Introduction
Cervical cancer is one of the most frequently
occurring malignant tumors and the fourth leading cause of
cancer-related mortality in females worldwide, causing about
300,000 deaths every year. More than 75% of these cases and deaths
occur in developing countries (1).
The disproportionately high burden of cervical cancer in developing
countries and elsewhere medically underserved populations is
principally because of lacking screening that can prevention and
detection of early-stage cervical cancer (2). Therefore, it is very important to study
a method to reliably predict disease outcome and reduce risk of
treatment failures.
Protein-coding genes account for only about two
percent of the human genome, whereas the overwhelming majority of
transcripts are non-coding RNAs (ncRNAs), including lncRNAs.
LncRNAs are defined as >200 nt in length (3). Their roles include regulating gene
expression at the epigenetic, transcriptional, and
post-transcriptional level in cellular homeostasis (4). As we know that lncRNA studies are still
in their infancy and the functions remain unclear. Up to now, more
and more literature has proved that lncRNA expressions are involved
in cancer metastasis (5,6). Brain cytoplasmic RNA 1 (BCYRN1), also
called BC200, ordinarily cannot be detected in normal tissue,
except for in the primate nervous system (7). Accordance to the literature, lncRNA
BCYRN1is strongly expressed in some carcinomas of the cervic,
oesophagus and lung, but not detected in normal tissues (8,9).
MicroRNAs (miRNAs), a class of short ncRNA molecules
ranging in size from 19 to 25 nucleotides (nt), is one of the
well-characterized classes of ncRNAs. miRNAs have been recognized
as a new class of genes involved in human cancer and have recently
been shown to be biomarkers of diagnostic, prognostic and
therapeutic (10,11). Several studies have identified some
miRNAs that were significantly altered and have the potential to
restrain proliferation of cervical cancer (12,13).
Previous studies suggest that miRNA-138 restrains cervical cancer
cells proliferation (14). Although a
large number of ncRNA research has focused on the regulation of
protein-coding genes mediated by them, it suggests that miRNAs and
lncRNAs can interact with each other, especially in transcriptional
regulation.
In the present study, we demonstrated that BCYRN1
was upregulated in cervical cancer tissues and cell lines. BCYRN1
was then knockdown by transfecting small interfering RNA (siRNA) in
HeLa cells. BCYRN1 siRNA suppressed the viability and mobility of
cervical cancer in vitro and in vivo and may through
targeting miR-138. BCYRN1 may serve as a novel target for cervical
cancer treatment.
Materials and methods
Patients and materials
The experiments were undertaken with the
understanding and written consent of each subject. Informed consent
was obtained from each patient recruited, and the study protocol
was approved by the Human Ethics Committee of Wuzhong People's
Hospital in Suzhou (Jiangsu, China). Cervical cancer tissues were
collected from 25 cases of cervical cancer patients who underwent
curative surgery from January 2005 to May 2014 at Wuzhong People's
Hospital in Suzhou (Jiangsu, China) (Table I). And normal cervix healthy subjects
were as normal control. On removal of the surgical specimen,
researchers immediately transferred the tissues to the surgical
lab. Each sample was snap-frozen in liquid nitrogen before RNA
analysis.
 | Table I.Clinicopathological variables in
cervical cancer patients. |
Table I.
Clinicopathological variables in
cervical cancer patients.
| Variable | Cases |
|---|
| Age,
yearsa |
|
|
<55 | 15 |
| ≥55 | 10 |
| Sex |
|
|
Female | 25 |
| Pathological
classification |
|
| Squamous
classification | 16 |
|
Adenoma | 7 |
|
Other | 2 |
| Clinical stage |
|
| 0 | 3 |
| I | 7 |
| II | 10 |
|
III | 4 |
| IV | 1 |
| Response to
chemotherapy |
|
| No or
poor | 12 |
|
Moderate | 5 |
| Chemotherapy
regimen |
|
|
5-fluorouracil | 12 |
|
Cyclophosphamide | 7 |
|
Cisplatin | 11 |
|
Doxorubicin | 6 |
Cell culture
Three human cervical cancer cell lines (SiHa, HeLa
and CaSki) were cultured in this study. They were purchased from
Cell Bank of the Chinese Academy of Science (Shanghai, China). A
non-cancerous ectocervical epithelial cell line (Ect1/E6E7) was
purchased from the Health Science. All cells were cultured in DMEM
(Gibco-BRL, Grand Island, NY, USA), including 10% fetal bovine
serum (FBS; Hyclone; Invitrogen, Camarillo, CA, USA), as well as
100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen,
Carlsbad, CA, USA). Cells were maintained in a humidified incubator
(37°C, 5% CO2).
RT-PCR analysis
Total RNA from cervical cancer tissues and cells was
extracted using Trizol reagent (Invitrogen). RNA was transcribed
into cDNAs using the PrimerScript one-step RT-PCR kit (Takara,
Dalian, China). Then cDNA template was amplified by RT-PCR (SYBR
Premix Dimmer Eraser kit; TaKaRa). GADPH expression level was as a
standardization for each gene expression. The primer sequences were
as follows: BCYRN1 forward, 5′-CTGGGCAATATAGCGAGAC-3′ and reverse,
5′-TGCTTTGAGGGAAGTTACG-3′; GAPDH forward,
5′-GTCAACGGATTTGGTCTGTATT-3′ and reverse,
5′-AGTCTTCTGGGTGGCAGTGAT-3′. The condition of RT-PCR was 95°C for 2
min, followed by 40 cycles of 15 sec at 95°C, and at last 1 min at
55°C. The 2−ΔΔCt method was used to calculate the
relative expression fold change of mRNAs.
Northern blot analysis
Total RNAs were extracted by the TRIzol (Gibco-BRL)
method. Poly(A)+ RNA was isolated using an Oligotex mRNA
midi kit (Qiagen GmbH, Hilden, Germany) following the
manufacturer's instructions. Analysis of RNA expression was
performed by Northern blotting as previously described (15). Total RNA was fractionated on a
denaturing 12% polyacrylamide gel containing 8 M urea, transferred
to Nytran N membrane (Schleicher and Schuell, Germany, Dassell,
Germany) by capillary method and fixed by ultraviolet
cross-linking. Prehybridization of the filters was carried out in
50% formamide, 0.5% SDS, 5X SSPE, 5X Denhardt's solution and 20
µg/ml sheared, denatured salmon sperm DNA. Filters were washed at
70°C in 2X SSC and 1% SDS for 10 rain, in 0.2X SSC and 0.5% SDS for
the next 10 rain, and then in 0.1X SSC and 0.l% SDS for 20 min. The
filter for detecting the EP4 mRNA was treated with 1 pg/ml RNase A
at 20°C for 10 min, and washed again at 70°C in 0.1X SSC and 0.1%
SDS for 10 rains.
Cell transfection
hsa-miRNA-138 mimic/negative-control mimic and
hsa-miRNA-138 inhibitor/negative-control inhibitor were purchased
from Genechem (Shanghai, China). The BCYRN1 siRNAs were synthesized
by Invitrogen. BCYRN1 siRNA (targeted region) sequences were as
follows: siRNA_1, CGCCUGUAAUCCCAGCUCUCA; siRNA_2,
AUAAGCGUAACUUCCCUCAAA; siRNA_3
CGUAACUUCCCUCAAAGCAACAACC. pcDNA-3.1(+)-BCYRN1
(lncRNA-BCYRN1), the overexpression plasmid of BCYRN1 was
constructed from Genechem. According to the manufacturer's
instructions, transfections were performed using the Lipofectamine
2000 kit (Invitrogen).
