Introduction
Esophageal cancer (ESCC) has become one of the
leading causes of cancer-associated mortality in China over the
past few decades (1). Despite the
marked improvements made in chemotherapy and radiotherapy in the
past few decades, metastases and tumor recurrence in ESCC remain
associated with a poor prognosis (2).
To improve patient survival, there is a pressing requirement to
understand the mechanism of pathogenesis and to identify novel
biomarkers and therapeutic targets in patients with ESCC. One
potential approach to cancer diagnosis and treatment is through the
assessment and targeting of non-coding RNAs (ncRNAs), which are
widely transcribed in the eukaryotic genome (2). Long ncRNAs (lncRNAs), which have become
a focus of the study of ncRNAs, are a class of non-coding RNAs
characterized by a length of >200 nucleotides (2). Evidence indicates that lncRNAs may
exhibit a marked effect on a number of molecular genetics and
cellular processes, including chromatin modification, cellular
differentiation and cell cycle regulation (3). Concurrently, lncRNAs have been
demonstrated to be dysregulated in various tumors and to serve as
promoters or suppressors in multiple signaling pathways (4). Previous studies have indicated that
lncRNAs are crucial regulators of pathways involved in
tumorigenesis and the progression of ESCC, including
metastasis-associated lung adenocarcinoma transcript 1 (MALAT1)
(5), sprouty RTK signaling antagonist
4-intronic transcript 1 (SPRY4-IT1) (6) and colon cancer-associated transcript 2
(7). A previous study demonstrated
that lncRNA MALAT1 expression was increased in ESCC tissues
compared with adjacent normal tissues (5). MALAT1 knockdown may inhibit ESCC cell
proliferation, migration and tumor-sphere formation, but increase
cell apoptosis (5). Downregulation of
MALAT1 decreased the expression of β-catenin, Lin28 homolog A and
enhancer of zeste 2 polycomb repressive complex 2 subunit (Ezh2),
whereas overexpression of Ezh2 reversed the small interfering
(si)-MALAT1-mediated repression (5).
In addition, Zhang et al (8)
identified that the expression of lncRNA SPRY4-IT1 was increased in
ESCC cell lines compared with normal esophageal epithelial cells.
Overexpression of SPRY4-IT1 may increase ESCC cell motility via
induction of the epithelial-mesenchymal transition (8). On the contrary, other studies also
revealed that several lncRNAs may serve as tumor suppressor genes
in ESCC, including the lncRNAs low expression in tumor (9) and urothelial cancer associated 1
(10). However, despite the progress
made in understanding lncRNAs, the non-protein-coding genes
encoding small nucleolar RNAs (snoRNAs) have received little
attention.
snoRNAs, which are ncRNAs, are small RNAs measuring
60–300 nucleotides in length (11).
Owing to their nucleolar localization, the majority of snoRNAs
serve as guide RNAs for post-transcriptional modifications, to
ensure the production of efficient and accurate ribosomes (12). It was initially assumed that the
non-coding genes encoding snoRNAs have no function, but may host
coding sequences in their introns (13). However, previous studies have
indicated that snoRNA host genes (SNHGs) may serve critical roles
in cancer, with roles documented for SNHG1 in non-small cell lung
cancer (14), SNHG3 in hepatocellular
carcinoma (15) and SNHG12 in human
osteosarcoma (16). A previous study
by Makarova and Kramerov (17)
proposed the existence of the unusual snoRNA gene small nucleolar
host gene 6 (U87HG), also termed SNHG6, which is a housekeeping
gene of the 5′-terminal oligopyrimidine tract (TOP) family and
associated with ribosomes. SNHG6 demonstrated a high degree of
conservation and was ineffectively degraded by nonsense-mediated
mRNA decay (NMD), which indicated that it served additional
functions separate from the production of U87 RNA (17). The present study used reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), MTT
assays, flow cytometry and subcellular fraction assays to
investigate the expression and functional roles of lncRNA SNHG6 in
ESCC. The results indicated that the expression of SNHG6 was
significantly upregulated in ESCC tissues and cell lines compared
with normal esophageal epithelial cells. Inhibition of SNHG6 may
result in diminished cell growth and increased apoptosis, which
indicates the potential role served by SNHG6 in ESCC.
