Introduction
Colorectal cancer (CRC) is the third most common
type of malignancy and the fourth most common cause of
cancer-associated mortality worldwide (1). In 2015, there were 376,300
newly-diagnosed CRC cases and 191,000 CRC-associated mortalities in
China projected by the National Office for Cancer Prevention and
Control, National Cancer Center (2).
Despite improved precancerous screening, surgical resection,
chemotherapy and radiotherapy, patients with CRC, particularly
those in the advanced stages, exhibit poor prognosis with
significant morbidity and mortality rates, thereby constituting a
major burden on global health (3).
For example, a survey from the United States of America suggested
that the 5-year survival rate is 70.4% in patients with CRC with
regional invasion, while in patients with distant metastasis the
rate is 12.5% (4). During the
development of CRC, multiple genetic mutations accumulate, and also
involve genes that regulate cell proliferation and survival,
thereby making CRC a biologically heterogeneous disease (5,6).
Therefore, CRC exhibits diverse treatment responses in patients
with similar clinicopathological parameters. Furthermore, different
molecular drivers may exist in patients with CRC at the same stage,
leading to varied prognosis. Thus, there is an urgent requirement
to investigate the molecular markers underlying CRC and identify
novel therapeutic targets for CRC treatment.
Estrogen-related receptor (ERR) is a member of the
steroid nuclear hormone receptor superfamily and is involved in
energy homeostasis regulation (7).
ERR consists of three closely associated members ERRα, ERRβ and
ERRγ. No natural estrogens have been identified to activate ERR,
therefore they are classified as orphan receptors (8). Combined with transcriptional
coactivator, peroxisome proliferator-activated receptor γ
coactivator-1α (PGC1α), ERRα regulates key genes coding for
components of energy homeostasis, including fatty acid and glucose
metabolism, mitochondrial biogenesis, and oxidative stress
(7). In a previous study, ERRα was
demonstrated to be expressed in 100% of the patients with CRC, and
ERRα mRNA expression was elevated in tumor tissue compared with
normal mucosa (9). In addition, a
significantly increasing association was observed between ERRα
expression in tumor tissues and TNM stages II to IV (9).
The present study aimed to investigate whether ERRα
acts as an effective prognostic marker for patients with CRC.
Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and immunohistochemistry were performed to detect the
expression of ERRα in CRC tissues, and their adjacent normal
tissues. Statistical analysis was applied to evaluate the
associations between ERRα expression, and clinicopathological
parameters and prognosis. As a result, a high expression of ERRα
was revealed to be associated with local recurrence and reduced
5-year survival rates. ERRα was identified as an independent
prognostic factor for patients with CRC. The results of the present
study confirm that ERRα demonstrates clinical and prognostic
significance, and may also be a novel therapeutic target for CRC
treatment.
Materials and methods
Patients and tissue samples
A total of 15 fresh primary CRC tissues, and their
adjacent normal tissues were obtained for RT-qPCR between July 2015
and December 2015. A total of 128 paraffin-embedded primary CRC
tissues and their adjacent normal tissues that were obtained
between January 2005 and December 2010 were used for the
immunohistochemistry assay. All specimens were collected from
patients with CRC that underwent curative resection at the
Department of General Surgery in Affiliated Tumor Hospital of
Guangxi Medical University (Nanning, China). All the patients
received no preoperative chemotherapy or radiotherapy. The 128
patients with paraffin-embedded samples included 72 males and 56
females, with a mean age of 56 years and range of 27–84 years. The
histological type was determined by two experienced pathologists
who reviewed the slides of CRC biopsies stained with hematoxylin
and eosin according to the World Health Organization classification
(10). Tumor differentiation status
was divided into three types: Well, moderately and poorly
differentiated. Tumor invasion was classified into T1, T2, T3 and
T4 stages, and clinical status was classified into A, B, C and D
stages according to the Dukes classification system (11). Postoperative follow-up was performed
for each patient to monitor local recurrence and/or distal
metastasis using laboratory tests (once every 3 months) and
radiological examination (once every 6 months). Patients with
incomplete follow-up records were excluded. The fresh specimens
from 15 patients were snap-frozen and stored at −80°C for RNA
isolation, and the 128 specimens were fixed with 10% formalin at
4°C for 24 h and embedded in paraffin for immunohistochemistry. All
128 patients with CRC were followed up after surgery every three
months, with the follow-up deadline set at December 2015. Overall
survival (OS) was defined as the interval between surgery and
mortality or the last follow-up (censored data for living
patients). Disease-free survival (DFS) was defined as the interval
between surgery and the date of relapse. The present study was
approved by the Ethics Committee of Affiliated Tumor Hospital of
Guangxi Medical University and written informed consent was
obtained from patients whose tissue specimens were used.
