Introduction
Colorectal cancer (CRC) is the third leading cause
of cancer-associated mortality worldwide (1). Recurrence and metastasis are the major
causes of mortality in patients with CRC (2,3). Although
screening, surgical techniques and adjuvant chemotherapy for
patients with CRC have been developed to achieve a significant
improvement in survival time (4,5), the
5-year overall survival (OS) rate of CRC remains at ~65% (6). The stage of CRC at the time of diagnosis
is a key factor for deciding the therapeutic regimen and assessing
prognosis. However, at the time of diagnosis, the majority of
patients exhibit intermediate- or late-stage disease (7,8).
Therefore, efficient diagnostic and predictive biomarkers are
required for early-stage diagnosis in order to improve the
prognosis of patients with CRC.
The mitochondrial tumor necrosis factor
receptor-associated protein-1 (TRAP-1) is one of the 90 isoforms of
heat shock protein (HSP) and is also termed HSP75 (9,10). TRAP-1
has been demonstrated to serve an effective function in stabilizing
cellular homeostasis and regulating mitochondrial integrity
(11,12). By antagonizing cyclophilin D (CypD),
TRAP-1 serves a cytoprotective function for cells responding to
apoptotic stimuli and enables tumor cell survival in a hostile
environment (13). It has been
reported that TRAP-1 protects cancer cells from apoptosis by
blocking mitochondrial effects (14).
Additionally, increasing evidence has demonstrated that increased
TRAP-1 expression is observed in cancerous tissues of the breast,
lung, prostate and pancreas as compared with the corresponding
normal tissues (15–17). A previous study also demonstrated that
TRAP-1 expression level is associated with lymph node metastasis in
CRC (18). These aforementioned
results indicate that TRAP-1 is a potential candidate biomarker for
breast, lung and prostate cancer, as well as CRC, and may be a
cancer-specific therapeutic target. However, only a limited number
of studies have evaluated the prognostic relevance of TRAP-1
expression based on clinical data of CRC.
The aim of the present study was to investigate the
association between TRAP-1 expression and the clinicopathological
features of patients with CRC, including tumor-node-metastasis
(TNM) stage, degree of pathological differentiation, and lymph node
metastasis, as well as to demonstrate any clinical significance of
TRAP-1 expression in CRC prognosis.
Materials and methods
Patients and follow-up
A total of 256 patients diagnosed with CRC, and who
underwent surgical resection at PuAi Hospital of Tongji Medical
College, Huazhong University of Science and Technology of Wuhan
(Wuhan, China) between 1 January 2004 and 31 December 2009, were
investigated in the present study. None of the patients received
neoadjuvant chemotherapy or radiotherapy prior to surgery. Among
the 256 patients, 226 patients received 5-fluorouracil (5-FU)-based
chemotherapy following surgical resection, including 5-FU alone
(5-FU/leucovorin; 500 mg/m2 5-FU on days 1–5 and 200
mg/m2 leucovorin on days 1–5, capecitabine (a 5-FU
derivative) alone (1,250 mg/m2, twice daily on days
1–14), and 5-FU combined with oxaliplatin (500 mg/m2
5-FU on days 1–5, and 130 mg/m2 oxaliplatin on day 1).
These 226 cases underwent a 21-day cycle chemotherapy for 8
cycles.
By December 31st, 2014, at the end of follow-up, the
median follow-up time was 60 months (range, 1–60 months). The
outpatient and inpatient medical records, physical exams, complete
blood biochemical detection, serum carcinoembryonic antigen and
carbohydrate antigen 19-9 measurements, ultrasound and endoscopic
examinations, as well as computed tomography scans, were conducted
to monitor the status of patients every 3–6 months. Differentiation
grading and TNM classification for enrolled patients with
colorectal cancer were based on the 7th American Joint Committee on
Cancer TNM staging system (19).
The OS time was defined as the interval from the
date of CRC surgery until mortality from any cause.
Progression-free survival (PFS) was defined as the interval from a
particular treatment to the first evidence of progression or
recurrence, or to disease-associated mortality.
All procedures used in the present study involving
human participants were approved by the Ethics Committee of PuAi
Hospital of Tongji Medical College, Huazhong University of Science
and Technology, and were performed in accordance with the 1964
Declaration of Helsinki and its later amendments or comparable
ethical standards. Informed consent was obtained from all
individual participants included in the study.
Tissue specimens
Samples of 256 paired CRC tissues and paracancerous
tissues were collected and examined. To assess metastatic CRC, 33
metastatic CRC tissue samples from 25 cases were selected for
analysis (multiple-site metastases were present in 8 cases),
including 4 cases metastasized to the pelvis, 4 cases metastasized
to the liver, 2 cases metastasized to the lung, 2 cases
metastasized to the bladder, 15 cases metastasized to the
peritoneum and 6 cases metastasized to other organs, such as the
ovaries. To assess lymph node metastasis, >12 lymph nodes were
obtained and studied. A total of 53 cases of patients who underwent
colonoscopy were also examined, including 25 cases of colorectal
adenoma and 28 cases of colorectal high-grade intraepithelial
neoplasia. All specimens were fixed in 10% formalin for 24 h at
room temperature and embedded in paraffin.
