Introduction
Colorectal cancer (CRC) is the third most common
type of cancer in men and women, and the second leading cause of
cancer-associated mortality in Western countries (1). At the time of diagnosis, synchronous
metastases can be found in almost 20–25% of patients with CRC, and
the majority of patients with stage III disease have a poor
prognosis within 5 years of diagnosis. The mainstream drugs used
for advanced CRC include 5-fluorouracil, capecitabine, oxaliplatin,
irinotecan, vascular endothelial growth factor (VEGF) antibody and
epidermal growth factor receptor (EGFR) antibody, which may be used
as a single agent or in combination in the first or secondary line
of therapy (2,3). However, these therapies are limited in
application due to their toxic and adverse effects. Further
understanding of the pathogenesis of CRC may provide support for
investigating novel drugs and individualized treatments for CRC
(4).
Human epidermal growth factor receptor 4
(HER4/ErbB4) belongs to the EGFR family, a group of transmembrane
receptor tyrosine kinases (RTKs). At least four HER4 variants
(JM-a/CYT1, JM-a/CYT2, JM-b/CYT1 and JM-b/CYT2) can be generated by
different HER4 mRNA splicing (5,6).
Therefore, seven different human EGF RTKs have been found to be
expressed in various normal and malignant cells: HER1 (EGFR/ErbB1),
HER2 (ErbB2/Neu), HER3 (ErbB3), and four HER4 isoforms (JM-a/CYT-1,
JM-a/CYT-2, JM-b/CYT-1 and JM-b/CYT-2) (7). Agents targeting EGFR and/or HER2 have
been approved for clinical use. In addition, the overexpression or
mutation of HER3 is associated with malignant cell growth,
contributing to enhanced tumor progression and poor patient
outcomes (8). There are potentially
oncogenic ERBB4 mutations in non-small cell lung cancer (9), and it has been reported that HER4 is
overexpressed in human colon cancer and enhances cellular
transformation (10). In addition,
HER4 promotes breast cancer cell proliferation, mediates acquired
resistance to ERBB2 inhibitors and may serve as a prognostic marker
in patients with breast cancer (11–14).
However, the role of HER4 in CRC remains to be fully elucidated.
The alternative splicing of HER4 yields four major isoforms, which
differ in the extracelluar juxtamembrane domain (JM-a, vs. JM-b)
and cytoplasmic domain (CYT-1, vs. CYT-2). Failure to account for
isoform-specific roles in previous studies may have led to
controversial reports on the role of HER4 in cancer. Therefore, it
is important to definitively determine the expression of HER4
isoforms in CRC.
Neuregulins (NRGs) are HER4 ligands, and comprise a
large family of EGF-like signaling molecules involved in cell-cell
communication during development and disease. NRG1 is a
high-affinity ligand of HER4, which is classified into at least
three subgroups (types I–III) with 30 isoforms as a result of
splicing variants (15). NRG1 type I
and type II are processed at the membrane by metalloproteinases
ADAM17 and ADAM19, whereas the NRG1 type III contains a
cysteine-rich domain, which binds to and activates HER3 and HER4
(16).
The aim of the present study was to evaluate the
expression of HER4 isoforms and the isoforms of the ligand NRG1 in
human CRC tissues, and to analyze the correlation between their
expression and the clinicopathological parameters of patients with
CRC.
Materials and methods
Patient selection and biopsy
collection
A total of 76 fresh-frozen samples (38 cancer
tissues and 38 paired adjacent normal tissues) were obtained from
patients with CRC who were treated at the Second Department of
Surgery, The Fourth Hospital of Hebei Medical University (Hebei,
China) between November 2013 and August 2014. The surgery was
performed on patients by the same surgeon, and the samples were
collected during primary surgery prior to chemotherapy or
radiation. The tissues were diagnosed as CRC preoperatively by
endoscopic biopsy, and the normal tissues were 5 cm from the tumor
edge. All patients had a pathological diagnosis and complete
clinical data. The detailed clinical data, including gender, age,
tumor size, tumor location, histological type, tumor
differentiation, serum carcinoembryonic antigen (CEA) level, gene
mutation, lymph node metastasis status, and tumor-node-metastasis
(TNM) stage were collected from patient's medical records. Clinical
staging was performed in accordance with the TNM staging system,
formulated jointly by the American Joint Committee on Cancer and
the Union for International Cancer Control (1). All experiments were approved by the
Ethics Committee of The Fourth Hospital of Hebei Medical
University. Written informed consent was obtained from each
patient. The endpoints were the assessments of the association
between the expression of HER4 and NRG1 with the
clinicopathological parameters of patients with CRC.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from the cryopreserved
tissues using TRIzol (Takara Bio, Inc., Otsu, Japan). Total RNA (5
µg) was used for the synthesis of cDNA using a reverse
transcription kit. The isoform-specific primers for HER4 and NRG1
are listed in Table I. RT-qPCR
analysis was performed in triplicate with 1 µg cDNA and 2.5 µm
primers in 25 µl buffer using SYBR Premix Ex Taq (Takara Bio, Inc.)
on a Light Cycler 480 as follows: 94°C for 4 min; 94°C for 30 sec,
56°C for 30 sec (40 cycles), and 72°C for 30 sec. The mRNA
expression level was normalized to β-actin and calculated using the
2−ΔΔCq method (17).
 | Table I.Primers used in the present study. |
Table I.
