Introduction
It is well established that human solid tumors
frequently contain a substantial fraction of hypoxic cells. Hypoxia
is a direct cause of resistance to radiotherapy and the majority of
chemotherapeutic agents (1). In
addition, hypoxia can lead to a more aggressive tumor phenotype
(2). It has been demonstrated that
the presence of measurable hypoxia is associated with poor outcome
in many types of tumor (3–5). Since the mid 1970's, clinical research
in overcoming tumor hypoxia was mainly focus on the use of
nitroimidazoles and its derivatives as hypoxic cell sensitizers
(6,7).
Several compounds have been developed for hypoxia detection
(misonidazole and pimonidazole) and radiosensitization (etanidazole
and nimorazole) in clinical settings (8,9).
The randomized double-blind phase III study (DAHANCA
5 trial) demonstrated that nimorazole significantly improved the
effect of radiotherapy for head and neck carcinoma (10). However, the randomized multicenter
study of nimorazole concomitant with accelerated radiotherapy in
head and neck squamous cell carcinoma was incomplete, and the
number of patients involved in the study was small. Nevertheless,
the results suggested an improvement in loco-regional tumor control
and overall survival with given nimorazole in addition to
accelerated fractionation radiation therapy (8).
Hypoxic radiosensitizers have also been successfully
developed in China. Sodium glycididazole
(C18H22N7NaO10·3H2O;
also called CMNa) was approved by the China Food and Drug
Administration (11). Preliminary
study indicated that CMNa was able to improve short-term
locoregional control and was well tolerated in patients with
locoregionally advanced laryngeal cancer (12). The phase II randomized trial
demonstrated that CMNa was able to improve curative effects without
increasing adverse side effects when treating patients with locally
advanced nasopharyngeal carcinoma (13).
Ample data exist to support a high level of evidence
for the benefit of hypoxic modification (5–15).
However, hypoxic modification remains to have less impact on
general clinical practice (16). One
of main reason for this is the difficulty in detecting clinical
status of tumor hypoxia and its correlation with the
radiosensitizing effect of the target agent. Retrospective analysis
of DAHANCA 5 trial also revealed that high concentrations of plasma
osteopontin (OPN) might be useful in identifying patients who would
benefit from modification of hypoxia (17). However, Lim et al (18) reported that high plasma OPN levels
were not predictive of benefit with hypoxic cell cytotoxin,
tirapazamine (TPZ). However, the correlation of pretreatment
hypoxia status with radiosensitization effects was not defined in
the study.
In the present study, the pharmacokinetics and
pharmacodynamics of CMNa in different human cancer xenografts were
evaluated, and whether tumor hypoxia status is correlated with the
radiosensitizing effect of CMNa was investigated.
Materials and methods
Drug and chemicals
CMNa and its main metabolite, metronidazole, were
provided by Luye Pharmaceutical Co., Ltd (Yantai, Shandong, China).
Analytical grade methanol, acetonitrile and oxamide were purchased
from Zhaoshang Industry and Trade Ltd. (Shanghai, China). CMNa was
dissolved in saline (0.9% NaCl) to the required concentration and
stored at 4°C in the dark for subsequent experiments.
Cell culture
Human esophageal carcinoma cell EC-109, lung
carcinoma cell A549, and squamous cell FaDu were purchased from the
Chinese Academy of Sciences, Shanghai Institute of Cell Bank
(Shanghai, China) and cultured in Dulbecco's modified Eagle's
medium (DMEM, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and penicillin (100 U/ml) with streptomycin (100
µg/ml). Cultures were kept in a humidified atmosphere of 95% air
and 5% CO2 incubator at 37°C.
Animal xenograft
The female mice (nu/nu, 18–22 g; 4–6 weeks old) were
obtained from Huafukang Biotechnology Co., Ltd., (Beijing, China).
The total number of mice used was 500–600. Housing conditions were
as follows: Sealed plastic cage with air filter, no pathogen
condition room, temperature 26–28°C, air laminar flow apparatus, 10
h light/14 h dark cycle, sterilized food and water. All animal
experimental protocols were approved by the Institutional Animal
Experimentation Committee of Shandong Cancer Hospital (Shandong,
China). Tumor xenografts were formed by injecting 5×106
cells subcutaneously into the right hind legs of the mice. Each
tumor was measured with digital caliper in three orthogonal
dimensions (a, b and c). Tumor volume was calculated as πabc/6.
Experiments were performed when the tumors reached a volume of ~500
mm3 for the pharmacokinetics study, or a volume of ~150
mm3 for the tumor growth delay study.
Blood sample preparation
CMNa solution (171.9, 57.3 or 19.1 mg/kg) was
injected through the tail vein of the mice bearing EC109
xenografts. The blood was collected from eye vein under anesthesia
at 0.5, 1, 2, 3, 4, 5, 10, 15, 30, 60, 120 and 240 min following
injection (five or six animals were used for each time point). The
blood sample was mixed with 3% (v/v) sodium heparin immediately.
Subsequently, 0.2 ml acetonitrile was added and then centrifuged
(25°C, 1,200 × g, 2–3 min). All steps were carried out under dark
conditions.
Normal tissue and tumor sample
preparation
Mice bearing EC109, A549 or FaDu xenografts were
injected through the tail vein with CMNa (171.9, 57.3 or 19.1
mg/kg). Tissues (tumor, muscle, heart, liver, spleen, lungs,
kidneys, brain, bile and intestine) of each mouse was rapidly
excised following sacrifice at 2, 5, 10, 20, 30, 40, 50, 60, 70,
80, 90, 100, 110 and 120 min, respectively. The samples were washed
twice with 0.9% saline, wiped with filter paper, weighed and
homogenized with 0.9% saline to the same weight of tissues.
Homogenates were spiked with oxamide (0.2 ml) and grinded.
Following centrifugation for 2 min (25°C, 1,200 × g), 20 µl
supernatant was injected into the high-performance liquid
chromatography (HPLC) system for analysis. All the processes were
performed rapidly in the dark. A total of five animals were used
for each time point.