Immunohistochemistry (IHC) for
TRAP-1
Sections from formalin-fixed, paraffin embedded
human colorectal tissues measuring 3-µm thick were sectioned on
slides. The sections were then deparaffinized at room temperature
with xylene, hygradated with ethyl alcohol in the descending
gradients (100, 95 and 75%) and washed in distilled water several
times. Antigen retrieval was conducted using Heat Induced Epitope
Retrieval with a pressure-cooker for 10 min after the pressure
starting beating in 0.01 M citrate antigen retrieval solution (pH
6.0). Subsequent to cooling to room temperature, those sections
were processed with 0.3% H2O2 methanol for 10
min to remove endogenous peroxidase activity at room temperature.
IHC staining for TRAP-1 was conducted as previously described
(20,21). Briefly, slides were incubated with a
monoclonal rabbit anti-TRAP-1 primary antibody (clone EPR5381, cat.
no. ab109323, dilution 1:200; Abcam, Cambridge, MA, USA;) at 4°C
overnight, followed by incubation with a horseradish
peroxidase-labeled goat anti-rabbit IgG secondary antibody (cat.
no. A-11035; dilution 1:500; Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA;) at 37°C for 2 h. Slides were scored by two
experienced pathologists according to intensity and proportion of
immunostaining using an upright optical microscope (Olympus
Corporation, Tokyo, Japan). Immunostaining was assessed in 5
high-powered fields at magnification, ×200. Cytoplasmic staining of
epithelial cells was considered positive. The intensity of
immunohistochemical staining (0, negative; 1, weak; 2,
intermediate; 3, strong) and the percentage of positive cells (0,
<5% positive cells; 1, 5–25% positive cells; 2, 26–50% positive
cells; 3, >50% positive cells) were evaluated. As for
calculating the percentage of positive cells of each slide, 5
fields of high power lens were selected for observation. The final
score for each section was assessed by multiplying the scores of
staining intensity and percentage of positive cells. The
immunohistochemical staining score was calculated by taking the
average of the staining scores assigned by each pathologist,
according to the formula [(score 1: Percentage of area stained ×
staining intensity) + (score 2: Percentage of area stained ×
staining intensity)]/2, as previously described (18). The two scores were evaluated by two
pathologists respectively. For statistical analysis, tissue
specimens were divided into the following four groups according to
their immunohistochemical score: Negative (staining score, 0);
positive (staining score, 1–12); low (staining score, 1–4);
intermediate (staining score, 5–8); and high (staining score,
9–12).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 13.0; SPSS Inc., Chicago, IL, USA). For
comparative analysis of qualitative variables, Pearson's
χ2 test and Fisher's exact test were used to evaluate
the association between the expression levels of TRAP-1 and
clinical factors. OS and PFS were analyzed using Kaplan-Meier Plot,
and the log-rank test was performed to detect the significance of
prognostic factors. Cox's proportional hazards model was applied to
identify independent predictive factors for survival. All P-values
were two-sided, and P<0.05 were considered to indicate a
statistically significant difference.
Results
Clinical characteristics of patients