RT-qPCR
Total RNA was extracted from 15 frozen CRC tissues
and their corresponding adjacent normal tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The SuperScript III Reverse Transcriptase
kit (Promega Corporation, Madison, WI, USA) was used to synthesize
obtained RNA into cDNA according to the manufacturer's
instructions, with the temperature protocol as follows: 42°C for 30
min, 85°C for 5 sec, then holding at 4°C. The Step One Plus
Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was applied for the qPCR assay using SYBR Green mix (Takara
Bio, Inc., Otsu, Japan). The thermocycling conditions were as
follows: Initial denaturation at 95°C for 5 min; followed by 40
amplification cycles of 95°C for 5 sec; annealing at 60°C for 15
sec; and elongation at 72°C for 15 sec. Ribosomal protein L13a
(RPL13A) served as the internal control and the relative gene
expression levels was determined by 2−ΔΔCq method
(12). The primer sequences used in
the present study were as follows: ERRα forward,
5′-TGCTCAAGGAGGGAGTGC-3′ and reverse,
5′-GGCGACAATTTCTGGTTCGGGTCAGGCATGGCATAG-3′; RPL13A forward,
5′-CCTGGAGGAGAAGAGGAAAGAGA-3′ and reverse,
5′-TTGAGGACCTCTGTGTATTTGTCAA-3′. Three independent experiments were
performed.
Immunohistochemical staining and
evaluation
The CRC tissue specimens were paraffin-embedded and
processed for 4 µm-thick sections. The sections were dewaxed in
xylene and rehydrated with descending series of ethanol gradient
(100, 95, 90, 80 and 70%). The sections were incubated with 0.3%
hydrogen peroxidase at room temperature for 25 min to block
endogenous peroxidase activity, and were heated at 100°C in a 700W
microwave oven for 15 min for antigen retrieval. In order to
prevent nonspecific staining, the sections were pre-incubated with
10% normal goat serum (cat. no. 71-00-27; KPL; Seracare Life
Sciences, Milford, MA, USA) in PBS at room temperature for 30 min.
The sections were incubated with rabbit anti-ERRα primary antibody
(cat. no. ab227944,1:500; Abcam, Cambridge, UK) overnight at 4°C,
followed by incubation with goat anti-rabbit secondary antibody
conjugated to horseradish peroxidase (cat. no. ab97051,1:200;
Abcam) for 30 min at room temperature. The 3,3′-diaminobenzidine