CCK-8 analysis
Cervical cancer cells were seeded in a 96-well plate
(5×103 cells/well) for 24 h. Then the cultured cells
were transfected with miR-138 mimic or BCYRN1 siRNA for 48 h. Then
CCK-8 reagent (5 mg/ml) was added to each well and incubated in
dark at 37°C for 2 h. Finally, the absorbance was determined with
the wavelength of 490 nm.
Flow cytometric analysis
Cells transfected with desired plasmid or negative
control were plated in 6-well plates. After incubation for 48 h, 3
µg/ml Annexin V-FITC and 5 µg/ml propidium iodide was used to
staining at RT for 30 min in the dark. Then cultures were collected
and analyzed using a FACSCalibur™ flow cytometer (BD
Biosciences, San Jose, CA, USA) with Beckman CXP software (Beckman
Coulter, Inc., Brea, CA, USA).
Western blot analysis
The proteins, extracted from tissues and cultured
cells, were separated through SDS-PAGE and then were transferred
onto polyvinylidene fluoride (PVDF) membranes (Millipore,
Billerica, MA, USA). The membranes were blocked in PBST (PBS with
0.1% Tween-20) containing 5% non-fat milk for 2 h at room
temperature, and then were incubated with the primary antibodies:
Anti-Ki67, anti-proliferation cell nuclear antigen (PCNA),
anti-caspase-3, anti-caspase-9, anti-matrix metalloproteinase
(MMP)-9, anti-vascular endothelial cell growth factor (VEGF),
anti-SOX-4, anti-GAPDH and corresponding HRP-conjugated secondary
antibodies. Membranes were extensively washed several times with
PBST. Proteins were detected using a ChemiDoc XRS imaging system
and Quantity One analysis software (Bio-Rad, San Francisco, CA,
USA). GAPDH (Abcam, Cambridge, UK) was used as an endogenous
reference.
Luciferase reporter assays
The 3′-UTR of BCYRN1 was PCR amplified from human
genomic DNA, and cloned downstream of the firefly luciferase coding
region of the pMIR-GLO™Luciferase vector (Promega, Madison, WI,
USA). The recombinant vector was called pMIR-BCYRN1-wild-type.
Mutations in miR-138 binding sites were introduced by site-directed
mutagenesis and the resulting vector was called pMIR-BCYRN-mutant.
Cells were seeded into 24-well plates and co-transfected with 200
ng of pMIR-BCYRN1 or pMIR-BCYRN1-mut vector and miR-138 mimic or
miR-138 inhibitor. Cells were harvested and then lysed (lysis
buffer; Promega), after 36 h. The luciferase reporter gene assay
was detected by the Dual-Luciferase Reporter Assay system (Promega,
Shanghai, China), according to the manufacturer's instructions.
Invasion and migration assays
For the invasion assay, after 48 h of transfection,
2×104 HeLa cells were cultured without FBS in the upper
chamber of an insert (Chemicon, Temecula, CA, USA). Matrigel
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to precoat
the chamber. Then the lower chambers were incubated for 24 h in
culture medium with 10% FBS, before examination. On the upper
surface, HeLa cells were scraped and washed away, whereas the
invaded cells on the lower surface were fixed and stained with
Diff-Quik (Sysmex, Kobe, Japan) at RT for 2 h. The invading cells
were observed and counted under a DMLB2 light microscope (Leica,
Wetzlar, Germany) at a magnification of ×200 in 10 random fields in
each well. For the migration assay, approximately
1.5×106 cells/well pretreated with BCYRN1 siRNA or
siRNA-scramble were seeded in 6-well plate and cultured overnight
until the cells reached 90% confluence. Then a straight scratch was
created by a sterile pipette tip. After rinsing off the destroyed
cells with PBS, the plate was cultured in medium for another 24 h.
Cell migration was observed using an Olympus IX 70 microscope and
imaged at 0 and 24 h with a digital camera (Leica DFC300FX).
Subcutaneously xenografted mouse
model
All animal experiments were carried out in
accordance with a protocol approved by the Institutional Animal
Care and Use Committee (IACUC). HeLa cells were transfected with
BCYRN1 siRNA or siRNA-scramble for 24 h. Then, 4×106
cells were subcutaneously inoculated into 6–8 weeks old male
athymic nude mice. After tumors (100–150 mm3) had
established, the tumor volume was measured every 5 days using the
same protocol, and calculated in length ×
(width2)/2.
Immunohistochemistry
Formalin-fixed paraffin-embedded sections (5 µM)
from tissue microarrays were prepared. They were deparaffinized in
xylene and rehydrated then were incubated in 30%
H2O2 to quench the activity of endogenous
peroxidase. Then the sections were incubated with primary
antibodies directed against MMP-2 and VEGF overnight at 4°C.
Proteins were visualized under a light microscopy.
Statistical analysis
All data were processed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). For statistical analysis, quantitative data from
at least three experiments were compared and are expressed as the
mean ± SD. Analysis of variance (ANOVA) was used for comparison
among groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
BCYRN1 is upregulated in cervical
cancer
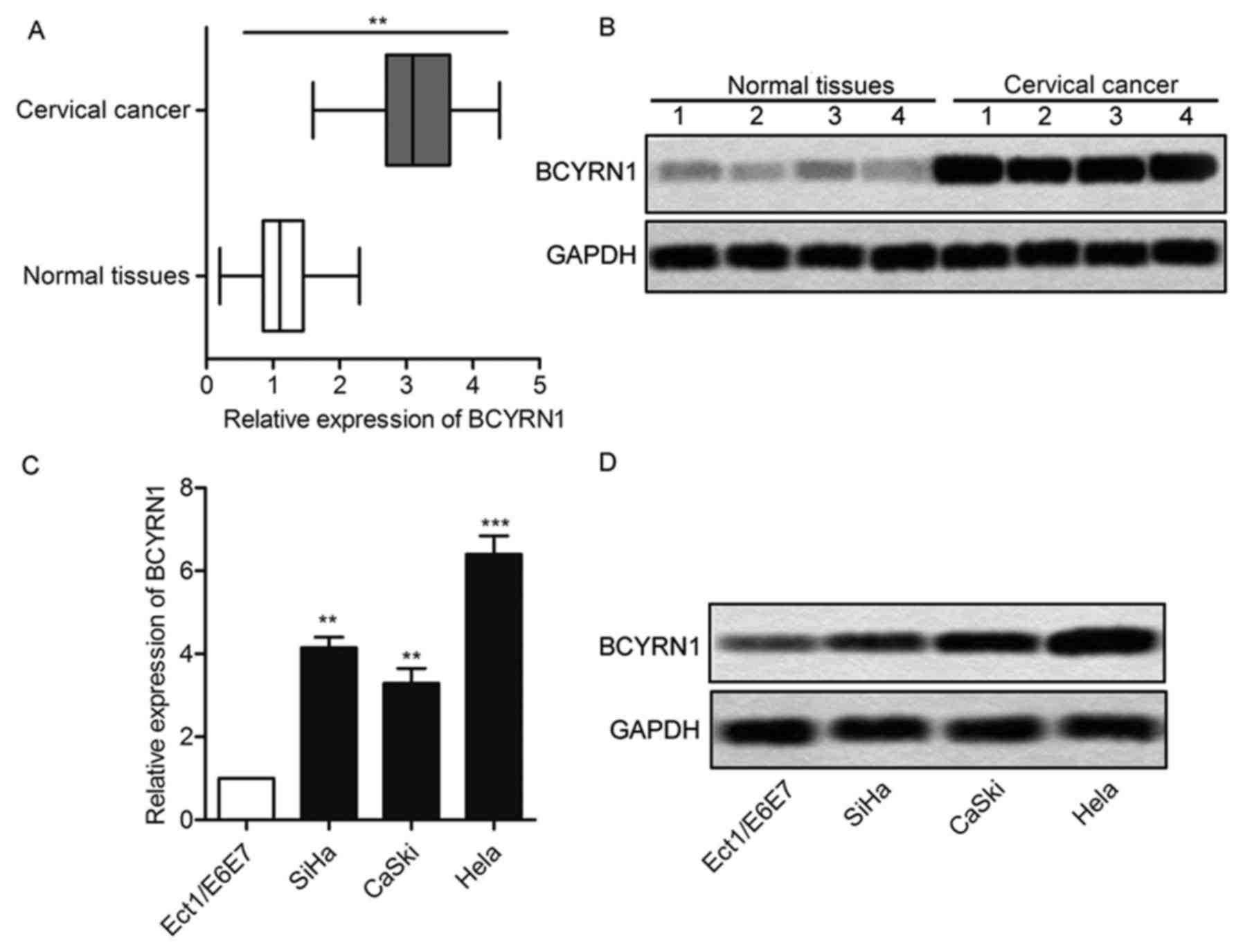
BCYRN1 relative expression was evaluated using
RT-PCR and northern blot from 25 cervical cancer patients. BCYRN1
was upregulated in cervical cancer tissues compared with normal
tissues (Fig. 1A and B; P<0.01).
