Introduction
Hypopharyngeal carcinoma has one of the poorest
prognoses of all head and neck carcinomas as it is frequently
diagnosed at an advanced stage and exhibits aggressive and distant
metastasis (1). Although there have
been significant advancements in surgical techniques and
chemoradiotherapy recently, the prognosis of hypopharyngeal
carcinoma remains unsatisfactory (2).
At present, the most common diagnostic tools include
different types of laryngoscopy, computed tomography (CT), and
routine magnetic resonance imaging (MRI). CT may reveal the extent
of infiltration of hypopharyngeal carcinoma, but it also has
certain limitations, including over estimation of invasion of the
vocal cords, underestimation of invasion of the upper esophagus,
difficulty in displaying mild invasion of thyroid cartilage, and
uncertainty with regard to the normal size of the cervical lymph
nodes and whether they are subject to metastasis. Routine MRI has
high resolution in soft tissue, enabling it to accurately determine
the infiltration extent of tumors; however, it remains a challenge
to diagnose small lesions or micrometastatic nodes (3). Positron emission tomography (PET) and
PET/CT may supply functional information and differentiate
malignant tumors from benign lesions; however, they are expensive
with low availability and are limited by relatively low spatial
resolution and risk of radiation exposure (3).
Recently, diffusion-weighted MRI (DWI) has emerged
as a relatively novel functional imaging tool that records the
molecular motion of protons corresponding to Brownian motion within
living tissue (4). The extent of
molecular diffusion may be estimated and quantified in terms of the
apparent diffusion coefficient (ADC) (5). DWI is widely used in clinical practice
to differentiate benign masses from malignant tumors, to diagnose
lymph node metastasis, to detect recurrent lesions following
radiotherapy/chemotherapy, and to predict the effect of treatment
using DWI and ADC values. DWI is able to detect changes in tumor
size and shape prior to them becoming visible to the naked eye, and
the ADC value is affected by cell size, density and integrity
(6).
Initially, DWI was primarily used in neurology to
identify intracranial lesions. However, the complex anatomical
structure and functions occurring in the head and neck, including
respiration, swallowing and phonation, limit the use of DWI in
these regions. Nonetheless, with the advances in MRI technical
performance and MRI machinery, the application of DWI to head and
neck oncology is increasing (7,8). Head and
neck cancer occurs at multiple sites, including the oral cavity,
oropharynx and larynx (9,10). Recently, Driessen et al
(10) used single-shot spin-echo
echo-planar DWI of 1.5T MRI to investigate the association between
histological characteristics of laryngeal and hypopharyngeal
squamous cell carcinoma and ADC values (10). However, to the best of our knowledge,
few previous studies have investigated the role of DWI at a single
site in the head and neck regions. To the best of our knowledge,
our previous study was the first to successfully use DWI and 3.0-T
MRI to differentiate preoperative laryngeal carcinomas from
precursor lesions (11), and there
have been no previous studies regarding the use of ADC values and
single-shotecho-planar imaging sequence (EPI) of 3.0-T MRI alone in
hypopharyngeal carcinoma.
The aim of the present study was to assess the role
of DWI and ADC values in hypopharyngeal carcinoma in order to
determine: i) Whether ADC has diagnostic value in discriminating
carcinomas from benign lesions, or discriminating metastatic lymph
nodes from reactive cervical lymph nodes; and ii) whether ADC
values are associated with the prognosis of hypopharyngeal
carcinomas.
Materials and methods
Patients
The present study was approved by the Institutional
Review Board of the First Affiliated Hospital, College of Medicine,
Zhejiang University, Hangzhou, China (IRB no. 2015428), and written
informed consent was obtained from all enrolled patients.
Between June 2012 and July 2015, patients with
hypopharyngeal lesions who had undergone preoperative
laryngostroboscopy (Endo-stroboscope L; Atmos Medizin Technik GmbH
& Co. KG, Lenzkirch, Germany) and DWI were considered for
inclusion in the present study. The inclusion criteria were as
follows: i) Suspicious lesions in the hypopharynx on
laryngostroboscopy; ii) 3.0-T MRI (including DWI, b=0 and 1,000
sec/mm2) prior to treatment; iii) surgery, concurrent
chemo-radiotherapy (CCR) and pathological confirmation of diagnoses
(including frozen sections and routine pathological results); and
iv) availability of complete clinical data. Exclusion criteria were
as follows: i) Incomplete clinical data; ii) 3.0-T MR without DWI
prior to treatment; iii) undetermined Tumor-Node-Metastasis (TNM)
stage of hypopharyngeal carcinoma (11) or recurrent hypopharyngeal carcinoma
requiring a second surgery.
Consequently, 63 patients (62 males and 1 female)
were included. The mean age of was 55.3 years (range, 30–81 years).
Of these patients, 4 were excluded owing to susceptibility
artifacts (due to linear blurring, geometric distortion or imaging
distortion) that compromised image quality. A total of 4 patients,
who waived any treatment, were also excluded. In the remaining 55
patients, pathological results revealed 40 cases of hypopharyngeal
carcinoma (Table I) and 15 benign
lesions. In cases where a suspicious mass at the primary site or a
neck mass was observed on physical examination or CT/MRI, a biopsy
or needle biopsy was performed. The tumor volume (TV) of samples
was calculated as follows: TV=XxYxZ/2 (X, greatest length; Y,
greatest width and Z, greatest depth of tumor samples). Patients
were followed up every 1 month over the first year, every 3 months
over the second year, every 6 months over the next 3 years and
annually thereafter. The last follow-up date was March 2015.
 | Table I.The clinicopathological
characteristics of 40 patients with hypopharyngeal carcinoma. |
Table I.
The clinicopathological
characteristics of 40 patients with hypopharyngeal carcinoma.
| Patient | Sex | Age, years | Site | TNM stage | Treatment | H | R | MET | Follow-up |
|---|
| 1 | M | 67 | Right PS | T3N0M0 |
ND+surgery+preservation of LF | M | No | No | 9 months, NED |
| 2 | M | 45 | Right PS | T4N2cM0 | PRT(50
Gy)+ND+surgery+preservation of LF | M | 3 months after
surgery | No | 9 months, mortality
due to hemorrhea |
| 3 | F | 42 | Left PS, involving
the upper esophagus | T4N0M0 |
ND+surgery+preservation of LF | M-P | 9 months after
surgery | Lung metastasis 9
months after surgery | 10 months, AWD |
| 4 | M | 57 | Left PS | T3N2cM0 |
ND+surgery+preservation of
LF+postoperative CCR | M | No | No | 16 months, NED |
| 5 | M | 80 | Left PS | T3N0M0 |
ND+surgery+preservation of
LF+postoperative CCR | M-P | 10 months after
surgery | Pulmonary
metastasis, 10 months after surgery | 10 months, AWD |
| 6 | M | 71 | Left PS | T3N1M0 |
ND+surgery+preservation of
LF+postoperative CCR | M | No | Pulmonary
metastasis after surgery 13 months after surgery | 13 months, AWD |
| 7 | M | 59 | Left PS | T4N2bM0 |
ND+surgery+preservation of
LF+postoperative CCR | P | No | No | 15 months, NED |
| 8 | M | 78 |
Retropharyngeal | T2N0M0 |
ND+surgery+preservation of LF | W | No | No | 13 months, NED |
| 9 | M | 61 | PR | T3N1M0 |
ND+surgery+TFO+postoperative CCR | M | No | No | 14 months, NED |
| 10 | M | 58 | Right PS | T4N2aM0 | ND+surgery+total
laryngectomy+ postoperative CCR | W-M | No | No | 15 months, NED |
| 11 | M | 66 | Left PS | T4aN2aM0 | ND+surgery+TFO | M | No | No | 6 months until
mortality |
| 12 | M | 70 |
Retropharyngeal | T4N0M0 | Postoperative
CCR | M | No | No | 15 months, NED |
| 13 | M | 60 | Right PS | T4N2aM0 |
ND+surgery+preservation of
LF+postoperative CCR | M-P | No | No | 13 months until
mortality |
| 14 | M | 74 | Right PS | T4N1M0 |
ND+surgery+preservation of
LF+postoperative CCR | M-P | No | No | 18 months, NED |
| 15 | M | 48 | PR | T4aN2CM0 |
ND+surgery+TFO+postoperative CCR | M-P | No | No | 19 months, NED |
| 16 | M | 75 | Left PS | T4bN2M0 | Postoperative
CCR | W-M | No | No | 5 months until
mortality |
| 17 | M | 65 | Light PS | T3N0M0 |
ND+surgery+preservation of
LF+postoperative CCR | P | No | No | 19 months, NED |
| 18 | M | 63 | Light PS | T4aN1M0 |
ND+surgery+preservation of