tetra-hydrochloride (liquid DAB+; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) was used to reveal
antigen-antibody reactions. Finally, after the tissues were
counterstained with hematoxylin at room temperature for 5 min, the
sections were dehydrated and mounted. Sections incubated with PBS
instead of the primary antibody served as negative controls.
Two pathologists independently evaluated the
staining semi-quantitatively, who were blind to the clinical data.
Any discrepancy between the two observers was assessed by a
pathologist to reach the consensus. In each section, five visual
fields were randomly selected for evaluation. The expression of
ERRα was evaluated according to the labeling index (LI), which
indicates the positive immunoreactivity of carcinoma cells. For
statistical analyses, the cases that exhibited LI <10% were
considered to have a low expression of ERRα, and the cases with LI
>10% were considered to have a high expression of ERRα (13).
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation. Statistical analysis was performed using SPSS
19.0 statistical software (IBM Corp., Armonk, NY, USA). The
unpaired two-tailed student's t-test was applied to compare the
ERRα mRNA expression in primary CRC tumors and adjacent
non-tumorous tissues. The Chi-square test was applied to assess the
association between clinicopathological parameters and ERRα
expression. The Kaplan-Meier estimator was performed to construct
survival and local recurrence curves, and the log-rank test was
applied to compare differences between groups. Significant
independent prognostic factors for patients with CRC were analyzed
using univariate and multivariate analysis based on the Cox
proportional hazard model. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of ERRα in CRC tissues and
adjacent normal tissues
The mRNA expression of ERRα was determined by
RT-qPCR analysis in 15 fresh CRC tissues and matched normal
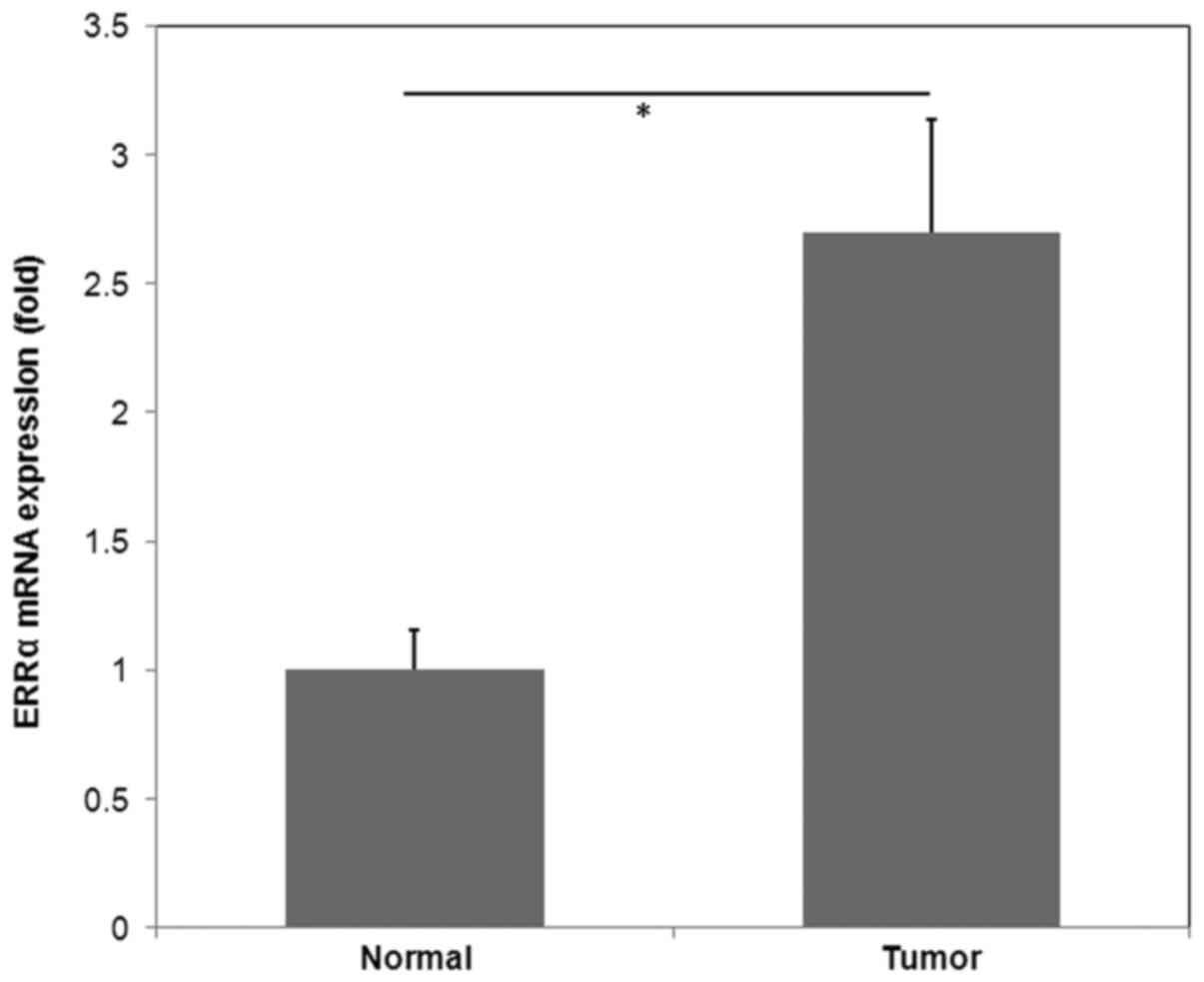
tissues. The results demonstrated that CRC tissues exhibited
significantly higher ERRα expression compared with normal tissues
(P<0.05; Fig. 1).
The protein expression of ERRα was determined by
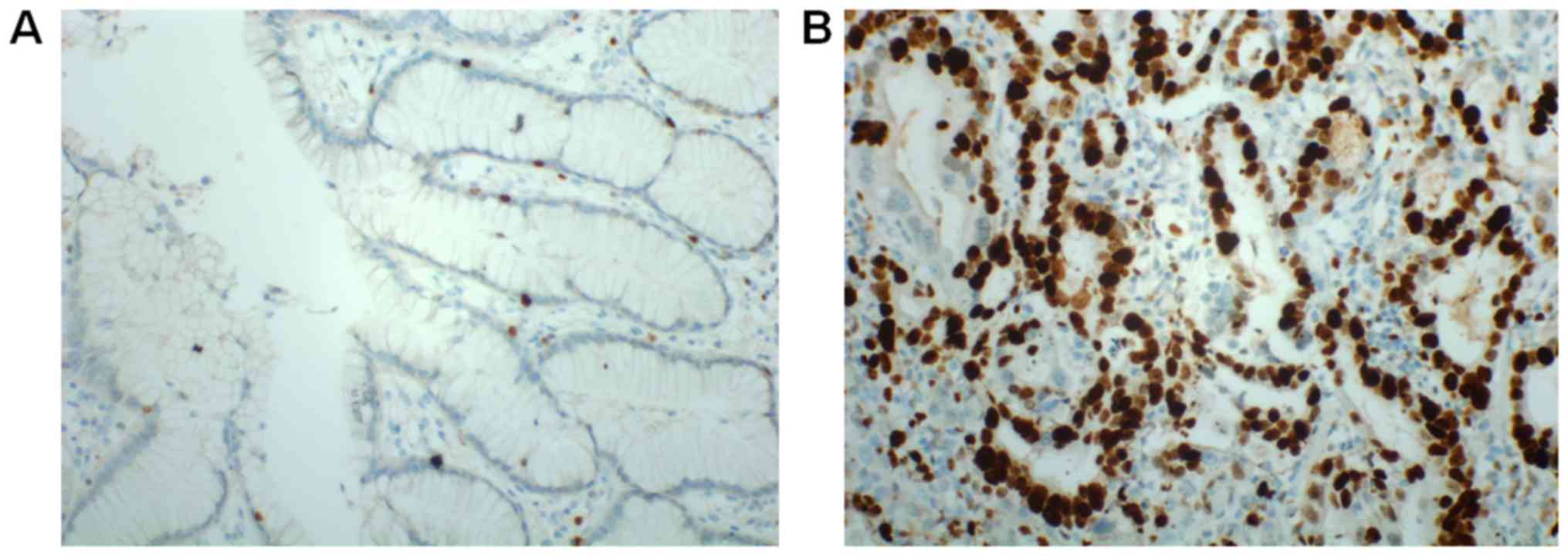
immunohistochemistry in 128 CRC tissues and matched normal tissues.