Primers used in the present study.
| Gene | Direction | Primer sequence |
|---|
| CYT1 | Forward |
5′-GGATGAAGAGGATTTGGAAG-3′ |
|
| Reverse |
5′-TCCTGACATGGGGGTGTA-3′ |
| CYT2 | Forward |
5′-GAATAGGAACCAGTTTGTATACCG-3′ |
|
| Reverse |
5′-ACAGCAGGAGTCATCAAAAATC-3′ |
| JMa | Forward |
5′TAACGGTCCCACTAGTCA-3′ |
|
| Reverse |
5′-CATGTTGTGGTAAAGTGG-3′ |
| JMb | Forward |
5′-ATAGGCTCAAGTATTGAAG-3′ |
|
| Reverse |
5′-CCATCAGGCCGATGC-3′ |
| NRG1 I | Forward |
5′-AGGGCAAGAAGAAGGAGCG-3′ |
|
| Reverse |
5′-CCTTCAGTTGAGGCTGGCATA-3′ |
| NRG1 II | Forward |
5′-CGCCTTCCGAGCCTCTTTC-3′ |
|
| Reverse |
5′-CCTTCTCCGCACATTTTACAAGA-3′ |
| NRG1 III | Forward |
5′-CCGGCCTCAAGTGGGTATT-3′ |
|
| Reverse |
5′-CCCAGTGGTGGATGTAGATGTAGA-3′ |
| β-actin | Forward |
5′-CGTGACATTAAGGAGAAGCTG-3′ |
|
| Reverse |
5′-CTAGAAGCATTTGCGGTGGAC-3′ |
Statistical analysis
The gene expression levels between the cancer and
adjacent tissues were compared using the Wilcoxon rank sum test.
Two groups of independent samples were compared using the
Mann-Whitney test. Spearman's correlation method was used to
analyze the correlation between HER4 isoforms and the
clinicopathological data. To identify variables, which were
independent predictors of CRC, univariate analysis and logistic
regression analysis with backward stepwise selection were employed.
The data were processed using SPSS 22.0 software (IBM SPSS, Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of HER4 and NRG1 isoforms
in CRC tissues and adjacent normal tissues
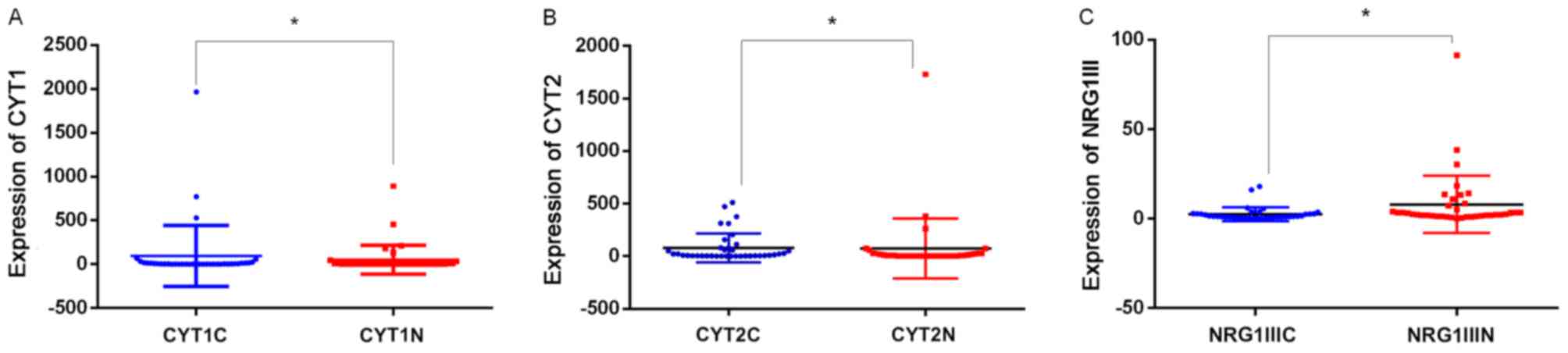
The mRNA levels of CYT1 (P=0.002), CYT2 (P=0.002,
and NRG1 type III (P<0.001) were significantly higher in the CRC
tissues, compared with those in adjacent normal tissues (P<0.05;
Fig. 1A-C). No significant
differences in the mRNA levels of JM-a, JM-b, NRG1 type I or NRG1
type II were found between the cancer tissues and the adjacent
normal tissues.
