Introduction
Primary hepatocellular carcinoma is a common type of
malignant tumor in the digestive system with the characteristics of
high incidence, rapid progression and high mortality (1,2). The
occurrence and development of hepatocellular carcinoma is very
complex with various genes and signaling transduction pathways
involved, and the pathogenesis remains unclear (3). At present, the main clinical treatment
of hepatocellular carcinoma is surgery. However, the early clinical
symptoms of hepatocellular carcinoma are not obvious, which in turn
leads to misdiagnosis. Therefore, most hepatocellular carcinoma
patients are at an advanced stage at the time of diagnosis, and
approximately 90% of patients no longer have the option of surgical
resection, leading to the utilization of radiotherapy and
chemotherapy (4,5).
The phosphatidylinositol 3-kinase/protein kinase B
pathway (PI3K/Akt) can affect the normal physiological activity of
cells. Findings of previous studies have shown that the abnormal
activation of the PI3K/Akt signaling pathway plays pivotal roles in
the occurrence and development of breast, ovarian, gastric, and
lung cancers and other malignant tumors (6–9). In
addition, the PI3K/Akt signaling pathway plays an important role in
the development and progression of hepatocellular carcinoma, and
activation of the PI3K/Akt signaling pathway can affect the
proliferation, invasion and apoptosis of hepatocellular carcinoma
cells (10).
Apatinib is an antitumor drug that is used for the
clinical treatment of advanced gastric cancer (11). In addition, previous findings showed
that apatinib has therapeutic effects on breast and non-small cell
lung cancer, as well as other types of cancer (12–14).
Apatinib can specifically bind to ATP binding sites of VEGFR-2 in
tumor cells to block the downstream signaling transductions of
c-Kit, Ret and c-Src genes, thereby inhibiting
neovascularization in tumor tissues (15).
The aim of the present study was to investigate the
inhibitory effects of apatinib on the proliferation of the
SMMC-7721 human hepatocellular carcinoma cell line, and to
investigate the effects of apatinib on the expression of
apoptotic-related genes Bcl-2, Bax and caspase-9 and
the PI3K/Akt signaling pathway-related proteins, and to explore the
mechanism of the effects of apatinib on hepatocellular carcinoma in
order to lay the foundation for the clinical treatment of
hepatocellular carcinoma with apatinib.
Materials and methods
Materials
Materials used in the present study were: Apatinib
and DMSO (Aladdin, Shanghai, China); SMMC-7721 hepatocellular
carcinoma cell line (The Cell Bank of Type Culture Collection of
Chinese Academy of Sciences, Shanghai, China); RPMI-1640 medium
(Gibco Life Technologies, Carlsbad, CA, USA); MTT (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany); Annexin V/PI apoptosis detection
kit (Beyotime Institute of Biotechnology, Nantong, China); TRIzol,
reverse transcription kit and RT-qPCR kit (all from Invitrogen,
Carlsbad, CA, USA); primer synthesis (Takara Biotechnology Co.,
Ltd., Dalian, China); rabbit anti-human PIAK, rabbit anti-human
pPI3K, rabbit anti-human Akt, rabbit anti-human pAkt, rabbit
anti-human Bcl-2, rabbit anti-human Bax, rabbit anti-human
caspase-9, and rabbit anti-human GAPDH primary antibodies, and
HRP-labeled goat anti-rabbit secondary antibody (all from
Proteintech Group, Inc., Wuhan, China).
Cell culture
SMMC-7721 cells were incubated with RPMI-1640 medium
containing 100 U/ml penicillin, 100 µg/ml streptomycin and 10%
fetal bovine serum (FBS) in an incubator (37°C, 5% CO2).
The cells were collected during the logarithm growth period, and
digested with trypsin to produce single cell suspension. The cells
were subjected to different treatments according to the
experimental design.
MTT assay to detect SMMC-7721 cell
proliferation
SMMC-7721 cells (100 µl, 1×105/ml) were
inoculated into 96-well plates and cultured (37°C, 5%
CO2) for 24 h. Then, apatinib was added at a
concentration of 0, 0.5, 1, 2, 4, 8 and 16 µmol/l. After incubation
for 48 h, 10 µl of MTT solution (5 mg/ml) was added into each well,
followed by incubation for 4 h. Absorbance values (OD) at 570 nm
were measured using a microplate reader (Model 680; Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and the inhibition rate of
apatinib on cell proliferation was calculated using the formula:
Inhibition rate (%) = (OD value of experimental group - OD value of
normal control group)/(OD value of normal control group) ×
100%.
