Introduction
Cervical cancer originates from the epithelium of
cervix uteri. It is one of the most common malignant tumors in
women and the fourth leading cause of cancer-associated mortality
worldwide (1). Persistent infection
of high-risk human papillomaviruses (HPVs) is associated with the
progression of cervical cancer (2).
There have been a number of efficient measures for the diagnosis
and prevention of cervical cancer, including screening programs and
prophylactic HPV vaccine injections for patients with cervical
cancer (3). Surgery, chemotherapy and
radiotherapy are the primary therapies for cervical cancer;
however, for patients with advanced cervical cancer, these methods
are ineffective due to distant metastases. Consequently, the
metastasis and recurrence of cervical cancer remain the principal
causes of mortality. Therefore, there is a pressing requirement to
elucidate the underlying molecular mechanisms, and to explore
potential biomarkers and drug targets to develop novel options for
the early diagnosis and treatment of cervical cancer.
S100A6 is a member of the S100 calcium-binding
protein family and its gene is located at human chromosome 1q21,
where chromosomal abnormalities occur frequently (4). Chromosome 1q21 polyploidy was considered
a high-risk characteristic for patients with multiple myeloma
receiving bortezomib therapy (5).
Chromosome 1q21 abnormalities were also associated with
intellectual disability, mild dysmorphic features and congenital
heart disease (6). Previous studies
have demonstrated that S100A6 is associated with tumorigenesis and
tumor progression. S100A6 promotes the proliferation and migration
of human colorectal cancer cells, hepatocellular carcinoma cells,
osteosarcoma cells and epithelial-mesenchymal transition (EMT) of
pancreatic cancer cells (7–10). Furthermore, S100A6 is associated with
the poor prognosis of patients with gastric cancer (11). S100A6 has also been demonstrated to be
involved in a number of signaling pathways such as phosphoinositide
3-kinase (PI3K)/protein kinase (Akt) (8), Wnt/β-catenin (10,12),
p38/mitogen-activated protein kinase (13) and nuclear factor-κB signaling
(14). However, to the best of our
knowledge, there have not been any studies pertaining to the effect
and underling molecular mechanism of S100A6 on cervical cancer at a
cellular level.
The PI3K/Akt pathway is a key driver in
carcinogenesis (15). Akt is a key
signaling molecule that phosphorylates numerous downstream targets,
connecting it to multiple interrelated signaling pathways. Thus, it
is responsible for modulating several processes including cell
survival, cell cycle progression, protein synthesis, angiogenesis
and cellular migration (16–18). Akt is known to be overexpressed in
many types of human cancer, and is associated with poor outcome in
some cancer types (19). Therefore,
it is hypothesized that the PI3K/Akt pathway is involved in
progression of cervical cancer.
EMT is key regulator of metastasis as it facilitates
tumor cell invasion and dissemination to distant organs.
Standardized analysis of EMT markers in tumor biopsies may serve as
a promising strategy for future clinical application (20). E-cadherin, N-cadherin, vimentin are
EMT markers and Snail, Twist are EMT transcription factors and
therefore the detection of these markers will reflect the status of
EMT (20).
The aim of the present study was to investigate the
effect of S100A6 on the proliferation and migration of cervical
cancer cells and its underlying mechanisms. The present study
hypothesizes that S100A6 exhibits the ability to promote the
proliferation, migration and EMT of cervical cancer cells through
activating PI3K/Akt signaling.
Materials and methods
Cell culture
The human cervical cancer cell lines HeLa, SiHa and
CaSki were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). Cells were maintained in Dulbecco's
modified Eagle's medium (DMEM; HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
incubated at 37°C with 5% CO2.
Recombinant adenoviruses (Ad),
recombinant proteins and reagents
AdS100A6 and AdsiS100A6 [carrying human S100A6 gene
or S100A6-short interfering (si)RNA gene, respectively] and their
controls [Ad-green fluorescent protein (GFP) or Ad-red fluorescent
protein (RFP)] were gifts from Dr He (Medical Center, The
University of Chicago, Chicago, IL, USA). Untreated cells were used
as blank controls. Recombinant glutathione transferase (GST) and
GST-S100A6 proteins were prepared according to the protocol of Miao
et al (21). The following
antibodies were used: Anti-human S100A6 (cat. no. sc-50409),
anti-β-catenin (cat. no. sc-59737), anti-phosphorylated (p)-Akt
(cat. no. sc-33437) and anti-total (t-)Akt (cat. no. sc-8312) (all
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-epithelial (E)-cadherin (cat. no. 14472), anti-neuronal
(N)-cadherin (cat. no. 13116), anti-p-glycogen synthase kinase 3β
(GSK3β) (cat. no. 9323), anti-t-GSK3β (cat. no. 9315) (all from
Cell Signaling Technology, Inc., Danvers, MA, USA), anti-E-cadherin
for HeLa cells (cat. no. YM3353; ImmunoWay Biotechnology Company,
Plano, TX, USA), anti-β-actin (cat. no. TA-09; OriGene
Technologies, Inc., Beijing, China) and goat anti-rabbit antibody
(cat. no. ZB-2301) or goat anti-mouse antibody (cat. no. ZB-2305)
(OriGene Technologies, Inc.). MTT (Beyotime Biotechnology,
Shanghai, China). Hoechst 33258 (Beyotime Institute of
Biotechnology, Haimen, China) and the PI3K inhibitor LY294002
(MedChemExpress, Monmouth Junction, NJ, USA) were dissolved in
dimethyl sulfoxide (DMSO) to a concentration of 10 mM.
RNA extraction, reverse transcription
(RT), qPCR and semi-qPCR
Total RNA was extracted from the cervical cancer
cell lines using RNAiso Plus (Takara Biotechnology Co., Ltd.,
Dalian, China). RT reactions were performed according to the
manufacturer's instructions (Takara Biotechnology Co., Ltd.).