ERRα was primarily detected in the nuclei of tumor cells. According
to the results of staining evaluation, the mean values of ERRα LI
were 28.7% (range, 0–83%) and 12.1% (range, 0–31%) in the 128 CRC
cancer tissues and 127 adjacent normal tissues, respectively. The
number of ERRα high expression colorectal cancer (ERRα LI >10%)
was 50/128 cases (39.1%) (Table I),
compared with 14/128 cases (10.9%) in adjacent normal tissues. ERRα
expression was significantly higher in colorectal cancer tissues
compared with that in adjacent normal tissues (P<0.05) (data not
shown). The representative results of immunohistochemistry are
presented in Fig. 2.
 | Table I.Association between ERRα expression
and clinicopathological parameters in patients with CRC. |
Table I.
Association between ERRα expression
and clinicopathological parameters in patients with CRC.
|
|
| ERRα expression
(n=128) |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
features | All cases | Low (n=78) | High (n=50) | χ2
test | P-value |
|---|
| Sex |
|
|
| 0.469 | 0.494 |
| Male | 72 | 42 | 30 |
|
|
|
Female | 56 | 36 | 20 |
|
|
| Age, years |
|
|
| 1.297 | 0.255 |
|
<60 | 77 | 50 | 27 |
|
|
| ≥60 | 51 | 28 | 23 |
|
|
| Tumor location |
|
|
| 0.340 | 0.560 |
|
Colon | 65 | 38 | 27 |
|
|
|
Rectum | 63 | 40 | 23 |
|
|
| Tumor size, cm |
|
|
| 2.053 | 0.152 |
|
<5 | 74 | 49 | 25 |
|
|
| ≥5 | 54 | 29 | 25 |
|
|
| Tumor
differentiation |
|
|
| 6.386 | 0.012 |
| Well,
moderate | 69 | 49 | 20 |
|
|
|
Poor | 59 | 29 | 30 |
|
|
| Tumor invasion |
|
|
| 5.330 | 0.021 |
|
T1+T2 | 70 | 49 | 21 |
|
|
|
T3+T4 | 58 | 29 | 29 |
|
|
| Lymph node
status |
|
|
| 8.412 | 0.004 |
|
Absent | 74 | 53 | 21 |
|
|
|
Present | 54 | 25 | 29 |
|
|
| Dukes stage |
|
|
| 7.691 | 0.006 |
|
A+B | 78 | 55 | 23 |
|
|
|
C+D | 50 | 23 | 27 |
|
|
Associations between ERRα expression
and CRC clinicopathological parameters
To further investigate the clinical significance of
ERRα in CRC, the associations between ERRα expression and CRC
clinicopathological characteristics in 128 patients were
statistically analyzed (Table I).
Significant associations were identified between ERRα expression,
and tumor differentiation (P=0.012), tumor invasion (P=0.021),
lymph node status (P=0.004) and Dukes stage (P=0.006). However, no
statistically significant associations between ERRα expression and
other clinicopathological parameters, including gender (P=0.494),
age (P=0.255), tumor location (P=0.560) and tumor size (P=0.152),
were identified.