Annexin V/PI double staining to detect
apoptosis of SMMC-7721 cells
The cells were collected during the logarithm growth
period. After digestion with 0.25% trypsin, the cells were
inoculated into 6-well plates, and then randomly divided into the
control and apatinib treatment groups (1, 2 and 4 µmol/l). After
treatment with apatinib for 48 h, the cells were digested with
0.25% trypsin, followed by centrifugation at 3,000 × g for 10 min
to collect cells. The cells were washed twice with
phosphate-buffered saline (PBS) and 5 µl Annexin V and 5 µl PI were
added and mixed gently, followed by incubation in the dark for 15
min. Cell apoptosis was detected by flow cytometry
(Becton-Dickinson, Franklin Lakes, NJ, USA).
RT-qPCR used to detect the expression
of related genes in SMMC-7721 cells
After the process described, TRIzol reagent was used
to extract total RNA from each group of cells. Only the RNA samples
with a ratio of A260/A280 between 1.8 and 2.0 were used for reverse
transcription to synthesize cDNA. Primer sequences used in PCR
reactions are listed in Table I. PCR
reaction conditions were as follows: 94°C for 3 min followed by 30
cycles of 94°C for 30 sec, 57°C for 30 sec and 72°C for 1 min. The
experiment was repeated 3 times. The data were processed using
2−ΔΔCq method: ΔCq (target gene) = target gene Cq -
control gene Cq; ΔΔCt = ΔCq (target gene) - ΔCq (standard value).
Relative expression levels of Bax, caspase-9 and Bcl-2 were
calculated according to endogenous control GAPDH.
 | Table I.Primer sequences used in RT-qPCR. |
Table I.
Primer sequences used in RT-qPCR.
| Genes | Primer sequences |
|---|
| Bcl-2 | F:
5′-TGGGATGCCTTTGTGGAAC-3′ |
|
| R:
5′-CATATTTGTTTGGGGCAGGTC-3′ |
| Bax | F:
5′-TGCTACAGGGTTTCATCCAG-3′ |
|
| R:
5′-ATCCACATCAGCAATCATCC-3′ |
| Caspase-9 | F:
5′-AGCCAGATGCTGTCCCATAC-3′ |
|
| R:
5′-CAGGAGACAAAACCTGGGAA-3′ |
| GAPDH | F:
5′-GGAAAGCTGTGGCGTGAT-3′ |
|
| R:
5′-AAGGTGGAAGAATGGGAGTT-3′ |
Western blot analysis used to detect