HPLC
The HPLC system (Dionex; Thermo Fisher Scientific,
Inc.) consisted of a DGP-3600A pump, a VW-3100 detector and a
TCC-3000 column (4.6×250 mm; particle size, 5 µm). The mobile phase
consisted of a mixture of methanol and ammonium oxalate solution
0.02 mol/l (40:60 v/v), and the elution was performed at 30°C at a
flow rate of 1.0 ml/min. CMNa and its metabolites were detected
using a UV detector at 320 nm and quantified by peak area. The CMNa
blood concentration-time data were simulated using the 3p87
software (Pharmacological Society of China, Beijing, China) and
fitted to a linear open two-compartment model.
Tumor irradiation and growth delay
assay
The tumors were irradiated under anesthesia using
the X-ray irradiator (X-rad225Cx; National Instruments Corp,
Austin, TX, USA). A total of 30 Gy in 6 fractions (5 Gy every other
day) were delivered in 3 weeks. Tumor bearing mice were injected
intravenously with CMNa at a dose of 171.9, 57.3, or 19.1 mg/kg 30
min prior to irradiation. The mice received radiation alone, and
those that were not treated were used as the control. The relative
tumor volume (RTV) was calculated as RTV=Vt/V0, where Vt
is the tumor volume at any given time and V0 is the
volume at the time of initial treatment. The tumor growth time
(TGT) was defined as the time required following the first day of
treatment for a tumor to reach twice the initial volume, and the
tumor growth delay time (TGDT) was defined as the TGT in each
treated mouse minus the mean TGT in the control group. A total of
six animals were used in each group.
Plasma osteopontin concentration
test
Prior to the start of the first irradiation, the
blood of tumor-bearing mice (50 µl) was collected from the oculi
rimae of mice. The plasma samples were centrifuged at 300 × g and
25°C for 10 min and supernatant (20 µl) was collected and stored.
The concentration of OPN was quantified by ELISA using the OPN test
kit (JK0235; Meilian Biological Ltd., Shanghai, China) according to
the manufacturer's instructions.
Immunohistochemical staining of
HIF-1α
Following excision, the tumors were fixed overnight
(within 12 h at 25°C) in 10% formalin and embedded in paraffin
blocks, from which 4 µm thick sections were prepared for
immunohistochemical staining. To analyze the expression of HIF-1α,
the slides were incubated with mouse monoclonal antibodies against
HIF-1α (WL01607; OriGene Technologies, Inc., Beijing, China) and
secondary antibodies (IgG-horseradish peroxidase, WLA023; Leica
Biosystems Newcastle Ltd., Newcastle, UK) diluted with PBS (29 g
Na2HPO4, 3 g NaH2PO4,
85 g NaCl dissolved in 500 ml distilled water, fixed to 1,000 ml).
Finally, the sections were stained with hematoxylin (10 min) and
eosin (1–3 min) at room temperature. Ratios of positive HIF-1Α
staining and intensity were compared among different groups. The
degree of staining score of positive cells was defined by counting
100 cells in the ×20 field of view. The positive cell number 0–25,
26–50, 51–75 and 76–100% was defined as (−), (+), (++) and (+++),
respectively.
Statistical analysis
Analyses were performed using the Statistical
Package for Social Sciences, (version 16.0; SPSS, Chicago, IL,
USA). Quantitative data are expressed as the mean ± standard error.
Comparisons of histological parameters between groups were
calculated using one-way analysis of variance followed by the
Bonferroni post hoc test or the Mann-Whitney U-test. A Pearson's
correlation coefficient and linear regression analysis were
performed for analysis of the tumor growth delay assay and OPN
concentration. All P-values were two-sided, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Serum concentrations of CMNa and
metabolites
The chromatographic baseline characteristics of CMNa
and its main metabolite, metronidazole, were far apart, with
retention times 6.5 and 4.4 min, respectively. No endogenous
components interfered the analysis. The linearity of the
calibration curve was determined with concentrations in the range
of 0.269–68.8 ng/ml with a regression equation Y=0.0923+4.2352X
(r=0.9997, n=5). The inter-day variation coefficients of CMNa were
<10% overnight, and the mean recovery was 88.1% for high
concentration of CMNa. CMNa was rapidly eliminated from the blood,
and the distribution half-life at three doses of CMNa were 0.765,
0.613 and 0.293 min, respectively. The relevant pharmacokinetic
parameters were listed in Table I.
The maximum CMNa concentration in blood (Cmax) and area
under the curve (AUC) values were directly proportional to doses,
which indicates first-order kinetics.
 | Table I.Main pharmacokinetic parameters of
CMNa. |
Table I.
Main pharmacokinetic parameters of
CMNa.
|
| CMNa (mg/kg) |
|---|
|
|
|
|---|
| Parameter | 171.9 | 57.3 | 19.1 |
|---|
| A | 734.99 | 107.148 | 12.011 |
| α | 0.906 | 1.131 | 2.367 |
| B | 0.01 | 19.504 | 2.544 |
| β | 0.01 | 1.131 | 0.588 |
| Vd/l/kg | 0.234 | 0.452 | 1.312 |
|
Cmax/mg/l | 466.79 | 71.27 | 5.575 |
| T1/2α,
min | 0.765 | 0.613 | 0.293 |
| T1/2β,
min | 69.315 | 0.613 | 1.179 |
| K10,
min−1 | 0.602 | 0.663 | 1.04 |
| K12,
min−1 | 0.304 | 0.467 | 1.017 |
| K21,
min−1 | 0.01 | 1.131 | 0.899 |
| AUC, mg/min | 802.008 | 122.163 | 9 |
| CL, min/kg | 0.141 | 0.3 | 1.364 |
Distribution of CMNa in tumors and
normal tissues
CMNa was distributed into the peripheral compartment
2 min following intravenous injection. A total of 5 min following
CMNa administration, it was possible to detect CMNa in different
organs (liver, intestine, kidney, lung, heart and brain), tumor
adjacent tissues (muscle), and the tumors. The concentration of
CMNa in the heart, liver, spleen, lungs, kidneys, brain, bile and
intestine at different time points following intravenous
administration determined by HPLC was listed in Table II.
 | Table II.Concentration of CMNa in normal
tissues. |
Table II.