Materials and methods
Tissue samples and patient data
Patients (n=70; age range, 24–84; median age, 64;
admitted between January and December 2012) diagnosed with primary
ESCC and scheduled for routine surgery at Huai'an First People's
Hospital, Nanjing Medical University (Huai'an, China) were included
in the present study. All the patients with a histological
diagnosis had not received preoperative therapy. Clinical
information, including age, gender, history of smoking and
drinking, tumor size and pathological Tumor-Node-Metastasis (TNM)
stage [according to the 7th AJCC TNM staging system (18)], was collected from clinical data and
personal interviews. ESCC and adjacent non-cancerous tissues
following resection were snap-frozen in liquid nitrogen and stored
at −80°C. All procedures performed in the present study involving
human participants were conducted in accordance with the ethical
standards of Huai'an First People's Hospital, Nanjing Medical
University and with the 1964 Helsinki declaration and its later
amendments. Informed consent was obtained from all individual
participants included in the present study.
Cell culture
The ESCC ECA-109 and TE-1 cell lines were purchased
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and cultured in high-glucose Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing, penicillin-streptomycin and 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). A normal human
esophageal epithelial cell line (HEEC) was obtained from ScienCell
Research Laboratories, Inc. (San Diego, CA, USA) and grown in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.),
containing penicillin-streptomycin and 10% FBS. All cells were
maintained in humidified incubators under standard conditions
(37°C, 5% CO2).
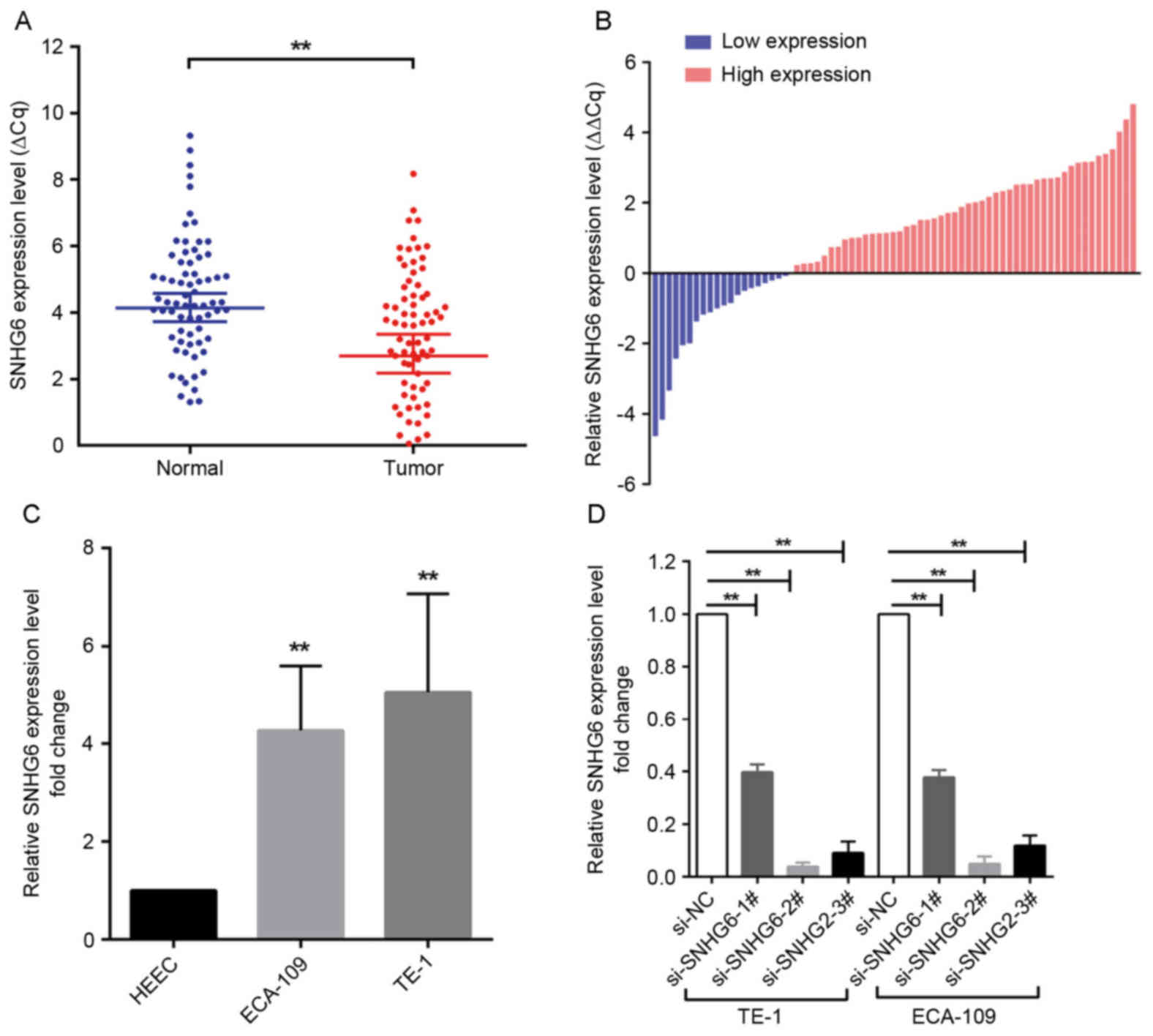
Cell transfection
ECA-109 and TE-1 cells were transfected with
specific small interfering RNA (siRNA) oligonucleotides. A total of
3 different siRNAs (sequences are listed in Table I) were designed to ensure the
efficiency of interference, 2 of which (si-SNHG6-2# and
si-SNHG6-3#) were considered appropriate for SNHG6 knockdown as