the expression-related proteins in SMMC-7721 cells
After the process described, the total protein was
collected from each group of cells after cell lysis using RIPA cell
lysate. Protein concentration was quantified, and protein samples
were subjected to 10% SDS-PAGE electrophoresis, followed by
transmembrane to PVDF membrane. The membranes were blocked with 5%
skimmed milk, followed by incubation with primary antibodies of
PI3K, pPI3K, Akt, pAkt, Bcl-2, Bax, caspase-9 and GAPDH (1:1,000)
overnight at 4°C. After washing with TBST for 30 min, membranes
were incubated with labeled secondary antibody (1:500) at room
temperature for 2 h. Color development was performed with ECL
solution and the results were photographed. Images were analyzed
using Image Lab 4.0.1 software, and the relative expression of each
protein was normalized to endogenous control GAPDH.
Statistical analysis
Data are expressed as mean ± standard deviation and
processed by SPSS 17.0 (IBM, Corp., Armonk, NY, USA). Single factor
analysis of variance was used to analyze the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of apatinib on the
proliferation of SMMC-7721 cells
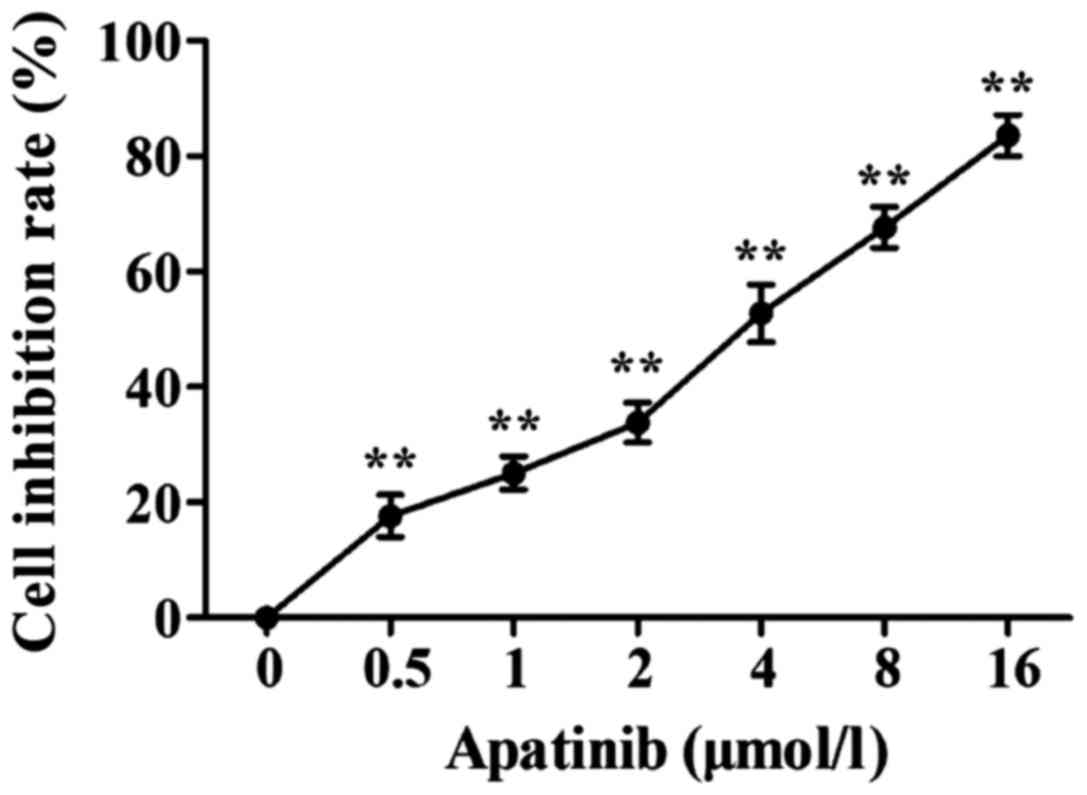
MTT results showed that apatinib (0.5, 1, 2, 4, 8
and 16 µmol/l) was able to inhibit the activity of SMMC-7721 cells
in a dose-dependent manner (Fig. 1).
For the following experiments, apatinib at the concentrations of 1,
2 and 4 µmol/l, which showed 50% inhibitory rate on cell
proliferation were used, and the action time was 48 h.
Effects of apatinib on the apoptosis
of SMMC-7721 cells
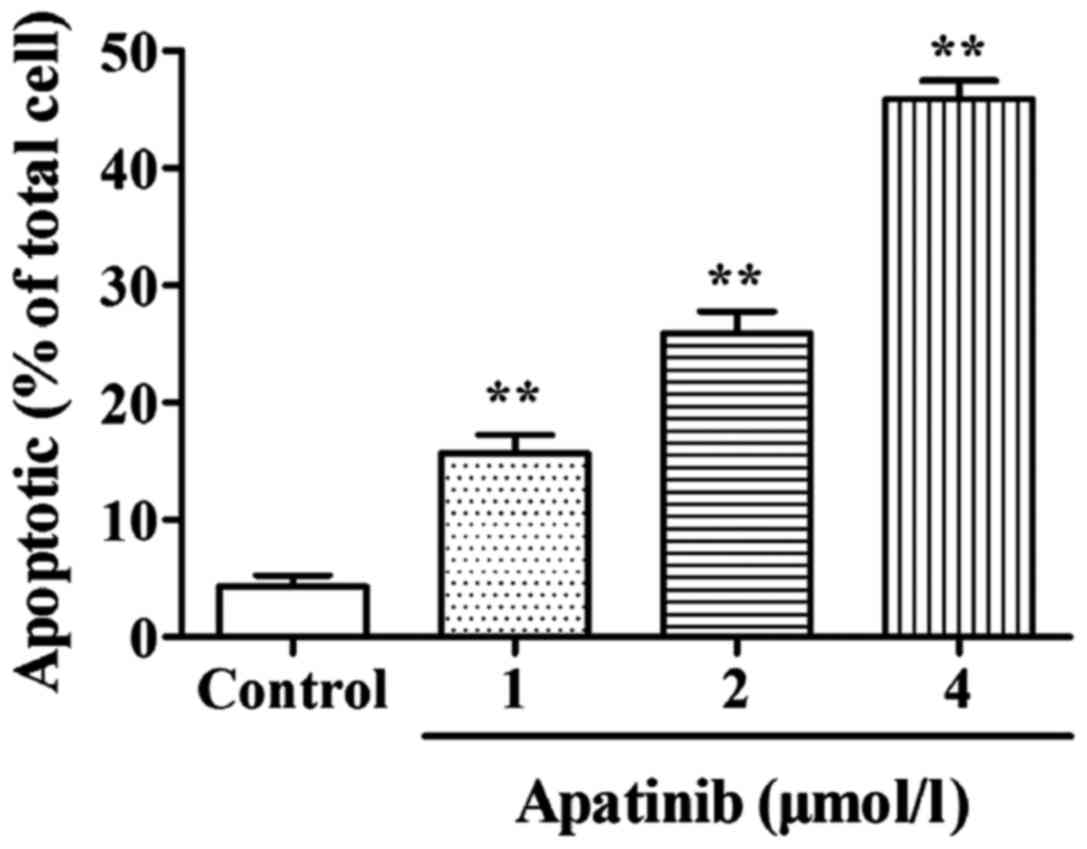
Effect of apatinib on the apoptosis of SMMC-7721
cells was detected by AV/PI double staining. As shown in Fig. 2, compared with the control group, the
cell apoptotic rate in the apatinib treatment groups were
significantly increased (p<0.01), indicating that apatinib can
significantly induce apoptosis of SMMC-7721 cells.