Semi-qPCR was performed according to the protocol of Duan et
al (22). Taq DNA polymerase was
purchased from Takara Biotechnology Co., Ltd., and the cDNA
products were further diluted 5-fold and used in subsequent
experiments. Cycling conditions were as follows: 94°C for 5 min,
94°C for 30 sec, 68°C for 30 sec and 72°C for 12 cycles with a
decrease in 1°C/cycle; then, 94°C for 30 sec, 55°C for 30 sec, and
72°C for 30 sec for 18–27 cycles depending on the abundance of the
target genes. The PCR products were separated using 2% agarose gel
and stained with ethidium bromide. The results were recorded using
the Gel imaging system (GelDoc 1000; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and analyzed using Quantity One (version 4.5.0;
Bio-Rad Laboratories, Inc.).
qPCR was performed on the CFX96 real-time PCR
detection system from Bio-Rad Laboratories, Inc. using SYBR Premix
Ex Taq™ II (Takara Biotechnology Co., Ltd.). Data were collected
and analyzed using the comparative 2−ΔΔCt method
(23). GAPDH was used as control. All
primers used in the present study are presented in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer
sequence | Product size,
bp |
|---|
| S100A6 | Forward,
5′-ATGGCATGCCCCTGGATCAGG-3′ | 272 |
|
| Reverse,
5′-TCAGCCCTTGAGGGCTTCAT-3′ |
|
| Vimentin | Forward,
5′-TTGAACGCAAAGTGGAAT-3′ | 136 |
|
| Reverse,
5′-AGGTCAGGCTTGGAAACA-3′ |
|
| Snail | Forward,
5′-ACCCCACATCCTTCTCACTG-3′ | 217 |
|
| Reverse,
5′-TACAAAAACCCACGCAGACA-3′ |
|
| Twist | Forward,
5′-TCTTACGAGGAGCTGCAGAC-3′ | 406 |
|
| Reverse,
5′-TATCCAGCTCCAGAGTCTCT-3′ |
|
| GAPDH | Forward,
5′-CAGCGACACCCACTCCTC-3′ | 120 |
|
| Reverse,
5′-TGAGGTCCACCACCCTGT-3′ |
|
Western blot analysis
Total protein was extracted using
radioimmunoprecipitation assay lysis buffer (Beyotime
Biotechnology) in the presence of protease inhibitor (Complete
EasyPack; Roche Applied Science, Penzberg, Germany) and phosphatase
inhibitor (PhosSTOP; Roche Applied Science) at 72 h after treatment
with recombinant adenoviruses or recombinant proteins. Protein was
quantified using spectrophotometry (Thermo Fisher Scientific, Inc.)
at 280 nm. Protein (300 µg loaded per lane) was separated using
SDS-PAGE (10% gel) and transferred onto polyvinylidene fluoride
membranes (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The
membranes were blocked with 5% bovine serum albumin (Solarbio,
Beijing, China) at 37°C for 2 h and incubated with primary
antibodies (dilution 1:1,000) overnight at 4°C. The membranes were
washed with Tris-buffered saline with Tween-20 and incubated with
horseradish peroxidase (HRP) conjugated secondary antibody
(dilution 1:5,000) for 1 h at 37°C. Finally, the membranes were
exposed using Immobilon western chemiluminescent HRP substrate (EMD
Millipore, Billerica, MA, USA). Results were recorded using the
Bio-Rad electrophoresis documentation system (Gel Doc 1000; Bio-Rad
Laboratories, Inc.) and Quantity One software (version 4.5.0;
Bio-Rad Laboratories, Inc.).
Cell viability assay
Cell viability was determined using the MTT assay
according to the protocol of Duan et al (7).
Hoechst staining assay
Cells were seeded (3×104 cells/well) in a
24-well plate and incubated at 37°C overnight, then treated with
recombinant adenoviruses for 72 h when cells were ~30% confluent.
Cells were then fixed with 4% paraformaldehyde for 15 min, washed
with PBS three times, stained with 100 µl Hoechst 33258 solution
(dilution 1:1,000; Beyotime Institute of Biotechnology) for 30 min
and washed with PBS a further three times. Images were captured
under an inverted fluorescence microscope (magnification, ×100 and
×400). When the cell was apoptotic, the nucleus was stained bright
blue. The ratio of the number of apoptotic cells to the total
number of cells in a randomly given field of view was calculated as
the rate of apoptosis.
Wound healing assay
Cells were seeded (3×105 cells/well) into
a six-well plate and treated with recombinant adenoviruses for 72 h
when cells reached 95% confluency. The wound healing assay was
performed according to the protocol of Duan et al (7).
Transwell assay
The Transwell inserts were purchased from EMD
Millipore. Cells were seeded (1.5×105 cells/well) in a
6-well plate and treated with recombinant adenoviruses or
recombinant proteins for 72 h when cells were ~30% confluent. The
treated cells were collected and 4×104 cells were
resuspended in 400 µl serum-free DMEM and seeded in the upper
chamber, another 600 µl medium with 20% FBS was added to the lower
chamber. Following incubation for 24 h, chambers were fixed with 4%
paraformaldehyde for 15 min and then stained with 0.05% crystal
violet for 15 min at room temperature. The transmembrane cells were
counted using inverted microscopy (magnification, ×100) in 10
different views for each insert and images were captured. The
relative cell number per field was calculated as follows; number of
cells in each group/number of cells in the blank group.
Statistical analysis
Results are presented as the mean ± standard
deviation. A one-way analysis of variance, followed by the
Student-Newman-Keuls test was used to analyze the statistical
significance of differences among groups, and all analyses were
performed using GraphPad Prism software (version 5; GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
S100A6 promotes the proliferation of
cervical cancer cells
To examine the effect of S100A6 on cervical cancer
cells, the expression of S100A6 was detected in the three cervical
cancer cell lines HeLa, SiHa and CaSki. The qPCR results
demonstrated that the S100A6 mRNA level was comparatively lower in
HeLa cells. As presented in Fig. 1A,
the mRNA levels of S100A6 in SiHa and CaSki cells were increased
6.92- and 7.54-fold compared with those of HeLa cells respectively
(P<0.005). The S100A6 level of HeLa and CaSki cells was
upregulated by AdS100A6, and the S100A6 level of SiHa and CaSki
cells was downregulated by AdsiS100A6 as was demonstrated by
western blot analysis (Fig. 1B).