with CRC
In the 256 cases of CRC analyzed, patient age at
diagnosis ranged from 29–80 years (mean ± SD, 61.9±11.0 years).
Among these cases, 122 patients (47.7%) were male and 134 (52.3%)
patients were female. The primary tumor site was the colon in 151
(59.0%) patients and the rectum in 105 (41.0 %) patients. Lymph
node metastases were present in 117 (45.7%) patients (Table I).
 | Table I.Baseline characteristics of patients
with colorectal cancer (n=256). |
Table I.
Baseline characteristics of patients
with colorectal cancer (n=256).
| Characteristics | n (%) |
|---|
| Age, years |
|
|
<65 | 140 (54.7) |
| ≥65 | 116 (45.3) |
| Sex |
|
| Male | 122 (47.7) |
|
Female | 134 (52.3) |
| Site of primary
tumor |
|
|
Colon | 151 (59.0) |
|
Rectum | 105 (41.0) |
| N staging |
|
| N0 | 139(54.3) |
|
N1-N2 | 117(45.7) |
| TNM
stagea |
|
| I | 25 (9.8) |
| II | 113 (44.1) |
|
III | 93 (36.3) |
| IV | 25 (9.8) |
| Surgery |
|
|
Curative resection intent | 230 (89.8) |
|
Palliative resection | 26 (10.2) |
| Chemotherapy |
|
|
Yes | 226 (88.3) |
| No | 30 (11.7) |
|
Differentiationa |
|
|
Well-differentiated | 58 (22.6) |
|
Moderately differentiated | 129 (50.4) |
| Poorly
differentiated | 69 (27.0) |
| ECOG performance
status |
|
| 0 | 159 (62.1) |
| 1 | 73 (28.5) |
| 2 | 24 (9.4) |
Expression of TRAP-1 in CRC and its
clinicopathological association
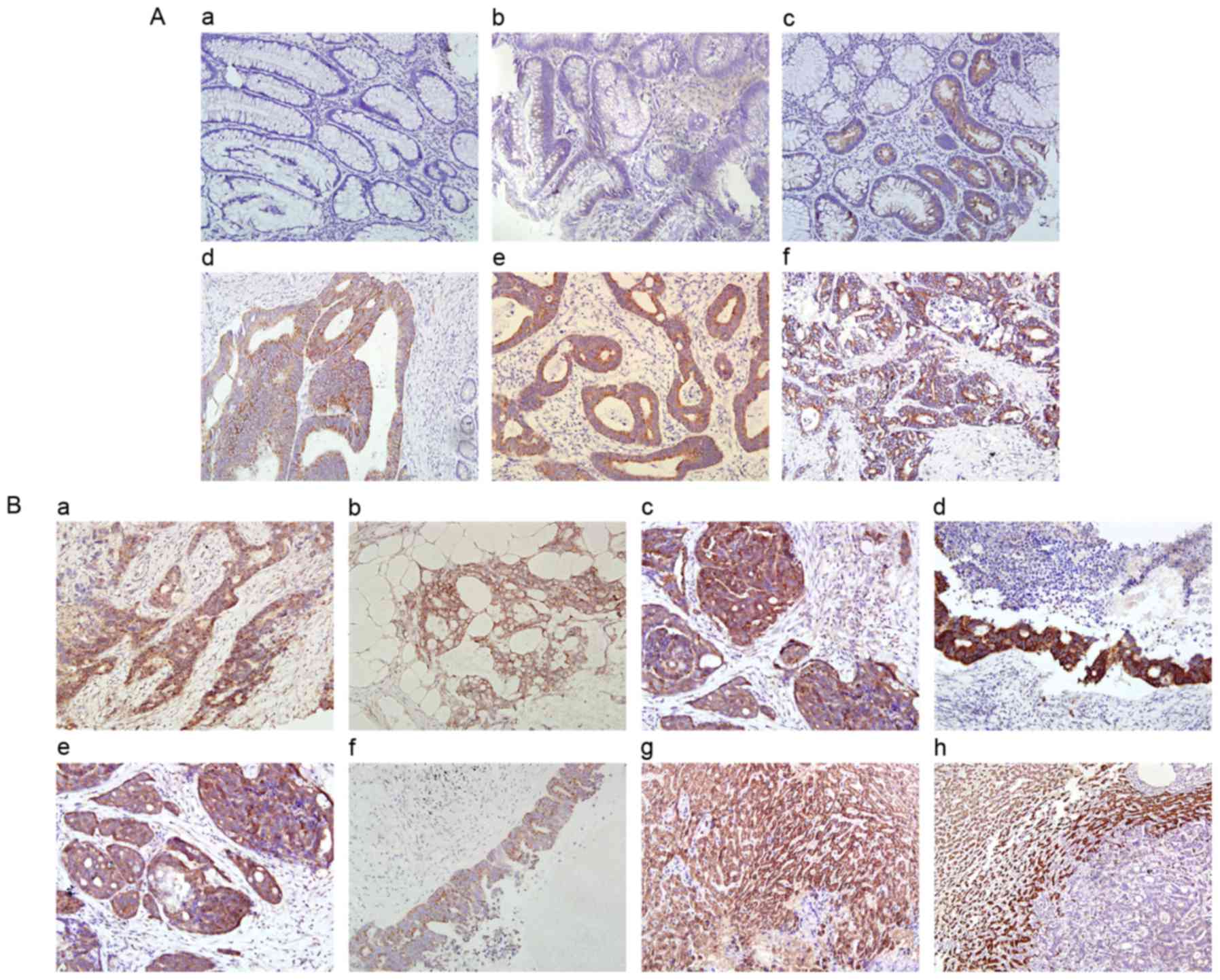
TRAP-1 expression was investigated in paired
cancerous and paracancerous tissue sections in 256 patients with
CRC. Positive expression was observed in 203/256 (79.3%) cancerous
tissue samples and in 25/256 (9.8%) paracancerous tissue samples.
In positive samples, TRAP-1 was primarily localized in the
cytoplasm of colorectal epithelial cells. The positive expression
levels were significantly increased in cancerous tissues compared
with paracancerous tissue samples (P<0.001; Fig. 1A). A total of 77 (30.1%) samples of
cancerous tissue displayed high TRAP-1 expression, 86 (33.6%)
samples exhibited intermediate TRAP-1 expression, 40 (15.6%)
samples exhibited low TRAP-1 expression, and 53 (20.7%) samples
exhibited negative TRAP-1 expression.
In the present study, the associations between
TRAP-1 expression and clinicopathological features were analyzed.
For the purpose of this analysis, the high expression group
comprised patients who possessed a TRAP-1 expression score of 9–12
(n=77, 30.1%), while all other patients (with intermediate, low or
negative TRAP-1 expression) were assigned to the low expression
group (n=179, 69.9%). Clinicopathological features included sex
(male vs. female), age (<65 vs. ≥65 years), primary tumor
location (colon vs. rectum), TNM stage, [stage I/II (defined as
early stage) vs. stage III/IV (defined as advanced stage)], tumor
invasion (T1/T2 vs. T3/T4), histodifferentiation (well/moderate vs.
poor) and lymph node metastasis (N0 vs. N1/N2). The results
demonstrated that TRAP-1 expression levels were associated with the
degree of differentiation (P=0.011), depth of invasion (T stage;
P=0.006), lymph node metastasis (N stage; P<0.001) and TNM stage
(P<0.001; Table II; Fig. 1B). No significant association was
observed between TRAP-1 expression and sex, age or tumor location
(P>0.05; Table II).
 | Table II.Association between TRAP-1 expression
and clinicopathological features. |
Table II.
