells. Genet
Mol Res. 15:2016. View Article : Google Scholar
|
|
8
|
Suzuki A, Badger BL, Haase J, Ohashi T,
Erickson HP, Salmon ED and Bloom K: How the kinetochore couples
microtubule force and centromere stretch to move chromosomes. Nat
Cell Biol. 18:382–392. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wan X, Huang W, Yang S, Zhang Y, Pu H, Fu
F, Huang Y, Wu H, Li T and Li Y: Identification of
androgen-responsive lncRNAs as diagnostic and prognostic markers
for prostate cancer. Oncotarget. 7:60503–60518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang KH, Li R, Kuri B, Lotan Y, Roehrborn
CG, Liu J, Vessella R, Nelson PS, Kapur P, Guo X, et al: A
gain-of-function mutation in DHT synthesis in castration-resistant
prostate cancer. Cell. 154:1074–1084. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang P, Gao K, Tang Y, Jin X, An J, Yu H,
Wang H, Zhang Y, Wang D, Huang H, et al: Destruction of DDIT3/CHOP
protein by wild-type SPOP but not prostate cancer-associated
mutants. Hum Mutat. 35:1142–1151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
An J, Ren S, Murphy SJ, Dalangood S, Chang
C, Pang X, Cui Y, Wang L, Pan Y, Zhang X, et al: Truncated ERG
oncoproteins from TMPRSS2-ERG fusions Are resistant to
SPOP-mediated proteasome degradation. Mol Cell. 59:904–916. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Wang J, Wang Y, Zhang Y, Castro
P, Shao L, Sreekumar A, Putluri N, Guha N, Deepak S, et al: MNX1 Is
oncogenically upregulated in African-American prostate cancer.
Cancer Res. 76:6290–6298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Das M: MNX1: A novel prostate cancer
oncogene. Lancet Oncol. 17:e5212016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie JJ, Zhuo YJ, Zheng Y, Mo RJ, Liu ZZ,
Li BW, Cai ZD, Zhu XJ, Liang YX, He HC and Zhong WD: High
expression of ASPM correlates with tumor progression and predicts
poor outcome in patients with prostate cancer. Int Urol Nephrol.
49:817–823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schlomm T: Re: The molecular taxonomy of
primary prostate cancer. Eur Urol. 69:11572016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cancer Genome Atlas Research Network, .
The molecular taxonomy of primary prostate cancer. Cell.
163:1011–1025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buyyounouski MK, Choyke PL, McKenney JK,
Sartor O, Sandler HM, Amin MB, Kattan MW and Lin DW: Prostate
cancer-major changes in the American Joint Committee on Cancer
eighth edition cancer staging manual. CA Cancer J Clin. 67:245–253.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rees MA, Resnick MI and Oesterling JE: Use
of prostate-specific antigen, Gleason score, and digital rectal
examination in staging patients with newly diagnosed prostate
cancer. Urol Clin North Am. 24:379–388. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dirks WG and Drexler HG: STR DNA typing of
human cell lines: Detection of intra- and interspecies
cross-contamination. Methods Mol Biol. 946:27–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nabzdyk CS, Chun M, Pradhan Nabzdyk L,
Yoshida S and LoGerfo FW: Differential susceptibility of human
primary aortic and coronary artery vascular cells to RNA
interference. Biochem Biophys Res Commun. 425:261–265. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tusher VG, Tibshirani R and Chu G:
Significance analysis of microarrays applied to the ionizing
radiation response. Proc Natl Acad Sci USA. 98:pp. 5116–5121. 2001;
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taylor BS, Schultz N, Hieronymus H,
Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva
B, et al: Integrative genomic profiling of human prostate cancer.
Cancer Cell. 18:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin H, Meng T, Zhou L, Chen H and Song D:
SPC24 is critical for anaplastic thyroid cancer progression.
Oncotarget. 8:21884–21891. 2017.PubMed/NCBI
|
|
25
|
Pathania R, Ramachandran S, Mariappan G,
Thakur P, Shi H, Choi JH, Manicassamy S, Kolhe R, Prasad PD, Sharma
S, et al: Combined Inhibition of DNMT and HDAC blocks the
tumorigenicity of cancer stem-like cells and attenuates mammary
tumor growth. Cancer Res. 76:3224–3235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsaur I, Hennenlotter J, Oppermann E, Munz
M, Kuehs U, Stenzl A and Schilling D: PCA3 and PSA gene activity
correlates with the true tumor cell burden in prostate cancer lymph
node metastases. Cancer Biomark. 15:311–316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prensner JR, Rubin MA, Wei JT and
Chinnaiyan AM: Beyond PSA: The next generation of prostate cancer
biomarkers. Sci Transl Med. 4:127rv32012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wan X, Yang S, Huang W, Wu D, Chen H, Wu
M, Li J, Li T and Li Y: UHRF1 overexpression is involved in cell
proliferation and biochemical recurrence in prostate cancer after
radical prostatectomy. J Exp Clin Canc Res. 35:342016. View Article : Google Scholar
|
|
29
|
D'Amico AV, Whittington R, Malkowicz SB,
Wu YH, Chen M, Art M, Tomaszewski JE and Wein A: Combination of the
preoperative PSA level, biopsy gleason score, percentage of
positive biopsies, and MRI T-stage to predict early PSA failure in
men with clinically localized prostate cancer. Urology. 55:572–577.
2000. View Article : Google Scholar : PubMed/NCBI
|