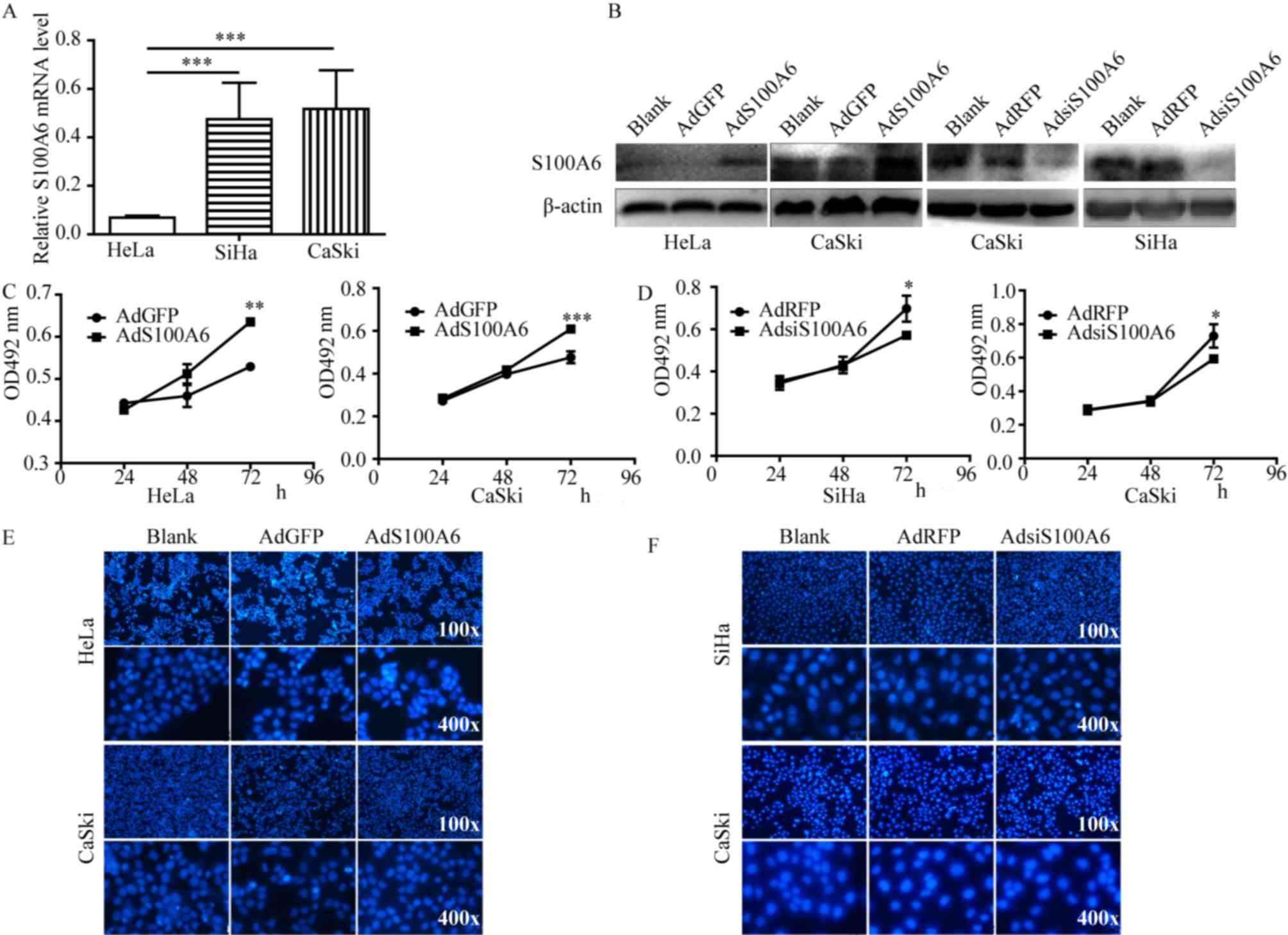 | Figure 1.S100A6 promotes the proliferation of
cervical cancer cells (A) qPCR was used to analyze the mRNA levels
of S100A6 in the cervical cancer cell lines HeLa, SiHa and CaSki.
(B) AdS100A6 infected HeLa and CaSki cells, AdsiS100A6 infected
CaSki and SiHa cells. The infection efficiency was determined by
western blotting. Cell proliferation was detected using an MTT
assay in (C) HeLa and CaSki cells following treatment with AdS100A6
and in (D) SiHa and CaSki cells following treatment with
AdsiS100A6. Cell apoptosis was determined by Hoechst staining assay
in (E) HeLa and CaSki cells after treatment with AdS100A6 and in
(F) SiHa and CaSki cells following treatment with AdsiS100A6. As no
significant changes were identified between all groups in number of
apoptosis cells, no quantitative data was provided. *P<0.05,
**P<0.01, ***P<0.005 compared with the AdGFP or AdRFP groups.
All the experiments were repeated three times. Results were
presented as the mean ± standard deviation. A one-way analysis of
variance, followed by the Student-Newman-Keuls test was used to
analyze the statistical significance of differences among groups.
OD, optical density; nm, nanometer; AdGFP, adenovirus carrying
green fluorescent protein; AdS100A6, adenovirus carrying S100A6;
AdRFP, adenovirus carrying red fluorescent protein; AdsiS100A6,
adenovirus carrying S100A6-short interfering RNA gene; E-cadherin,
epithelial cadherin; N-cadherin, neuronal cadherin. |
To investigate whether S100A6 is involved in the
proliferation of cervical cancer cells, the MTT assay was
performed. Cell proliferation ability was significantly increased
following treatment with AdS100A6 for 72 h in HeLa and CaSki cells
(P<0.01 and P<0.005 respectively; Fig. 1C), whereas it was markedly decreased
following treatment with AdsiS100A6 for 72 h in SiHa and CaSki
cells (P<0.05; Fig. 1D). The
effect of S100A6 on cellular apoptosis was investigated using
Hoechst staining and no marked changes were identified between the
examined groups in number of apoptosis cells (Fig. 1E and F). These results demonstrated
that S100A6 is able to promote the proliferation of cervical cancer
cells and had no significant effect on the apoptosis of cells.
S100A6 promotes the migration and EMT
of cervical cancer cells
Wound healing and Transwell assays were performed to
investigate the effect of S100A6 on cell migration. In the present
study, AdGFP was a control of AdS100A6 and AdRFP was a control of
AdsiS100A6. Furthermore, CaSki cells were treated with AdS100A6 and
AdsiS100A6 in order to upregulate or downregulate the level of
S100A6, respectively. The present study used AdS100A6 to upregulate
the level of S100A6 in HeLa cells, and AdsiS100A6 to down-regulate
the level of S100A6 in SiHa cells. Following the upregulation of
S100A6 by AdS100A6 for 72 h, the wound healing rates were increased
2.1- (P<0.05) and 3.6-fold (P<0.01) compared with those of
the AdGFP groups in HeLa and CaSki cells (Fig. 2A). Conversely, following the
downregulation of S100A6 by AdsiS100A6 for 72 h, the wound-healing
rates were decreased by 41% (P<0.05) and 44% (P<0.05) of that
in their respective AdRFP groups (Fig.
2B). The migratory ability of cells was further confirmed by a
Transwell assay. Following treatment with AdS100A6, the number of
transmembrane cells were increased ~5- (P<0.005) and 2-fold
(P<0.01) compared with that in the AdGFP groups of HeLa and
CaSki cells (Fig. 2C). However,
following treatment with AdsiS100A6, the number of transmembrane
cells were decreased by 66% (P<0.005) and 65% (P<0.01)
compared with those of their respective AdRFP groups (Fig. 2D). These results indicated that S100A6
promoted cell migration.