Concentration of CMNa in normal
tissues.
|
|
Time
(min) |
|---|
|
|
|
|---|
| Concentration
(ng/ml) | 0 | 5 | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 |
|---|
|
Bile |
5.8±2.1 |
9.2±4.5 |
30.9±18.1 |
117.8±58.7 |
515.3±119.1 |
236.9±100.2 |
144.7±67.8 |
234.2±127.9 |
123.6±56.9 |
51.0±14.5 |
|
Intestinal |
22.4±7.4 |
16.5±8.5 |
8.7±3.8 |
0.7±0.5 |
2.8±1.3 |
3.7±2.1 |
6.3±1.8 |
4.1±2.3 |
0.2±0.07 |
0.2±0.05 |
|
Liver |
44.5±15.0 |
29.1±11.5 |
15.4±6.7 |
2.9±1.6 |
2.6±1.1 |
18.4±7.7 |
3.4±1.4 |
2.5±2.2 |
1.6±0.8 |
1.4±0.8 |
|
Kidney |
347.4±212.0 |
12.2±5.6 |
1.2±1.0 |
0.2±0.08 |
0.7±0.03 |
0.2±0.1 |
0.8±0.7 |
0.2±0.1 |
0.2±0.07 |
0.1±0.05 |
|
Heart |
3.4±1.4 |
2.1±0.9 |
0.2±0.09 |
0.2±0.11 |
0.2±0.05 |
0.2±0.1 |
0.2±0.1 |
0.2±0.05 |
0.2±0.05 |
0.2±0.05 |
|
Lung |
4.6±1.6 |
1.8±1.5 |
0.2±0.06 |
0.4±0.2 |
0.4±0.18 |
0.2±0.04 |
0.2±0.04 |
0.2±0.03 |
0.2±0.05 |
0.2±0.05 |
|
Spleen |
5.9±2.4 |
2.9±2.3 |
0.3±0.2 |
0.3±0.2 |
0.2±0.04 |
0.2±0.03 |
0.2±0.1 |
0.2±0.04 |
0.2±0.08 |
0.2±0.08 |
|
Brain |
0.3±0.2 |
0.2±0.1 |
0.1±0.02 |
0.2±0.07 |
0.2±0.08 |
0.2±0.05 |
0.2±0.05 |
0.2±0.04 |
0.2±0.1 |
0.2±0.04 |
The concentration of CMNa immediately following
intravenous administration in the kidney was the highest, followed
by intestinal, liver, heart, lung, spleen and brain tissues. A
total of 15 min following CMNa administration, the drug
concentration significantly decreased and was very low in the
kidney, spleen, heart, lung and brain. It was possible to detect
CMNa again in intestinal and liver tissues approximately 60–80 min
following injection, and the concentration-time curves were
biphasic. After 120 min, CMNa was undetectable in intestinal and
liver tissues.
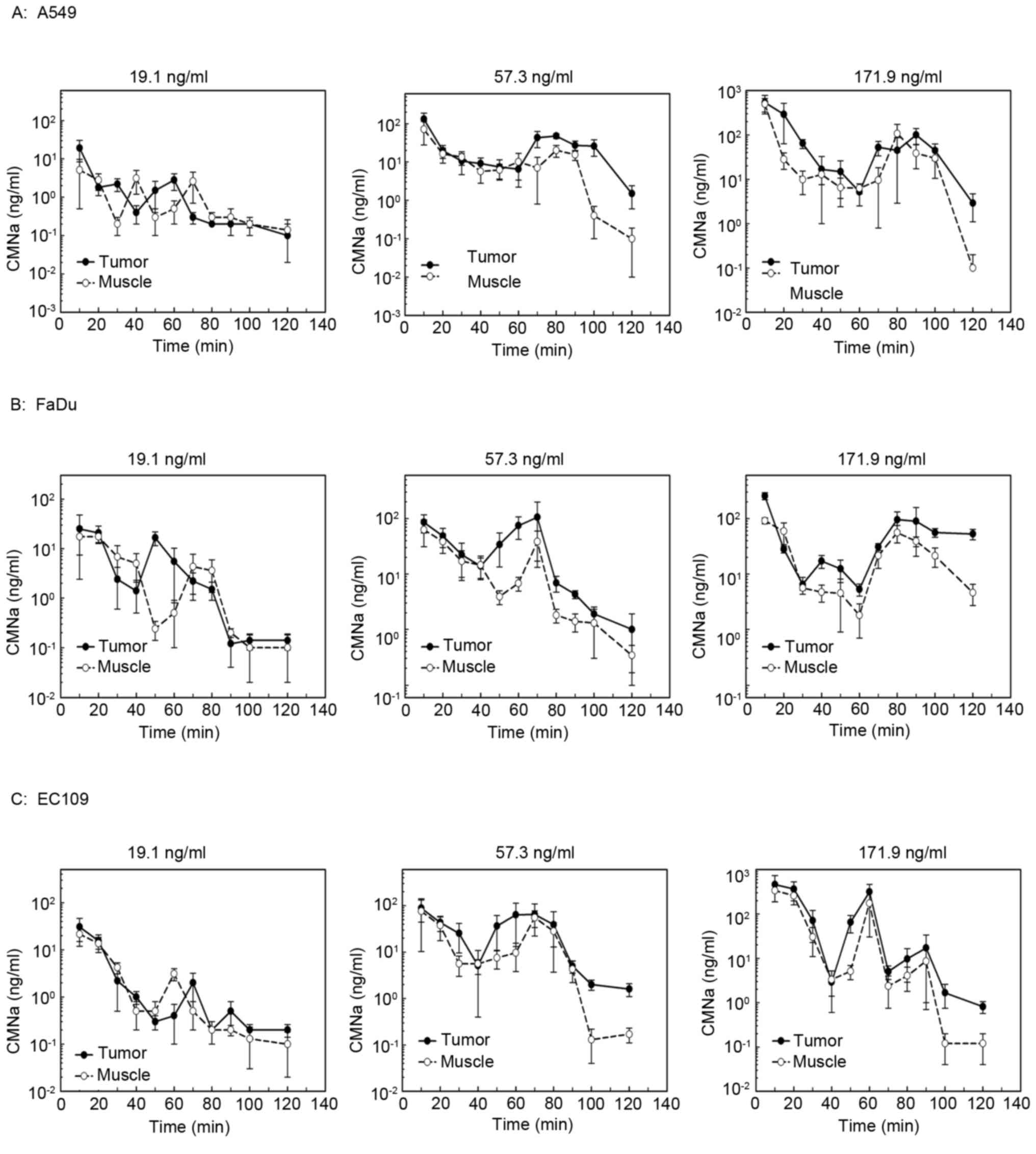
The levels of CMNa in the tumors were measured and
compared with adjacent muscle in different tumor xenografts at
high, medium and low doses of CMNa (Fig.