Prognostic significance of ERRα
expression in CRC
The Kaplan-Meier estimator model was applied to
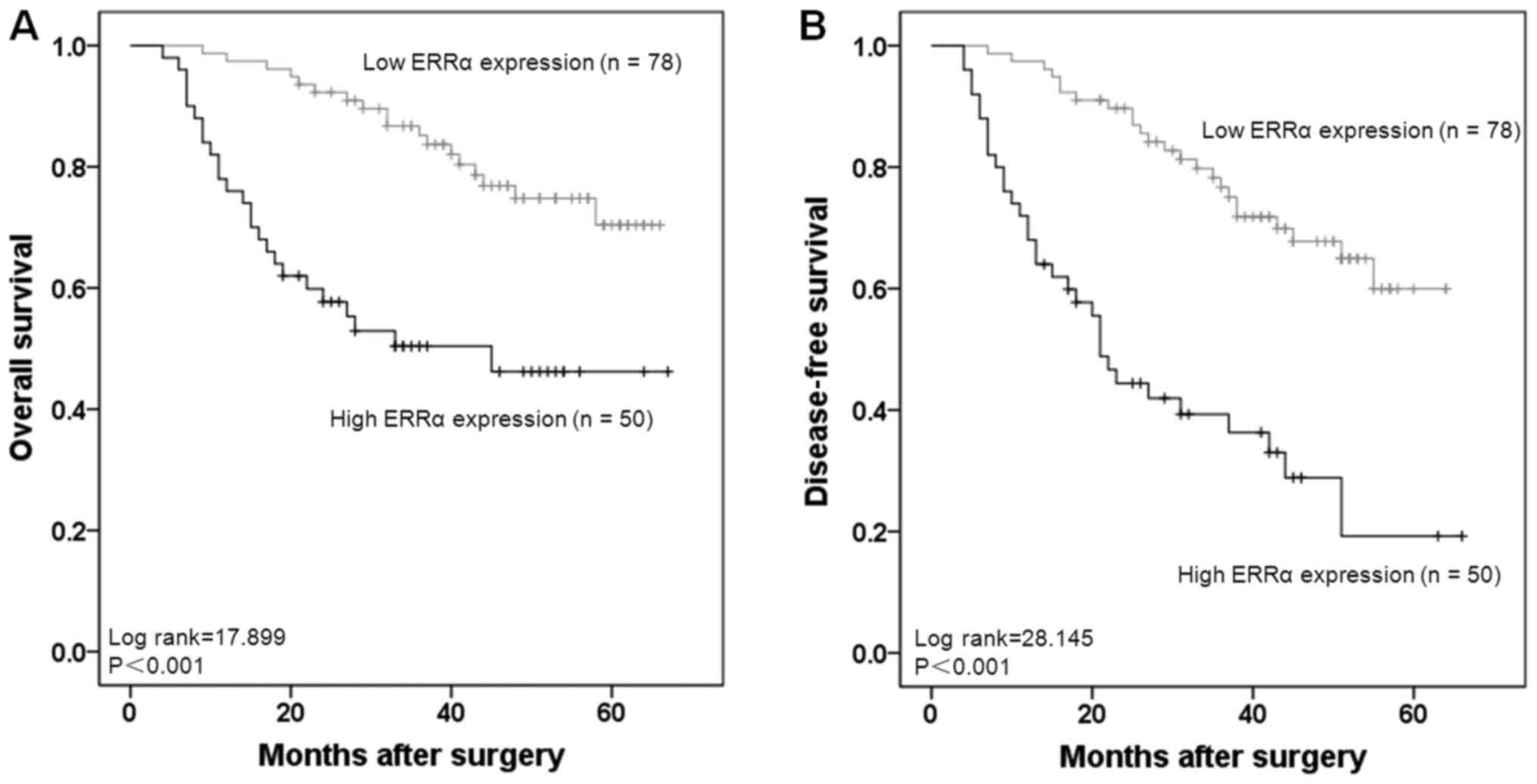
evaluate the prognostic significance of ERRα expression. The 5-year
OS rates in patients with high ERRα expression and low expression
were 50.0 and 76.9%, respectively. Patients with high ERRα
expression had significantly lower OS and DFS rates compared with
patients with low ERRα expression (both P<0.001; Fig. 3A and B).
The univariate analysis was conducted to identify
prognostic factors for CRC patients. It was revealed that in
patients with CRC, ERRα expression was significantly associated
with decreased rates of OS and DFS (P<0.05; Table II). Furthermore, tumor
differentiation, tumor invasion, lymph node status and Dukes stage
were also significantly associated with decreased rates of OS and
DFS in patients with CRC (Table II).
These results suggested that ERRα is a valuable prognostic factor
in CRC. Therefore, multivariate analysis was performed using the
Cox proportional hazards model, which demonstrated that ERRα
expression was an independent prognostic factor for survival in
patients with CRC [OS: Hazard ratio (HR), 2.022; 95% confidence
interval (CI), 1.067–3.835; DFS: HR, 2.375; 95% CI, 1.365–4.133;
Table III].
 | Table II.Univariate Cox regression analyses
for overall survival and disease-free survival in patients with
CRC. |
Table II.
Univariate Cox regression analyses
for overall survival and disease-free survival in patients with
CRC.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Clinical
features | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex | 1.289
(0.671–2.475) | 0.446 | 1.300
(0.730–2.318) | 0.373 |
| Age | 1.215
(0.644–2.292) | 0.548 | 1.201
(0.698–2.067) | 0.507 |
| Tumor location | 1.387
(0.728–2.644) | 0.320 | 1.121
(0.641–1.962) | 0.689 |
| Tumor size | 1.409
(0.718–2.768) | 0.319 | 1.267
(0.718–2.236) | 0.413 |
| Tumor
differentiation | 2.217
(1.108–4.434) | 0.024 | 1.947
(1.096–3.458) | 0.023 |
| Tumor invasion | 2.156
(1.098–4.233) | 0.026 | 1.809
(1.026–3.189) | 0.041 |
| Lymph node
status | 2.035
(1.037–3.995) | 0.039 | 2.012
(1.136–3.563) | 0.016 |
| Dukes stage | 2.192
(1.149–4.181) | 0.017 | 2.386
(1.350–4.217) | 0.003 |
| ERRα
expression | 2.023
(1.024–3.997) | 0.043 | 2.323
(1.292–4.176) | 0.005 |
 | Table III.Multivariate Cox regression analyses
for overall survival and disease-free survival in patients with
CRC. |
Table III.