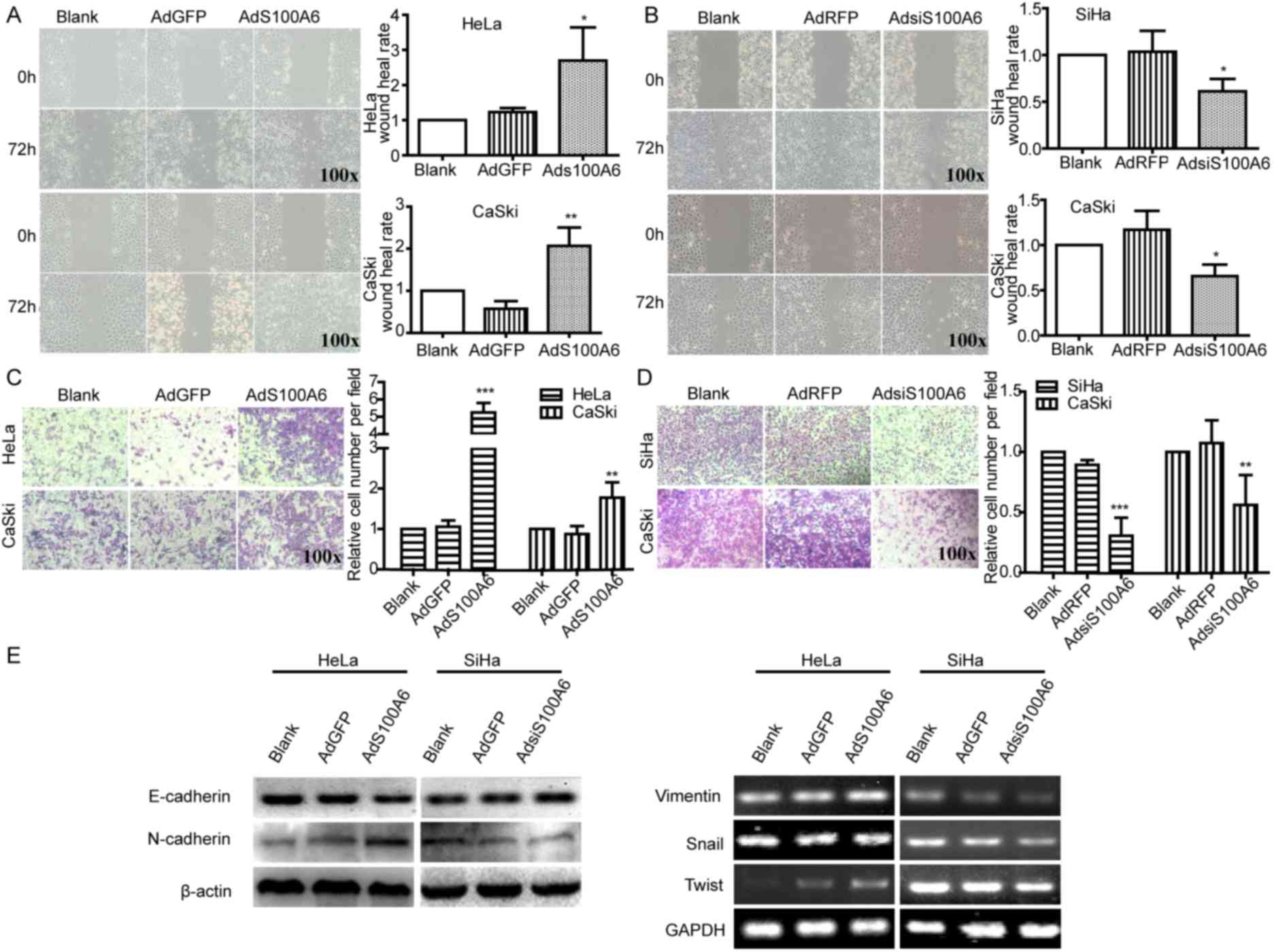 | Figure 2.S100A6 promotes the migration and EMT
of cervical cancer cells. Cell migration ability was detected using
a wound healing assay. The wound width of sites was measured and
the wound healing rate of (A) HeLa and CaSki cells with AdGFP, and
(B) SiHa and CaSki with AdRFP was calculated. The wound healing
rate was calculated as follows; (width at 0 h-width at 72 h)/width
at 0 h ×100%. Cell migration ability of (C) HeLa and CaSki cells
with AdGFP, and (D) SiHa and CaSki cells with AdRFP were detected
using a Transwell assay, where the number of transmembrane cells
were counted and the relative cell number per field was calculated.
The relative cell number per field = number of cells in per field
of treatment group/number of cells in per field of blank group. (E)
The protein levels of E-cadherin and N-cadherin were determined
using western blotting and the mRNA levels of vimentin, Snail and
Twist were determined using semi-quantitative PCR in HeLa and SiHa
cells. *P<0.05, **P<0.01, ***P<0.005 compared with the
AdGFP or AdRFP groups. All experiments were repeated for three
times. Results were presented as the mean ± standard deviation. A
one-way analysis of variance, followed by the Student-Newman-Keuls
test was used to analyze the statistical significance of
differences among groups. EMT, epithelial-mesenchymal transition;
AdGFP, adenovirus carrying green fluorescent protein; AdS100A6,
adenovirus carrying S100A6; AdRFP, adenovirus carrying red
fluorescent protein; AdsiS100A6, adenovirus carrying S100A6-short
interfering RNA gene; E-cadherin, epithelial cadherin; N-cadherin,
neuronal cadherin. |
Additionally, upregulation of S100A6 in HeLa cells
increased the mRNA levels of N-cadherin, vimentin, Snail and Twist,
and decreased the protein level of E-cadherin, whereas the
downregulation of S100A6 in SiHa cells induced the opposite effect
(Fig. 2E). Collectively, these
results indicated that S100A6 was able to induce EMT in cervical
cancer cells.
S100A6 activates the PI3K/Akt
signaling pathway and promotes the viability and migration of
cervical cancer cells
To explore the predominant downstream signaling
pathway of S100A6, the alteration of several pathway proteins were
evaluated using western blotting. The phosphorylation level of Akt
and its downstream target genes p-GSK3β and β-catenin were
demonstrated to be increased in the AdS100A6 group of HeLa cells,
and was consistent with activation of the PI3K/Akt pathway
(Fig. 3A). To further validate the
involvement of PI3K/Akt activation in S100A6-enhanced proliferation
and migration of cervical cancer cells, a pharmacological approach
was adopted. HeLa cells were treated with the PI3K inhibitor
LY294002 (10 µM) alone, or combined with GST-S100A6 (10 µg/ml). The
level of p-Akt, p-GSK3β and β-catenin were decreased when the
PI3K/Akt pathway was blocked using the PI3K inhibitor LY294002
(Fig. 3B). Furthermore, LY294002
significantly impaired the S100A6-enhanced proliferation and
migration of HeLa cells (P<0.005; Fig.
3C and D). Taken together, these results suggest that S100A6
promotes the proliferation and migration of cervical cancer cells
through the PI3K/Akt signaling pathway.