Then, we examined the mRNA expression level of BCYRN1 in cervical
cancer cells. The non-cancerous ectocervical epithelial cell line
(Ect1/E6E7) was used as normal control. Among the cervical cancer
cells, the BCYRN1 expression were notably upregulated as compared
with that in the Ect1/E6E7 cells (Fig. 1C
and D; P<0.01). We selected HeLa cells for BCYRN1 knockdown
and the following experimental study, as it harbored the highest
expression level of BCYRN1.
Silencing BCYRN1 attenuates the
viability and motility of cervical cancer
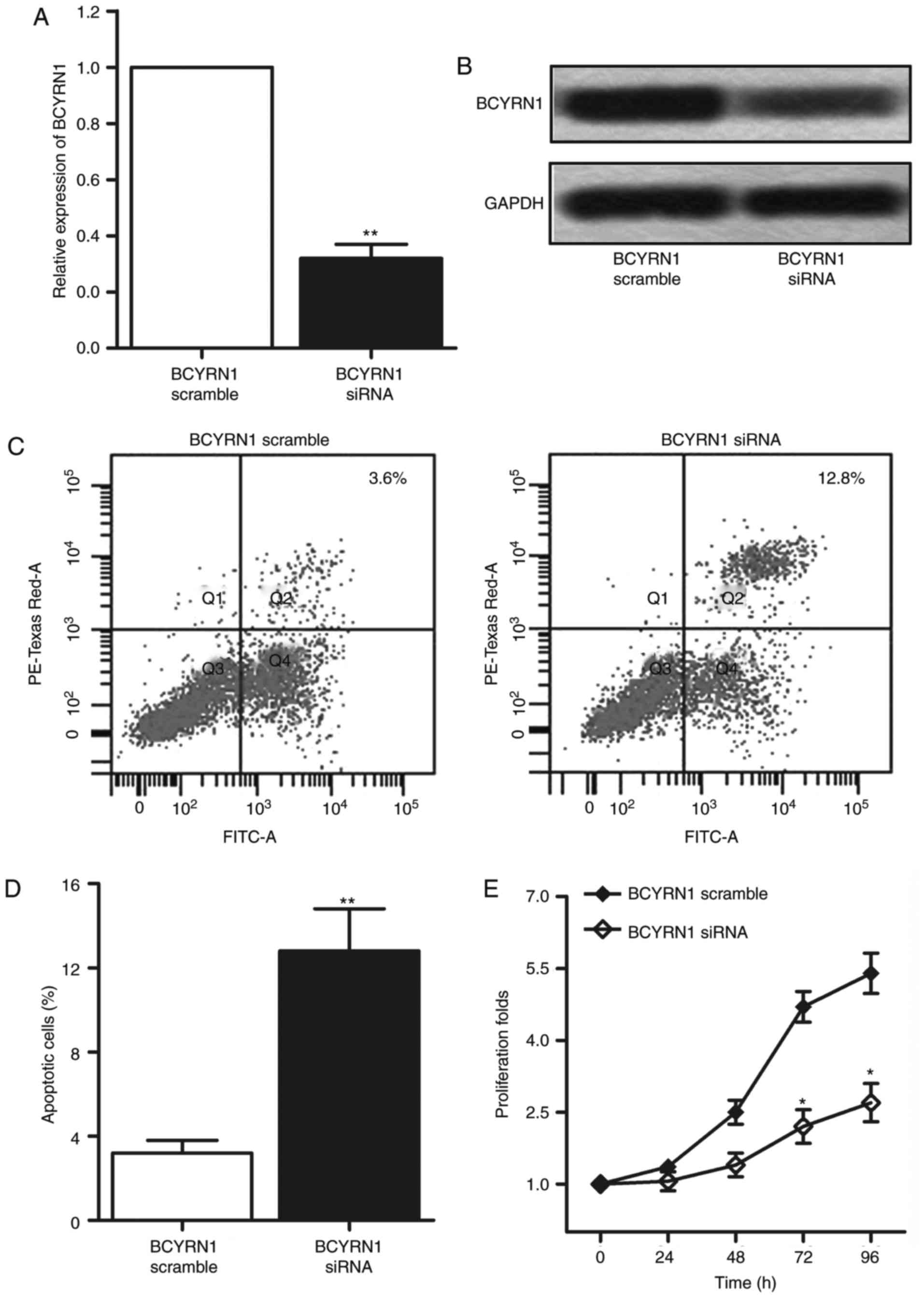
Then we evaluated the in vitro effects of
BCYRN1 in cervical cancer by transfection of 3 different siRNAs of
BCYRN1 into HeLa cells. Then an optimal knockdown siRNA (siRNA_1)
was chosen in this study. The BCYRN1 siRNA caused a significant
downregulation of the mRNA expression of BCYRN1 (Fig. 2A and B; P<0.01). Flow cytometric
analysis showed that HeLa cells apoptosis was markedly increased
after transfection with BCYRN1 siRNA (Fig. 2C and D; P<0.01). Next, CCK-8 assay
was performed to detect the cell viability. Data showed that
transfection with BCYRN1 siRNA distinctly suppressed the
proliferation of HeLa cells (Fig. 2E;