Multivariate Cox regression analyses
for overall survival and disease-free survival in patients with
CRC.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Clinical
features | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Tumor
differentiation | 2.347
(1.208–4.560) | 0.012 | 1.952
(1.124–3.391) | 0.018 |
| Tumor invasion | 2.300
(1.184–4.467) | 0.014 | 1.955
(1.123–3.402) | 0.018 |
| Lymph node
status | 2.299
(1.197–4.417) | 0.012 | 2.122
(1.216–3.702) | 0.008 |
| Dukes stage | 2.209
(1.187–4.111) | 0.012 | 2.474
(1.436–4.263) | 0.001 |
| ERRα
expression | 2.022
(1.067–3.835) | 0.031 | 2.375
(1.365–4.133) | 0.002 |
Discussion
ERRα is a key regulator in energy-driven cellular
processes through the modulation of mitochondrial function and
metabolism. Cancer cells demonstrate high proliferating
capabilities and require constant biosynthesis of macromolecules as
building blocks for the generation of new cells, thereby resulting
in a high-energy demand for cancer cells (14). ERRα demonstrates key regulatory roles
in a variety of malignant processes, including proliferation,
invasion, metastasis and chemotherapy resistance (15–17). Since
ERRα serves a crucial role in cancer initiation, development and
progression, it may be speculated that ERRα holds significant
clinical significance for patients with cancer. ERRα expression was
reported to be elevated in a variety of cancer types. A previous
study revealed increased expression of ERRα mRNA in ovarian cancer
compared with healthy ovaries (18).
In addition, the ERRα-positive group exhibited statistically
significant reduced OS rates compared with the ERRα-negative group
(18). In another study, the levels
of ERRα mRNA increased with the clinical stage of ovarian cancer,
thus making ERRα a prognostic factor for ovarian cancer (19). In endometrial adenocarcinoma, the
expression of ERRα mRNA was positively correlated with the
International Federation of Gynecology and Obstetrics stage and
myometrial invasion, indicating that ERRα participates in the
tumorigenesis of endometrial adenocarcinoma, and may be a promising
prognostic factor (20). Through the
use of immunohistochemistry on prostate cancer specimens,
researchers demonstrated that nuclear ERRα expression was
significantly higher in the cancerous lesions compared with that in
benign epithelia (21). Elevated ERRα
expression in cancerous lesions was significantly correlated with
poor cancer-specific survival rates in patients with prostate
cancer (21). In human breast
carcinoma, ERRα immunoreactivity was significantly associated with
increased recurrence and shorter survival times (22). Although ERRα expression has been
reported to be associated with CRC tumor progression (9), the clinical and prognostic significance
of ERRα in CRC remains unclear.
In the present study, RT-qPCR was performed to
detect the mRNA expression of ERRα in 15 primary CRC tissues and
adjacent normal tissues. The result demonstrated that ERRα mRNA
expression was significantly higher in primary CRC tissues compared
with in adjacent normal tissues. Furthermore, immunohistochemistry
confirmed the results of the RT-qPCR analysis whereby an increased
number of CRC tissues (81/127, 63.8%) exhibited high ERRα protein
expression compared with adjacent normal tissues (34/127, 26.8%).
These results were consistent with a previous study whereby
elevated levels of ERRα mRNA were detected in CRC tumor tissues
when compared with normal mucosa (9).
Furthermore, immunohistochemistry from 127 patients with CRC
indicated that ERRα expression was significantly associated with
tumor differentiation, tumor invasion, lymph node status, Dukes
classification and distant metastasis, suggesting that ERRα may be
involved in the progression of CRC.
Prognostic biomarkers provide useful information for
doctors in the clinical treatment of patients with CRC, and they
are conducive for identifying patients who have a higher
probability of recurrence or developing chemoresistance. In the
current study, the potential for ERRα as a CRC prognostic biomarker
was demonstrated. High ERRα expression was associated with lower
5-year OS and DFS compared with patients with low ERRα expression.
Multivariate analysis was performed, which identified ERRα
expression as an independent prognostic factor for patients with
CRC. Thus, ERRα detection may be valuable for prognosis evaluation
and personalized therapy in patients with CRC. Tumor recurrence is
an important cause for poor survival of patients with CRC. In the
present study, compared with patients with low ERRα expression,
patients with high ERRα expression exhibited a higher recurrence
rate. These results are in accordance with another study whereby
high ERRα expression was associated with an increased risk of
recurrence in patients with breast cancer (22).
A variety of molecular mechanisms may underlie the
associations between ERRα expression, and recurrence and survival
in CRC. Firstly, ERRα contributes to CRC recurrence through
promoting CRC cell proliferation. A recent study demonstrated that
ERRα significantly enhanced CRC cell proliferation, colony
formation and accelerated cell cycle transition from the G1 to the
S phase (23). ERRα also promotes the
growth of human lung cancer cells, which is not a hormone-dependent
cancer (16), indicating the effect
of ERRα on cell proliferation is universal in various cancer types.