1). The AUC of drug concentration curves was compared among
different tumor types and different drug groups. The results
indicated that the drug concentration in the tumors was 1.6–2.8
times higher compared with muscle at the high and medium dose, but
not in the low dose groups (Table
III). The concentration-time curves of CMNa were biphasic,
which were similar compared with those in the liver and intestine
(Table II). The concentration
decreased of CMNa quickly following injection, increased slightly
at 60–80 min following injection and decreased to the lowest
afterwards.
 | Table III.Comparison of AUC values. |
Table III.
Comparison of AUC values.
| Tumor | CMNa dose
(mg/kg) | Tumor (AUC) | Muscle (AUC) | P-value |
|---|
| A549 | 171.9 |
9039.3±805.7 |
4979.7±513.1 | 0.145 |
|
| 57.3 |
2631.7±213.8 |
1303.6±139.8 | 0.034 |
|
| 19.1 |
192.7±25.6 |
128.7±20.6 | 0.397 |
| EC109 | 171.9 |
10832.0±1505.1 |
6552.4±804.4 | 0.022 |
|
| 57.3 |
3306.7±423.0 |
1924.1±290.8 | 0.023 |
|
| 19.1 |
375.2±57.0 |
335.9±30.9 | 0.415 |
| FaDu | 171.9 |
4953.5±238.9 |
2607.2±111.8 | 0.068 |
|
| 57.3 |
3550.5±452.1 |
1550.7±49.6 | 0.032 |
|
| 19.1 |
632.9±70.5 |
468.2±65.9 | 0.339 |
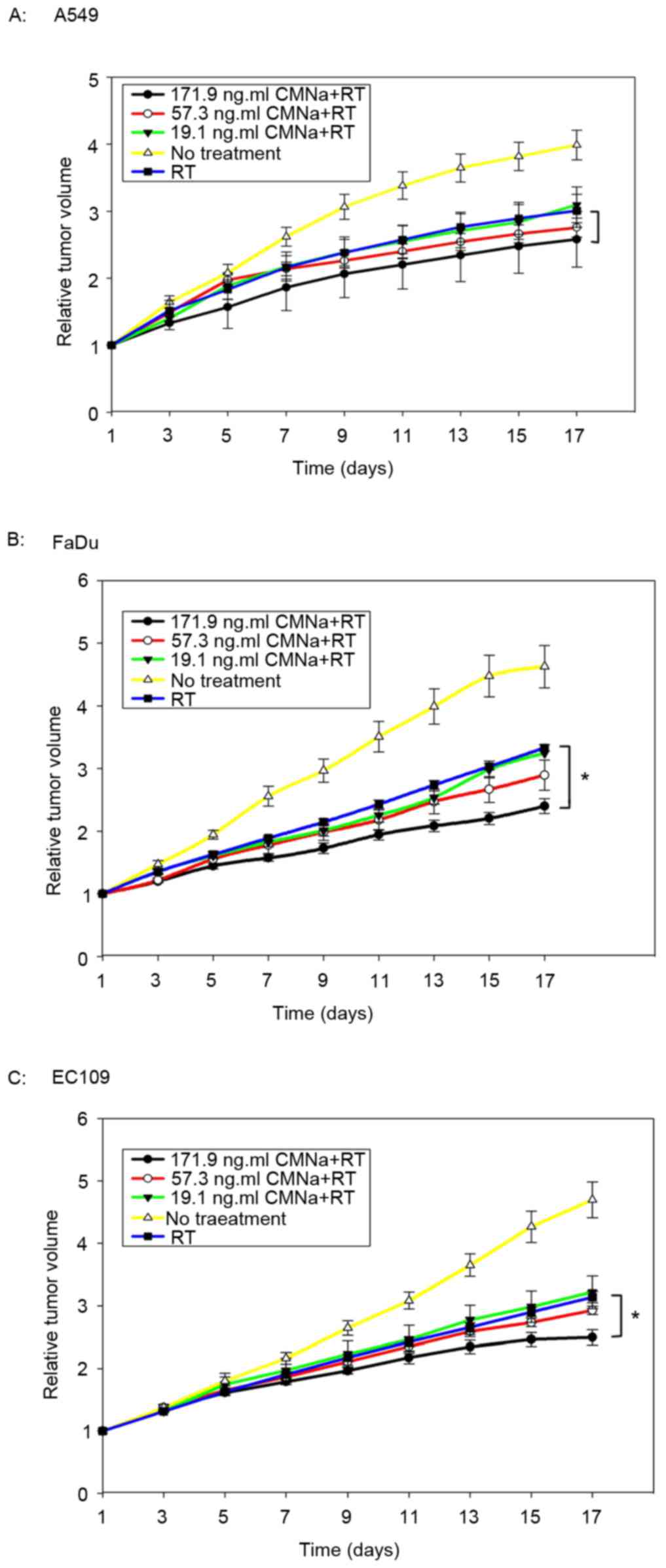
Radiosensitizing effects of CMNa
The radiosensitizing effects of CMNa were evaluated
for three types of xenografts at three dose levels. It was
demonstrated that CMNa was able to sensitize tumors to irradiation
for all three cancer types (A549, FaDu and EC109) at different
doses (Fig. 2). Tumor growth delay
time (TGDT) was quantified and compared within the groups (Table IV). For EC109 and FaDu xenografts,
TGDT in high dose groups was significantly longer compared with
TGDT values in the irradiation control groups (P<0.05). However,
these were no statistical differences between medium, low dose and
irradiation control groups (P>0.05). For A549 xenografts, no
statistical differences were observed between high, medium, low
dose groups and irradiation control groups (P>0.05).