Association between TRAP-1 expression
and clinicopathological features.
|
|
| Expression
intensity of TRAP-1, n (%) |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Total, n | Low | High | P-value |
|---|
| All patients | 256 | 179 (69.9) | 77 (30.1) |
|
| Sex |
|
|
| 0.240 |
|
Male | 122 | 81 (66.4) | 41 (33.6) |
|
|
Female | 134 | 98 (73.1) | 36 (26.9) |
|
| Age, years |
|
|
| 0.605 |
|
<65 | 140 | 96 (68.6) | 44 (31.4) |
|
|
≥65 | 116 | 83 (71.6) | 33 (28.4) |
|
| Tumor location |
|
|
| 0.872 |
|
Rectum | 105 | 74 (70.5) | 31 (29.5) |
|
|
Colon | 151 | 105 (69.5) | 46 (30.5) |
|
|
Differentiationa |
|
|
| 0.011 |
|
Well/moderately
differentiated | 187 | 139 (74.3) | 48 (25.7) |
|
| Poorly
differentiated | 69 | 40 (58.0) | 29 (42.0) |
|
| Depth of invasion
(pT status)a |
|
|
| 0.006 |
|
T1/T2 | 32 | 29 (90.6) | 3 (9.4) |
|
|
T3/T4 | 224 | 150 (67.0) | 74 (33.0) |
|
| Lymph node
metastasis (pN status)a |
|
|
| <0.001 |
| N0 | 139 | 114 (82.0) | 25 (18.0) |
|
|
N1/N2 | 117 | 65 (55.6) | 52 (44.4) |
|
| pTNM
stagea |
|
|
| <0.001 |
|
I/II | 138 | 107 (77.5) | 31 (22.5) |
|
|
III/IV | 118 | 65 (55.1) | 53 (44.9) |
|
In addition, 25 colorectal adenoma tissues and 28
colorectal high-grade intraepithelial neoplasia tissues were
analyzed. The results demonstrated weakly positive TRAP-1
expression (staining score, 1–3) in all 28 high-grade
intraepithelial neoplasia tissues as well as in 2/25 colorectal
adenoma tissues, whereas 23 of 25 colorectal adenoma tissues
exhibited negative TRAP-1 expression (Fig. 1A).
Cancer recurrence/metastasis according
to TRAP-1 expression status
During the follow-up period, cancer recurrences or
metastases were detected in 144/256 (56.3%) patients and at 239
different sites, including anastomotic stoma/pelvic (63 cases),
liver (47 cases), lung (34 cases), abdominal cavity/peritoneum (43
cases), regional lymph nodes (26 cases), bone/brain (13 cases) and
other unusual sites (13 cases). In these cases, tissues of multiple
metastatic sites were detected. The TRAP-1 expression levels in
distant metastatic tissues of 22 patients were analyzed, including
11 cases of liver, 6 cases of lung and 5 cases of peritoneal
metastasis. High TRAP-1 expression was determined in all metastatic
peritoneal tissues. However, in contrast to peritoneal and lymph
node metastases, low TRAP-1 expression was detected in all
metastatic liver tissues with respect to normal liver tissues. This
indicated an inverse association between TRAP-1 expression levels
and liver metastasis. In addition, all metastatic lung tissues
exhibited low TRAP-1 expression, in comparison with normal lung
tissues, which exhibited negative TRAP-1 expression (Fig. 1B).
Survival analysis according to TRAP-1
expression or expression intensity
Among the 256 patients, 128 mortalities (50.0%)
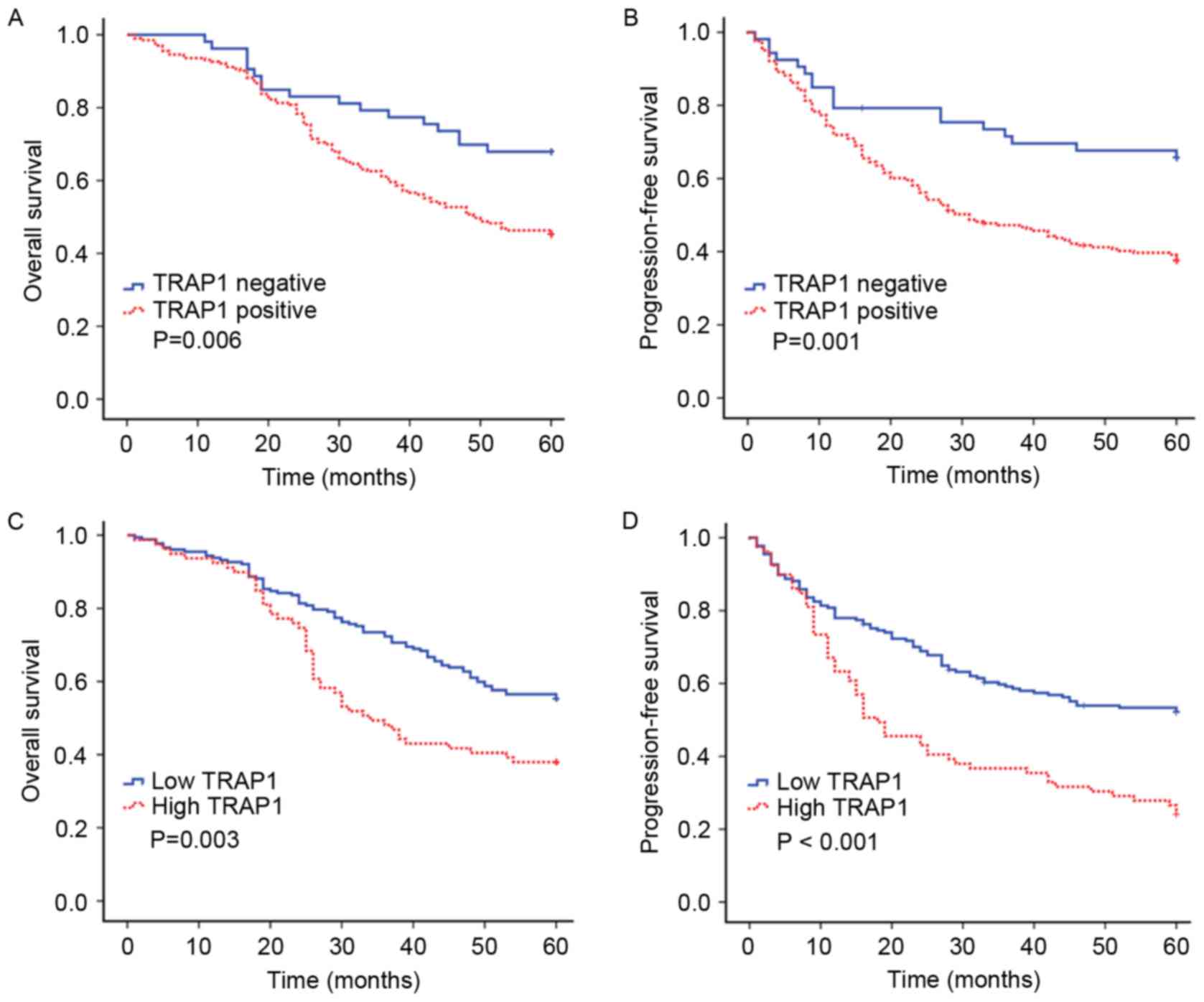
occurred within 5 years. Kaplan-Meier curves and log-rank tests
were applied to determine the association between TRAP-1 expression
and 5-year OS and PFS rates, respectively (Fig. 2). As is demonstrated in Fig. 2A and B, TRAP-1 positive expression was
significantly associated with decreased 5-year OS (P=0.006) and PFS
(P=0.001) rates in patients with CRC.
Patients with CRC were analyzed according to high
and low TRAP-1 expression groups. Patients with high TRAP-1
expression experienced a 5-year OS rate of 38.0%, compared with