Effects of apatinib on the expression
of apoptosis-related genes in SMMC-7721 cells
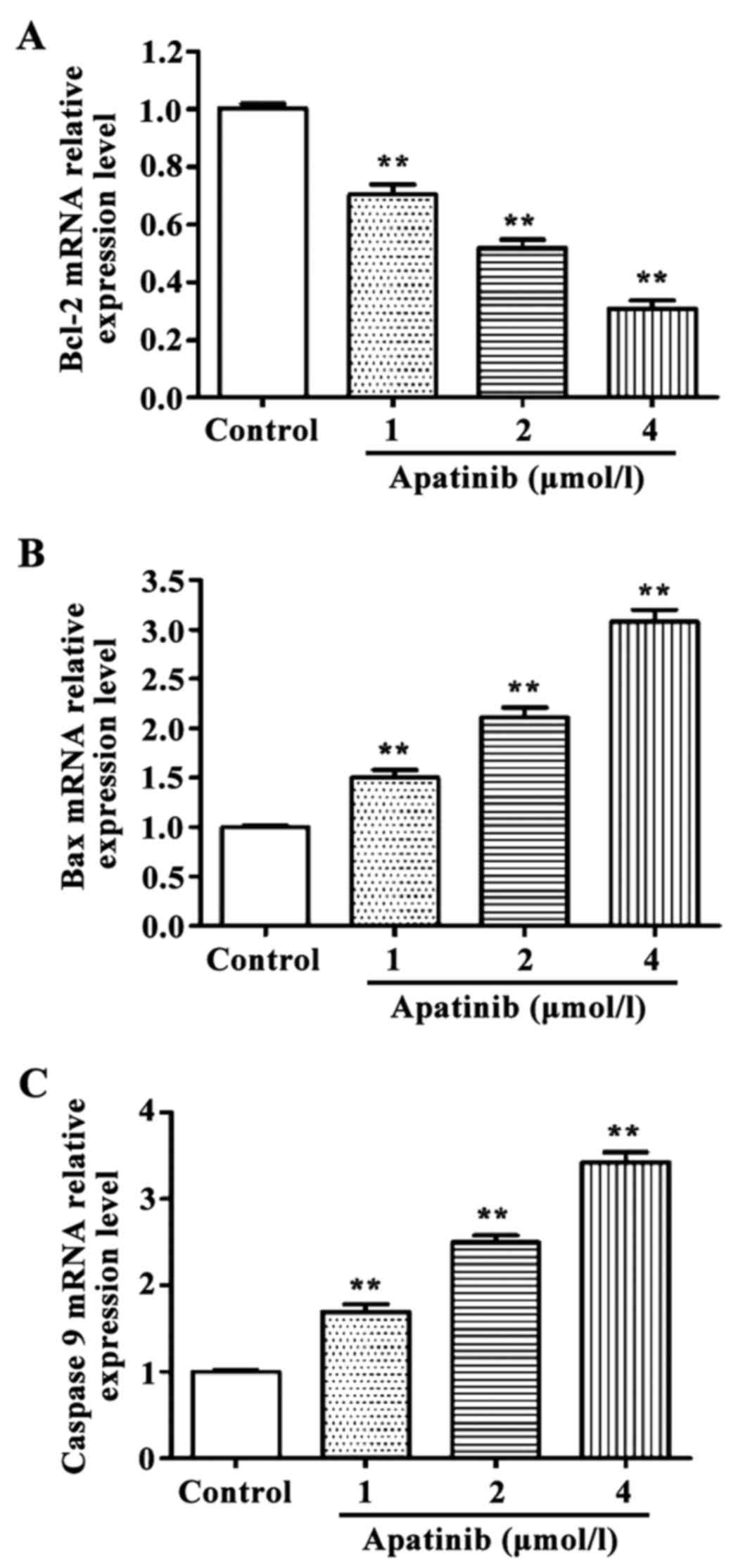
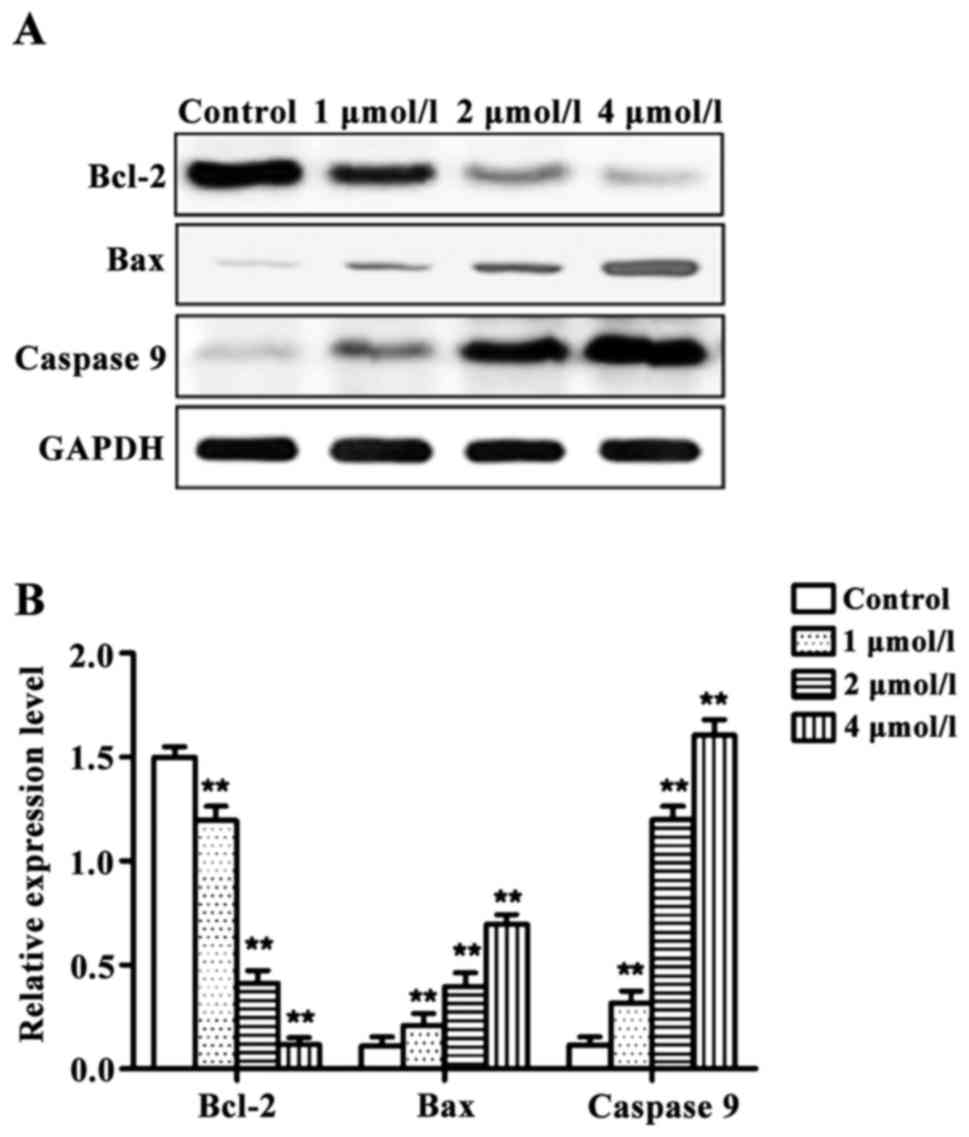
RT-qPCR and western blot results revealed that the
expression levels of pro-apoptotic genes Bax and
caspase-9 were significantly higher, and the expression
level of the anti-apoptotic gene Bcl-2 was significantly
lower in cells treated with different concentrations of apatinib
compared with the control group at the mRNA and protein levels
(Figs. 3 and 4).