MicroRNAs may negatively regulate ERRα gene expression at the
post-transcriptional level and by silencing ERRα expression,
miR-137 reduced the proliferation of breast cancer cells and
miR-125a reduced the proliferation of oral squamous cell carcinoma
cells (24,25). Secondly, ERRα may promote invasion and
metastasis of colorectal cancer cells through
epithelial-mesenchymal transition (EMT) process. Invasion and
metastasis are important clinicopathological factors for patients
with CRC with unfavorable prognosis, and they are induced by EMT at
the molecular level (26). Previous
studies have reported that ERRα promotes invasion and metastasis by
inducing EMT in lung cancer, and ovarian cancer cells (16,27).
Therefore, we hypothesize that EMT mediated by ERRα underlies the
mechanism for the association between high ERRα expression and poor
prognosis in patients with CRC. Thirdly, ERRα may be involved in
the activation of CRC stem cells. In CRC, EMT induces the
generation of CRC stem cells, which have high metastatic potential
(28). As a positive regulator of EMT
in several cancer types (16,27), ERRα may increase the number of CRC
stem cells from residual cancer cells in patients with CRC
following curative resection, thereby contributing to recurrence. A
previous study revealed that ERRα, combined with PGC1α, activates
the promoter of osteopontin (OPN) gene and leads to elevated OPN
expression in CRC cells (29). OPN is
a glycoprotein secreted by a variety of tissues and enhances cancer
stem cell phenotypes in various cancer types (30,31).
Higher levels of OPN are produced by macrophages when co-cultured
with cluster of differentiation 44-positive CRC stem cells,
subsequently increasing the tumorigenicity of the CRC cells
(32). Given the fact that ERRα is
able to increase the tumorigenic capacity of CRC cells (23), we hypothesize that ERRα-induced OPN
expression may enhance the CRC cell phenotype and promote the tumor
recurrence in patients. However, the detailed mechanisms require
further investigation.
In conclusion, the results of the present study
revealed that ERRα expression was higher in CRC tissues compared
with in adjacent normal tissues, and ERRα expression was associated
with the progression of CRC. In addition, high ERRα expression was
associated with lower OS and local recurrence, and was identified
as an independent prognostic factor for patients with CRC. These
results suggest that ERRα is a promising therapeutic target for
patients with CRC.
Acknowledgements
The present study was supported by Regional Science
Fund Project of China Natural Science Foundation (grant nos.
81660498, 81360347 and 81560493), by China Postdoctoral Science
Foundation, the 60th Grant Funding of General Program for the
Post-Doctoral Funding Program in the Western Region (grant no.
2016M602919XB), by Guangxi Natural Science Foundation (grant nos.
2016GXNSFBA380090 and 2015GXNSFAA139128), by Self-raised Scientific
Research Funds of Ministry of Health of Guangxi Province (grant
nos. Z2015605 and Z2016480), by Funds of Development of Appropriate
Civilization Health Technologies of Guangxi (grant no.
S2017101).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karsa LV, Lignini TA, Patnick J, Lambert R
and Sauvaget C: The dimensions of the CRC problem. Best Pract Res
Clin Gastroenterol. 24:381–396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kriza C, Emmert M, Wahlster P,
Niederländer C and Kolominsky-Rabas P: Cost of illness in
colorectal cancer: An international review. Pharmacoeconomics.
31:577–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berg M and Søreide K: Genetic and
epigenetic traits as biomarkers in colorectal cancer. Int J Mol
Sci. 12:9426–9439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pritchard CC and Grady WM: Colorectal
cancer molecular biology moves into clinical practice. Gut.
60:116–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giguère V: Transcriptional control of
energy homeostasis by the estrogen-related receptors. Endocr Rev.
29:677–696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horard B and Vanacker JM: Estrogen
receptor-related receptors: Orphan receptors desperately seeking a
ligand. J Mol Endocrinol. 31:349–357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cavallini A, Notarnicola M, Giannini R,
Montemurro S, Lorusso D, Visconti A, Minervini F and Caruso MG:
Oestrogen receptor-related receptor alpha (ERRalpha) and oestrogen
receptors (ERalpha and ERbeta) exhibit different gene expression in
human colorectal tumour progression. Eur J Cancer. 41:1487–1494.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bosman FT, Carneiro F, Hruban R and Theise
N: WHO Classification of Tumours of the Digestive System. 3. 4th.
IARC; Lyon: 2010
|
|
11
|
Sarma DP: The Dukes classification of
colorectal cancer. JAMA. 256:14471986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rudolph A, Toth C, Hoffmeister M, Roth W,
Herpel E, Jansen L, Marx A, Brenner H and Chang-Claude J:
Expression of oestrogen receptor β and prognosis of colorectal
cancer. Br J Cancer. 107:831–839. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deblois G, St-Pierre J and Giguère V: The
PGC-1/ERR signaling axis in cancer. Oncogene. 32:3483–3490. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bianco S, Sailland J and Vanacker JM: ERRs
and cancers: Effects on metabolism and on proliferation and
migration capacities. J Steroid Biochem Mol Biol. 130:180–185.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang JW, Guan BZ, Yin LH, Liu FN, Hu B,
Zheng QY, Li FL, Zhong YX and Chen Y: Effects of estrogen-related
receptor alpha (ERRα) on proliferation and metastasis of human lung
cancer A549 cells. J Huazhong Univ Sci Technolog Med Sci.