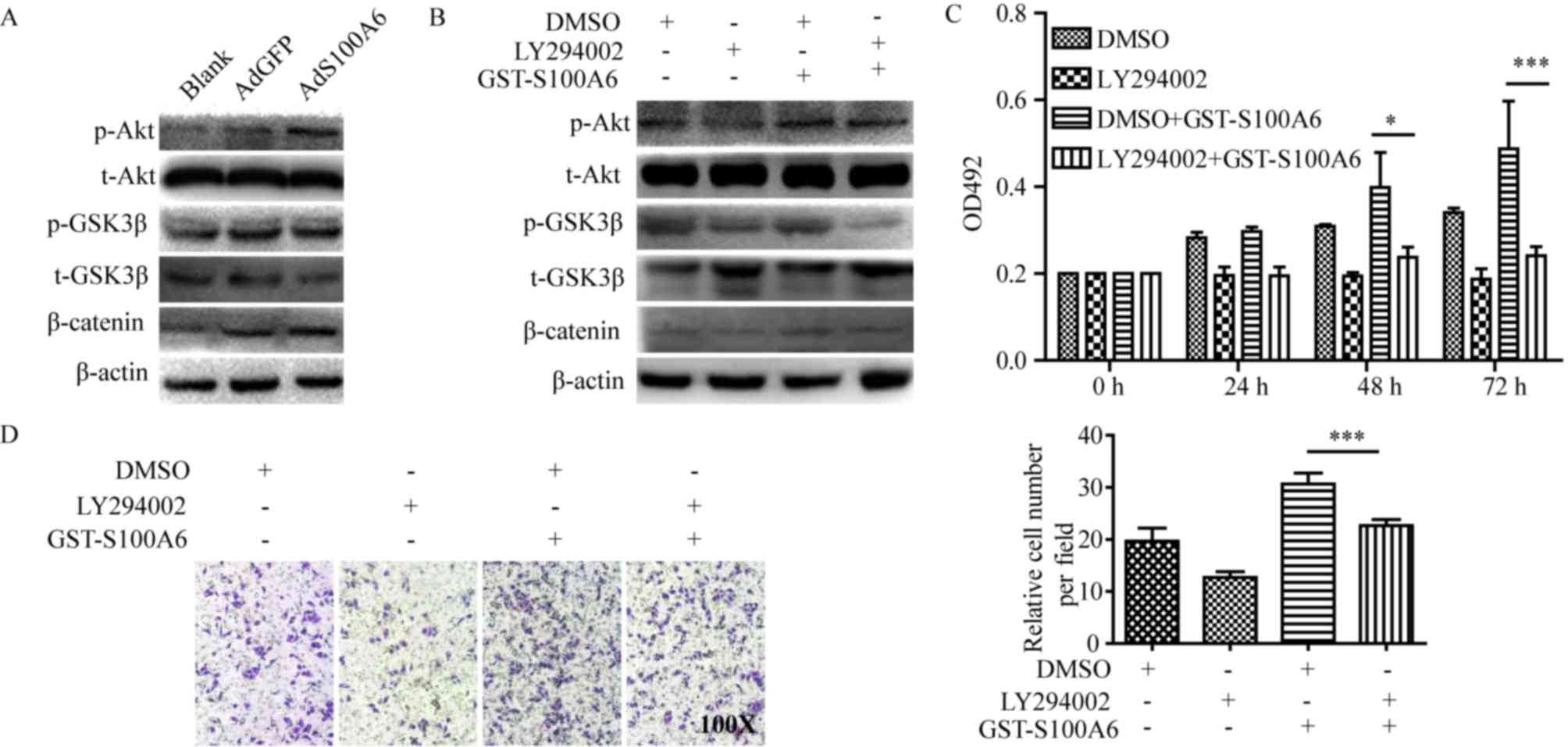 | Figure 3.S100A6 activates the PI3K/Akt
signaling pathway and promotes the viability and migration of
cervical cancer cells. (A) HeLa cells were treated with AdS100A6 or
AdGFP for 72 h. Total Akt, total GSK3β, β-catenin, p-Akt and
p-GSK3β were detected using western blotting. (B) HeLa cells were
treated with GST-S100A6 and LY294002 for 72 h, and total Akt, total
GSK3β, β-catenin, p-Akt and p-GSK3β were detected using western
blotting. (C) The effect of LY294002 on S100A6-enhanced
proliferation of HeLa cells was determined using an MTT assay. HeLa
cells were treated with GST-S100A6 (10 µg/ml) and LY294002 (10 µM)
for 72 h. (D) The effect of LY294002 on S100A6-enhanced migration
of HeLa cells was determined by a Transwell assay (magnification,
×100). HeLa cells were treated with GST-S100A6 (10 µg/ml), LY294002
(10 µM) or DMSO for 72 h. The number of transmembrane cells were
counted and calculated. All experiments were repeated three times.
Results were presented as the mean ± standard deviation. A one-way
analysis of variance, followed by the Student-Newman-Keuls test was
used to analyze the statistical significance of differences among
groups. *P<0.05, ***P<0.005, DMSO + GST-S100A6 group compared
with LY2940002 + GST-S100A6 group. p, phosphorylated; t, total;
AdGFP, adenovirus carrying green fluorescent protein; AdS100A6,
adenovirus carrying S100A6; OD, optical density; GST, glutathione
transferase; DMSO, dimethyl sulfoxide. |
Discussion
Cervical cancer is a common tumor of the female
reproductive system with a high metastatic potential and mortality.
Although infection with HPV is considered to be a principal cause
of cervical cancer, there are a variety of risk factors associated
with the morbidity of cervical cancer, including reproductive and
nutritional factors, and other types of pathogen infection
including chlamydia trachomatis, herpes simplex virus type II and
trichomonad. The presence of these additional risk factors provides
additional difficulties in the identification of novel potential
biomarkers for the detection and therapy of cervical cancer.
There have been a number of studies investigating
the role of S100A6 in tumorigenesis and tumor development. The
results provide evidence that S100A6 promotes proliferation and
migration in colorectal cancer cells (7), pancreatic cancer cells (24) and other malignant tumor cells
(9). A previous study indicated that
the level of S100A6 differs in different cervical lesion tissues,
and its expression is positively associated with the degree of
cervical lesion, invasion and metastasis (25). S100A6 was markedly expressed in
cervical cancer tissues, and is predominantly expressed in
fibroblasts and epithelial cells of the breast, heart, intestine,
kidney, liver, ovary, placenta, stomach, thymus and uterus
(26). Furthermore, increased
expression of S100A6 has been identified in tumors (27). To the best of our knowledge, no
previous studies have been performed that investigate the level of
S100A6 in normal epithelial cell lines of cervix uteri. This was a
limitation of the present study and it is therefore a
recommendation for future studies in this field. The results of the
present study demonstrated that S100A6 serves an important role in
promoting the proliferation and migration of cervical cancer cells
through the PI3K/Akt signaling pathway.