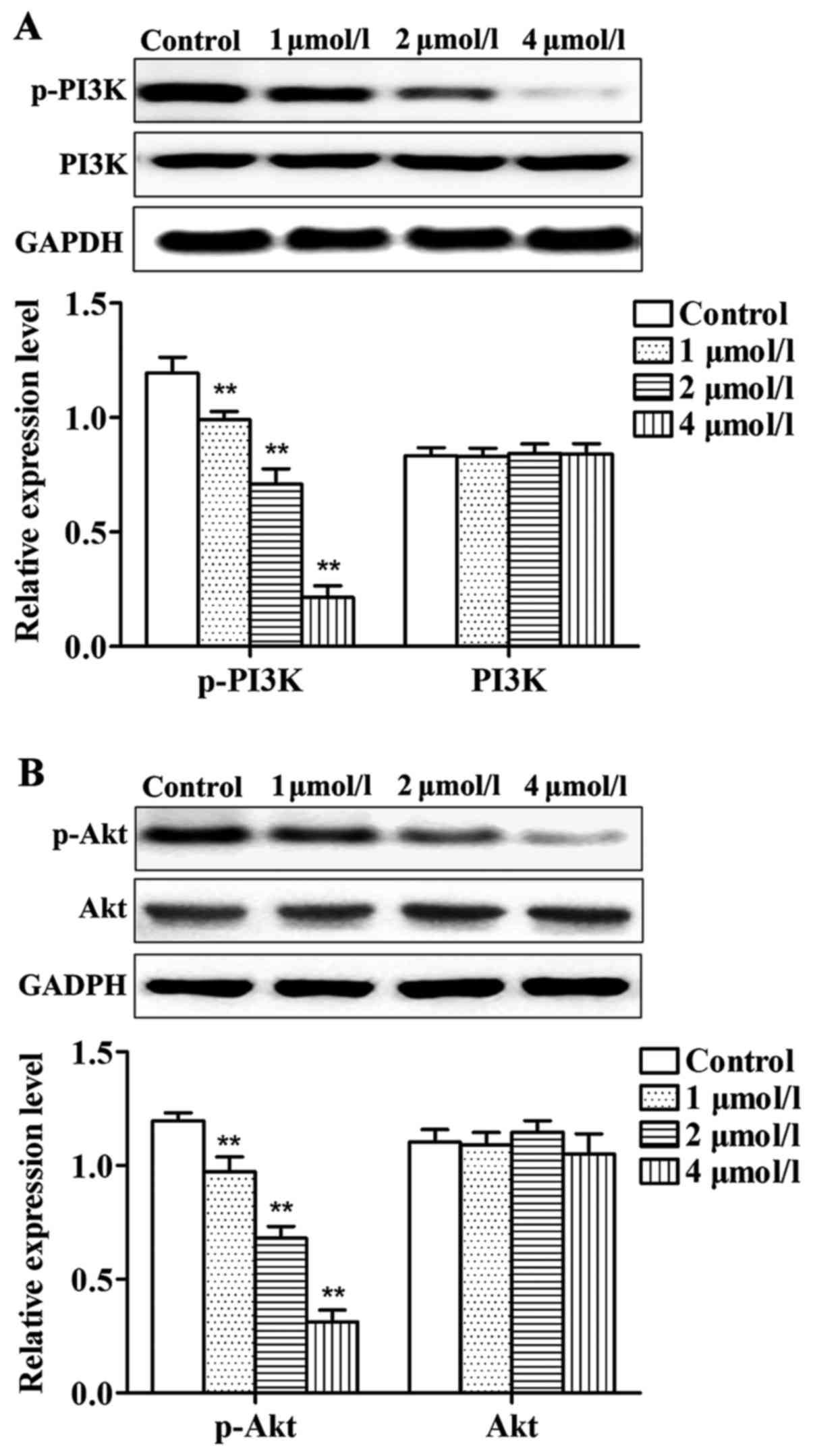
Effects of apatinib on PI3K/Akt
pathway in SMMC-7721 cells
As shown in Fig. 5,
compared with control group, levels of p-PI3K and p-Akt in cells
treated with different concentrations of apatinib were
significantly reduced. No significant differences were identified
in the total protein levels of PI3K and Akt. Thus, apatinib can
inhibit the phosphorylation of PI3K and Akt protein.
Discussion
In China, a large portion of the population is
affected by hepatocellular carcinoma, which shows both a high
incidence and mortality rate (16).
At present, the preferred treatment is surgical resection. However,
the early symptoms of hepatocellular carcinoma are not clear, and
most patients are diagnosed with distant metastasis, which can only
be treated with radiotherapy or chemotherapy (17). Apatinib, or N-[4-(1-cyanocyclopentyl)
phenyl]-2-(4-pyridylmethyl) amino-3-pyridinecarboxamide
methanesulfonate, is a new type of small molecule VEGFR-2 tyrosine
kinase inhibitor that can be taken orally. Apatinib has been
approved by the China Food and Drug Admistration to treat patients
with recurrent advanced gastric adenocarcinoma and
gastric-esophageal junction adenocarcinoma. In addition, the
therapeutic effects of apatinib on non-small cell lung, breast,
liver and rectal cancers are now being tested clinically (18–20).