LF+postoperative CCR | M | No | No | 19 months, NED |
| 19 | M | 70 | Right PS | T3N1M0 | ND+surgery+total
laryngectomy+postoperative CCR | M | No | No | 10 months,
mortality due tohemorrhea |
| 20 | M | 67 | PR | T4N0M0 |
ND+surgery+TFO+postoperative CCR | M-P | No | No | 23 months, NED |
| 21 | M | 77 | Left PS | T3N2bM0 |
ND+surgery+preservation of LF | M | No | No | 25 months, NED |
| 22 | M | 54 | Right PS | T2N2M0 |
ND+surgery+preservation of LF+
postoperative CCR | NA | No | No | 27 months, NED |
| 23 | M | 67 | Left PS | T2N1M0 |
ND+surgery+preservation of
LF+postoperative CCR | M-P | No | No | 26 months, NED |
| 24 | M | 56 | Left PS | T2N2M0 | ND+surgery+total
laryngectomy | M-P | No | No | 15 months,
mortality due to accidental injury |
| 25 | M | 58 | PR | T1N1M0 |
ND+surgery+preservation of
LF+postoperative CCR | W-M | No | No | 27 months, NED |
| 26 | M | 59 | Right PS | T2N2M0 |
ND+surgery+TFO+postoperative CCR | M-P | No | No | 28 months, NED |
| 27 | M | 50 | Left PS | T2N2M1 | Postoperative
CCR | M | NA | NA | NA |
| 28 | M | 57 | Left PS | T4N1M0 |
ND+surgery+preservation of
LF+postoperative CCR | M | No | No | 33 months, NED |
| 29 | M | 57 | PR | T4N2cM0 |
ND+surgery+TFO+postoperative CCR | M | No | No | 9 months, NED |
| 30 | M | 45 | Right PS | T2N1M0 |
ND+surgery+preservation of
LF+postoperative CCR | P | No | No | 8 months, NED |
| 31 | M | 55 | PR | T4N1M0 |
ND+surgery+TFO+postoperative CCR | M-P | No | No | 8 months, NED |
| 32 | M | 68 |
Retropharyngeal | T4N1M0 |
ND+surgery+preservation of
LF+postoperative chemotherapy | M-P | No | Mediastinal lymph
node 8 months metastasis after surgery | 7 months, AWD |
| 33 | M | 50 |
Retropharyngeal | T4N0M0 |
ND+surgery+preservation of
LF+postoperative CCR | M | No | No | 5 months, NED |
| 34 | M | 69 | Right PS | T2N0M0 |
ND+surgery+preservation of LF | M | No | No | 6 months, NED |
| 35 | M | 53 |
Retropharyngeal | T4N0M0 | ND+surgery+TFO | W-M | No | No | 3 months, NED,
continues CCR treatment |
| 36 | M | 81 | Right PS | T2N0M0 |
ND+surgery+preservation of LF | M | No | No | 3 months, NED, in
the CCR |
| 37 | M | 60 | Left PS | T4N1M0 | ND+surgery+TFO | W-M | No | No | 2 months, NED, in
the CCR |
| 38 | M | 68 | Right PS | T3N2M0 |
ND+surgery+preservation of LF | M | No | No | 2 months, NED, in
the CCR |
| 39 | M | 62 | Left PS | T3N2M0 |
ND+surgery+preservation of LF | M-P | No | No | 3 months, NED, in
the CCR |
| 40 | M | 62 | Left PS | T4aN2M0 |
ND+surgery+preservation of LF | W-M | No | No | 1 months, NED, in
the CCR |
MRI protocol
All MRI examinations were performed using a 3.0-T
MRI scanner (Philips Achieva® 3.0T; Royal Philips
Electronics, Amsterdam, Netherlands) with a 16-channel head and
neck coil. Conventional MRI included an axial T1-weighted turbo
spin echo (TSE) sequence with the following parameters (11): Slice thickness, 4 mm; 24 slices;
intersection gap, 1 mm; repetition time/echo time (TR/TE), 450
ms/10 ms; matrix, 320×224; field of view (FOV), 240×180 mm; and an
axial T2-weighted TSE sequence (slice thickness, 4 mm; 24 slices;
intersection gap, 1 mm; TR/TE, 400 ms/10 ms; matrix, 320×224; FOV,
240×180 mm). The coronal T2-weighted TSE sequence included the
following parameters: Slice thickness, 4 mm; 24 slices;
intersection gap, 1 mm; TR/TE, 400 ms/10 ms; matrix 320×224; FOV,
240×220 mm; and two signals were acquired, covering the larynx.
Following gadolinium injection, T1-weighted fat-saturated sequences
were performed in the axial plane (using identical parameters to
pre-contrast medium administration) and in the coronal plane (24
slices; slice thickness, 4 mm; intersection gap, 1 mm; TR/TE, 540
ms/9.2 ms; matrix, 320×224; FOV, 240×220 mm; and two signals were
acquired using fat suppression).
DWI was performed with a single-shot EPI-DWI). The
parameters were as follows: TR/TE, 8,000 ms/60 ms; FOV, 240×240 mm;
matrix, 124×124; 24 slices; slice thickness, 4 mm; and b=0 or 1,000
sec/mm2). ADC maps were generated using Extended MR
Workspace (EWS). In order to minimize susceptibility artifacts, the
patients were encouraged not to swallow, speak or cough during
imaging. In addition, thin slices were obtained, meticulous
shimming was applied and sight fixation (to steady the patient's
head) were used.
Image analysis
The imaging data were reviewed by two radiologists
from the Department of Radiology (the First Affiliated Hospital,
Zhejiang University, Hangzhou, P.R. China) with specific experience
in head and neck imaging, but with no knowledge of the primary
lesion. They reached an agreed opinion prior to reviewing the
pathology results. The lesion contour, size and internal
architecture were documented. An ADC map was generated by DWI with
EWS and the ADC value was measured.
All hypopharyngeal lesions were characterized based
on the signal intensity in T1- and T2-weighted MRIs and enhancement
characteristics. DW-MRI at a native b-value of 1,000
sec/mm2 (b-1,000 images) and the corresponding ADC maps
were matched to and evaluated with the morphological images as
previously described (11). A
hyperintense signal on the native b-1,000 image compared with the
surrounding tissue with a corresponding low signal intensity in the
matching ADC map was considered positive for a tumor. A high signal
intensity on the b-1,000 image with a corresponding high signal
intensity on the matching ADC map was considered to represent T2
shine-through and therefore, the absence of a tumor. The absence of
hyper intensity on the b-1,000 image was also considered negative
for a tumor. The region of interest (ROI) on single slice was
determined by a single radiologist (Department of Radiology (the
First Affiliated Hospital, Zhejiang University, Hangzhou, P.R.
China) with 20 years of experience. The ROI was determined as
previously described (12). In brief,
the ROIs were placed on the axial ADC 1,000 maps following referral
to contrast-enhanced T1-weighted images obtained in 3 orthogonal
planes. The investigator (Department of Radiology (the First
Affiliated Hospital, Zhejiang University, Hangzhou, P.R. China) had
no knowledge of the final pathological or clinical results when he
interpreted the images. The ROIs were drawn on the largest or the
highest conspicuity of the lesion of the ADC map. The boundary of
the ROI contained the visible tumor in that section of the ADC map
corresponding to T1-weighted, T2-weighted, or contrast-enhanced
T1-weighted images, but any necrotic portions and normal osseous
structures were avoided where possible. The ADC value in the same
section was calculated three times. The mean ± standard deviation
(SD) of the ADC values for the hypopharyngeal lesions and cervical
lymph nodes were calculated.
Statistical analyses
Statistical analyses were performed using SPSS
software 20.0 (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference. All
variables were assessed for normality by using the
Kolmogorov-Smirnov test. Spearman's rank correlation was performed
to evaluate the correlation between tumor size and ADC value.
Differences in ADC (mean ± SD) of the hypopharyngeal lesions
between patients with malignant lesions and those with benign
lesions were tested using an independent samples t-test. A 95%
confidence interval was used. In the univariate survival analysis,
the curves for overall survival were estimated using the
Kaplan-Meier method, and the log rank test was used to test
differences between groups. Multivariate analysis was performed
using a Cox proportional hazard test.
Receiver operating characteristic (ROC) curve
analysis was used to investigate the discriminatory ability of the
ADC values in differentiating hypopharyngeal carcinomas from benign
lesions. The area under the ROC curve (AUC) was calculated. The ADC
value that corresponded to the highest Youden index
(sensitivity+specificity-1) was chosen as the optimal ADC threshold
value as it optimized the sensitivity and specificity. The AUC was
used as an alternative global measure of test performance.
Results
Patient clinical characteristics
The mean age of the patients with benign
hypopharyngeal lesions was 56.5 years (range, 34–74 years). There
were 13 males and 2 females. The main symptoms included a sensation
of a foreign body in the throat and pharyngalgia. The benign
hypopharyngeal lesions included 10 cases of chronic inflammation,
two vascular lesions, two hypopharyngeal polyps and one cyst.