HPV16 and HPV18 are the most common types of
cervical cancer and account for ~67% of all cervical carcinomas
worldwide (28). HeLa cells are
HPV18+ whereas SiHa cells are HPV16+, and
HPV16+ and HPV18+ cervical cancer exhibit
different biological behavior in vivo (29). This is due to the E6 and E7 gene
products of HPV contributing to the pathogenesis of cancer. HPV
integrates with the DNA within the host cell nucleus and thereby
dysregulates the expression of the oncoproteins E6 and E7.
Degradation of p53 is induced by E6, which results in a loss of p53
activity. Its degradation is accomplished through the formation of
a complex with p53, E6 and E6-associated protein (30). In a physiological state, p53 functions
to arrest cells in the G1 phase of the cell cycle and
induces apoptosis to allow the repair of host DNA (30). Furthermore, E7 binds to the
cyclin-dependent kinase inhibitor, which results in a loss of
control over the cell cycle (30).
HeLa and SiHa are two different cell types, with different types of
HPV; this is a potential explanation for why, in the present study,
the S100A6 levels varied between the two cell lines.
In the present study, cervical cancer cells were
treated with AdS100A6 and AdsiS100A6 to upregulate and downregulate
the level of S100A6. The results of the present study suggest that
S100A6 promotes the proliferation of cervical cancer cells, and
these results were consistent with those of previous studies in
colorectal cancer (7), hepatocellular
carcinoma (8,31) and osteosarcoma (9). A Hoechst staining assay was performed to
provide a visual result; however, there are human errors associated
with the manual analysis and counting of cells. Consequently, the
absence of flow cytometric data to detect apoptosis is a limitation
of the present study. Furthermore, S100A6 was demonstrated to
significantly promote the migration of cervical cancer cells, which
is in accordance with a previous study on pancreatic duct
adenocarcinoma (10). These results
imply that S100A6 facilitates the metastasis of cervical cancer
cells, as cell invasion and migration are the basic steps for tumor
metastasis. Therefore, S100A6 may contribute to cervical cancer
metastasis and may be a potential target for the treatment of
cervical cancer, in order to increase the survival period of
patients. However, the absence of data regarding the regulatory
effect of S100A6 on cell invasion is a limitation of the present
study.
A number of studies have reported that EMT has
become a principal factor for tumor malignancy, as EMT contributes
to the motility and invasiveness of tumor cells, and therefore
contributes to distant metastasis (32–34).
Cancer-associated EMT is a complicated and comprehensive
reprogramming process that is involved in the metabolism,
epigenetics and differentiation of cells (35). Previous studies have demonstrated that
claudin-1, oncostatin-M receptor and lysine-specific demethylase 1
are associated with EMT and a poor outcome in cervical cancer
(36–38). The results of the present study
confirmed that S100A6 induced EMT in cervical cancer cells.
Therefore, EMT serves an important role in the progression of
cervical cancer. HeLa cells expressed lower levels of S100A6 and
SiHa cells expressed higher levels of S100A6. Consequently, an
increased level of S100A6 was attributed to the higher mesenchymal
properties of SiHa cells. The level of E-cadherin was increased in
HeLa cells compared with that of SiHa cells, whereas the level of
N-cadherin was the opposite. These are consistent with the
hypothesis that a function of S100A6 is to induce EMT in cervical
cancer cells. In the present study, the expression of these
molecules were determined by western blotting or semi-qPCR. A
limitation of the present study is that the expression of these
molecules was determined only at the mRNA or protein level.
A number of studies have demonstrated the
involvement of S100A6 in a number of signaling pathways in many
types of cancer cell (7–14). In cervical cancer cells, a number of
pathways are implicated in cellular proliferation, apoptosis,
migration and invasion, including Wnt/β-catenin, mTOR and ERK
signaling (39–42). In the present study, the PI3K/Akt
signaling pathway was selected to determine the effect of S100A6 on
cell proliferation and migration of cervical cancer cells. This is
due to the PI3K/Akt signaling pathway serving an important role in
tumorigenesis and the regulation of critical cellular functions,
including survival, proliferation and metabolism (43). An increasing amount of evidence has
demonstrated that the PI3K/Akt pathway is involved in the
proliferation, apoptosis and autophagy of cervical cancer cells
(44–46). It has been reported that Akt and
matrix metalloproteinase 3 are key regulators of EMT in cervical
cancer (47). It has also been
suggested that the PI3K/Akt signaling pathway promotes metastasis
and EMT in nasopharyngeal carcinoma (48). In our previous study, it was
demonstrated that S100A6 may promote the proliferation and
migration of osteosarcoma cells via the PI3K/Akt signaling pathway
(49). Therefore, an aim of the
present study was to determine whether the PI3K/Akt signaling
pathway was also involved in S100A6-enhanced proliferation and
migration of cervical cancer cells. GST-S100A6 and LY294002 were
chosen to further validate the involvement of PI3K/Akt activation
in S100A6-enhanced proliferation and migration of cervical cancer
cells. The results demonstrated that S100A6 activated the PI3K/Akt
signaling pathway and inhibition of the PI3K/Akt pathway markedly
impaired S100A6-enhanced proliferation and migration of HeLa cells.
Similar experiments were performed using AdS100A6; however, when
cells were co-treated with AdS100A6 and LY294002, a large cohort of
cells immediately underwent apoptosis due to the toxicity of
polybrene and DMSO. Consequently, GST-S100A6 was used as it has a
similar effect to AdS100A6 and is less toxic (50). Receptor for advanced glycation
end-products (RAGE), Toll-like receptor 4 and epidermal growth
factor receptors are receptors of the S100 protein, and S100A6
activates RAGE through distinct signal transduction pathways
(51–53). Previous research of the present study
cohort has demonstrated that S100A6 is an exocrine protein that can
be secreted to the extracellular space, and the secreted S100A6
protein in the cell culture medium upregulated the mRNA level of
endogenous S100A6 through Wnt/β-catenin signaling. This
subsequently affected the biological characteristics of tumor cells
(50) and GST-S100A6 was demonstrated
to act similarly to AdS100A6 (50).
The secreted S100A6 protein was able to promote the proliferation
and migration of colorectal cancer cells (50). Further research is required to explore
the effects of LY294002 on EMT. Collectively, these results suggest
that S100A6 promotes the proliferation and migration of cervical
cancer cells through the PI3K/Akt signaling pathway.
In conclusion, the results of the present study
demonstrate that S100A6 serves a pivotal role in the malignancy of
cervical cancer cells, including promoting the proliferation,
migration and EMT of cervical cancer cells by activating the
PI3K/Akt signaling pathway. The present study provides evidence to
improve the understanding of the pathophysiological process of
cervical cancer as S100A6 may be a promising molecular target for
patients with cervical cancer. However, the absence of in
vivo data and data regarding the expression and clinical
significance of S100A6 in cervical cancer tissues are limitations
of the present study. Therefore, further in vivo studies and
data regarding the expression and clinical significance of S100A6
in cervical cancer tissues based on in vivo studies and
databases including Oncomine are required to verify these issues
and confirm the role of S100A6 as a novel biomarker for the
detection and therapy of cervical cancer.