PI3K is an important kinase of phosphatidylinositol
and inositol. PI3K can be activated by receptor on cell surface to
form p-PI3K, and p-PI3K can activate Akt and other downstream
effector to transduct the signals of various growth factors and
cytokines to cells (21,22). Phosphorylated Akt can act on proteins
in Bcl-2 family and the caspase family to inhibit cell apoptosis,
which in turn lead to excessive cell growth and proliferation
(23). Studies have shown that PI3K
and Akt were highly expressed in various types of tumor cells, and
the activated PI3K/Akt pathway can promote the proliferation and
invasion of tumor cells and induce resistance of tumor cells to
chemotherapeutic drugs (24,25).
As an anti-apoptotic protein, Bcl-2 plays a key role
in the apoptotic signaling pathway. Bcl-2 can inhibit cell
apoptosis and improve cell proliferation (26). As a pro-apoptotic protein, Bax has
opposite functions to Bcl-2. Bax and Bcl-2 belong to the same
family. Bax, not only antagonizes the inhibitory effects of Bcl-2
on cell apoptosis, but also directly promotes tumor cell apoptosis
(27). Caspase-9 is a key factor in
the mitochondrial apoptosis pathway. Activated caspase-9 can
further activate downstream caspase-3, which in turn leads to tumor
cell apoptosis (28,29).
In the present study, different concentrations of
apatinib were used to treat SMMC-7721 cells for 48 h. The MTT assay
showed that apatinib significantly inhibited the proliferation of
SMMC-7721 cells. Annexin V/PI double staining results showed that
apatinib was able to induce the apoptosis of SMMC-7721 cells in a
concentration-dependent manner. Results of RT-qPCR and western blot
analysis showed that apatinib induced the expression of
pro-apoptotic genes Bax and caspase-9 and inhibited
the expression of anti-apoptotic gene Bcl-2, indicating that
apatinib can induce tumor cell apoptosis. In this study, the
possible involvement of the PI3K/Akt signaling pathway was also
investigated. Western blot analysis revealed that apitatinib
significantly reduced the levels of p-PI3K and p-Akt in SMMC-7721
cells, but showed no significant effect on total protein levels of
PI3K and Akt. Similar results were found in a study carried out by
Yin et al, that is, apatinib can induce the apoptosis of
HCT-116 colon cancer cells by inhibiting the MAPK/Erk pathway,
reducing the phosphorylation of p-ERK and p-AKT in the PI3K/Akt
pathway, and inducing the expression of Bax and caspase-3 (30).
In conclusion, the present findings have shown that
apatinib inhibited the proliferation and induced the apoptosis of
human hepatocellular carcinoma cells possibly by inhibiting the
PI3K/Akt signaling transduction pathway, upregulating the
expression of Bax and caspase-9, and downregulating the expression
of Bcl-2.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and YCa analyzed and interpreted the patient
data. HZ wrote the manuscript. YCh and HY collected the patient
data and revised the manuscript for important intellectual content.
HZ and GL contributed to the conception and design of the study.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Heze Municipal Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gomaa AI, Khan SA, Toledano MB, Waked I
and Taylor-Robinson SD: Hepatocellular carcinoma: Epidemiology,
risk factors and pathogenesis. World J Gastroenterol. 14:4300–4308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Yue M, Shu R, Cheng H and Hu P:
Recent advances in the management of hepatocellular carcinoma. J
BUON. 21:307–311. 2016.PubMed/NCBI
|
|
5
|
Ling CQ, Liu Q, Li DT, Yue XQ, Hou FG, Zhu
DZ, Yu CQ, Chen Z, Zhai XF and Yu Y: Study of a qualitative
diagnostic criterion for basic syndromes of traditional Chinese
medicine in patients with primary liver cancer. Zhong Xi Yi Jie He
Xue Bao. 3:95–98. 2005.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu P, Liu T and Hu Y: PI3K inhibitors for
cancer therapy: What has been achieved so far? Curr Med Chem.
16:916–930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mabuchi S, Kuroda H, Takahashi R and
Sasano T: The PI3K/AKT/mTOR pathway as a therapeutic target in
ovarian cancer. Gynecol Oncol. 137:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu HY, Kim SO, Jin CY, Kim GY, Kim WJ, Yoo
YH and Choi YH: β-lapachone-induced apoptosis of human gastric
carcinoma AGS cells is caspase-dependent and regulated by the
PI3K/Akt pathway. Biomol Ther (Seoul). 22:184–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu YL, Zhang QH, Wang XW and He H:
Antidiabetic drug metformin mitigates ovarian cancer SKOV3 cell
growth by triggering G2/M cell cycle arrest and inhibition of
m-TOR/PI3K/Akt signaling pathway. Eur Rev Med Pharmacol Sci.