56.5% for patients with low TRAP-1 expression (P=0.003). Median OS
times were 35.7 and 60.0 months for the high and low TRAP-1
expression groups, respectively. Similarly, the 5-year PFS rate was
26.6% for patients with high TRAP-1 expression and 53.3% for
patients with low TRAP-1 expression (P<0.001), and the median
PFS times were 21.5 and 60.0 months for the high and low TRAP-1
expression groups, respectively. Patients with CRC with high TRAP-1
expression exhibited shorter OS and PFS times compared with
patients with low TRAP-1 expression (Fig.
2C and D).
Prognostic factors associated with
TRAP-1 expression status
Univariate analysis demonstrated a significant
association between positive TRAP-1 expression and poor OS
(P=0.008) and PFS (P=0.001) times. Multivariate analyses using
Cox's regression model revealed positive TRAP-1 expression as an
independent prognostic factor for both OS [hazard ratio (HR),
1.914; 95% confidence interval (CI), 1.133–3.233; P=0.015] and PFS
(HR, 2.534; 95% CI, 1.534–4.212; P<0.001) (Table III).
 | Table III.Univariate and multivariate analyses
for OS and PFS of patients with colorectal cancer (n=256). |
Table III.
Univariate and multivariate analyses
for OS and PFS of patients with colorectal cancer (n=256).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) |
|
|
|
|
|
|
| OS | 0.815 | 0.576–1.153 | 0.248 | ND | ND | ND |
|
PFS | 0.741 | 0.534–1.028 | 0.073 | ND | ND | ND |
| Age (≥65 vs. <65
years) |
|
|
|
|
|
|
| OS | 1.619 | 1.143–2.294 | 0.007 | 1.022 | 0.700–1.494 | 0.910 |
|
PFS | 1.551 | 1.118–2.152 | 0.009 | 1.182 | 0.837–1.668 | 0.342 |
| Location (rectum
vs. colon) |
|
|
|
|
|
|
| OS | 0.936 | 0.658–1.331 | 0.712 | ND | ND | ND |
|
PFS | 0.932 | 0.668–1.299 | 0.676 | ND | ND | ND |
| TNM stage (I/II vs.
III/IV)a |
|
|
|
|
|
|
| OS | 5.144 | 3.491–7.581 | <0.001 | 11.325 | 2.580–49.713 | 0.001 |
|
PFS | 4.215 | 2.963–5.995 | <0.001 | 2.511 | 1.661–3.795 | <0.001 |
| Differentiation
(well/moderate vs. poor)a |
|
|
|
|
|
|
| OS | 3.777 | 2.656–5.372 | <0.001 | 2.641 | 1.773–3.933 | <0.001 |
|
PFS | 3.335 | 2.372–4.690 | <0.001 | 2.219 | 1.514–3.252 | <0.001 |
| ECOG performance
status (0 vs. 1 vs. 2) |
|
|
|
|
|
|
| OS | 3.399 | 2.655–4.352 | <0.001 | 1.591 | 1.169–2.165 | 0.003 |
|
PFS | 2.922 | 2.305–3.705 | <0.001 | 1.585 | 1.171–2.146 | 0.003 |
| Surgery (radical
surgery vs. palliative surgery) |
|
|
|
|
|
|
| OS | 0.135 | 0.086–0.212 | <0.001 | 0.345 | 0.202–0.589 | <0.001 |
|
PFS | 0.165 | 0.105–0.261 | <0.001 | 0.386 | 0.226–0.658 | <0.001 |
| Chemotherapy (yes
vs. no) |
|
|
|
|
|
|
| OS | 0.456 | 0.283–0.735 | 0.001 | 0.323 | 0.192–0.542 | <0.001 |
|
PFS | 0.592 | 0.369–0.949 | 0.030 | 0.548 | 0.35–0.899 | 0.017 |
| TRAP-1 expression
(negative vs. positive) |
|
|
|
|
|
|
| OS | 1.997 | 1.198–3.328 | 0.008 | 1.914 | 1.133–3.233 | 0.015 |
|
PFS | 2.253 | 1.374–3.695 | 0.001 | 2.534 | 1.534–4.212 | <0.001 |
Furthermore, multivariate analysis demonstrated that
an advanced stage, poor Eastern Cooperative Oncology Group
(20) performance status, poor tumor
differentiation, and the absence of surgery or chemotherapy were
independent prognostic factors for cancer-specific OS and PFS
times. In addition, no significant association of other factors,
such as sex and primary tumor site, with either cancer-specific OS
or PFS were identified (P>0.05; Table III).