Acknowledgements
The authors would like to thank Dr T.C. He (Medical
Center, The University of Chicago, Chicago, IL, USA) for the gifts
of AdS100A6, AdsiS100A6, AdGFP and AdRFP.
Funding
The present study was supported by the Chongqing
Graduate Student Research Innovation Project Funding (grant no.
CYS15133) and Chongqing Yuzhong District Science and Technology
Project Funding (Basic and Frontier Research) (grant no.
20160106).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on request.
Authors' contributions
AL performed most of the research and was a major
contributor in writing the manuscript. YG, XL, HS, JX, JZ, MH, LC
and QP prepared experimental materials and reviewed the article.
HZ, YZ, YW and LZ made substantial contributions to the design of
the work, drafting the manuscript or revising it critically for
important intellectual content. LZ gave final approval of the
version to be published.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu C, Lin J, Li L, Zhang Y, Chen W, Cao
Z, Zuo H, Chen C and Kee K: HPV16 early gene E5 specifically
reduces miRNA-196a in cervical cancer cells. Sci Rep. 5:76532015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Das BC, Hussain S, Nasare V and Bharadwaj
M: Prospects and prejudices of human papillomavirus vaccines in
India. Vaccine. 26:2669–2679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heizmann CW, Fritz G and Schafer BW: S100
proteins: Structure, functions and pathology. Front Biosci.
7:1356–1368. 2002. View
Article : Google Scholar
|
|
5
|
An G, Xu Y, Shi L, Shizhen Z, Deng S, Xie
Z, Sui W, Zhan F and Qiu L: Chromosome 1q21 gains confer inferior
outcomes in multiple myeloma treated with bortezomib but copy
number variation and percentage of plasma cells involved have no
additional prognostic value. Haematologica. 99:353–359. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ha K, Shen Y, Graves T, Kim CH and Kim HG:
The presence of two rare genomic syndromes, 1q21 deletion and Xq28
duplication, segregating independently in a family with
intellectual disability. Mol Cytogenet. 9:742016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duan L, Wu R, Zou Z, Wang H, Ye L, Li H,
Yuan S, Li X, Zha H, Sun H, et al: S100A6 stimulates proliferation
and migration of colorectal carcinoma cells through activation of
the MAPK pathways. Int J Oncol. 44:781–790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Tang M, Ling B, Liu S, Zheng Y, Nie
C, Yuan Z, Zhou L, Guo G, Tong A and Wei Y: Increased expression of
S100A6 promotes cell proliferation and migration in human
hepatocellular carcinoma. J Mol Med (Berl). 92:291–303. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Wagner ER, Yan Z, Wang Z, Luther G,
Jiang W, Ye J, Wei Q, Wang J, Zhao L, et al: The Calcium-Binding
Protein S100A6 accelerates human osteosarcoma growth by promoting
cell proliferation and inhibiting osteogenic differentiation. Cell
Physiol Biochem. 37:2375–2392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Liu X, Lang H, Zhang S, Luo Y and
Zhang J: S100 calcium-binding protein A6 promotes
epithelial-mesenchymal transition through β-catenin in pancreatic
cancer cell line. PloS One. 10:e01213192015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XH, Zhang LH, Zhong XY, Xing XF, Liu
YQ, Niu ZJ, Peng Y, Du H, Zhang GG, Hu Y, et al: S100A6
overexpression is associated with poor prognosis and is
epigenetically up-regulated in gastric cancer. Am J Pathol.
177:586–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kilańczyk E, Graczyk A, Ostrowska H,
Kasacka I, Leśniak W and Filipek A: S100A6 is transcriptionally
regulated by β-catenin and interacts with a novel target, lamin
A/C, in colorectal cancer cells. Cell Calcium. 51:470–477. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li A, Shi D, Xu B, Wang J, Tang YL, Xiao
W, Shen G, Deng W and Zhao C: S100A6 promotes cell proliferation in
human nasopharyngeal carcinoma via the p38/MAPK signaling pathway.
Mol Carcinog. 56:972–984. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joo JH, Kim JW, Lee Y, Yoon SY, Kim JH,
Paik SG and Choe IS: Involvement of NF-kappaB in the regulation of
S100A6 gene expression in human hepatoblastoma cell line HepG2.
Biochem Biophys Res Commun. 307:274–280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robbins HL and Hague A: The PI3K/Akt
pathway in tumors of endocrine tissues. Front Endocrinol
(Lausanne). 6:1882016.PubMed/NCBI
|
|
16
|
Saji M, Vasko V, Kada F, Allbritton EH,
Burman KD and Ringel MD: Akt1 contains a functional leucine-rich
nuclear export sequence. Biochem Biophys Res Commun. 332:167–173.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martelli AM, Tabellini G, Bressanin D,
Ognibene A, Goto K, Cocco L and Evangelisti C: The emerging
multiple roles of nuclear Akt. Biochim Biophys Acta.
1823:2168–2178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ocana A, Vera-Badillo F, Al-Mubarak M,
Templeton AJ, Corrales-Sanchez V, Diez-Gonzalez L, Cuenca-Lopez MD,
Seruga B, Pandiella A and Amir E: Activation of the PI3K/mTOR/AKT
pathway and survival in solid tumors: Systematic review and
meta-analysis. PloS One. 9:e952192014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santamaria PG, Moreno-Bueno G, Portillo F
and Cano A: EMT: Present and future in clinical oncology. Mol
Oncol. 11:718–738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miao JK, Lai TX, Zuo GW, et al: Expression
and purification of human S100A6-GST fusion protein. J Chongqing
Med Univ. 32:1009–1012. 2007.
|
|
22
|
Duan L, Wu R, Ye L, Wang H, Yang X, Zhang
Y, Chen X, Zuo G, Zhang Y, Weng Y, et al: S100A8 and S100A9 are
associated with colorectal carcinoma progression and contribute to
colorectal carcinoma cell survival and migration via Wnt/b-catenin
pathway. PloS One. 8:e620922013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song Q, Zhong L, Chen C, Tang Z, Liu H,
Zhou Y, Tang M, Zhou L, Zuo G, Luo J, et al: miR-21 synergizes with
BMP9 in osteogenic differentiation by activating the BMP9/Smad
signaling pathway in murine multilineage cells. Int J Mol Med.
36:1497–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nedjadi T, Kitteringham N, Campbell F,
Jenkins RE, Park BK, Navarro P, Ashcroft F, Tepikin A, Neoptolemos
JP and Costello E: S100A6 binds to annexin 2 in pancreatic cancer
cells and promotes pancreatic cancer cell motility. Br J Cancer.