they had an interference efficiency of >70% (Invitrogen; Thermo
Fisher Scientific, Inc.). Negative control siRNA (si-NC) was also
purchased from Invitrogen; Thermo Fisher Scientific, Inc. ESCC
cells were seeded at 6-well plates for 24 h. Cells were then
transfected with either SNHG6-siRNA (100 nM) or si-NC (100 nM)
using Lipofectamine RNAiMAX transfection reagent®
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Following incubation for 48 h, transfected
cells were used for further experiments.
 | Table I.Sequence for primers and siRNAs. |
Table I.
Sequence for primers and siRNAs.
| Experimental
method | Sequence, 5′-3′ |
|---|
| RT-qPCR |
|
|
GAPDH |
|
|
Forward |
GGGAGCCAAAAGGGTCAT |
|
Reverse |
GAGTCCTTCCACGATACCAA |
|
SNHG6 |
|
|
Forward |
TTAGTCATGCCGGTGTGGTG |
|
Reverse |
AATACATGCCGCGTGATCCT |
| U1 |
|
|
Forward |
GGGAGATACCATGATCACGAAGGT |
|
Reverse |
CCACAAATTATGCAGTCGAGTTTCCC |
| Transfection |
|
| siRNA
oligonucleotides |
|
|
si-NC |
UUCUCCGAACGUGUCACGUTT |
| si-
SNHG6-1# |
UUCACCUCAAAGGCUUUCUUGCACC |
| si-
SNHG6-2# |
AAAUGCUGCAUGCCACACUUGAGGU |
| si-
SNHG6-3# |
GCGGCAUGUAUUGAGCAUAUAGGUU |
RNA extraction and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from tissues specimens or
cultured cells with TRIzol® reagent (Life Technologies;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. A total of 1 µg total RNA was used for the reverse
transcription reaction in a final volume of 20 µl, using random
primers from the PrimeScript RT Reagent kit with gDNA Eraser
(Takara Biotechnology Co., Ltd., Dalian, China) and 1 µl cDNA was
used, according to the manufacturer's protocols, for subsequent
RT-qPCR reactions (SYBR Premix Ex Taq, Takara Bio, Inc., Otsu,
Japan) of denaturation at 95°C for 5 sec, annealing at 60°C for 34
sec, elongation at 68°C for 20 sec for 40 cycles. The
constitutively expression gene GAPDH was used to normalize target
gene expression and U1 was used to indicate nuclear expression. The
RT-qPCR analysis was performed on ABI 7500 Real-Time PCR system
using the 2−ΔΔCt method (19) (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The assay was run in triplicate for each sample.