 | Table IV.Comparison of tumor growth delay
time. |
Table IV.
Comparison of tumor growth delay
time.
| Tumor type | Drug dose and
treatment | TGDT, mean ±
standard error (days) | P-value |
|---|
| A549 | RT alone |
2.91±0.54 | 0.116 |
|
| 171.9 mg/kg; CMNa
plus RT |
4.46±1.73 |
|
|
| 57.3 mg/kg; CMNa
plus RT |
6.48±2.30 |
|
|
| 19.1 mg/kg; CMNa
plus RT |
3.76±1.40 |
|
| EC109 | RT alone |
1.60±0.44 | 0.032 |
|
| 171.9 mg/kg; CMNa
plus RT |
3.12±0.80 | vs. RT alone
0.033 |
|
| 57.3 mg/kg; CMNa
plus RT |
2.04±0.41 | vs. RT alone
0.604 |
|
| 19.1 mg/kg; CMNa
plus RT |
2.70±0.52 | vs. RT alone
0.721 |
| FaDu | RT alone |
2.99±0.30 | 0.007 |
|
| 171.9 mg/kg; CMNa
plus RT |
7.47±1.54 | vs. RT alone
0.032 |
|
| 57.3 mg/kg; CMNa
plus RT |
5.10±1.73 | vs. RT alone
0.095 |
|
| 19.1 mg/kg; CMNa
plus RT |
6.66±1.51 | vs. RT alone
0.448 |
OPN concentration and tumor HIF1-α
expression
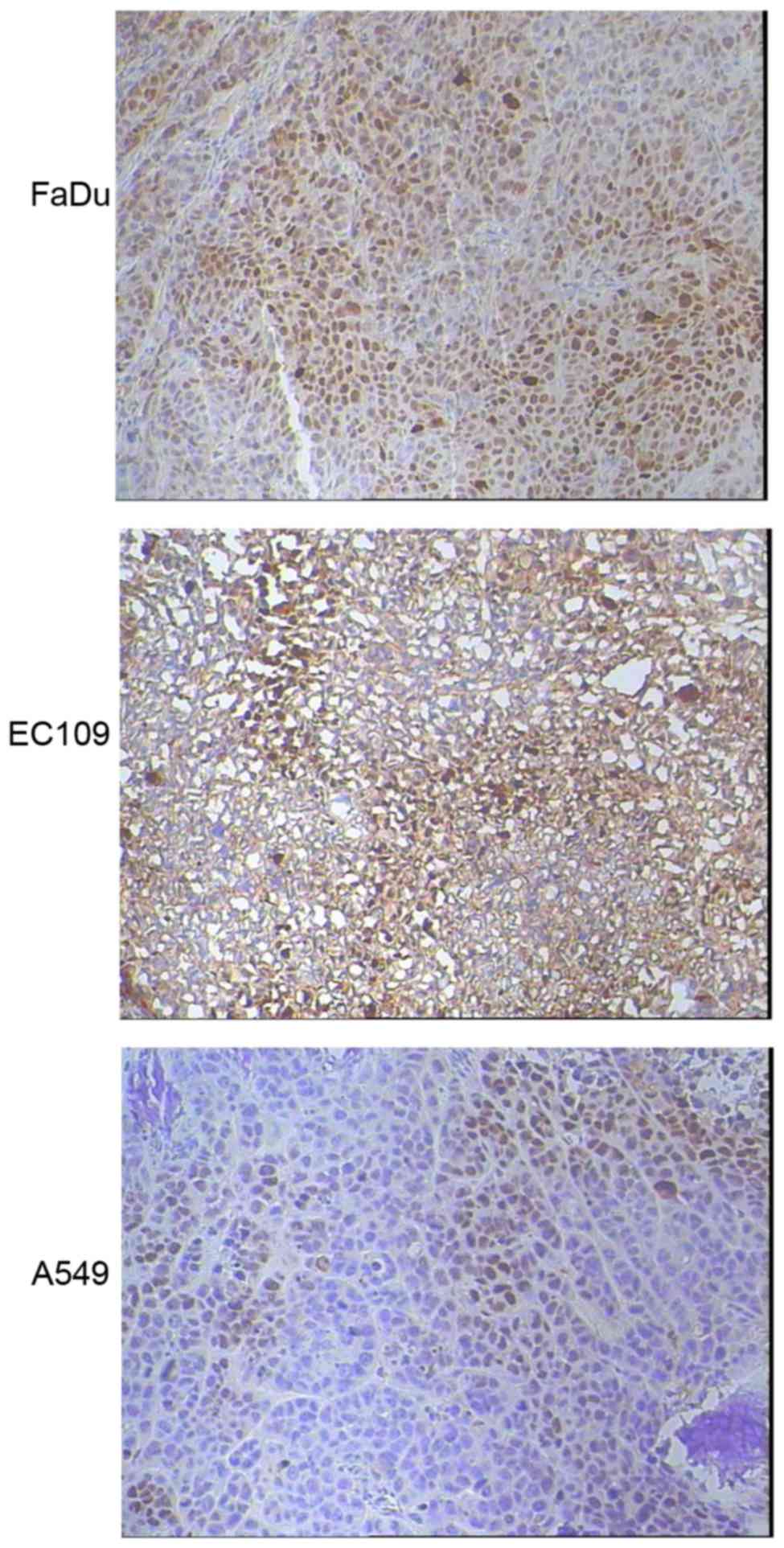
Tumor HIF1-α expression was evaluated by
immunohistochemical staining for three types of xenografts (n=5 for
each type). Markedly increased HIF1-α expression were detected in
FaDu (3+, 70%) and EC109 (2+~3+, 50%) xenografts compared with A549
tumors (1+, 40%), as shown in Fig.