P<0.05). Given that the inhibition of BCYRN1 reduced cell
viability in HeLa cells, further experiments was conducted to
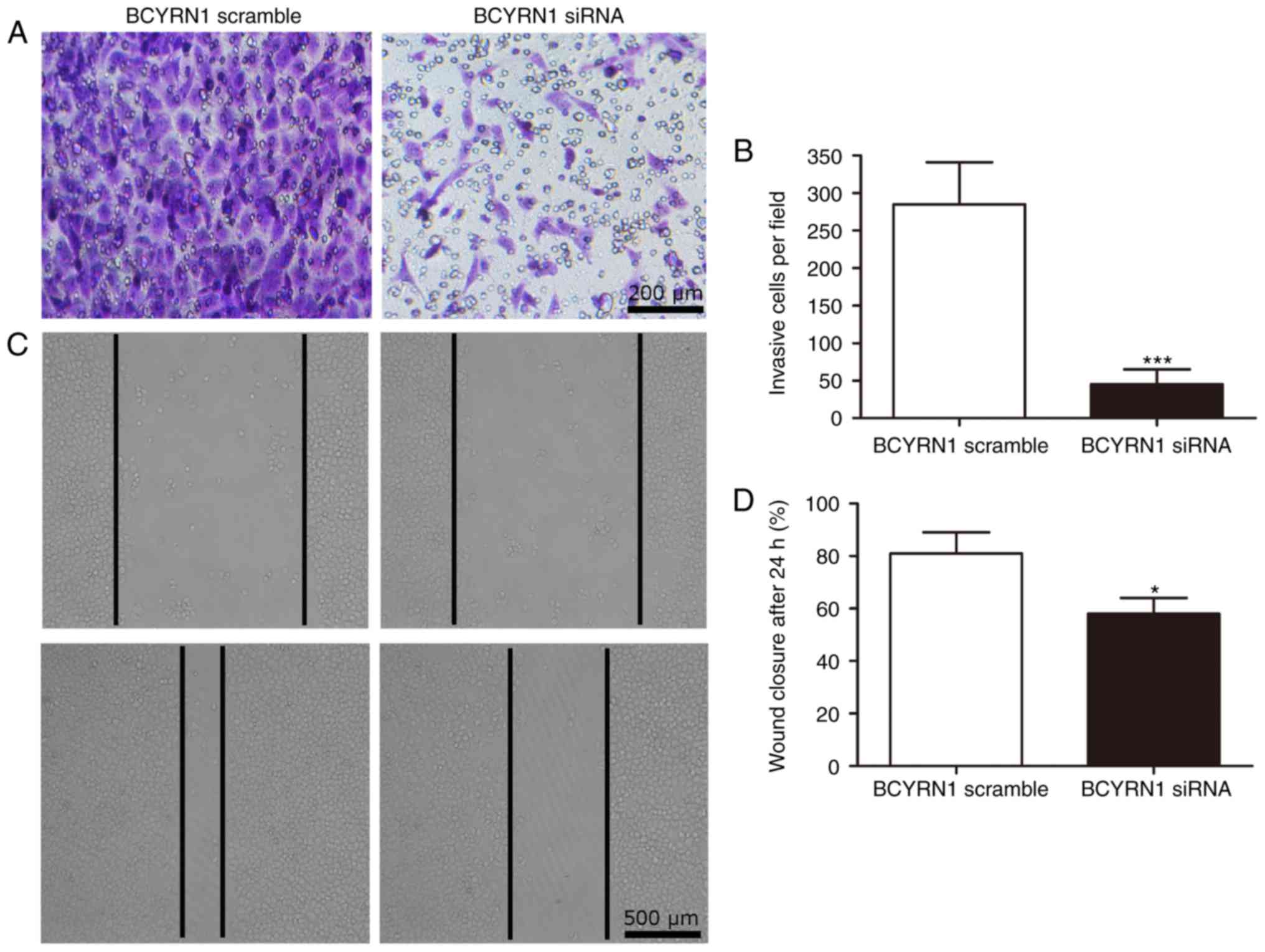
examine the effect of BCYRN1 on cell motility. The result of
transwell invasion assay showed that invasion cells was noticeably
declined in HeLa cells transfected with BCYRN1siRNA (P<0.001;
Fig. 3A and B). By comparing the
closure of the gap at 0 and 24 h later after transfection, a
significantly decreased closing rate of scratch wounds was detected
in BCYRN1 siRNA group compared with the siRNA scramble group
(P<0.05; Fig. 3C and D). The
results above indicated that inhibition of BCYRN1 suppressed cell
viability and motility in cervical cancer.
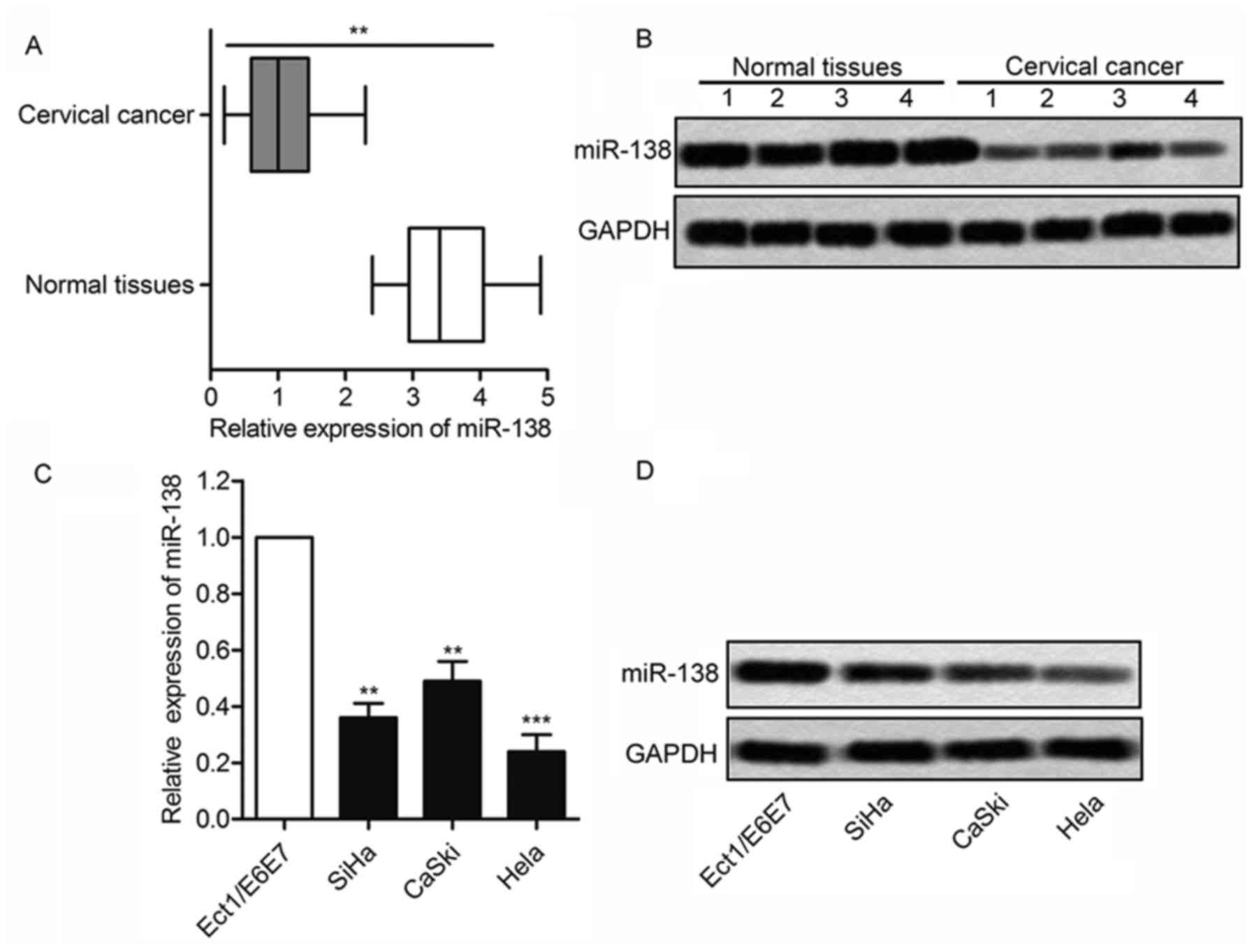
MiRNA-138 is downregulated in cervical
cancer
As shown in Fig. 4A and
B, there was a 3–4-fold decrease in the expression level of
miR-138 in the 25 tumors than the normal tissues (P<0.01).
miR-138 level also was downregulated in cervical cancer cell lines
compared with noncancerous ectocervical epithelial cell Ect1/E6E7
(Fig. 4C and D; P<0.01). Above
data indicated that miR-138 may be as an important role in cervical
cancer.