The primer sequences are summarized in Table I. The specimens were divided into a
high-expression group (n=50) and a low-expression group (n=20)
according to the expression of SNHG6 (a fold-change ≥1 represents
high expression of SNHG6, while a fold-change <1 indicates low
expression of SNHG6 in ESCC tissues.).
Cell proliferation assays
Each cell line was seeded in flat-bottomed 96-well
plates (3,000 cells/well) 24 h following siRNA transfection. The
absorbance was measured using a microplate reader at a wavelength
of 490 nm. Cell proliferation was evaluated using an MTT Cell
Proliferation Reagent kit I (Roche Diagnostics, Basel,
Switzerland). For the colony-formation assay, 500 transfected cells
were plated in a 6-well plate and maintained in RPMI-1640
containing 10% FBS. After 14 days, cells were washed twice with
PBS, then fixed with 4% methanol at room temperature for 15 min and
stained with 0.1% crystal violet (dissolved using 95% ethanol) at
room temperature for 15 min (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The colony formation was determined by counting the
number of stained colonies under a light Olympus microscope (×40).
All the experiments were performed in triplicate. The formula for
the colony formation rate was as follows: Rate (%)=numbers of
colony/initial cell population × 100.
Cell apoptosis analysis
Subsequent to transfection with si-SNHG6 or si-NC,
cells were harvested using centrifugation (4°C at 7,500 × g for 5
min) and stained using an annexin V-Fluorescein
Isothiocyanate/Propidium Iodide Apoptosis Detection kit (BD
Biosciences, Franklin Lakes, NJ, USA). A flow cytometry system
(FACScan®, BD Biosciences) equipped with CellQuest Pro
Software version 5.1 (BD Biosciences, USA) was applied to analyze
cell apoptosis, in accordance with the manufacturer's protocol.
Cells were divided into viable cells, dead cells, early apoptotic
cells and late apoptotic cells. The relative proportion of early
apoptotic cells and apoptotic cells were expressed as the mean ±
standard deviation.
Subcellular fractionation
The separation of the nuclear and cytosolic
fractions of ECA-109 and TE-1 cells was performed using a PARIS™
kit (Life Technologies; Thermo Fisher Scientific, Inc.), according
to the manufacturer's protocol.
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was
used to perform statistical analyses. All experiments were
performed in triplicate. Student's t-test was performed to evaluate
the difference in SNHG6 expression between ESCC and adjacent
non-cancerous tissues and was used to compare two independent
groups. One-way analysis of variance, followed by a Tukey's post
hoc test, was used to compare three or more groups. The association
between SNHG6 expression and clinicopathological features was
evaluated using Pearson's χ2 test. P<0.05 was
considered to be indicate a statistically significant
difference.
Results
SNHG6 is upregulated in ESCC tissues
and associated with clinicopathological features
SNHG6 expression was analyzed in 70 human ESCC
specimens and matched non-cancerous tissues by RT-qPCR. The
expression level of SNHG6 was significantly increased in ESCC
tissues compared with matched adjacent tissues (50 of the 70
tissues, an increase of ≥1.0-fold; P<0.01; Fig. 1A and B). Subsequently, the specimens
were divided into a high-expression group (n=50) and a
low-expression group (n=20) according to the expression of SNHG6 (a
fold-change ≥1 represents high expression of SNHG6, while a
fold-change <1 indicates low expression of SNHG6 in ESCC
tissues.). Furthermore, the prognostic role of SNHG6 in determining
the clinical significance of patients with ESCC was investigated by
analyzing the association between SNHG6 expression and
clinicopathological features (Table
II). The data indicated that SNHG6 expression was associated
with tumor size (P=0.040) and TNM stage (P<0.01), but was not
associated with other parameters.
 | Table II.Association between SNHG6 expression
and clinicopathological characteristics in esophageal squamous cell
carcinoma. |
Table II.