Of the 40 patients with hypopharyngeal carcinoma, 39
were male and one was female. The mean age was 61.4 years (range,
42–81 years). All 40 hypopharyngeal carcinomas were squamous cell
carcinoma (SCC). A total of 29 tumors (72.5%) were located in the
pyriform sinus, 8 (20.0%) were located in the posterior pharyngeal
wall and 3 (7.5%) were located in the postcricoid area. A total of
36 patients (90.0%) had a history of smoking, 32 (80.0%) had a
history of drinking (any alcohol consumption), and 27 (67.5%) had a
history of smoking and drinking. Signs and symptoms included
odynophagia (32.5%), difficulty swallowing (30.0%), sensation of a
foreign body in the throat (20.0%), neck mass (10.0%) and
hoarseness (2.5%). According to the International Union Against
Cancer TNM classification system (2007, 7th edition), 1 patient
(2.5%) exhibited stage T1N1M0
disease, 3 (7.5%) exhibited stage
T2N0M0 disease, 3 (5.0%) exhibited
stage T2N1M0 disease, 3 (7.5%)
exhibited stage T2N2M0 disease, 3
(7.5%) exhibited stage T3N0M0
disease, 3 (7.5%) exhibited stage
T3N1M0 disease, 4 (10.0%)
exhibited stage T3N2M0 disease, 5
(12.5%) exhibited stage T4N0M0
disease, 6 (15.0%) exhibited stage
T4N1M0 disease, 9 (22.5%)
exhibited stage T4aN2M0 disease, 1
(2.5%) exhibited stage T2N2M1
disease, and one patient developed lung metastasis. With regards to
clinical stage (11), 2 patients
(5.0%) exhibited stage II disease, 8 (20.0%) exhibited stage III
disease, and 30 (75.0%) exhibited stage IV disease (Table I). According to current
classifications, 95.0% of patients were at an advanced stage of
disease. A total of 3 patients received CCR, while 37 patients
received surgery and neck dissection (18 ipsilateral and 19
bilateral). One of the 37 patients received preoperative CCR and 36
received postoperative CCR.
The mean longest diameter of the tumor samples was
4.7 cm and ranged between 2.0 and 10.0 cm. The mean tumor volume
was 38.8 cm3 and ranged between 4.0 and 210
cm3 (Table II).
 | Table II.Tumor sizes and ADC values. |
Table II.
Tumor sizes and ADC values.
| Patient | Tumordiameter,
cm | Tumor volume,
cm3 | ADC,
10−3 mm2/sec |
|---|
| 1 | 4.0×3.0×2.50 | 15.00 | 0.89 |
| 2 | 3.5×3.0×1.0 | 5.25 | 0.96 |
| 3 | 3.0×3.0×1.2 | 6.75 | 1.32 |
| 4 | 4×2.5×1.5 | 7.50 | 0.92 |
| 5 | 3.0×3.0×2.0 | 9.00 | 1.06 |
| 6 | 3.4×3.0×3.0 | 15.30 | 1.05 |
| 7 | 4.0×4.0×2.5 | 20.00 | 0.88 |
| 8 | 5.0×5.0×3.5 | 43.75 | 1.06 |
| 9 | 7.5×4.5×2 | 33.75 | 0.92 |
| 10 | 3.0×4.0×4.0 | 24.00 | 0.70 |
| 11 | 5.0×4.5×1.5 | 16.88 | 0.80 |
| 12 | 2.0×3.5×3.5 | 12.25 | 0.97 |
| 13 | 4.0×5.0×5.0 | 50.00 | 0.45 |
| 14 | 3.0×3.0×3.0 | 13.50 | 1.05 |
| 15 | 5.0×5.0×5.5 | 68.75 | 0.98 |
| 16 | 5.0×5.0×5.0 | 62.50 | 0.80 |
| 17 | 4.0×3.0×3.0 | 18.00 | 1.41 |
| 18 | 4.0×4.0×5.0 | 40.00 | 0.97 |
| 19 | 4.0×4.0×5.5 | 44.00 | 0.96 |
| 20 | 4.0×5.5×5.5 | 60.50 | 1.00 |
| 21 | 3.0×5.5×7.0 | 57.75 | 0.89 |
| 22 | 2.0×2.0×2.0 | 4.00 | 1.14 |
| 23 | 4.0×3.0×3.0 | 18.00 | 1.16 |
| 24 | 3.0×3.0×3.0 | 13.50 | 0.88 |
| 25 | 5.0×5.0×4.5 | 56.25 | 0.90 |
| 26 | 4.0×4.5×4.5 | 40.50 | 0.91 |
| 27 | 2.0×2.0×2.0 | 4.00 | 0.91 |
| 28 | 4.0×4.0×4.0 | 32.00 | 1.24 |
| 29 | 4.0×4.5×4.5 | 40.50 | 1.07 |
| 30 | 7.3×3.0×2.1 | 23.00 | 1.04 |
| 31 | 4.0×4.5×5.5 | 49.50 | 1.66 |
| 32 | 7.0×5.0×5.0 | 87.50 | 1.06 |
| 33 | 7.0×5.0×5.0 | 87.50 | 1.34 |
| 34 | 2.0×3.0×3.0 | 9.00 | 1.07 |
| 35 | 9.0×7.0×5.0 | 157.50 | 1.09 |
| 36 | 3.0×2.0×2.0 | 6.00 | 0.95 |
| 37 | 10.0×7.0×6.0 | 210.00 | 1.07 |
| 38 | 3.0×4.5×4.5 | 30.38 | 1.18 |
| 39 | 3.0×3.0×3.0 | 13.50 | 1.37 |
| 40 | 4.0×4.0×5.5 | 44.00 | 1.06 |
The mean follow-up time was 12.9 months (range, 1–33
months). A total of 3 patients developed local recurrence and one
patient developed lung metastasis. A total of 6 patients succumbed
to hypopharyngeal carcinoma, and one mortality occurred as a result
of accidental trauma.
Laryngostroboscopy in discriminating
hypopharyngeal lesions
Of the 40 patients with hypopharyngeal SCC, 37 were
diagnosed with hypopharyngeal carcinoma. Of the 15 patients with
hypopharyngeal benign lesions, 3 were diagnosed with hypopharyngeal
carcinoma. The sensitivity, specificity and accuracy were 92.5,
80.0 and 89.1%, respectively.
Conventional MRI, DWI, and ADC values:
The diagnostic value of conventional MRI in hypopharyngeal
lesions
A total of 10/40 hypopharyngeal SCCs (25.0%) were
hyperintense on the T1-weighted image, 17 (42.5%) were isointense,
11 (27.5%) were hypointense and 2 (5.0%) gave heterogeneous
signals. On the T2-weighted images, 38 (95.0%) were hyperintense,
one (2.5%) was isointense and one (2.5%) gave a heterogeneous
signal. In gadopentetic acid contrast-enhanced T1-weighted MRI, 15
(37.5%) exhibited heterogeneous enhancement, 24 (60.0%) were
strongly enhanced and 1 (2.5%) was slightly enhanced. A total of
39/40 pathologically proven hypopharyngeal SCCs were diagnosed as
hypopharyngeal carcinoma according to these observations (Table III; sensitivity, 97.5%).
 | Table III.Conventional MRI observations in 40
hypopharyngeal lesions. |
Table III.
Conventional MRI observations in 40
hypopharyngeal lesions.
| Sequence | Signal | Patients (%) |
|---|
| T1W |
|
|
|
| Hyperintense | 10 (25.0) |
|
| Isointense | 17 (42.5) |
|
| Hypointense | 11 (27.5) |
|
| Heterogeneous | 2 (5.0) |
| T2W |
|
|
|
| Hyperintense | 38 (95.0) |
|
| Isointense | 1 (2.5) |
|
| Heterogeneous | 1 (2.5) |
| Contrast-enhanced
T1W |
|
|
|
| Heterogeneous
enhancement | 15 (37.5) |
|
| Strong
enhancement | 24 (60.0) |
|
| Slight
enhancement | 1 (2.5) |
In the T1-weighted images of 15 benign
hypopharyngeal lesions, 3 (20.0%) exhibited no abnormal signal, 7
(46.7%) were hypointense, 4 (26.7%) were hyperintense and 1 (6.6%)
was isointense. In the T2-weighted images, 14 (93.3%) were
hyperintense and 1 (6.7%) exhibited no abnormal signal. In the
contrast-enhanced T1-weighted MR images, 12 (80.0%) were strongly
enhanced and three (20.0%) exhibited no enhancement. A total of
5/15 pathologically proven benign hypopharyngeal lesions were
diagnosed as hypopharyngeal carcinoma. The specificity and accuracy
were 66.7 and 89.1%, respectively.