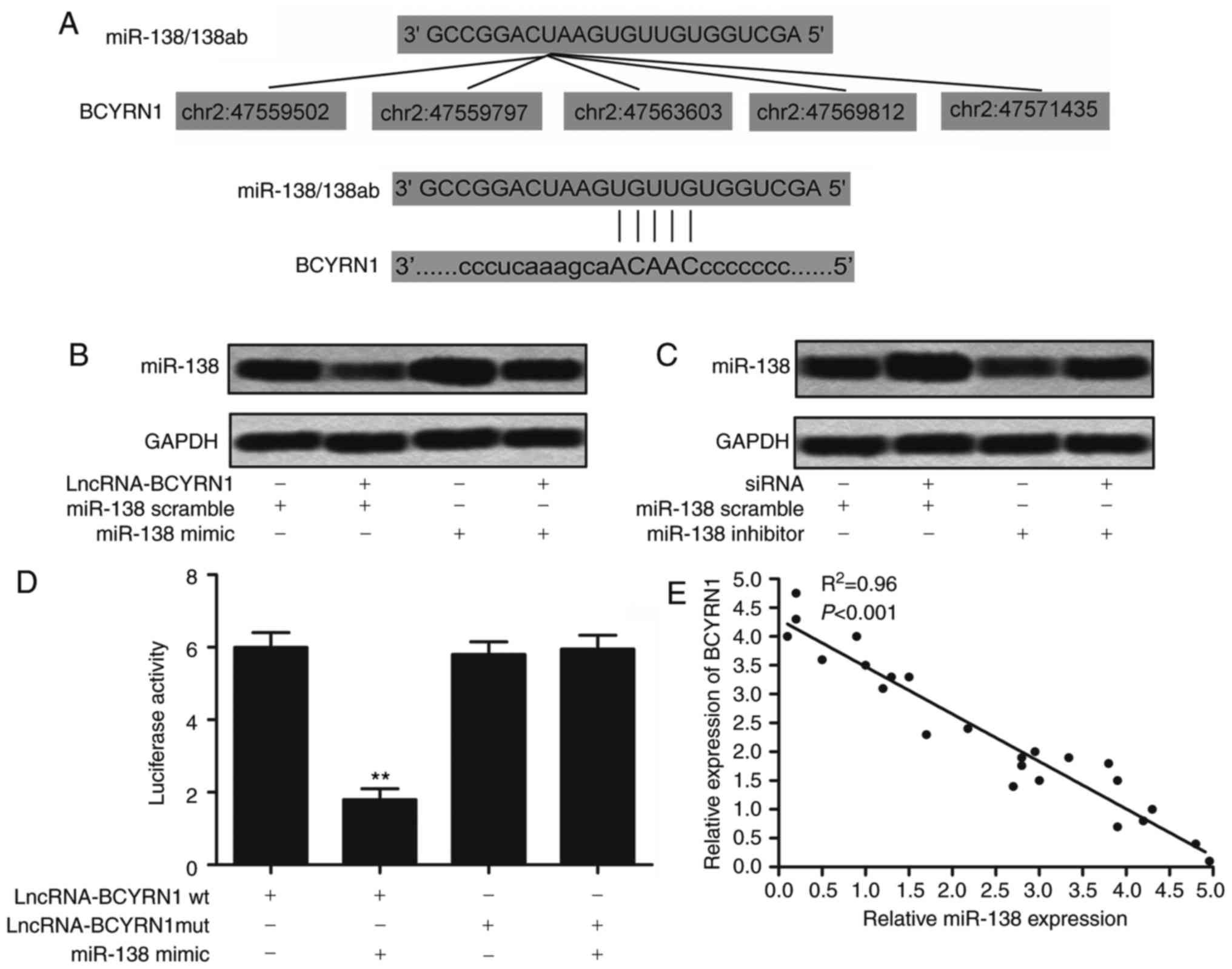
Identification of potential
BCYRN1-targeting miRNA-138
According to the prediction results, we identified
that BCYRN1 contained five seed sequences of miR-138 (Fig. 5A). That meant miRNA-138 may be a
potential targeting of BCYRN1. BCYRN1 was then overexpressed by
transfecting lncRNA BCYRN1 into HeLa cells. It showed that
overexpression of BCYRN1 could restrain the expression of miR-138
(Fig. 5B). But the level of miR-138
was upregulated after silencing of BCYRN1 (Fig. 5C). Luciferase reporter constructs were
generated to further confirm the direct binding between BCYRN1 and
miRNA-138. We observed that miRNA-138 mimic decreased the
luciferase activities of BCYRN1 wt reporter vector. But luciferase
activities in cells transfected with BCYRN1 mut and the miRNA-138
mimic were almost comparable to that of control cells (Fig. 5D; P<0.01). Trend relation of BCYRN1
and miR-138 in 25 patients indicated that BCYRN1 and miR-138 showed
the reverse changes (Fig. 5E;
P<0.001). It suggests that the regulation between miRNA-138 and
BCYRN1 may be in a way similar to the miRNA-mediated silencing of
protein-coding genes. These results confirmed the direct binding
between BCYRN1 and miRNA-138 and negative regulation
relationship.