34:875–881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen P, Wang H, Duan Z, Zou JX, Chen H, He
W and Wang J: Estrogen-related receptor alpha confers methotrexate
resistance via attenuation of reactive oxygen species production
and P53 mediated apoptosis in osteosarcoma cells. Biomed Res Int.
2014:6160252014.PubMed/NCBI
|
|
18
|
Sun P, Sehouli J, Denkert C, Mustea A,
Könsgen D, Koch I, Wei L and Lichtenegger W: Expression of estrogen
receptor-related receptors, a subfamily of orphan nuclear
receptors, as new tumor biomarkers in ovarian cancer cells. J Mol
Med (Berl). 83:457–467. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujimoto J, Alam SM, Jahan I, Sato E,
Sakaguchi H and Tamaya T: Clinical implication of estrogen-related
receptor (ERR) expression in ovarian cancers. J Steroid Biochem Mol
Biol. 104:301–314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao M, Sun P, Wang J, Zhao D and Wei L:
Expression of estrogen receptor-related receptor isoforms and
clinical significance in endometrial adenocarcinoma. Int J Gynecol
Cancer. 16:827–833. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujimura T, Takahashi S, Urano T, Kumagai
J, Ogushi T, Horie-Inoue K, Ouchi Y, Kitamura T, Muramatsu M and
Inoue S: Increased expression of estrogen-related receptor alpha
(ERRalpha) is a negative prognostic predictor in human prostate
cancer. Int J Cancer. 120:2325–2330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki T, Miki Y, Moriya T, Shimada N,
Ishida T, Hirakawa H, Ohuchi N and Sasano H: Estrogen-related
receptor alpha in human breast carcinoma as a potent prognostic
factor. Cancer Res. 64:4670–4676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bernatchez G, Giroux V, Lassalle T,
Carpentier AC, Rivard N and Carrier JC: ERRα metabolic nuclear
receptor controls growth of colon cancer cells. Carcinogenesis.
34:2253–2261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Li Y, Lou G, Zhao L, Xu Z, Zhang Y
and He F: miR-137 targets estrogen-related receptor alpha and
impairs the proliferative and migratory capacity of breast cancer
cells. PLoS One. 7:e391022012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tiwari A, Shivananda S, Gopinath KS and
Kumar A: MicroRNA-125a reduces proliferation and invasion of oral
squamous cell carcinoma cells by targeting estrogen-related
receptor α: Implications for cancer therapeutics. J Biol Chem.
289:32276–32290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Todosi AM, Gavrilescu MM, Aniţei GM, Filip
B and Scripcariu V: Colon cancer at the molecular level-usefulness
of epithelial-mesenchymal transition analysis. Rev Med Chir Soc Med
Nat Iasi. 116:1106–1111. 2012.PubMed/NCBI
|
|
27
|
Lam SS, Mak AS, Yam JW, Cheung AN, Ngan HY
and Wong AS: Targeting estrogen-related receptor alpha inhibits
epithelial-to-mesenchymal transition and stem cell properties of
ovarian cancer cells. Mol Ther. 22:743–751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Findlay VJ, Wang C, Watson DK and Camp ER:
Epithelial-to-mesenchymal transition and the cancer stem cell
phenotype: Insights from cancer biology with therapeutic
implications for colorectal cancer. Cancer Gene Ther. 21:181–187.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boudjadi S, Bernatchez G, Beaulieu JF and
Carrier JC: Control of the human osteopontin promoter by ERRα in
colorectal cancer. Am J Pathol. 183:266–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pietras A, Katz AM, Ekström EJ, Wee B,
Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT and
Holland EC: Osteopontin-CD44 signaling in the glioma perivascular
niche enhances cancer stem cell phenotypes and promotes aggressive
tumor growth. Cell Stem Cell. 14:357–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao L, Fan X, Jing W, Liang Y, Chen R, Liu
Y, Zhu M, Jia R, Wang H, Zhang X, et al: Osteopontin promotes a
cancer stem cell-like phenotype in hepatocellular carcinoma cells
via an integrin-NF-κB-HIF-1α pathway. Oncotarget. 6:6627–6640.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao G, Wang H, Li B, Huang L, Xue D, Wang
X, Jin H, Wang J, Zhu Y, Lu Y, et al: Reciprocal interactions
between tumor-associated macrophages and CD44-positive cancer cells
via osteopontin/CD44 promote tumorigenicity in colorectal cancer.
Clin Cancer Res. 19:785–797. 2013. View Article : Google Scholar : PubMed/NCBI
|