3.
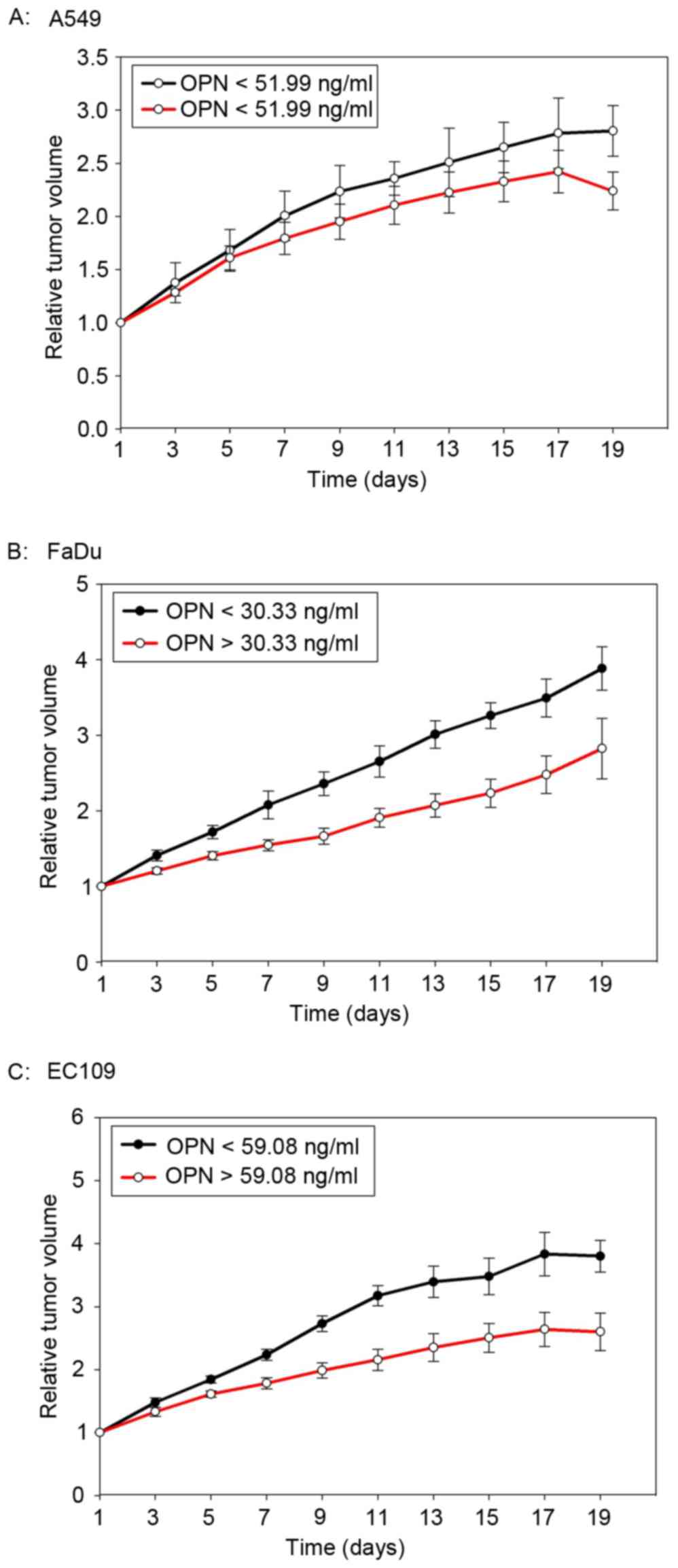
The median plasma concentration of OPN prior to the
start of radiotherapy was 59.08 ng/ml (23.09–111.04 ng/ml), 60.33
pg/ml (25.69–113.01 pg/ml) and 51.99 pg/ml (16.99–93.72 pg/ml) for
EC109, FaDu and A549 tumor-bearing mice, respectively. The median
OPN plasma level was used as a cut-off value. As shown in Fig. 4, mice with high OPN plasma levels had
a better tumor local control after radiotherapy. However, the
difference was not statistically significant (P=0.10, 0.117 and
0.374 for EC109, FaDu and A549, respectively).
Discussion
The novel hypoxic radiosensitizer, CMNa, has been
approved for use in combination with radiotherapy for the treatment
of nasopharyngeal cancer in China (10,11).
Perspective trials for lung cancer and esophageal cancer have also
been performed with encouraging results (19,20).
However, experimental data on CMNa, particularly on the in
vivo pharmacokinetic and pharmacodynamic parameters in
different tumor models were relatively limited. In the present
preclinical study, it was confirmed that high doses of CMNa was
able to sensitize human cancer xenograft to irradiation,
particularly for head and neck cancer and esophageal cancer. The
in vivo effects of CMNa might be associated with high
tumor/muscle drug concentration ratio and high tumor hypoxia status
(as detected by immunostaining for HIF-1α) in xenograft models.
Furthermore, it was identified that plasma OPN concentration was
correlated the radiosensitizing effect of CMNa in these tumor
xenografts. The present study provided useful information to define
optimal CMNa dose for personalizing radiosensization in further
translational studies in different types of cancer.