Association between SNHG6 expression
and clinicopathological characteristics in esophageal squamous cell
carcinoma.
|
| SNHG6 expression |
|
|---|
|
|
|
|
|---|
| Clinical
parameters | High (fold-change
≥1) | Low (fold-change
<1) | P-valuea |
|---|
| Age, years |
|
| 0.449 |
| ≤64 | 25 | 12 |
|
|
>64 | 25 | 8 |
|
| Sex |
|
| 0.451 |
|
Male | 37 | 13 |
|
|
Female | 13 | 7 |
|
| Smoking |
|
| 0.880 |
|
Yes | 24 | 10 |
|
| No | 26 | 10 |
|
| Drinking state |
|
| 0.508 |
| Yes
(frequent drinkers) | 41 | 15 |
|
| No
(never drinkers) | 9 | 5 |
|
| Tumor size, cm |
|
| 0.040 |
| ≤3 | 14 | 12 |
|
|
3–5 | 29 | 7 |
|
|
>5 | 7 | 1 |
|
| Tumor stage |
|
| <0.01 |
| I | 2 | 11 |
|
| II | 27 | 4 |
|
|
III | 21 | 5 |
|
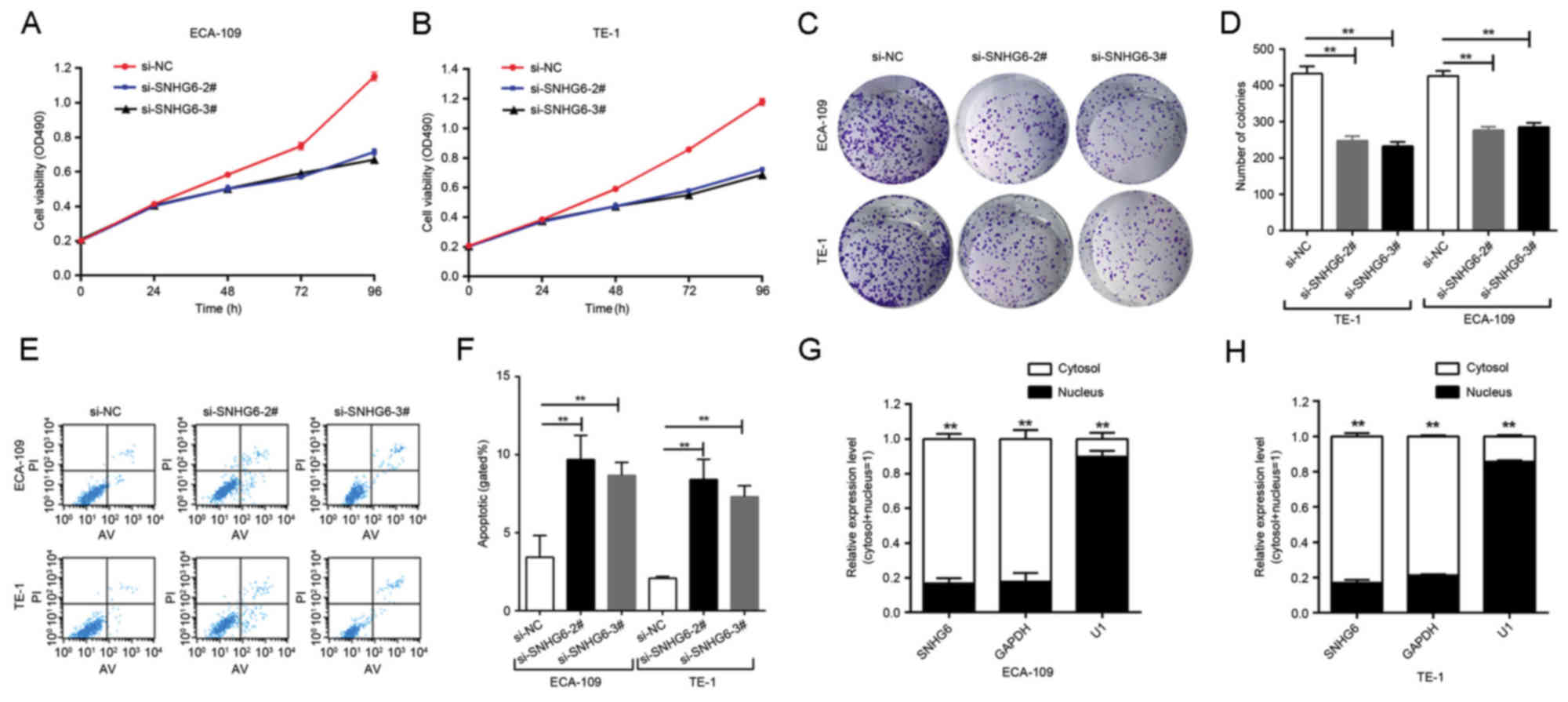
Effect of SNHG6 on cell
proliferation
Since SNHG6 was also upregulated in ESCC ECA109/TE-1
cell lines compared with expression in normal esophageal epithelial
HEEC cells (Fig. 1C), further
investigation was undertaken into the functional role of SNHG6 in
ESCC. To evaluate the role of SNHG6 in maintaining the malignant
phenotypes of ESCC cells, ECA109/TE-1 cells were transfected with
si-SNHG6-2# or si-SNHG6-3#, prior to being used for subsequent
experiments. The MTT assay demonstrated that the proliferation of
ECA109/TE-1 cells was markedly inhibited by the knockdown of SNHG6
(Fig. 2A and B). In addition, colony
formation assays were performed to assess the effect of SNHG6 on
ESCC cells, and indicated that clonogenic survival was markedly
decreased following SNHG6 knockdown in ECA109 and TE1 cell lines
(Fig. 2C and D).
Effect of SNHG6 on cell apoptosis
To examine the effect of SNHG6 on cell apoptosis,
flow cytometry analysis was utilized. The results of this analysis
indicated that the inhibition of SNHG6 markedly induced cell
apoptosis compared with the si-NC group (Fig. 2E and F).
Subcellular localization of SNHG6
Following separation of ECA-109 and TE-1 cells, RNA
was isolated from the nuclear and cytosolic fractions of the cells
and SNHG6 expression was measured by RT-qPCR. GAPDH was used as a
reference cytoplasm indicator and U1 was used as a reference
nucleus indicator. The results revealed that, in the 2 cell lines,