DWI and ADC values
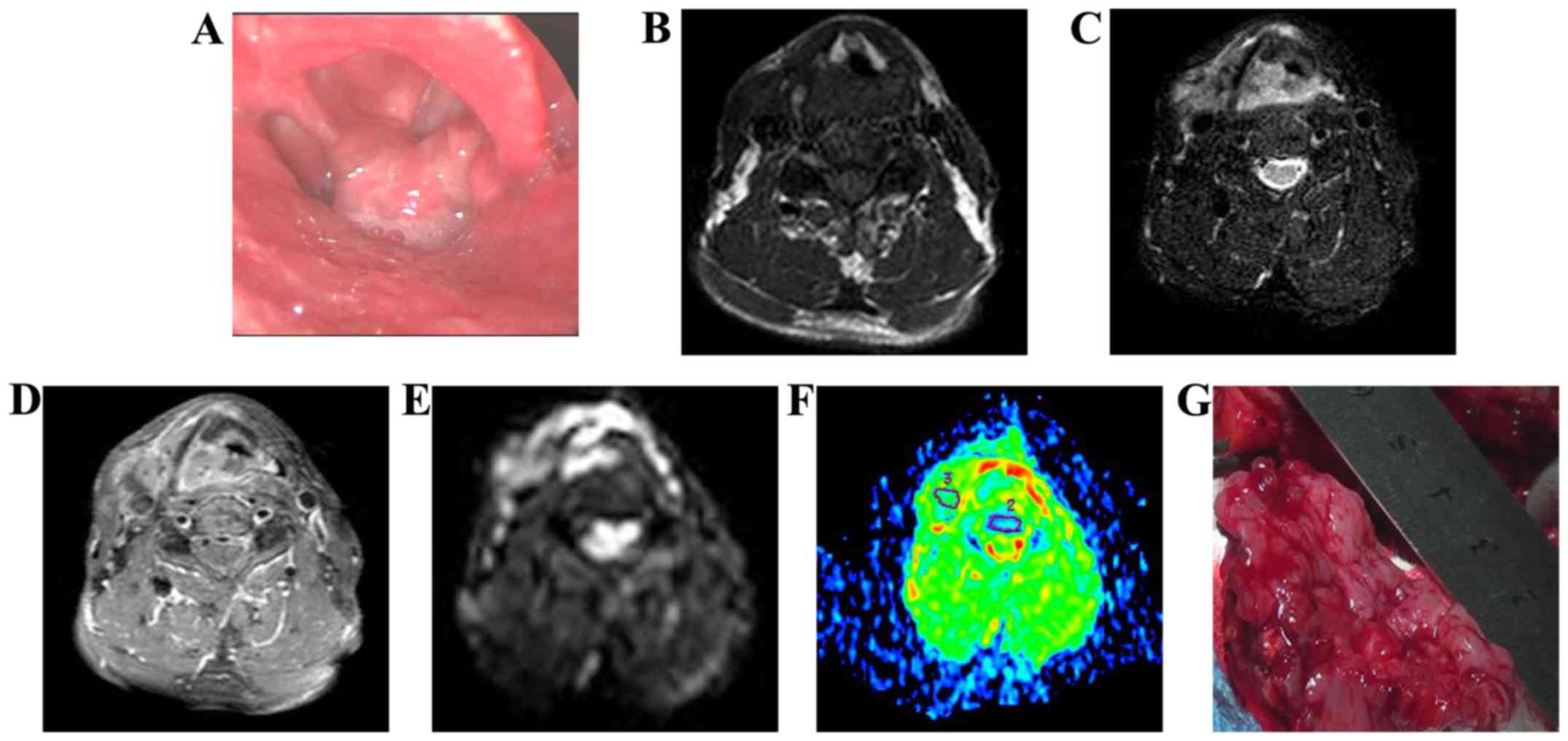
All 40 hypopharyngeal SCCs exhibited a high signal
in DWI (Fig. 1). The mean ADC value
was (1.03±0.0328)×10−3 mm2/sec. The ADCs of
all patients who succumbed to mortality were below the mean ADC
value. The tumor size (diameter and volume) was not significantly
correlated with the tumor ADC value (P=0.996 and P=0.900,
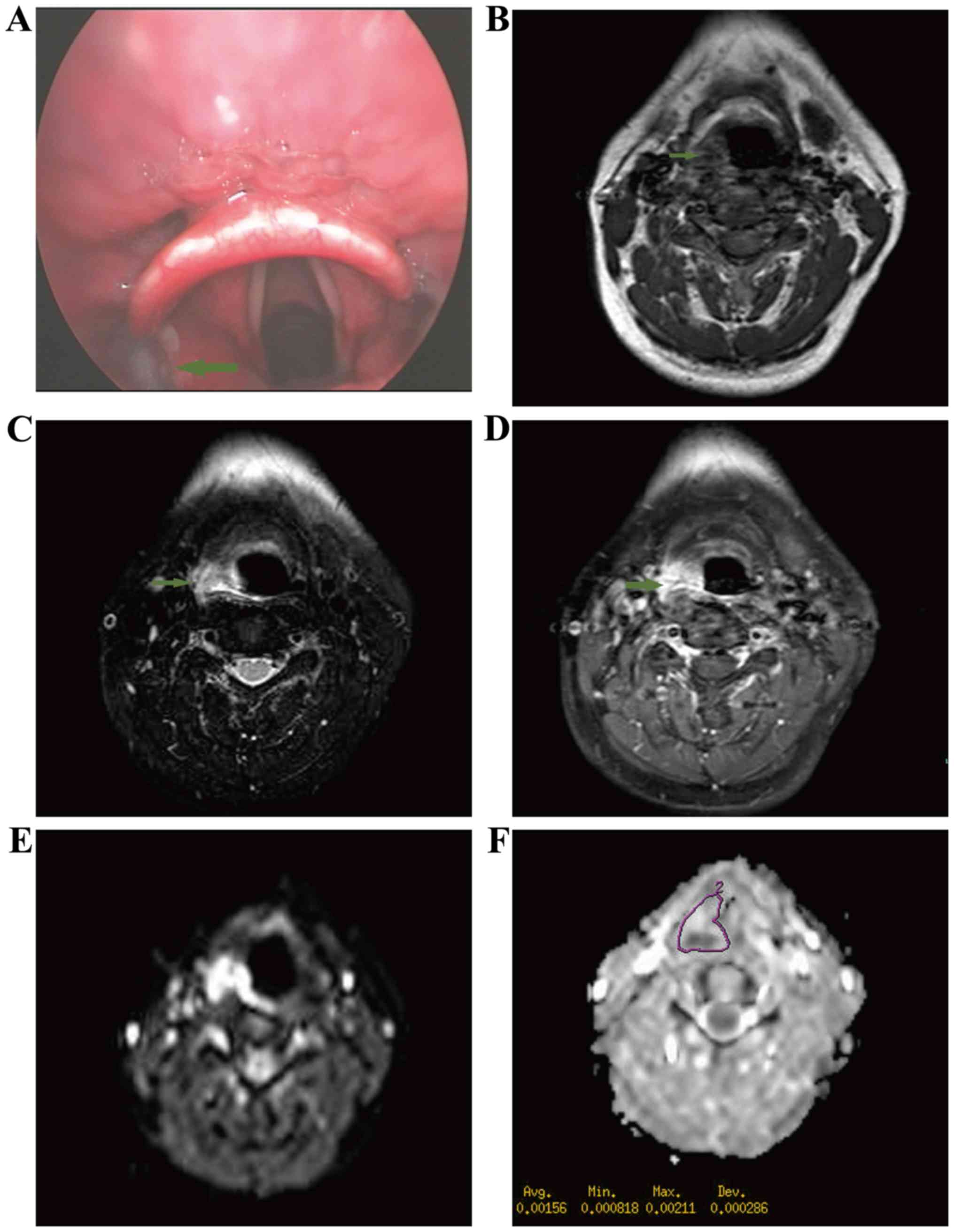
respectively). A total of 10/15 benign hypopharyngeal lesions
(66.7%) exhibited high signals in DWI (Fig. 2). The mean ADC value (b=1,000
sec/mm2) was (1.53±0.106)×10−3
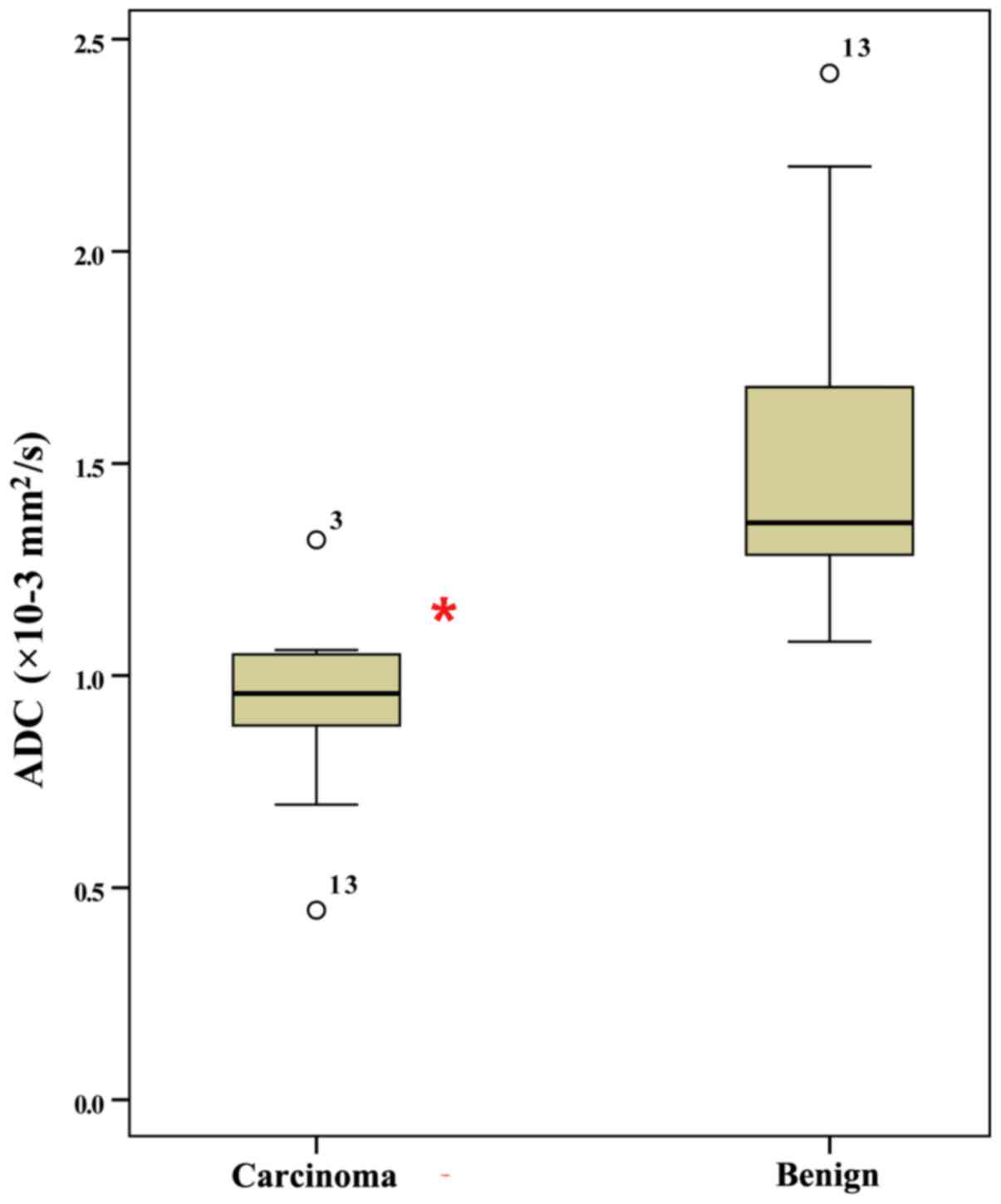
mm2/sec. The ADC values of hypopharyngeal SCCs were
significantly lower than those of benign lesions (P<0.001;
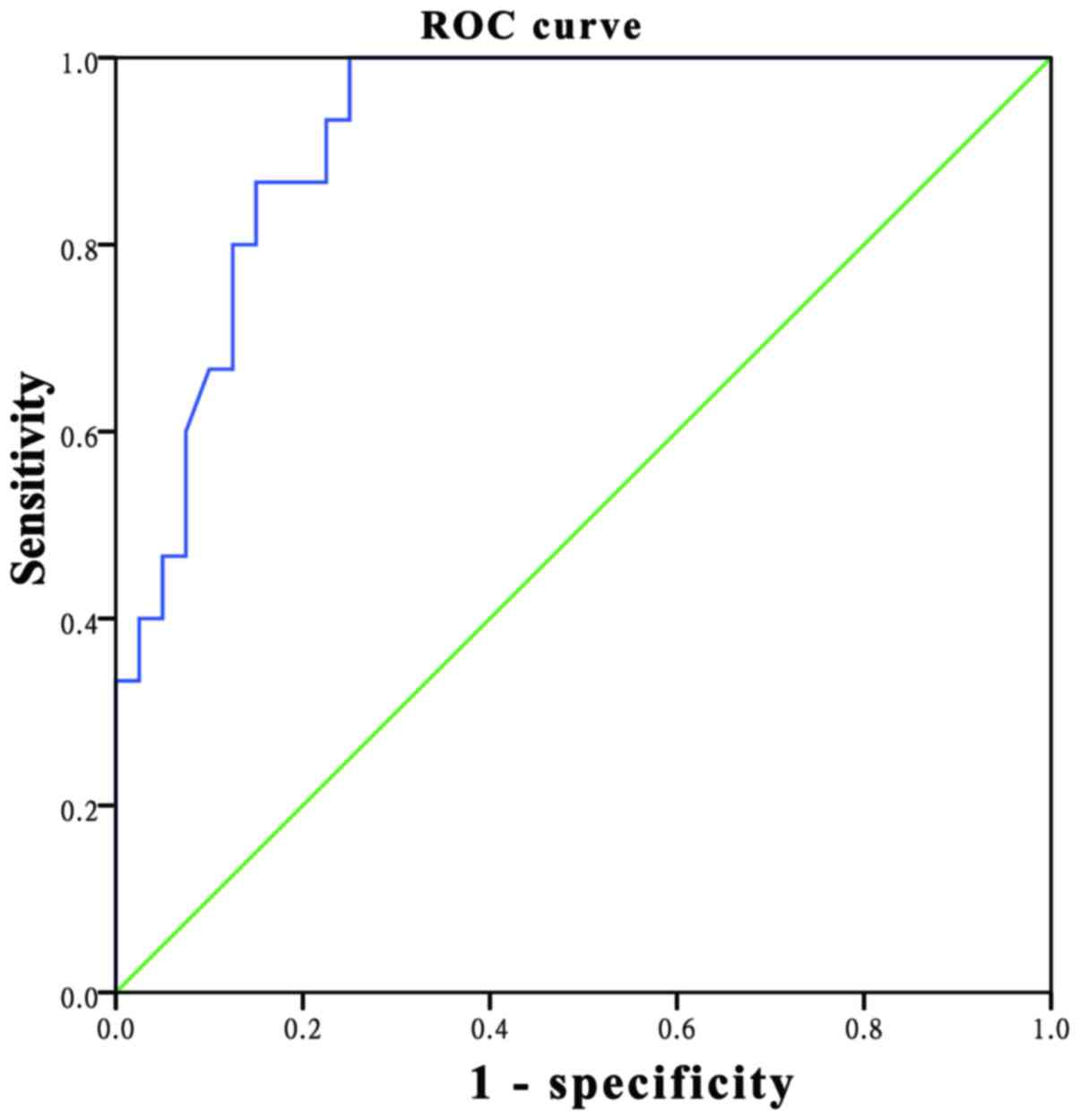
Fig. 3). ROC analysis revealed that
the AUC was 0.921, while the optimal threshold for the ADC cut-off