Normal tissue toxicity of hypoxic radiosensitizing
agents require attention in the clinical setting. In previous
clinical phase I–III trials, the main side effects associated with
the combination of CMNa and radiotherapy or chemoradiotherapy
included mild gastrointestinal reactions (nausea, vomiting and
constipation), mild reversible increases in serum alanine
aminotransferase and bilirubin. Higher doses of CMNa and
radiotherapy or chemoradiotherapy can also result in changes in
cardiac function and electrocardiogram, including ST-T depression,
arrhythmia and palpitation (11,12,21).
However, all the adverse effects were not statistically different
from the control group (11,12,21).
In present study, the distribution of levels of CMNa
was verified in normal tissues and in tumor xenografts. Similar to
a previous study (22), CMNa was
eliminated quickly from blood and other organs, including the
brain, heart and kidney. However, the concentration-time curves
were biphasic in the intestinal and liver tissues. This indirectly
confirmed that CMNa was excreted from the bile and re-absorbed from
the intestines to the liver (liver-intestinal circulation). From a
clinical point of view, this may lead to hepatotoxicity. Notably,
Liu et al (21) reported a
case of grade IV aminotransferase elevation in a trial for patients
with nasopharyngeal carcinoma receiving CMNa during radiotherapy.
This suggests that liver function should be monitored during CMNa
administration, particularly for patients with active
hepatitis.
Similar to other hypoxia radiosensitizers, CMNa was
primarily investigated and clinically used in patients with head
and neck cancer (6,18). Clinical trials in esophageal cancer
and lung cancer have also been performed (19,20). In
present study, significant radiosensitizing effects of CMNa were
observed in xenografts of human head and neck, and esophageal
cancer, but not in lung cancer. This finding was not surprising
because greater intrinsic tumor hypoxia was observed in FaDu and
EC109 tumors (Fig. 4). More
importantly, mice blood OPN concentration may predict the
radiosensitizing effects of CMNa. Mice blood OPN concentration may
provide novel information, to enable the selection of patients for
radiosensitizing based on tumor hypoxia condition, which had been
retrospectively reported in randomized trials. For example, in
DAHANCA 5 trial, elevated plasma OPN level was correlated with
poorer disease-specific survival and only patients with high OPN
level were able to benefit from nimorazole treatment (16). However, in another randomized trial,
which investigated hypoxic cytotoxin TPZ, patient plasma OPN levels
were not correlated with tumor control and survival (17). Le et al (23) evaluated 54 stage III–IV head and neck
squamous cell carcinoma patients and reported OPN to be a
hypoxia-regulated protein. Additionally, the levels of plasma OPN
were correlated with tumor pO2 (23). The present authors also observed the
positive correlation between tumor HIF-1 expression and OPN
expression in esophageal cancer patients and nude mice xenografts
(unpublished data). Therefore, further studies are required to
define the correlation between hypoxia parameters (plasma OPN,
hypoxia images and hypoxia gene expression profile) and the outcome
of radiosensitizing treatment.
The findings of the present pre-clinical study are
valuable for further clinical translational studies. Firstly, since
only higher dose of CMNa exhibited significant radiosensitization
for head and neck cancer, and esophageal cancer in the present
study, dose escalation clinical trials may be considered in further
studies. Secondly, in future clinical utilization, particularly in
dose escalation study, hepatotoxicity must be considered. Finally,
and most importantly, the hypoxia condition, either baseline or its
kinetics, should be tested using hypoxia imaging or hypoxia-driven
gene signatures/biomarkers (24,25). This
individualized radiosensitizing protocol should be developed in
future randomized trials.
There are some limitations in the present study.
Firstly, since CMNa has been previously tested during its early
development phase in vitro study (26), this was not repeated. Secondly, it was
not compared with other hypoxic radiosensitizing agents. Finally,
the tumor hypoxia conditions were only tested by detecting the
levels of plasma OPN and tissue HIF-1α expression and not verified
by other approaches, including hypoxia imaging or pimonidazole
staining.
In summary, higher tumor CMNa drug concentration was
detected in different tumor models. It was observed that CMNa was
able to sensitize tumors to irradiation, particularly at high does
for the treatment of head and neck, and esophageal cancer.
Furthermore, the levels of tumor HIF-1α and serum OPN concentration
may be used to predict the radiosensitizing effects. These findings
might be useful for future translational studies.
Acknowledgements
The present study was supported in part by the grant
from the National Natural Science Foundation of China (grant no.
81272502) and from the Shandong Provincial Natural Science
Foundation (grant no. ZR2015HZ004). Dr. Ligang Xing received a
research fund from Luye Pharmaceutical Co., Ltd. (Yantai, Shandong,
China).
References
|
1
|
Tatum JL, Kelloff GJ, Gillies RJ, Arbeit
JM, Brown JM, Chao KS, Chapman JD, Eckelman WC, Fyles AW, Giaccia
AJ, et al: Hypoxia: importance in tumor biology, noninvasive
measurement by imaging and value of its measurement in the
management of cancer therapy. Int J Radiat Biol. 82:699–757. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harris AL: Hypoxia-a key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nordsmark M, Overgaard M and Overgaard J:
Pretreatment oxygenation predicts radiation response in advanced
squamous cell carcinoma of the head and neck. Radiother Oncol.
41:31–39. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaupel P and Mayer A: Hypoxia in cancer:
Significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fyles A, Milosevic M, Hedley D, Pintilie
M, Levin W, Manchul L and Hill RP: Tumor hypoxia has independent
predictor impact only in patients with node-negative cervix cancer.