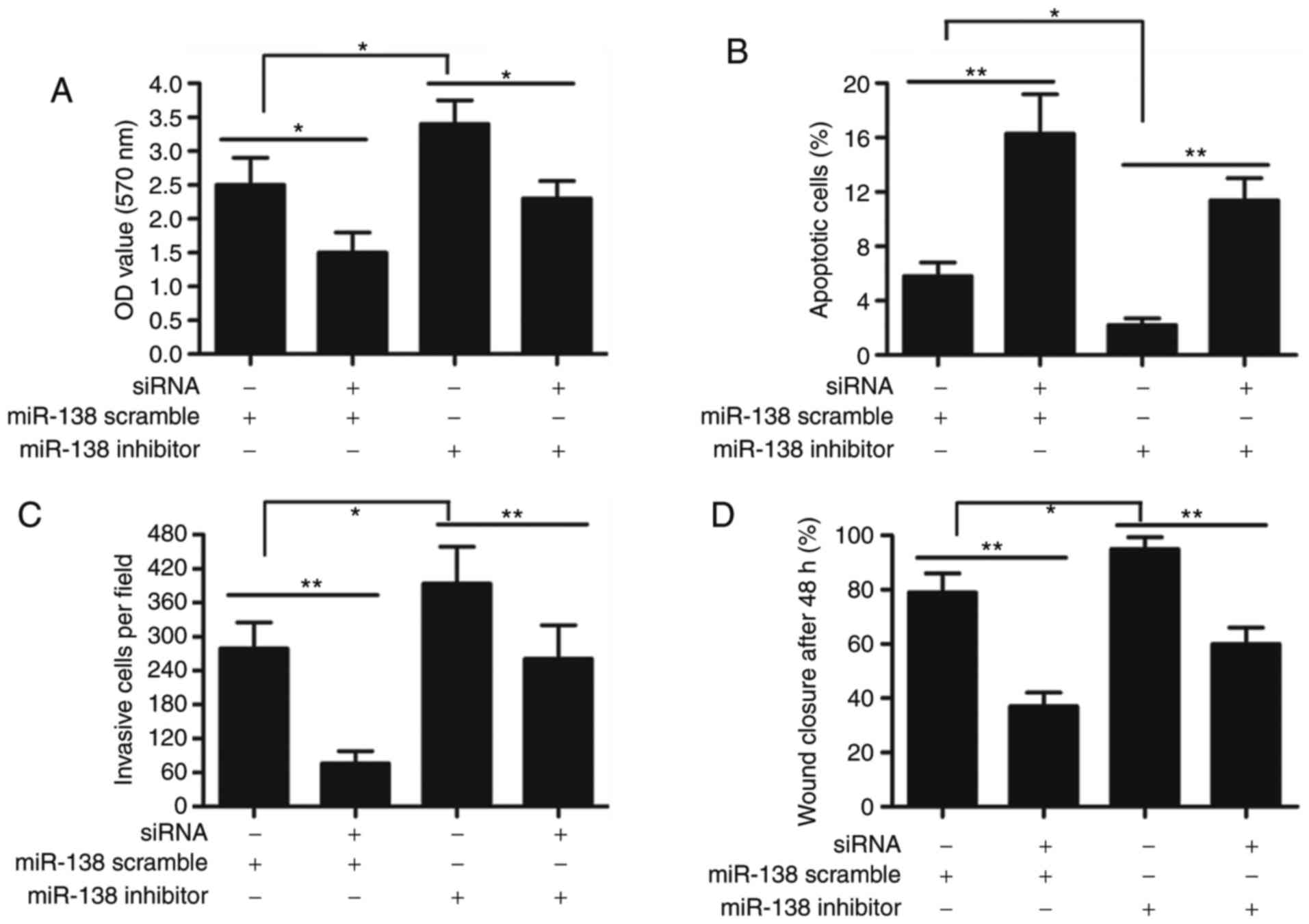
Bucking effect of miR-138 inhibitor on
cervical cancer cell proliferation, invasion and migration
To explore the underlying mechanism of such a
negative regulation between miR-138 and BCYRN1, we detected the
proliferation, apoptosis, invasion and migration in HeLa cells, in
response to BCYRN1 knockdown and miR-138 inhibitor. As illustrated
in Fig. 6, BCYRN1 siRNA significantly
restrained the proliferation, invasion and migration ability of
HeLa cells, but promoted apoptosis. The adding of miRNA-138
inhibitor enhanced cell proliferation, invasion and migration, but
BCYRN1 siRNA had bucking effect for miRNA-138 inhibitor in HeLa
cells (P<0.01).
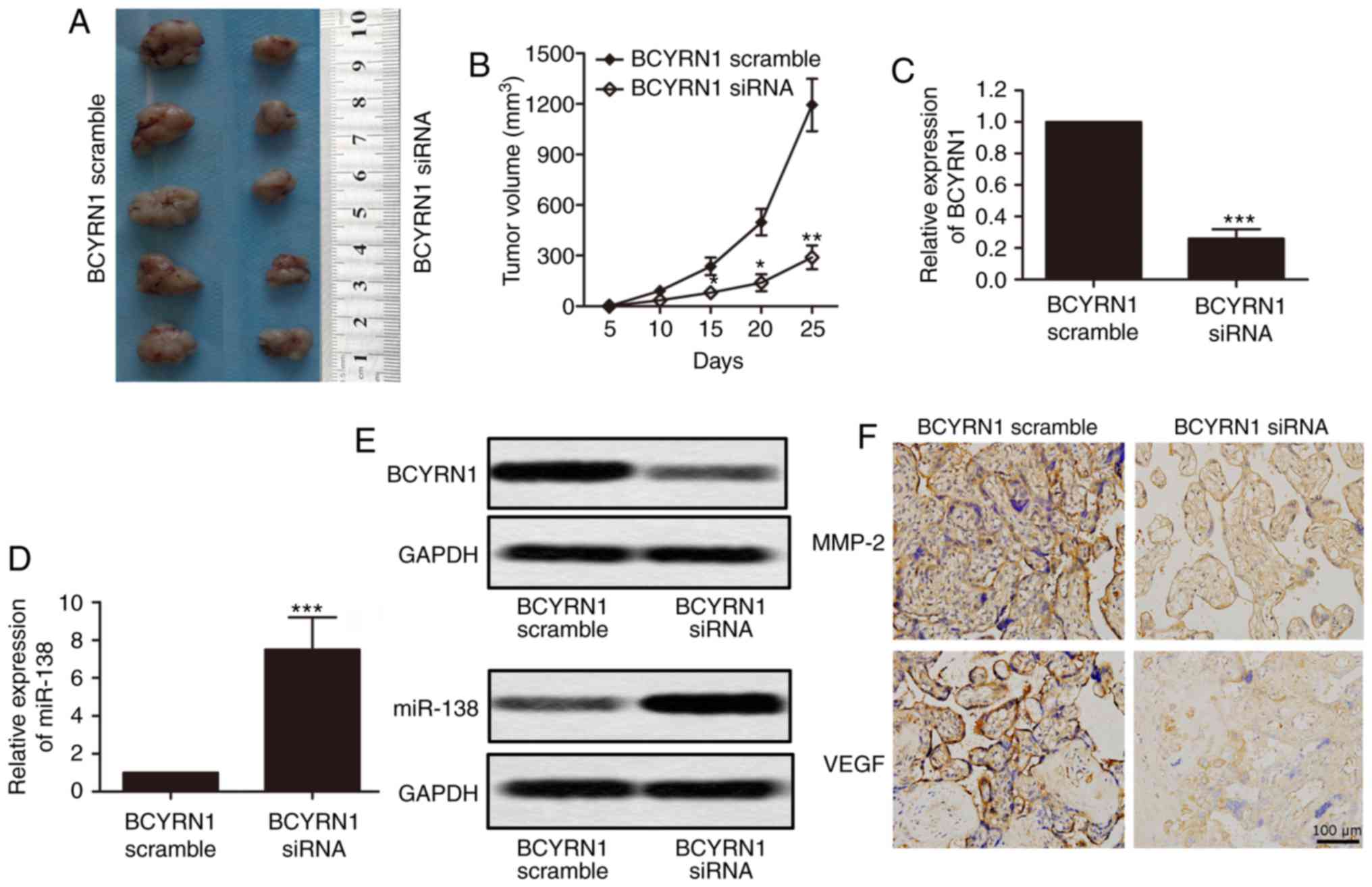
Negative regulation of miRNA-138 in
the oncogenic activity of BCYRN1 in vivo
To provide more evidence to the idea that BCYRN1′s
oncogenic activity is in part through the negative regulation of
miRNA-138, HeLa cells were transfected with BCYRN1 siRNA. The cells
were then injected subcutaneously into nude mice. After cancer cell
inoculation, mice were killed 4 weeks. We observed a decrease in
tumor growth in the BCYRN1 siRNA group, compared with the BCYRN1
scramble group (Fig. 7A and B;
P<0.05). Furthermore, the expression level of BCYRN1 from the
BCYRN1 siRNA group was lower but miR-138 was significantly
increased, compared with BCYRN1 scramble group (Fig. 7C-E; P<0.001). As shown in Fig. 7F, expression of MMP-2 and VEGF, which
are migration marker proteins, were all reduced in BCYRN1 siRNA
group. It indicated that BCYRN1 siRNA depressed cell migration. All
these data verified the previous results.