the expression level of SNHG6 was significantly increased in the
cytoplasm compared with the nucleus (Fig.
2G and H).
Discussion
ncRNAs, a class of genetic, epigenetic and
translational regulators without protein-coding capacity, contain
short and long transcripts and have the potential to be used as
biomarkers for multiple diseases (20). lncRNAs, which are >200 nucleotides,
are members of the ncRNA family. It has been demonstrated in a
variety of types of cancer that the expression of lncRNAs is
closely associated with the carcinogenesis, disease development,
metastasis and prognosis of patients with malignancy (4). lncRNAs may become targets for cancer
diagnosis and treatment.
A subset of small ncRNAs, snoRNAs, in the nucleoli
are involved in multiple steps of rRNA processing (21). According to their structure, snoRNAs
may be divided into 2 classes: C/D box snoRNAs, which serve as
guide RNAs in site-specific 2′-O-methylation of rRNAs, and H/ACA
box snoRNAs, which direct site-specific pseudouridylation of rRNAs
(20). In vertebrates, the majority
of snoRNA genes reside within the introns of protein-coding genes
and encode nucleolar proteins or proteins involved in translation
(22). However, a small number of
SNHGs contain multiple stop codons and do not code for proteins,
including UHG (23), U17HG (24), U19HG (25), gas5 (13) and U50HG (26). In 2005, a study reported the existence
of an unusual snoRNA host gene SNHG6, also termed U87HG, a novel
lncRNA 472 nucleotides in length and located within an intron of a
novel non-protein-coding gene (17).
SNHG6 is a housekeeping gene of the 5′TOP family and is associated
with ribosomes. The degree of conservation of SNHG6 RNA is similar
to those of the untranslated regions of protein-coding genes.
Additionally, SNHG6 RNA is ineffectively degraded by NMD. NMD
detects the mRNAs that contain premature termination codons and
triggers their degradation to prevent the accumulation of truncated
and potentially harmful proteins. Concomitant with the production
of U87 RNA, SNHG6 may also participate in translation or its
regulation (17). SNHG6 may be
functionally important, owing to the areas of high local similarity
to U87 RNA interspersed among the loosely-conserved sequence it
contains (17).