point was 1.075×10−3 mm2/sec, resulting in a
sensitivity of 100% and a specificity of 75% (Fig. 4).
The role of DWI and ADC values in
identifying metastatic lymph nodes in hypopharyngeal SCCs
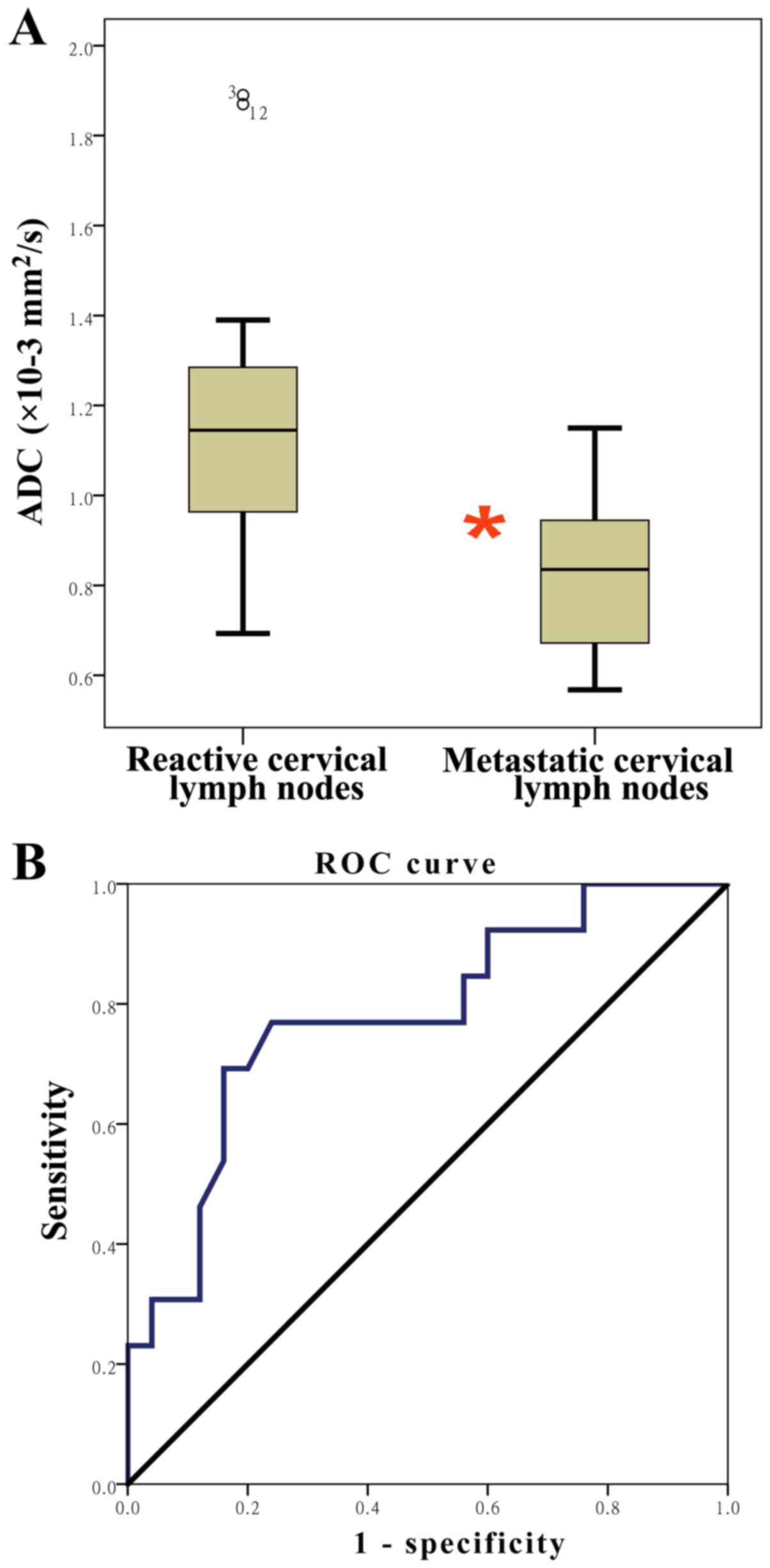
The agreed ADC values in lesions on metastatic
cervical lymph nodes and reactive cervical lymph nodes were
0.89×10−3 mm2/sec and 0.91×10−3
mm2/sec, respectively. Cervical lymph nodes were
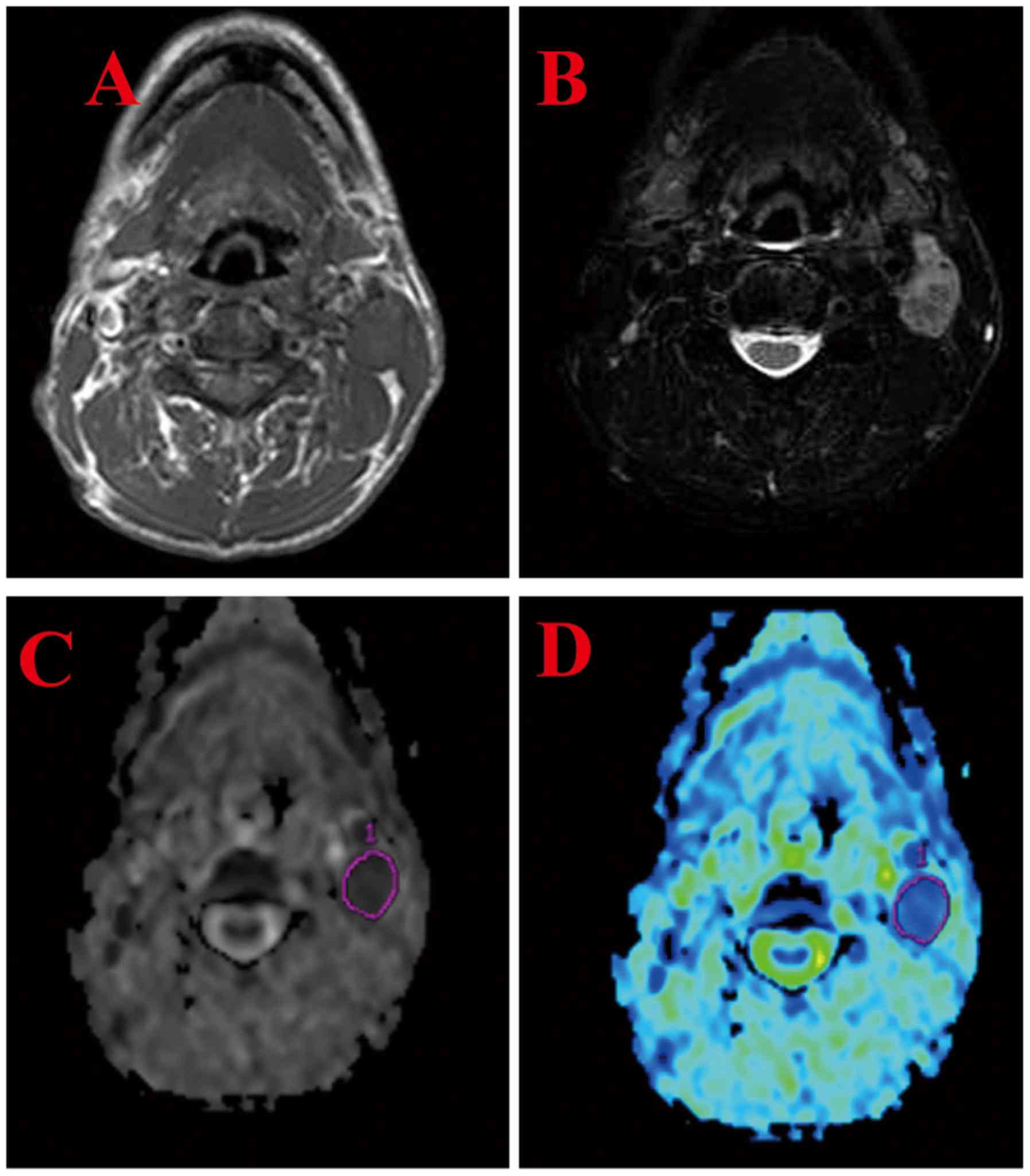
detected in 38/40 (95.0%) hypopharyngeal SCCs (Fig. 5). A total of 25 nodes were proven to
be histologically malignant and 13 were benign. The mean ADC value
of metastatic nodes was (0.918±0.0538)×10−3
mm2/sec, significantly lower than the mean value of the
benign nodes [1.25±0.115)×10−3 mm2/sec;
t=3.027; P=0.005). ROC curve analysis revealed that an optimal
threshold value of 1.075×10−3 mm2/sec was
suggested as the cut-off point, which results in 69.2% sensitivity