Discussion
As one of the most common diseases, cervical cancer
is seen to be a major cause of women mortality worldwide. Exploring
the molecular mechanisms of tumorigenesis is conducive to develop
new therapeutics for cervical carcinoma. In this study, it is the
first time to explore the expression pattern and biological
function of lncRNA BCYRN1 and miR-138 in cervical cancer.
There have been studies showing that lncRNAs are
very important in cancer pathogenesis and can provide new insights
into the therapy (16,17). Over the past years, the researches
about miRNAs have dominated the field of lncRNA regulation
(17,18). Nevertheless, the function of lncRNAs
in the tumorigenesis of cervical cancer is still unknown.
Understanding the precise molecular mechanism would accelerate the
development of lncRNA-directed diagnostics and therapeutics against
cancers. LncRNA BCYRN1 is normally not detected in normal tissue.
However, in this study we detected a low BCYRN expression level in
normal cervix tissues. Overexpression of BCYRN1 both in cervical
cancer tissues and cell lines were confirmed using RT-PCR and
northern blotting. The abnormal expression of cancer
metastasis-associated lncRNA may drive tumorigenesis via regulating
suppressive and oncogenic pathways. In order to make a further
exploration, the BCYRN1 siRNA was transfected into HeLa cells. Data
showed that BCYRN1 siRNA depressed cell proliferation, invasion and
migration, but apoptotic cells were increased. However, there might
be a limitation of the present study that unpredictable off-target
effects often happen in siRNAs transfection experiments. Therefore,
more siRNA oligonucleotides for BCYRN1 knockdown will be required
to verify the effects of BCYRN1 knockdown on HeLa cells in further
research.
miRNAs are considered as a group of small non-coding
RNA molecules that exhibit different roles in some biological
behaviors, for instance, cell apoptosis, differentiation, and
tumorigenesis (19). Recently some
studies have reported the role of miRNAs in modulating invasion and
metastasis of cervical cancer cells. For instance, Deng et
al (20) discovered that
miRNA-142-3p restrained cervical cancer cells proliferation and
invasion, indicating a potential therapeutic approach for cervical
cancer (20). Another study revealed
that miR-3156-3p is reduced in cervical cancer, suggesting as a
tumor-suppressive miRNA (21).
miR-138 has been reported to be inhibited in some cancers,
including aggressive papillary thyroid carcinoma, head and neck
squamous cell carcinoma, and lung cancer tumors (22,23).
Literature research reported that miR-138 inhibits proliferation,
migration and invasion of cervical cancer cells by targeting hTERT
(24,25). In this study, we get similar results
that miRNA-138 was evidently inhibited in the cervical cancer
tissues and cells. Taken together, combining these literature
reports and data we inferred that miR-138 performed as a tumor
suppressor in cervical cancer. However, the biological role of
miR-138 in cervical cancer had not been explored. Thus the next
study aimed to investigate the biological function of miR-138.
A growing number of reports suggest there is a
widespread interaction network between lncRNAs and miRNAs. lncRNAs
could modulate RNA by binding and titrating off their binding sites
on protein coding messengers (26).
An example of this type is lncRNA HULC, highly upregulated in liver
cancer. Its upregulation has inhibitory effects on the expression
and activity of miRNA-372 (27). Ma
et al (28) revealed that
lncRNA CCAT1 enhances gallbladder cancer development via negative
modulation of miRNA-218-5p. Liz et al focus on the interplay
between lncRNAs and miRNAs, and find the importance of such
interactions in the tumorigenic process, providing new insight into
the regulatory mechanisms of several ncRNA classes in cancer
(26). Similar report suggests that
miRNA-29 can regulate expression of the lncRNA gene MEG3 in
hepatocellular cancer and may contribute to human hepatocellular
cancer growth (29). Similar
phenomenon was observed between MALAT1 and miR-145 (30). The interaction between lncRNA and
miRNA remains to be explored.
We made a study for miRNAs that had complementary
base pairing with lncRNA BCYRN1 and fortunately some miRNAs were
identified. We focused on miRNA-138, as it has biological function
in cervical cancer. Inhibiting BCYRN1 increased miRNA-138
expression, while ectopic expression of BCYRN1 induced the
downregulation of miRNA-138 and the miRNA-138-binding site is
requisite for the BCYRN1-mediated repression. As we mentioned
earlier recent reports indicate lncRNAs may exert modulatory effect
via miRNAs. The miRNA-138 was demonstrated to inhibit tumor growth,
while BCYRN1 promoted tumor progression. We explored the biological
aspect of BCYRN1 and miRNA-138 in HeLa cells. Our study exhibited
that although silencing BCYRN1 expression suppressed the
proliferation and migration of HeLa cells, miRNA-138 inhibitor had
a reversed-effect for BCYRN1 siRNA. Thus it suggests that BCYRN1
may promote tumor development through regulating miRNA-138.
Consequently, we further explored the underlying mechanism between
them. The results showed that miRNA-138 inhibitor enhanced cell
proliferation, invasion and migration, but BCYRN1 siRNA had bucking
effect for miRNA-138 inhibitor on cervical cancer cell.
In conclusion, it demonstrated that BCYRN1 increases
cervical cancer cell proliferation and invasion by targeting
miR-138 in vitro and in vivo. Our findings show that
the regulation between BCYRN1 and miR-138 may represent a valuable
therapeutic target for cervical cancer therapy. It suggests another
layer of regulation involving lncRNAs and miRNAs in both molecular
and biological aspects. Further research is needed to identify the
more detailed signal pathways involved in the pathogenesis and
metastasis of cervical cancer that are supposedly regulated by
BCYRN1 and miR-138.
Glossary
Abbreviations
Abbreviations:
|
BCYRN1
|
brain cytoplasmic RNA 1
|
|
miR-138
|
microRNA 138
|
|
lncRNAs
|
long non-coding RNAs
|
|
siRNA
|
small interfering RNA
|
|
PCNA
|
proliferation cell nuclear antigen
|
|
MMP
|
matrix metalloproteinase
|
|
VEGF
|
vascular endothelial cell growth
factor
|
|
qRT-PCR
|
quantitative real-time polymerase
chain reaction
|
|
3′-UTR
|
3′-untranslated region
|
|
CCK-8
|
Cell Counting Kit-8
|
References
|
1
|
Barra F, Lorusso D, Leone Roberti Maggiore
U, Ditto A, Bogani G, Raspagliesi F and Ferrero S: Investigational
drugs for the treatment of cervical cancer. Expert Opin Investig
Drugs. 26:389–402. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
PDQ Screening and Prevention Editorial
Board: Cervical cancer prevention (PDQ®): Health
professional version = PDQ Cancer Information Summaries. National
Cancer Institute; Bethesda, MD: 2002
|
|
3
|
Chen J, Shishkin AA, Zhu X, Kadri S, Maza
I, Guttman M, Hanna JH, Regev A and Garber M: Evolutionary analysis
across mammals reveals distinct classes of long non-coding RNAs.