21:1169–1175. 2017.PubMed/NCBI
|
|
10
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geng R and Li J: Apatinib for the
treatment of gastric cancer. Expert Opin Pharmacother. 16:117–122.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roviello G, Ravelli A, Polom K, Petrioli
R, Marano L, Marrelli D, Roviello F and Generali D: Apatinib: A
novel receptor tyrosine kinase inhibitor for the treatment of
gastric cancer. Cancer Lett. 372:187–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H: Apatinib for molecular targeted
therapy in tumor. Drug Des Devel Ther. 9:6075–6081. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP,
Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, et al: Apatinib
(YN968D1) reverses multidrug resistance by inhibiting the efflux
function of multiple ATP-binding cassette transporters. Cancer Res.
70:7981–7991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y,
Li J and Lou L: YN968D1 is a novel and selective inhibitor of
vascular endothelial growth factor receptor-2 tyrosine kinase with
potent activity in vitro and in vivo. Cancer Sci. 102:1374–1380.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frau M, Biasi F, Feo F and Pascale RM:
Prognostic markers and putative therapeutic targets for
hepatocellular carcinoma. Mol Aspects Med. 31:179–193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y,
Sun G, Yang Y, Wang L, Xu N, et al: Apatinib for
chemotherapy-refractory advanced metastatic gastric cancer: Results
from a randomized, placebo-controlled, parallel-arm, phase II
trial. J Clin Oncol. 31:3219–3225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan M, Zhang J, Wang Z, Wang B, Zhang Q,
Zheng C, Li T, Ni C, Wu Z, Shao Z, et al: Phosphorylated VEGFR2 and
hypertension: Potential biomarkers to indicate VEGF-dependency of
advanced breast cancer in anti-angiogenic therapy. Breast Cancer
Res Treat. 143:141–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohtsu A, Shah MA, Van Cutsem E, Rha SY,
Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: A randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia M, Tong JH, Ji NN, Duan ML, Tan YH and
Xu JG: Tramadol regulates proliferation, migration and invasion via
PTEN/PI3K/AKT signaling in lung adenocarcinoma cells. Eur Rev Med
Pharmacol Sci. 20:2573–2580. 2016.PubMed/NCBI
|
|
22
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gondi CS, Kandhukuri N, Dinh DH, Gujrati M
and Rao JS: Down-regulation of uPAR and uPA activates
caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J
Oncol. 31:19–27. 2007.PubMed/NCBI
|
|
24
|
Roberts LR and Gores GJ: Hepatocellular
carcinoma: Molecular pathways and new therapeutic targets. Semin
Liver Dis. 25:212–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao JG, Zhang L, Xiang XJ, Yu F, Yu F, Ye
WL, Wu DP, Wang JF and Xiong JP: Erratum: Amarogentin secoiridoid
inhibits in vivo cancer cell growth in xenograft mice model and
induces apoptosis in human gastric cancer cells (SNU-16) through
G2/M cell cycle arrest and PI3K/Akt signalling pathway. J BUON.
21:13322016.PubMed/NCBI
|
|
26
|
Packham G and Cleveland JL: c-Myc and
apoptosis. Biochim Biophys Acta. 1242:11–28. 1995.PubMed/NCBI
|
|
27
|
Brady HJ and Gil-Gómez G: Bax. The
pro-apoptotic Bcl-2 family member, Bax. Int J Biochem Cell Biol.
30:647–650. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schroeder CP, Kadara H, Lotan D, Woo JK,
Lee HY, Hong WK and Lotan R: Involvement of mitochondrial and Akt
signaling pathways in augmented apoptosis induced by a combination
of low doses of celecoxib and N-(4-hydroxyphenyl) retinamide in
premalignant human bronchial epithelial cells. Cancer Res.
66:9762–9770. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agarwal S, Achari C, Praveen D, Roy KR,
Reddy GV and Reddanna P: Inhibition of 12-LOX and COX-2 reduces the
proliferation of human epidermoid carcinoma cells (A431) by
modulating the ERK and PI3K-Akt signalling pathways. Exp Dermatol.
18:939–946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin L, Wang J, Huang FC, Zhang YF, Xu N,
Wen ZQ, Li WL and Dong J: Inhibitory effect of apatinib on HCT-116
cells and its mechanism. Nan Fang Yi Ke Da Xue Xue Bao. 37:367–372.
2017.(In Chinese). PubMed/NCBI
|