J Clin Oncol. 20:680–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee DJ, Moini M, Giuliano J and Westra WH:
Hypoxic sensitizer and cytotoxin for head and neck cancer. Ann Acad
Med Singapore. 25:397–404. 1996.PubMed/NCBI
|
|
7
|
Shibamoto Y, Takahashi M and Abe M: A
phase I study of a hypoxic cell sensitizer KU-2285 in combination
with conventional radiotherapy. Radiother Oncol. 40:55–58. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hassan Metwally MA, Ali R, Kuddu M,
Shouman T, Strojan P, Iqbal K, Prasad R, Grau C and Overgaard J:
IAEA-HypoX. A randomized multicenter study of the hypoxic
radiosensitizer nimorazole concomitant with accelerated
radiotherapy in head and neck squamous cell carcinoma. Radiother
Oncol. 116:15–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drzymala RE, Wasserman TH, Won M, Shaw E,
Cmelak AJ, Loeffler J and Souhami L; Radiation Therapy Oncology
Group, : A phase I-B trial of the radiosensitizer: Etanidazole
(SR-2508) with radiosurgery for the treatment of recurrent
previously irradiated primary brain tumors or brain metastases
(RTOG Study 95–02). Radiother Oncol. 87:89–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Overgaard J, Hansen HS, Overgaard M,
Bastholt L, Berthelsen A, Specht L, Lindeløv B and Jørgensen K: A
randomized double-blind phase III study of nimorazole as a hypoxic
radiosensitizer of primary radiotherapy in supraglottic larynx and
pharynx carcinoma. results of the danish head and neck cancer study
(DAHANCA) Protocol 5–85. Radiother Oncol. 46:135–146. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He ZY, Li FY, Tong Q, Liao ZW, Guan XX and
Wang Y: Concurrent chemoradiotherapy with sodium glycididazole and
cisplatin for local advanced nasopharyngeal carcinoma. Nan Fang Yi
Ke Da Xue Xue Bao. 28:2038–2040. 2008.(In Chinese). PubMed/NCBI
|
|
12
|
Zeng YC, Wu R, Xu ZG, Zhang XY, Fan GL, Wu
LN, Wang YM, Hao SH, Zheng W, Chen XD, et al: Safety and
radiation-enhancing effect of sodium glycididazole in
locoregionally advanced laryngeal cancers previously treated with
platinum-containing chemotherapy regimens: A preliminary report.
Cancer Radiother. 14:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li MY, Liu JQ, Chen DP, Qi B, Liang YY and
Yin WJ: Glycididazole sodium combined with radiochemotherapy for
locally advanced nasopharyngeal carcinoma. Asian Pac J Cancer Prev.
15:2641–2646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nordsmark M, Bentzen SM, Rudat V, Brizel
D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J,
Terris DJ and Overgaard J: Prognostic value of tumor oxygenation in
397 head and neck tumors after primary radiation therapy. An
international multi-center study. Radiother Oncol. 77:18–24. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koukourakis MI, Bentzen SM, Giatromanolaki
A, Wilson GD, Daley FM, Saunders MI, Dische S, Sivridis E and
Harris AL: Endogenous markers of two separate hypoxia response
pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase
9) are associated with radiotherapy failure in head and neck cancer
patients recruited in the CHART randomized trial. J Clin Oncol.
24:727–735. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Overgaard J: Hypoxic radiosensitization:
Adored and ignored. J Clin Oncol. 25:1–4074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Overgaard J, Eriksen JG, Nordsmark M,
Alsner J and Horsman MR; Danish Head and Neck Cancer Study Group, :
Plasma osteopontin, hypoxia and response to the hypoxia sensitiser
nimorazole in radiotherapy of head and neck cancer: Results from
the DAHANCA 5 randomised double-blind placebo-controlled trial.
Lancet Oncol. 6:757–764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim AM, Rischin D, Fisher R, Cao H, Kwok
K, Truong D, McArthur GA, Young RJ, Giaccia A, Peters L and Le QT:
Prognostic significance of plasma osteopontin in patients with
locoregionally advanced head and neck squamous cell carcinoma
treated on TROG 02.02 phase III trial. Clin Cancer Res. 18:301–307.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Liu MZ, Cai L, Hu YH, Liu H, Li QQ
and Cui NJ: Phase II clinical trial of sodium glyci-didazole
(CM-Na) combined with concurrent radiochemotherapy for advanced
esophageal carcinoma. Ai Zheng. 27:622–666. 2008.(In Chinese).
PubMed/NCBI
|
|
20
|
Zhang Q, Wang DQ and Wu YF: Sodium
glycididazole enhances the efficacy of combined iodine-125 seed
implantation and chemotherapy in patients with non small-cell lung
cancer. Oncol Lett. 9:2335–2340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu MZ, He LR, Lu TX, Chen YY, Hu YH, Cui
NJ, Xu GZ, Gao L, Xiao GL, Zhang SW, et al: Effect of hypoxic
radiosensitizer sodium glycididazole on long-term result of
radiotherapy for nasopharyngeal carcinoma. Zhonghua Zhong Liu Za
Zhi. 28:932–937. 2006.(In Chinese). PubMed/NCBI
|
|
22
|
Liu CX, Wei GL and Xiao SH:
Pharmacokinetics of sodium bimetrondazole glycinate in mice and
rats. Yao Xue Xue Bao. 35:770–773. 2000.(In Chinese). PubMed/NCBI
|
|
23
|
Le QT, Sutphin PD, Raychaudhuri S, Yu SC,
Terris DJ, Lin HS, Lum B, Pinto HA, Koong AC and Giaccia AJ:
Identification of osteopontin as a prognostic plasma marker for
head and neck squamous cell carcinomas. Clin Cancer Res. 9:59–67.
2003.PubMed/NCBI
|
|
24
|
Toustrup K, Sørensen BS, Lassen P, Wiuf C,
Alsner J and Overgaard J; Danish Head and Neck Cancer Group
(DAHANCA), : Gene expression classifier predicts for hypoxic
modification of radiotherapy with nimorazole in squamous cell
carcinomas of the head and neck. Radiother Oncol. 102:122–129.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tran LB, Bol A, Labar D, Cao-Pham TT,
Jordan B, Grégoire V and Gallez B: Predictive value of (18)F-FAZA
PET imaging for guiding the association of radiotherapy with
nimorazole: A preclinical study. Radiother Oncol. 114:189–194.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng XL, Gao JG and Zhang H: In vitro
radiosensitization effect of sodium glycididazol on V79 cells.
Journal of Radiation Research and Radiation Processing. 13:213–218.
1994.
|