Genome Biol. 17:192016. View Article : Google Scholar
|
|
4
|
Seles M, Hutterer GC, Kiesslich T, Pummer
K, Berindan-Neagoe I, Perakis S, Schwarzenbacher D, Stotz M, Gerger
A and Pichler M: Current insights into long non-coding RNAs in
renal cell carcinoma. Int J Mol Sci. 17:5732016. View Article : Google Scholar
|
|
5
|
Zheng C, Hao H, Chen L and Shao J: Long
noncoding RNAs as novel serum biomarkers for the diagnosis of
hepatocellular carcinoma: A systematic review and meta-analysis.
Clin Transl Oncol. 19:961–968. 2017. View Article : Google Scholar
|
|
6
|
Gomes CC, de Sousa SF, Calin GA and Gomez
RS: The emerging role of long noncoding RNAs in oral cancer. Oral
Surg Oral Med Oral Pathol Oral Radiol. 123:235–241. 2017.
View Article : Google Scholar
|
|
7
|
Booy EP, McRae EK, Howard R, Deo SR, Ariyo
EO, Dzananovic E, Meier M, Stetefeld J and McKenna SA: RNA helicase
associated with AU-rich element (RHAU/DHX36) interacts with the
3′-tail of the long non-coding RNA BC200 (BCYRN1). J Biol Chem.
291:5355–5372. 2016. View Article : Google Scholar
|
|
8
|
Hu T and Lu YR: BCYRN1, a c-MYC-activated
long non-coding RNA, regulates cell metastasis of non-small-cell
lung cancer. Cancer Cell Int. 15:362015. View Article : Google Scholar
|
|
9
|
Zhang XY, Zhang LX, Tian CJ, Tang XY, Zhao
LM, Guo YL, Cheng DJ, Chen XL, Ma LJ and Chen ZC: LncRNAs BCYRN1
promoted the proliferation and migration of rat airway smooth
muscle cells in asthma via upregulating the expression of transient
receptor potential 1. Am J Transl Res. 8:3409–3418. 2016.
|
|
10
|
Lin Y, Liu J, Huang Y, Liu D, Zhang G and
Kan H: MicroRNA-489 plays an anti-metastatic role in human
hepatocellular carcinoma by targeting matrix metalloproteinase-7.
Transl Oncol. 10:211–220. 2017. View Article : Google Scholar
|
|
11
|
Jafri MA, Al-Qahtani MH and Shay JW: Role
of miRNAs in human cancer metastasis: Implications for therapeutic
intervention. Semin Cancer Biol. 44:117–131. 2017. View Article : Google Scholar
|
|
12
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2007. View Article : Google Scholar
|
|
13
|
Vrana D, Matzenauer M, Aujesky R, Vrba R,
Neoral C, Melichar B and Souček P: Potential predictive role of
microRNAs in the neoadjuvant treatment of esophageal cancer.
Anticancer Res. 37:403–412. 2017. View Article : Google Scholar
|
|
14
|
Jiang L, Liu X, Kolokythas A, Yu J, Wang
A, Heidbreder CE, Shi F and Zhou X: Downregulation of the Rho
GTPase signaling pathway is involved in the microRNA-138-mediated
inhibition of cell migration and invasion in tongue squamous cell
carcinoma. Int J Cancer. 127:505–512. 2010. View Article : Google Scholar
|
|
15
|
O'Donohue MF, Choesmel V, Faubladier M,
Fichant G and Gleizes PE: Functional dichotomy of ribosomal
proteins during the synthesis of mammalian 40S ribosomal subunits.
J Cell Biol. 190:853–866. 2010. View Article : Google Scholar
|
|
16
|
Qi P and Du X: The long non-coding RNAs, a
new cancer diagnostic and therapeutic gold mine. Mod Pathol.
26:155–165. 2013. View Article : Google Scholar
|
|
17
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar
|
|
18
|
Lin XC, Zhu Y, Chen WB, Lin LW, Chen DH,
Huang JR, Pan K, Lin Y, Wu BT, Dai Y and Tu ZG: Integrated analysis
of long non-coding RNAs and mRNA expression profiles reveals the
potential role of lncRNAs in gastric cancer pathogenesis. Int J
Oncol. 45:619–628. 2014. View Article : Google Scholar
|
|
19
|
Zhao M, Ang L, Huang J and Wang J:
MicroRNAs regulate the epithelial-mesenchymal transition and
influence breast cancer invasion and metastasis. Tumour Biol.
39:1010428317691682. 2017. View Article : Google Scholar
|
|
20
|
Deng B, Zhang Y, Zhang S, Wen F, Miao Y
and Guo K: MicroRNA-142-3p inhibits cell proliferation and invasion
of cervical cancer cells by targeting FZD7. Tumour Biol.
36:8065–8073. 2015. View Article : Google Scholar
|
|
21
|
Xia YF, Pei GH, Wang N, Che YC, Yu FS, Yin
FF, Liu HX, Luo B and Wang YK: miR-3156-3p is downregulated in
HPV-positive cervical cancer and performs as a tumor-suppressive
miRNA. Virol J. 14:202017. View Article : Google Scholar
|
|
22
|
Wang Q, Zhong M, Liu W, Li J, Huang J and
Zheng L: Alterations of microRNAs in cisplatin-resistant human
non-small cell lung cancer cells (A549/DDP). Exp Lung Res.
37:427–434. 2011. View Article : Google Scholar
|
|
23
|
Zhao X, Yang L, Hu J and Ruan J: miR-138
might reverse multidrug resistance of leukemia cells. Leuk Res.
34:1078–1082. 2010. View Article : Google Scholar
|
|
24
|
Zhou N, Fei D, Zong S, Zhang M and Yue Y:
MicroRNA-138 inhibits proliferation, migration and invasion through
targeting hTERT in cervical cancer. Oncol Lett. 12:3633–3639. 2016.
View Article : Google Scholar
|
|
25
|
Li B, Yang XX, Wang D and Ji HK:
MicroRNA-138 inhibits proliferation of cervical cancer cells by
targeting c-Met. Eur Rev Med Pharmacol Sci. 20:1109–1114. 2016.
|
|
26
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar
|
|
27
|
Wang J, Liu X, Wu H, Ni P, Gu Z, Qiao Y,
Chen N, Sun F and Fan Q: CREB up-regulates long non-coding RNA,
HULC expression through interaction with microRNA-372 in liver
cancer. Nucleic Acids Res. 38:5366–5383. 2010. View Article : Google Scholar
|
|
28
|
Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY,
Gong W and Quan ZW: Long non-coding RNA CCAT1 promotes gallbladder
cancer development via negative modulation of miRNA-218-5p. Cell
Death Dis. 6:e15832015. View Article : Google Scholar
|
|
29
|
Braconi C, Kogure T, Valeri N, Huang N,
Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM and Patel T:
MicroRNA-29 can regulate expression of the long non-coding RNA gene
MEG3 in hepatocellular cancer. Oncogene. 30:4750–4756. 2011.
View Article : Google Scholar
|
|
30
|
Lu H, He Y, Lin L, Qi Z, Ma L, Li L and Su
Y: Long non-coding RNA MALAT1 modulates radiosensitivity of
HR-HPV+ cervical cancer via sponging miR-145. Tumour
Biology. 37:1683–1691. 2016. View Article : Google Scholar
|