Discussion
The present study evaluated the clinical and
prognostic significance of TRAP-1 expression in 256 patients with
CRC. The results demonstrated that the expression level of TRAP-1
was increased in cancerous tissues compared with adjacent
noncancerous tissues, suggesting that TRAP-1 may exhibit a function
in CRC tumorigenesis and progression. This result is consistent
with reported studies from lung, breast, colorectal and pancreatic
adenocarcinomas (15–17). In addition, in the present study,
similar percentages of TRAP-1 overexpression were detected in
primary colon and rectal cancer tissues (30.5 and 29.5%,
respectively), indicating that TRAP-1 expression does not differ
between colon and rectal cancers.
Clinicopathological parameters serve important
functions for predicting prognosis and determining the most
suitable CRC therapy (22–24). In the present study, a significant
association was demonstrated between an increasing TRAP-1
expression and clinicopathological characteristics of CRC,
including differentiation, depth of cancer invasion and presence of
lymph node metastasis. Specifically, it was observed that TRAP-1
expression is a predictor for lymph node metastatic spread; this
result was in accordance with a previous study of CRC (18) where TRAP-1 positive samples in
patients with CRC were more frequently associated with lymphatic
metastasis compared with TRAP-1 negative samples. Additionally,
TRAP-1 expression was increased in metastatic lymph nodes compared
with matched in situ tumor tissues in the present study.
Therefore, the association between TRAP-1 expression and the
aforementioned clinicopathological factors may implicate TRAP-1 as
critical in CRC progression. In turn, TRAP-1 expression may serve
as a molecular marker for lymph node metastasis and poor prognosis.
However, no significant association was observed between TRAP-1
expression and the clinicopathological parameters of tumor
differentiation and depth of cancer invasion in a previous study
(18), and this is inconsistent with
the results of the present study. In addition, in the present study
no significant difference was indicated between the level of TRAP-1
expression in CRC tissues and other factors, including sex, age and
tumor localization (P>0.05).
However, in the present study, TRAP-1 expression
levels from different distant metastatic tissues revealed some
contradictory results. Metastatic cancerous tissue of the
peritoneum demonstrated an overexpression of TRAP-1, whereas
metastatic cancerous tissue of the lung exhibited low expression of
TRAP-1. These results suggested that TRAP-1 expression differs
between distinct metastatic CRC tissues, and the TRAP-1 expression
level in metastatic lung cancer tissues is inconsistent with TRAP-1
expression levels in primary lung tumor tissues (16). Furthermore, TRAP-1 was expressed
abundantly in normal liver tissues, whereas TRAP-1 expression was
decreased in metastatic liver cancer tissues, indicating that
TRAP-1 expression level is inversely associated with metastatic
liver cancer.
Notably, TRAP-1 expression levels increased
gradually from the colorectal mucosa of high-grade intraepithelial
neoplasia to CRC. This suggests that TRAP-1 expression may be
detected at the earliest stage of CRC tumorigenesis, and TRAP-1 may
serve a function not only in the progression, but also in the onset
of malignancy. This suggests that TRAP-1 may be gradually activated
during colorectal carcinogenesis. To the best of our knowledge,
there were several studies that noted TRAP-1 expression status in
colorectal mucosa of high-grade intraepithelial neoplasia. Previous
data obtained from CRC associated with ulcerative colitis
demonstrated increased TRAP-1 expression and the degree of
inflammation in CRC tissues only, but not in acute inflammation
tissues (25). These results, in
combination with those of the present study, suggest that TRAP-1
expression may occur in the earlier stages of tumorigenesis and
that acute inflammation is not likely to influence TRAP-1
expression.
The progression of CRC primarily involves tumor
differentiation, local infiltration, lymph node metastasis and
distant metastasis. These stages are closely associated with cancer
cell proliferation and invasion (26). A number of studies have identified
that TRAP-1 expression is upregulated in metastatic cancer cells
(15,22) and is involved in oncogenesis by
contributing to the inhibition of cancer cell apoptosis (27,28). Other
studies have identified that TRAP-1 is abundantly localized in the
mitochondria of tumor cells (13,17) and is
involved in protecting against oxidative stress and apoptosis
(27,29–31). These
results demonstrate the anti-apoptotic role of TRAP-1 in cancer
cells. In addition, functional studies on the lung adenocarcinoma
cell line A549 and the human breast cancer cell line MDA-MB-231
demonstrated that TRAP-1 expression is positively associated with
cell proliferation in vitro (28). These significant functions of TRAP-1
in promoting cell proliferation and inhibiting apoptosis may
explain the association between TRAP-1 expression levels and
clinicopathological characteristics of CRC observed in the present
study, including local infiltration, lymph node metastasis and
distant metastasis.