and 84.0% specificity; the AUC was 0.778 with a confidence interval
of 0.619–0.938 (P=0.005; Fig. 6).
ADC values, T stage and histological
grade
The mean ADC value of early stage
(T1+T2) hypopharyngeal SCCs was
(0.998±0.0367)×10−3 mm2/sec. The mean ADC
value of advanced stage (T3+T4)
hypopharyngeal SCCs was (1.04±0.0412)×10−3
mm2/sec. However, this difference was not statistically
significant (P>0.05). The mean ADC values of cases that were
well differentiated, moderately differentiated, and poorly
differentiated were (0.955±0.0590)×10−3
mm2/sec, (1.01±0.0337)×10−3
mm2/sec and (1.08±0.0686)×10−3
mm2/sec, respectively. However, these differences were
not statistically significant (P>0.05).
Prognosis and ADC values
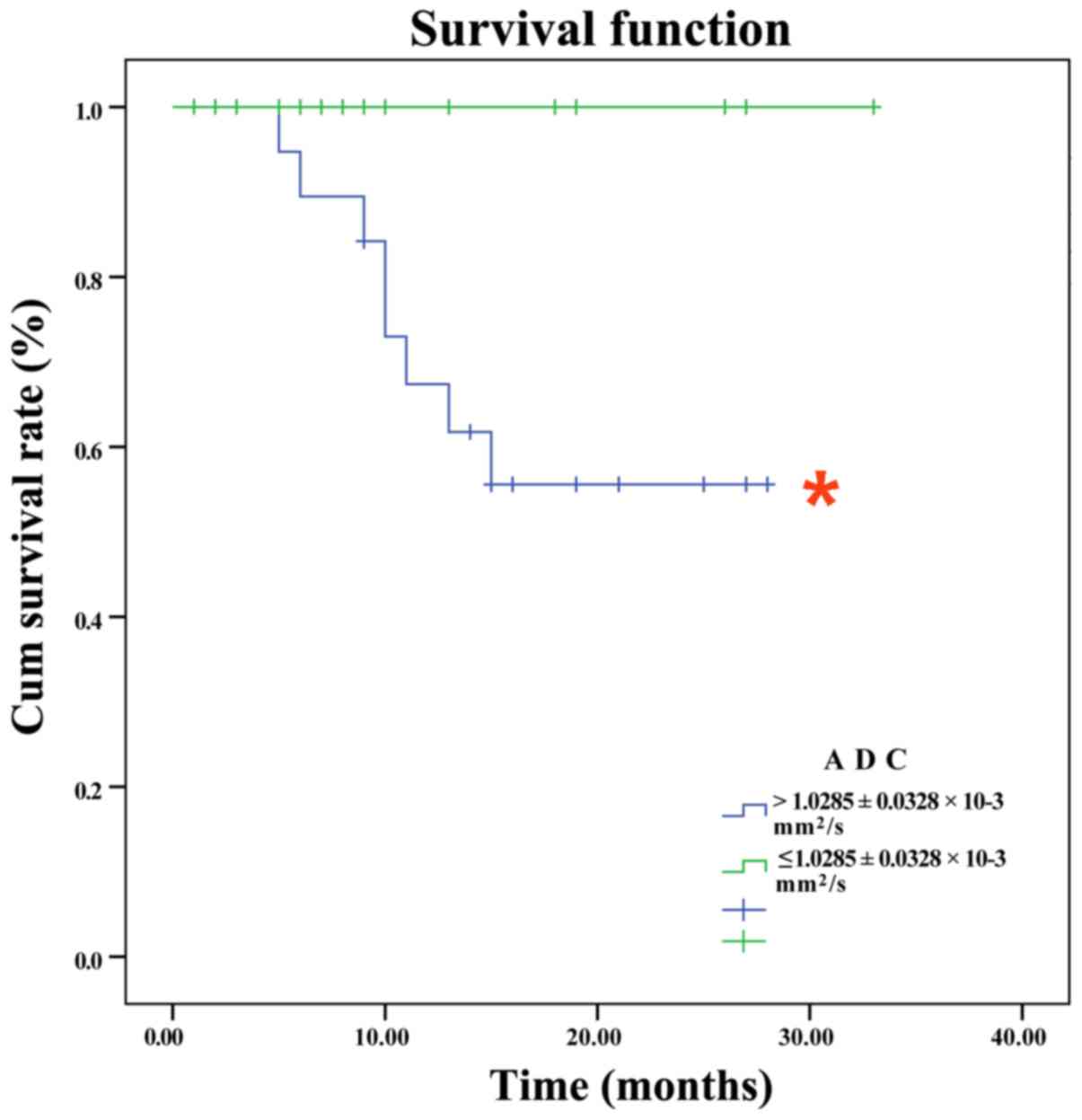
Cases were divided into two groups according to the
mean ADC values of hypopharyngeal SCCs
[≤(1.03±0.0328)×10−3 mm2/sec vs.