In the present study, the function of SNHG6 in ESCC,
and the potential association between its expression and
clinicopathological features was examined. Data analysis indicated
that the upregulation of SNHG6 was associated with tumor size and
TNM stage. The expression level of SNHG6 in ECA109 and TE-1 cells
was significantly increased compared with that in HEEC cells.
Induced downregulation of SNHG6 markedly inhibited the
proliferative and colony-forming abilities of, and induced
apoptosis in, ESCC cells, indicating its oncogenic role. This
result indicated the critical role of SNHG6 in tumorigenesis and
the progression of ESCC. Additional experimental results
demonstrated that SNHG6 RNA was predominately localized in the
cytoplasm, which was consistent with data from Makarova and
Kramerov (17). As it appears that
the regulatory mechanisms of lncRNAs are usually associated with
their localization (4), it can be
assumed that SNHG6 may serve as a microRNA sponge, which are able
to bind with several miRNAs to inhibit their expression and their
downstream pathways, or as an RNA-binding protein to regulate the
expression of target genes and participate in multiple pathways,
contributing to its oncogenic role in ESCC. Chaudhry (27) suggested that SNHG6 may be involved in
the ionizing radiation (IR)-induced stress response in a tumor
protein p53 (p53)-dependent manner. Similarly, a previous study
indicated that sno-microRNA-28, which directly targets the
p53-stabilizing gene TATA-box binding protein associated factor 9b,
was transcriptionally repressed by p53 through SNHG1 (28). These molecules form a regulatory loop
that affects p53 stability and downstream p53-regulated pathways
(28). Furthermore, Zhao et al
(29) identified that SNHG5 may
affect acetylation by trapping metastasis-associated 1 family
member 2 in the cytosol. SNHG5 overexpression may significantly
increase the acetylation levels of histone H3 and p53, thereby
interfering with the formation of the nucleosome remodeling and
histone deacetylation complex (29).
Therefore, it is possible that SNHG6 may also affect the cell cycle
and apoptosis through p53-associated pathways.
In conclusion, the present study revealed that SNHG6
may exhibit an important effect on the oncogenesis and development
of ESCC. As a potential predictor and a promising alternative
therapeutic target for future ESCC treatment, the detailed
molecular mechanism of SNHG6 involved in ESCC should be clarified
by additional studies.
Glossary
Abbreviations
Abbreviations:
|
lncRNA
|
long non-coding RNA
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deng G and Sui G: Noncoding RNA in
oncogenesis: A new era of identifying key players. Int J Mol Sci.
14:18319–18349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clark MB and Mattick JS: Long noncoding
RNAs in cell biology. Semin Cell Dev Biol. 22:366–376. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang W, Zhu Y, Li S, Chen X, Jiang G, Shen
Z, Qiao Y, Wang L, Zheng P and Zhang Y: Long noncoding RNA MALAT1
promotes malignant development of esophageal squamous cell
carcinoma by targeting β-catenin via Ezh2. Oncotarget.
7:25668–25682. 2016.PubMed/NCBI
|
|
6
|
Cui F, Wu D, He X, Wang W, Xi J and Wang
M: Long noncoding RNA SPRY4-IT1 promotes esophageal squamous cell
carcinoma cell proliferation, invasion, and epithelial-mesenchymal
transition. Tumour Biol. 37:10871–10876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Xu Y, He C, Guo X, Zhang J, He C,
Zhang L, Kong M, Chen B and Zhu C: Elevated expression of CCAT2 is
associated with poor prognosis in esophageal squamous cell
carcinoma. J Surg Oncol. 111:834–839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang CY, Li RK, Qi Y, Li XN, Yang Y, Liu
DL, Zhao J, Zhu DY, Wu K, Zhou XD and Zhao S: Upregulation of long
noncoding RNA SPRY4-IT1 promotes metastasis of esophageal squamous
cell carcinoma via induction of epithelial-mesenchymal transition.