A number of previous studies have demonstrated that
the main roles of TRAP-1 are in tumor progression, protection from
oxidative damage and cell survival (9,29). TRAP-1
interacts with CypD, a mitochondrial permeability transition pore
regulator, to suppress the main cell death pathway maintained by
CypD (10,27). Therefore, cytoprotection mediated by
the upregulated expression of TRAP-1 may be an indicator for the
onset of tumorigenesis. Furthermore, it has been reported that
TRAP-1 functions synergistically with tumor necrosis factor
receptor 1 to modulate the expression of the cell adhesion molecule
N-cadherin, while altering the inter-cellular adhesion of neuronal
cells through the signal transducer and activator of transcription
3 phosphorylation status (17,32). This
demonstrates the role of TRAP-1 in the processes of cell invasion
and motility, which are characteristics of tumorigenesis and
metastatic spread. In addition, previous studies have demonstrated
that TRAP-1 regulates genes involved in the cell cycle and
metastasis (28). The present study
also indicated that cytoprotective mitochondrial chaperone TRAP-1
may be viewed as a molecular target in localized and metastatic
CRC.
In the present study, follow-up analysis revealed
that TRAP-1 expression was associated with cancer-specific 5-year
OS and PFS rates in 256 patients with CRC, independent of the
degree of TRAP-1 expression. Patients with positive or high TRAP-1
expression experienced poorer rates of cancer-specific 5-year OS
and PFS compared with patients with negative or low TRAP-1
expression. Univariate and multivariate analyses revealed that
TRAP-1 expression is an independent prognostic factor for
cancer-specific OS and PFS of CRC. These observations support
previous studies suggesting that overexpression of TRAP-1 is
associated with a shortened OS time in other types of cancer,
including breast, lung and prostate cancer (15–17).
A major limitation of the present study is the
limited number of cases (n=256). A study on an increased scale is
required to confirm the clinical significance of TRAP-1 expression.
Due to the multiple roles of TRAP-1 in cellular functioning, future
research is required to elucidate the mechanisms underlying TRAP-1
expression and its role in cancer cell biology.
In conclusion, the present study demonstrated the
association between TRAP-1 expression and cancer-specific OS and
PFS of human CRC. As well as this, overexpression of TRAP-1 was
identified to be an adverse prognostic factor for patients with
CRC. Further studies on an increased scale will be required to
validate these results.
Acknowledgements
The authors wish to thank Dr Li-Jiang Liu (JiangHan
University Pathology Center, Jianghan University of China, Wuhan,
China) and Li-Hua Huang (Department of Pathology, PuAi Hospital of
Tongji Medical College, Huazhong University of Science and
Technology of Wuhan, Wuhan, China) for their technical assistance.
The present study was supported by the Natural Science Foundation
of Hubei (grant nos. 2012FFB05601 and 2016CFB340), Wuhan Yellow
Crane Medical Talent Program, Wuhan Science and Technology Bureau
(grant nos. 201260523197, 201271130460, 2013060602010259 and
2015061701011626) and National Natural Science Foundation of China
(grant no. 81502461).
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moniuszko T, Wincewicz A, Koda M,
Domysławska I and Sulkowski S: Role of periostin in esophageal,
gastric and colon cancer. Oncol Lett. 12:783–787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rogers AC, Winter DC, Heeney A, Gibbons D,
Lugli A, Puppa G and Sheahan K: Systematic review and meta-analysis
of the impact of tumour budding in colorectal cancer. Br J Cancer.
115:831–840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bond A, O'Toole P, Fisher G, Subramanian
S, Haslam N, Probert C, Cox T and Sarkar S: New-generation
high-definition colonoscopes increase adenoma detection when
screening a moderate-risk population for colorectal cancer. Clin
Colrectal Cancer. 16:44–50. 2017. View Article : Google Scholar
|
|
5
|
Chand M, Rasheed S, Heald R, Swift I, West
N, Rao S, Tekkis P and Brown G: Adjuvant chemotherapy may improve
disease-free survival in patients with persistently mrEMVI-positive
rectal cancer following chemoradiation. Colorectal Dis. 19:537–543.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan D, Li K, Zhu K, Yan R and Dang C:
Plasma miR-183 predicts recurrence and prognosis in patients with
colorectal cancer. Cancer Biol Ther. 16:268–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simon K: Colorectal cancer development and
advances in screening. Clin Interv Aging. 11:967–976. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matassa DS, Amoroso MR, Maddalena F,
Landriscina M and Esposito F: New insights into TRAP1 pathway. Am J
Cancer Res. 2:235–248. 2012.PubMed/NCBI
|
|
10
|
Neckers L and Ivy SP: Heat shock protein
90. Curr Opin Oncol. 5:419–424. 2003. View Article : Google Scholar
|
|
11
|
Lianos GD, Alexiou GA, Mangano A, Mangano
A, Rausei S, Boni L, Dionigi G and Roukos DH: The role of heat
shock proteins in cancer. Cancer Lett. 360:114–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hua G, Zhang Q and Fan Z: Heat shock
protein 75 (TRAP1) antagonizes reactive oxygen species generation
and protects cells from granzyme M-mediated apoptosis. J Biol Chem.
282:20553–20560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang BH, Plescia J, Dohi T, Rosa J, Doxsey
SJ and Altieri DC: Regulation of tumor cell mitochondrial
homeostasis by an organelle-specific Hsp90 chaperone network. Cell.