>(1.03±0.0328)×10−3 mm2/sec). The 2-year
overall survival rates of the groups were 55.6 and 100.0%,
respectively, a statistically significant difference (Fig. 7; χ2=5.073; P<0.02).
According to univariate analysis, other clinical parameters
including age, sex, site of tumor, T stage, N stage and distant
metastasis were not significant prognostic factors for survivalin
hypopharyngeal carcinoma (P>0.05). However, recurrence and
treatment modalities were prognostic factors for survival in
hypopharyngeal carcinoma (P=0.016 and P<0.001, respectively).
Patients who received surgical treatment in addition to
postoperative CCR exhibited a better survival rate than those who
received these treatment modalities alone. However, multivariate
analysis indicated that recurrence was the only independent risk
factor for survival in hypopharyngeal carcinoma (P<0.05).
Discussion
DWI has been widely used in the diagnosis,
differential diagnosis and evaluation of cancer differentiation,
clinical stage, outcome, recurrence and metastasis (9–14). Head
and neck cancer occurs at multiple sites, including the oral
cavity, oropharynx and larynx (9,10). In our
previous study, ADC values were revealed to be lower in patients
with T1 and T2 laryngeal carcinoma [mean,
(1.195±0.32)×10−3 mm2/sec] than in those with
laryngeal precancerous lesions [mean,(1.780±0.32)×10−3
mm2/sec; P<0.001] (12). ROC analysis revealed that the optimum
threshold for the ADC was 1.455×10−3 mm2/sec,
which may aid in distinguishing laryngeal carcinomas from laryngeal
precancerous lesions (12). The
present study continued to investigate the value of DWI in the
diagnosis of hypopharyngeal carcinoma and in predicting its
prognosis.
The results of the present study revealed that all
40 hypopharyngeal SCCs exhibited a high signal intensity in DWI.
Furthermore, the mean ADC value of hypopharyngeal SCCs was lower
than that of benign lesions (P<0.001).
These observations were similar to those from our
previous study on early laryngeal carcinoma (12) and to those of other previous studies
on head and neck carcinomas (4,10,15). Li et al (15) revealed that the mean ADC value of
malignant lesions of the tongue [(1.08±0.16)×10−3
mm2/sec] was lower than that of benign lesions
[(1.68±0.33)×10−3 mm2/sec] and cystic lesions
[(2.21±0.35)×10−3 mm2/sec; P<0.001]. ROC
analysis revealed that the AUC was 0.963 and the optimal threshold
for the ADC cut-off point was 1.31×10−3
mm2/sec for predicting malignancy (15). A meta-analysis investigating DWI as a
tool for differentiating malignancy from benign thyroid nodules
undertaken by Wu et al (9)
revealed that DWI sensitivity was 0.91 (95% CI, 0.86–0.94),
specificity was 0.92 (95% CI, 0.84–0.97), and ROC curves
demonstrated that AUC was 0.94 (95% CI, 0.92–0.96), indicating a
high level of overall accuracy. A systemic review by Driessen et
al (10) demonstrated that the
accuracy range of DWI was 66–86% in the detection of primary head
and neck squamous cell carcinoma (HNSCC). The mean of the ADC
values in malignant lesions was significantly lower than that in
benign lesions (4). This may be due
to increased cell density, which restricts diffusion thereby
decreasing ADC values in malignant lesions (4,6). Driessen
et al (10) investigated the
association between the ADC values of laryngeal and hypopharyngeal
carcinomas and histopathological observations. A significant
inverse correlation was observed between ADC values and cell
density, nuclear area and the nuclear-cytoplasmic ratio, and a
positive correlation was observed between ADC values and percentage
area of the stroma (10). However,
there is no standard ADC value that may be used as an optimal
threshold for differentiating malignant lesions from benign lesions
in the head and neck region (4,6,10,12). The
wide variation in ADC thresholds may be due to multiple factors,
including different b-values, field strengths, pathological types
and delineation methods (4,6).
The present study revealed that ADC values were not
correlated with tumor size. This observation was similar to that of
McVeigh et al (16), who
observed no correlation between ADC values and tumor volumes in
cervical cancer. However, Husby et al (17) observed that ADC values were negatively
correlated with tumor volume in endometrial carcinomas, suggesting
that tumor volume reflects tumor progression and prognosis in
endometrial carcinoma. The difference in the aforementioned results
may be due to the various measurement methods used and the
different b-values.
Another important feature of DWI is that ADC values
may aid in detecting metastatic lymph nodes in patients with HNSCC
(3,4,18,19). In the present study, 25 cervical lymph
nodes were proven to be histologically malignant and 13 nodes were
benign. The mean ADC value of metastatic nodes was significantly
lower than that of benign nodes (P=0.005). ROC curve analysis
revealed that an optimal threshold value of 1.075×10−3
mm2/sec was recommended as the cut-off point, resulting
in 69.2% sensitivity and 84.0% specificity; the AUC was 0.778 (95%
CI, 0.619–0.938; P=0.005). Additionally, Pekçevik et al
(18) analyzed 33 patients with 53
metastatic lymph nodes of HNSCC. The mean ADC values for nodal
metastases of nasopharyngeal carcinomas were significantly lower
than those for nodal metastases of laryngeal carcinomas. Zhong
et al (3) observed that 48
nodes were proven to be histologically malignant in 30 patients
with HNSCC and 17 nodes were benign. The mean ADC value of the
metastatic nodes (0.849×10−3 mm2/sec) was
significantly lower than that of the benign nodes
(1.443×10−3 mm2/sec; P<0.05). ROC curve
analysis revealed that the AUC was 0.83 and the optimal threshold
value was 0.960×10−3 mm2/sec, resulting in
89.58% sensitivity, 76.47% specificity and 86.15% accuracy. The
aforementioned study also revealed that DWI with ADC value
measurements may be more accurate than CT perfusion for the
preoperative diagnosis of cervical lymph node metastases in
patients with HNSCC (3). In oral
squamous cell carcinoma (OSCC), 21 nodes were proven to be
histologically malignant in 25 patients with OSCC and 30 nodes were
reactive (18). The mean ADC value of
the metastatic nodes [(0.702±0.197)×10−3
mm2/sec] was lower than that of the benign nodes
[(1.037±0.149)×10−3 mm2/sec; P<0.05). ROC
curve analysis revealed that the AUC was 0.887 and the optimal
threshold value was 0.887×10−3 mm2/sec,
resulting in 93.33% sensitivity, 80.95% specificity and 88.20%
accuracy (18). However, Lim et
al (20) revealed that the mean
ADC does not discriminate benign from metastatic cervical lymph
nodes in patients with HNSCC and non-necrotic, small lymph nodes.
The aforementioned study also suggested that metastatic small foci
in lymph nodes did not create sufficient architectural change to
affect ADC values. However, the patients included in the study were
examined using different MRI scanners (1.5-T, 3-T) (20). Sumi et al (21) observed the opposite results to those
of the present study; higher ADC values in metastatic nodes than in
benign lymphadenopathy (21). This
discrepancy may be due to the number of necrotic regions within the
metastatic nodes (21). Taken
together, these data suggest that further large-scale studies
focused on discriminating benign from metastatic cervical lymph
nodes using ADC values are required.
In the present study, univariate analysis revealed
that the mean ADC value was associated with the prognosis of
patients with hypopharyngeal carcinoma. However, no significant
correlation was observed between the mean ADC value and the
prognosis of patients with hypopharyngeal carcinoma according to
multivariate analysis, and the only independent risk factor for
survival was recurrence. Similarly, in other types of cancer, Zhang
et al (14) revealed that high
ADC values were a good prognostic indicator in 541 patients with
nasopharyngeal carcinoma (14).
Hatakenaka et al (22)
revealed found that ADC values were an independent prognostic
indicator in cases of HNSCC treated with radiotherapy. Yoshida
et al (23) also revealed that
low ADC values (<1.10×10−3 mm2/sec) were
significantly associated with shorter cancer-specific survival of
patients with upper urinary tract cancer. However, certain studies
have reported that ADC values were not associated with survival of
patients with HNSCC who were treated with radiotherapy (24,25). The
reason for these contradictory results remains unclear. Hatakenaka
et al (22) assessed possible
reasons and proposed that ADC values calculated from different
b-values may have an effect. For example, ADC values of 0 and 1,000
sec/mm2 are not significantly associated with overall
survival in patients with HNSCC treated with radiotherapy. In the
aforementioned study, patients with a higher ADC value (0–200
sec/mm2) exhibited a relatively good prognosis, while
those with a lower ADC value (300–1,000 sec/mm2)
exhibited a favorable prognosis (22). It was suggested that the spatial
distribution of photons in cancer cells is heterogeneous; certain
cancer cells with low ADC values were critically damaged following
radiotherapy, while other adjacent cells were not (22). These critically damaged cells
prevented chemical substances from diffusing to adjacent cancer
cells and resulted in damage to the adjacent cancer cells and radio
sensitivity. There may also be a converse effect in those cancer
cells with high ADC values (22). The
present study revealed that ADC values were not associated with T
stage or histological grade, observations with were similar to
those of previous reports (23,26).
Recently, it has been suggested that a histogram of ADC values may
better reflect ADC heterogeneity. Certain studies have demonstrated
that ADC histograms may be associated with T stage and prognosis of
certain types of solid cancer (27–29).