101:1145–1154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan T: The expression of tumour
metastasis-associated genes S100A6, Rab5a in cervical cancer and
its clinical significance. Guangxi Medical University. 2010.
|
|
26
|
Kuźnicki J, Kordowska J, Puzianowska M and
Woźniewicz BM: Calcyclin as a marker of human epithelial cells and
fibroblasts. Exp Cell Res. 200:425–430. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lesniak W, Slomnicki LP and Filipek A:
S100A6-new facts and features. Biochem Biophys Res Commun.
390:1087–1092. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao M, Xu Q, Li H, Gao H, Bie Y and Zhang
Z: Prevalence of human papillomavirus genotypes among women with
high-grade cervical lesions in Beijing, China. Medicine
(Baltimore). 95:e25552016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwartz SM, Daling JR, Shera KA,
Madeleine MM, McKnight B, Galloway DA, Porter PL and McDougall JK:
Human papillomavirus and prognosis of invasive cervical cancer: A
population-based study. J Clin Oncol. 19:1906–1915. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bansal A, Singh MP and Rai B: Human
papillomavirus-associated cancers: A growing global problem. Int J
Appl Basic Med Res. 6:84–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hua Z, Chen J, Sun B, Zhao G, Zhang Y,
Fong Y, Jia Z and Yao L: Specific expression of osteopontin and
S100A6 in hepatocellular carcinoma. Surgery. 149:783–791. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bedi U, Mishra VK, Wasilewski D, Scheel C
and Johnsen SA: Epigenetic plasticity: A central regulator of
epithelial-to-mesenchymal transition in cancer. Oncotarget.
5:2016–2029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baum B, Settleman J and Quinlan MP:
Transitions between epithelial and mesenchymal states in
development and disease. Semin Cell Dev Biol. 19:294–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li L and Li W: Epithelial-mesenchymal
transition in human cancer: Comprehensive reprogramming of
metabolism, epigenetics, and differentiation. Pharmacol Ther.
150:33–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang WN, Li W, Wang XL, Hu Z, Zhu D, Ding
WC, Liu D, Li KZ, Ma D and Wang H: CLDN1 expression in cervical
cancer cells is related to tumor invasion and metastasis.
Oncotarget. 7:87449–87461. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kucia-Tran JA, Tulkki V, Smith S, Scarpini
CG, Hughes K, Araujo AM, Yan KY, Botthof J, Pérez-Gómez E,
Quintanilla M, et al: Overexpression of the oncostatin-M receptor
in cervical squamous cell carcinoma is associated with
epithelial-mesenchymal transition and poor overall survival. Br J
Cancer. 115:212–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Wang Y, Chen C, Zhang J, Qian W,
Dong Y, Liu Z, Zhang X, Wang X, Zhang Z, et al: LSD1 binds to HPV16
E7 and promotes the epithelial-mesenchymal transition in cervical
cancer by demethylating histones at the Vimentin promoter.
Oncotarget. 8:11329–11342. 2016.
|
|
39
|
Chen Q, Cao HZ and Zheng PS: LGR5 promotes
the proliferation and tumor formation of cervical cancer cells
through the Wnt/β-catenin signaling pathway. Oncotarget.
5:9092–9105. 2014.PubMed/NCBI
|
|
40
|
Kong L, Hao Q, Wang Y, Zhou P, Zou B and
Zhang YX: Regulation of p53 expression and apoptosis by vault
RNA2-1-5p in cervical cancer cells. Oncotarget. 6:28371–28388.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rashmi R, DeSelm C, Helms C, Bowcock A,
Rogers BE, Rader JL, Grigsby PW and Schwarz JK: AKT inhibitors
promote cell death in cervical cancer through disruption of mTOR
signaling and glucose uptake. PloS One. 9:e929482014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu T, Liu Y, Bao X, Tian J, Liu Y and
Yang X: Overexpression of TROP2 predicts poor prognosis of patients
with cervical cancer and promotes the proliferation and invasion of
cervical cancer cells by regulating ERK signaling pathway. PloS
One. 8:e758642013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang SX, Polley E and Lipkowitz S: New
insights on PI3K/AKT pathway alterations and clinical outcomes in
breast cancer. Cancer Treat Rev. 45:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feng T, Zheng L, Liu F, Xu X, Mao S, Wang
X, Liu J, Lu Y, Zhao W, Yu X and Tang W: Growth factor progranulin
promotes tumorigenesis of cervical cancer via PI3K/Akt/mTOR
signaling pathway. Oncotarget. 7:58381–58395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qian S and Li M: Chamaejasmine induces
apoptosis in HeLa cells through the PI3K/Akt signaling pathway.
Anticancer Drugs. 28:40–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jeyamohan S, Moorthy RK, Kannan MK and
Arockiam AJ: Parthenolide induces apoptosis and autophagy through
the suppression of PI3K/Akt signaling pathway in cervical cancer.
Biotechnol Lett. 38:1251–1260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hagemann T, Bozanovic T, Hooper S, Ljubic
A, Slettenaar VI, Wilson JL, Singh N, Gayther SA, Shepherd JH and
Van Trappen PO: Molecular profiling of cervical cancer progression.
Br J Cancer. 96:321–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ke LR, Xiang YQ, Guo X, Lu J, Xia W, Yu Y,
Peng Y, Wang L, Wang G, Ye Y, et al: c-Src activation promotes
nasopharyngeal carcinoma metastasis by inducing the
epithelial-mesenchymal transition via PI3K/Akt signaling pathway: A
new and promising target for NPC. Oncotarget. 7:28340–28355. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang H, Zou Z, Duan L, Chen X, Li H, Yuan
S, He TC and Zhou L: S100A6 promotes proliferation and migration of
human osteosarcoma cell line 143B through PI3K/Akt signaling
pathway. Chin J Pathophysiol. 29:1928–1933. 2013.
|
|
50
|
Hui S: Exogenous S100A6 upregulates its
own expression in colorectal carcinoma cells and its mechanism.
Chongqing Medical University. 2013.
|
|
51
|
Sparvero LJ, Asafu-Adjei D, Kang R, Tang
D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ and Lotze
MT: RAGE (Receptor for Advanced Glycation Endproducts), RAGE
ligands, and their role in cancer and inflammation. J Transl Med.
7:172009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen H, Xu C, Jin Q and Liu Z: S100
protein family in human cancer. Am J Cancer Res. 4:89–115.
2014.PubMed/NCBI
|
|
53
|
Bresnick AR, Weber DJ and Zimmer DB: S100
proteins in cancer. Nat Rev Cancer. 15:96–109. 2015. View Article : Google Scholar : PubMed/NCBI
|

