Cell Biol Toxicol. 32:391–401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang PL, Liu B, Xia Y, Pan CF, Ma T and
Chen YJ: Long non-coding RNA-Low Expression in Tumor inhibits the
invasion and metastasis of esophageal squamous cell carcinoma by
regulating p53 expression. Mol Med Rep. 13:3074–3082. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Gao Z, Liao J, Shang M, Li X, Yin
L, Pu Y and Liu R: lncRNA UCA1 inhibits esophageal squamous-cell
carcinoma growth by regulating the Wnt signaling pathway. J Toxicol
Environ Health A. 79:407–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Williams GT and Farzaneh F: Are snoRNAs
and snoRNA host genes new players in cancer? Nat Rev Cancer.
12:84–88. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Decatur WA and Fournier MJ: rRNA
modifications and ribosome function. Trends Biochem Sci.
27:344–351. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith CM and Steitz JA: Classification of
gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member
of the 5′-terminal oligopyrimidine gene family reveals common
features of snoRNA host genes. Mol Cell Biol. 18:6897–6909. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
You J, Fang N, Gu J, Zhang Y, Li X, Zu L
and Zhou Q: Noncoding RNA small nucleolar RNA host gene 1 promote
cell proliferation in nonsmall cell lung cancer. Indian J Cancer. 3
Suppl 51:e99–e102. 2014. View Article : Google Scholar
|
|
15
|
Zhang T, Cao C, Wu D and Liu L: SNHG3
correlates with malignant status and poor prognosis in
hepatocellular carcinoma. Tumour Biol. 37:2379–2385. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruan W, Wang P, Feng S, Xue Y and Li Y:
Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12)
promotes cell proliferation and migration by upregulating
angiomotin gene expression in human osteosarcoma cells. Tumour
Biol. 37:4065–4073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Makarova JA and Kramerov DA: Noncoding RNA
of U87 host gene is associated with ribosomes and is relatively
resistant to nonsense-mediated decay. Gene. 363:51–60. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edge SB, Byrd DR and Compton CC: AJCC
Cancer Staging Manual. 7th. New York: Springer; 2009
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Busch A, Eken SM and Maegdefessel L:
Prospective and therapeutic screening value of non-coding RNA as
biomarkers in cardiovascular disease. Ann Transl Med. 4:2362016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bachellerie JP, Cavaillé J and Hüttenhofer
A: The expanding snoRNA world. Biochimie. 84:775–790. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maxwell ES and Fournier MJ: The small
nucleolar RNAs. Annu Rev Biochem. 64:897–934. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tycowski KT, Shu MD and Steitz JA: A
mammalian gene with introns instead of exons generating stable RNA
products. Nature. 379:464–466. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pelczar P and Filipowicz W: The host gene
for intronic U17 small nucleolar RNAs in mammals has no
protein-coding potential and is a member of the 5′-terminal
oligopyrimidine gene family. Mol Cell Biol. 18:4509–4518. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bortolin ML and Kiss T: Human U19
intron-encoded snoRNA is processed from a long primary transcript
that possesses little potential for protein coding. RNA. 4:445–454.
1998.PubMed/NCBI
|
|
26
|
Tanaka R, Satoh H, Moriyama M, Satoh K,
Morishita Y, Yoshida S, Watanabe T, Nakamura Y and Mori S: Intronic
U50 small-nucleolar-RNA (snoRNA) host gene of no protein-coding
potential is mapped at the chromosome breakpoint t(3;6)(q27;q15) of
human B-cell lymphoma. Genes Cells. 5:277–287. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chaudhry MA: Expression pattern of small
nucleolar RNA host genes and long non-coding RNA in X-rays-treated
lymphoblastoid cells. Int J Mol Sci. 14:9099–9110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu F, Bracken CP, Pillman KA, Lawrence DM,
Goodall GJ, Callen DF and Neilsen PM: p53 represses the oncogenic
Sno-MiR-28 derived from a SnoRNA. PLoS One. 10:e01291902015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao L, Guo H, Zhou B, Feng J, Li Y, Han
T, Liu L, Li L, Zhang S, Liu Y, et al: Long non-coding RNA SNHG5
suppresses gastric cancer progression by trapping MTA2 in the
cytosol. Oncogene. 35:5770–5780. 2016. View Article : Google Scholar : PubMed/NCBI
|