Therefore, future studies should investigate the association
between ADC histograms and prognosis in HNSCC.
The present study has certain limitations. To begin
with, the present study incorporated a small sample size with
relatively short follow-up periods. Additionally, only
advanced-stage hypopharyngeal carcinoma was investigated.
Furthermore, multiple b-values were not used to calculate ADCs.
Finally, the present study was retrospective in nature, resulting
in unavoidable bias. Therefore, large-scale prospective
multi-center studies are required for further study.
In conclusion, ADC values may discriminate
hypopharyngeal carcinomas from benign lesions and may also
discriminate metastatic lymph nodes of hypopharyngeal SCCs from
reactive cervical lymph nodes. Furthermore, mean ADC values may be
a useful prognostic factor in univariate analysis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372903) and the
Science and Technology Department of Zhejiang Province, China
(grant no. 2016C33G2010117).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim JW, Kim MS, Kim SH, Kim JH, Lee CG,
Kim GE and Keum KC: Definitive chemoradiotherapy versus surgery
followed by adjuvant radiotherapy in resectable stage III/IV
hypopharyngeal cancer. Cancer Res Treat. 48:45–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vandersteen C, Benezery K, Chamorey E,
Ettaiche M, Dassonville O, Poissonnet G, Riss JC, Pierre CS,
Hannoun-Lévi JM, Chand Me, et al: Contemporary therapeutic
management of locally advanced hypopharyngeal cancer: oncologic and
functional outcomes-a report on 100 cases. Acta Otolaryngol.
135:193–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhong J, Lu Z, Xu L, Dong L, Qiao H, Hua
R, Gong Y, Liu Z, Hao C, Liu X, et al: The diagnostic value of
cervical lymph node metastasis in head and neck squamous carcinoma
by using diffusion-weighted magnetic resonance imaging and computed
tomography perfusion. Biomed Res Int. 2014:2608592014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Driessen JP, van Kempen PM, van der
Heijden GJ, Philippens ME, Pameijer FA, Stegeman I, Terhaard CH,
Janssen LM and Grolman W: Diffusion-weighted imaging in head and
neck squamous cell carcinomas: A systematic review. Head Neck.
37:440–448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Y, Tong T, Cai S, Bi R, Xin C and Gu
Y: Apparent diffusion coefficient (ADC) value: A potential imaging
biomarker that reflects the biological features of rectal cancer.
PLoS One. 9:e1093712014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Payne KF, Haq J, Brown J and Connor S: The
role of diffusion-weighted magnetic resonance imaging in the
diagnosis, lymph node staging and assessment of treatment response
of head and neck cancer. Int J Oral Maxillofac Surg. 44:1–7. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chawla S, Kim S, Wang S and Poptani H:
Diffusion-weighted imaging in head and neck cancers. Future Oncol.
5:959–975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thoeny HC, De Keyzer F and King AD:
Diffusion-weighted MR imaging in the head and neck. Radiology.
263:19–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu LM, Xu JR, Liu MJ, Zhang XF, Hua J,
Zheng J and Hu JN: Value of magnetic resonance imaging for nodal
staging in patients with head and neck squamous cell carcinoma: A
meta-analysis. Acad Radiol. 19:331–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Driessen JP, Caldas-Magalhaes J, Janssen
LM, Pameijer FA, Kooij N, Terhaard CH, Grolman W and Philippens ME:
Diffusion-weighted MR imaging in laryngeal and hypopharyngeal
carcinoma: Association between apparent diffusion coefficient and
histologic findings. Radiology. 272:456–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee CC, Ho HC, Su YC, Yu CH and Yang CC:
Modified tumor classification with inclusion of tumor
characteristics improves discrimination and prediction accuracy in
oral and hypopharyngeal cancer patients who underwent surgery.
Medicine (Baltimore). 94:e11142015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shang DS, Ruan LX, Zhou SH, Bao YY, Cheng
KJ and Wang QY: Differentiating laryngeal carcinomas from precursor
lesions by diffusion-weighted magnetic resonance imaging at 3.0 T:
A preliminary study. PLoS One. 8:e686222013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang I, Choi SH, Kim YJ, Kim KG, Lee AL,
Yun TJ, Kim JH and Sohn CH: Differentiation of recurrent tumor and
posttreatment changes in head and neck squamous cell carcinoma:
Application of high b-value diffusion-weighted imaging. AJNR Am J
Neuroradiol. 34:2343–2348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Liu X, Zhang Y, Li WF, Chen L,
Mao YP, Shen JX, Zhang F, Peng H, Liu Q, et al: Prognostic value of
the primary lesion apparent diffusion coefficient (ADC) in
nasopharyngeal carcinoma: A retrospective study of 541 cases. Sci
Rep. 5:122422015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Cheng J, Zhang Y and Zhang Z:
Differentiation of benign and malignant lesions of the tongue by
using diffusion-weighted MRI at 3.0 T. Dentomaxillofac Radiol.
44:201403252015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McVeigh PZ, Syed AM, Milosevic M, Fyles A
and Haider MA: Diffusion-weighted MRI in cervical cancer. Eur
Radiol. 18:1058–1064. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Husby JA, Salvesen ØO, Magnussen IJ,
Trovik J, Bjørge L, Salvesen HB and Haldorsen IS: Tumour apparent
diffusion coefficient is associated with depth of myometrial
invasion and is negatively correlated to tumour volume in
endometrial carcinomas. Clin Radiol. 70:487–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pekçevik Y, Çukurova İ and Arslan İB:
Apparent diffusion coefficient for discriminating metastatic lymph
nodes in patients with squamous cell carcinoma of the head and
neck. Diagn Interv Radiol. 21:397–402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Si J, Huang S, Shi H, Liu Z, Hu Q, Wang G,
Shen G and Zhang D: Usefulness of 3T diffusion-weighted MRI for
discrimination of reactive and metastatic cervical lymph nodes in
patients with oral squamous cell carcinoma: A pilot study.
Dentomaxillofac Radiol. 43:201302022014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim HK, Lee JH, Baek HJ, Kim N, Lee H,
Park JW, Kim SY, Cho KJ and Baek JH: Is diffusion-weighted MRI
useful for differentiation of small non-necrotic cervical lymph
nodes in patients with head and neck malignancies? Korean J Radiol.
15:810–816. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sumi M, Sakihama N, Sumi T, Morikawa M,
Uetani M, Kabasawa H, Shigeno K, Hayashi K, Takahashi H and
Nakamura T: Discrimination of metastatic cervical lymph nodes with
diffusion-weighted MR imaging in patients with head and neck
cancer. AJNR Am J Neuroradiol. 24:1627–1634. 2003.PubMed/NCBI
|
|
22
|
Hatakenaka M, Nakamura K, Yabuuchi H,
Shioyama Y, Matsuo Y, Kamitani T, Yonezawa M, Yoshiura T, Nakashima
T, Mori M and Honda H: Apparent diffusion coefficient is a
prognostic factor of head and neck squamous cell carcinoma treated
with radiotherapy. Jpn J Radiol. 32:80–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshida S, Kobayashi S, Koga F, Ishioka J,
Ishii C, Tanaka H, Nakanishi Y, Matsuoka Y, Numao N, Saito K, et
al: Apparent diffusion coefficient as a prognostic biomarker of
upper urinary tract cancer: A preliminary report. Eur Radiol.
23:2206–2214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galban CJ, Mukherji SK, Chenevert TL,
Meyer CR, Hamstra DA, Bland PH, Johnson TD, Moffat BA, Rehemtulla
A, Eisbruch A and Ross BD: A feasibility study of parametric
response map analysis of diffusion-weighted magnetic resonance
imaging scans of head and neck cancer patients for providing early
detection of therapeutic efficacy. Transl Oncol. 2:184–190. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vandecaveye V, Dirix P, De Keyzer F, Op de
Beeck K, Vander Poorten V, Hauben E, Lambrecht M, Nuyts S and
Hermans R: Diffusion-weighted magnetic resonance imaging early
after chemoradiotherapy to monitor treatment response in
head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol
Phys. 82:1098–1107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamitani T, Matsuo Y, Yabuuchi H, Fujita
N, Nagao M, Jinnouchi M, Yonezawa M, Yamasaki Y, Tokunaga E, Kubo
M, et al: Correlations between apparent diffusion coefficient
values and prognostic factors of breast cancer. Magn Reson Med Sci.
12:193–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue H, Ren C, Yang J, Sun Z, Li S, Jin Z,
Shen K and Zhou W: Histogram analysis of apparent diffusion
coefficient for the assessment of local aggressiveness of cervical
cancer. Arch Gynecol Obstet. 290:341–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Downey K, Riches SF, Morgan VA, Giles SL,
Attygalle AD, Ind TE, Barton DP, Shepherd JH and De Souza NM:
Relationship between imaging biomarkers of stage I cervical cancer
and poor-prognosis histologic features: Quantitative histogram
analysis of diffusion-weighted MR images. AJR Am J Roentgenol.
200:314–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn SJ, Choi SH, Kim YJ, Kim KG, Sohn CH,
Han MH, Chang KH and Min HS: Histogram analysis of apparent
diffusion coefficient map of standard and high B-value diffusion MR
imaging in head and neck squamous cell carcinoma: A correlation
study with histological grade. Acad Radiol. 19:1233–1240. 2012.
View Article : Google Scholar : PubMed/NCBI
|