131:257–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian X, Ma P, Sui CG, Meng FD, Li Y, Fu
LY, Jiang T, Wang Y and Jiang YH: Suppression of tumor necrosis
factor receptor-associated protein 1 expression induces inhibition
of cell proliferation and tumor growth in human esophageal cancer
cells. FEBS J. 281:2805–2819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Wang J, Huang Z, Wei P, Liu Y,
Hao J, Zhao L, Zhang F, Tu Y and Wei T: Aberrantly upregulated
TRAP1 is required for tumorigenesis of breast cancer. Oncotarget.
6:44495–44508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agorreta J, Hu J, Liu D, Delia D, Turley
H, Ferguson DJ, Iborra F, Pajares MJ, Larrayoz M, Zudaire I, et al:
TRAP1 regulates proliferation, mitochondrial function, and has
prognostic significance in NSCLC. Mol Cancer Res. 12:660–669. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leav I, Plescia J, Goel HL, Li J, Jiang Z,
Cohen RJ, Languino LR and Altieri DC: Cytoprotective mitochondrial
chaperone TRAP-1 as a novel molecular target in localized and
metastatic prostate cancer. Am J Pathol. 176:393–401. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao JY, Song BR, Peng JJ and Lu YM:
Correlation between mitochondrial TRAP-1 expression and lymph node
metastasis in colorectal cancer. World J Gastroenterol.
18:5965–5971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rusch VW, Rice TW, Crowley J, Blackstone
EH, Rami-Porta R and Goldstraw P: The seventh edition of the
American Joint Committee on Cancer/International Union Against
Cancer Staging Manuals: The new era of data-driven revisions. J
Thorac Cardiovasc Surg. 139:819–821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hofheinz RD, Ronellenfitsch U, Kubicka S,
Falcone A, Burkholder I and Hacker UT: Treatment with
antiangiogenic drugs in multiple lines in patients with metastatic
colorectal cancer: Meta-analysis of randomized trials.
Gastroenterol Res Pract. 2016:291894832016. View Article : Google Scholar
|
|
21
|
Ou Y, Liu L, Xue L, Zhou W, Zhao Z, Xu B,
Song Y and Zhan Q: TRAP1 shows clinical significance and promotes
cellular migration and invasion through STAT3/MMP2 pathway in human
esophageal squamous cell cancer. J Genet Genomics. 41:529–537.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khanna A, Böckelman C, Hemmes A, Junttila
MR, Wiksten JP, Lundin M, Junnila S, Murphy DJ, Evan GI, Haglund C,
et al: MYC-dependent regulation and prognostic role of CIP2A in
gastric cancer. J Natl Cancer Inst. 101:793–805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elias E, Mukherji D, Faraj W, Khalife M,
Dimassi H, Eloubeidi M, Hattoum H, Abou-Alfa GK, Saleh A and
Shamseddine A: Lymph-node ratio is an independent prognostic factor
in patients with stage III colorectal cancer: A retrospective study
from the Middle East. World J Surg Oncol. 10:632012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chew MH, Teo JY, Kabir T, Koh PK, Eu KW
and Tang CL: Stage IV colorectal cancers: An analysis of factors
predicting outcome and survival in 728 cases. J Gastrointest Surg.
16:603–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen R, Pan S, Lai K, Lai LA, Crispin DA,
Bronner MP and Brentnall TA: Up-regulation of mitochondrial
chaperone TRAP1 in ulcerative colitis associated colorectal cancer.
World J Gastroenterol. 20:17037–17048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hauptman N and Glavač D: Colorectal cancer
blood-based biomarkers. Gastroenterol Res Pract. 2017:221953612017.
View Article : Google Scholar
|
|
27
|
Montesano Gesualdi N, Chirico G, Pirozzi
G, Costantino E, Landriscina M and Esposito F: Tumor necrosis
factor-associated protein 1 (TRAP-1) protects cells from oxidative
stress and apoptosis. Stress. 10:342–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakagawa T, Shimizu S, Watanabe T,
Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T and Tsujimoto Y:
Cyclophilin D-dependent mitochondrial permeability transition
regulates some necrotic but not apoptotic cell death. Nature.
434:652–658. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu D, Hu J, Agorreta J, Cesario A, Zhang
Y, Harris AL, Gatter K and Pezzella F: Tumor necrosis factor
receptor-associated protein 1(TRAP1) regulates genes involved in
cell cycle and metastases. Cancer Lett. 296:194–205. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Landriscina M, Laudiero G, Maddalena F,
Amoroso MR, Piscazzi A, Cozzolino F, Monti M, Garbi C, Fersini A,
Pucci P and Esposito F: Mitochondrial chaperone Trap1 and the
calcium binding protein Sorcin interact and protect cells against
apoptosis induced by antiblastic agents. Cancer Res. 70:6577–6586.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guzzo G, Sciacovelli M, Bernardi P and
Rasola A: Inhibition of succinate dehydrogenase by the
mitochondrial chaperone TRAP1 has anti-oxidant and anti-apoptotic
effects on tumor cells. Oncotarget. 5:11897–11908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kubota K, Inoue K, Hashimoto R, Kumamoto
N, Kosuga A, Tatsumi M, Kamijima K, Kunugi H, Iwata N, Ozaki N, et
al: Tumor necrosis factor receptor-associated protein 1 regulates
cell adhesion and synaptic morphology via modulation of N-cadherin
expression. J Neurochem. 110:496–508. 2009. View Article : Google Scholar : PubMed/NCBI
|