Association between HER4 and NRG1
expression and clinicopathological parameters in CRC
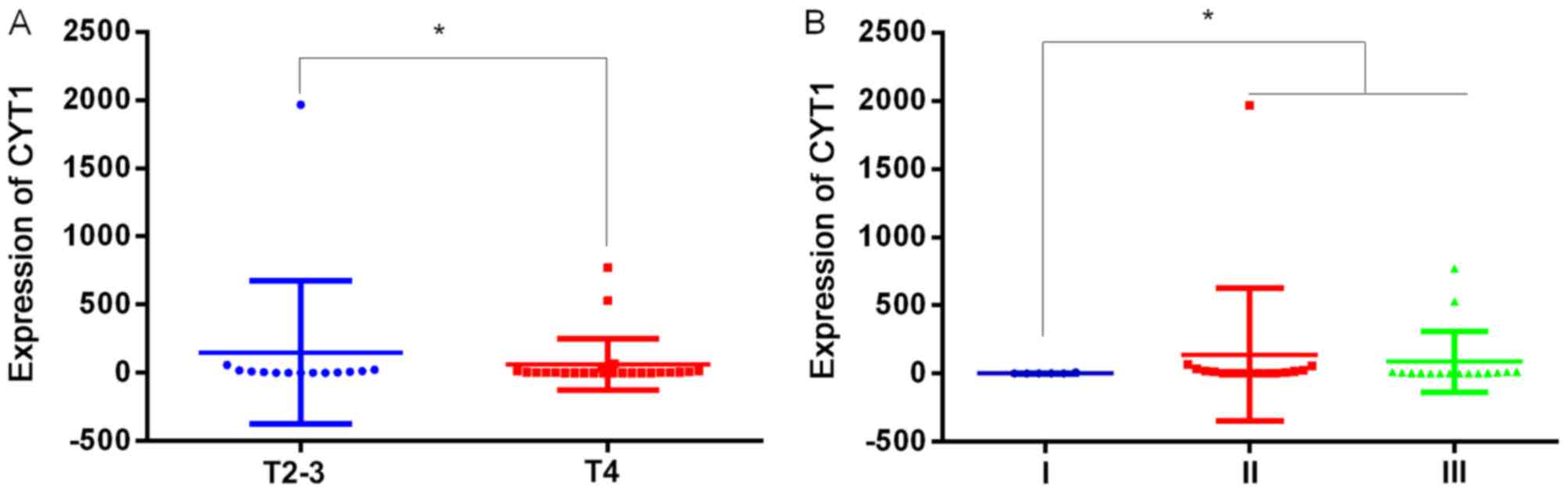
Of the 38 patients with CRC, the expression of CYT1
was significantly associated with the depth of invasion (P=0.027)
and TNM stage (P=0.033) in CRC (Fig. 2A
and B). The median expression of CYT1 in T2-3 CRC was lower,
compared with that of T4 (0.62, vs. 5.24, P=0.027). The median
expression of CYT1 in stage II CRC was increased significantly
compared with that of stage I (0.42, vs. 10.25). However, there was
no significant difference in the expression of CYT1 between stage
II and stage III CRC.
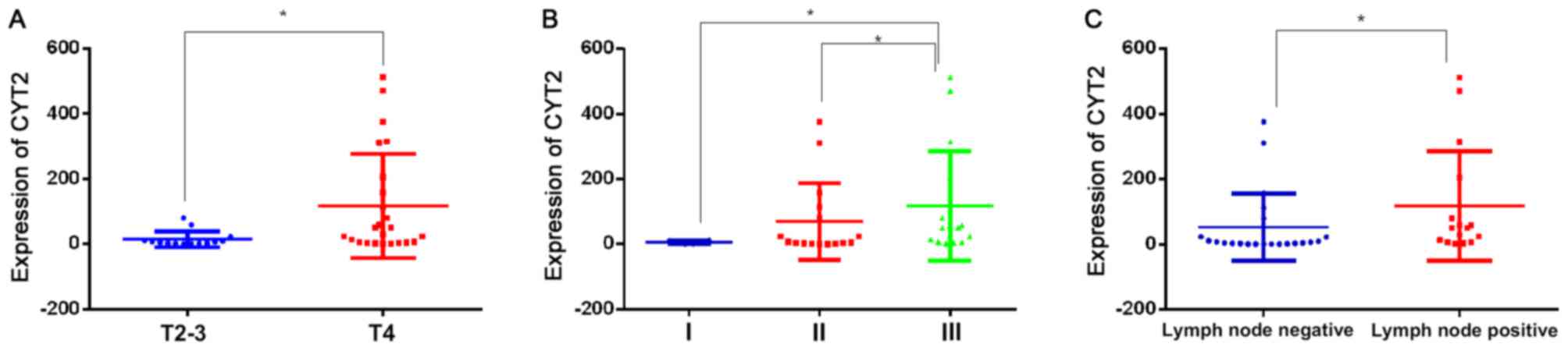
The expression of CYT2 was associated with T
(P=0.018), N (P=0.015), and TNM stage (P=0.038) in CRC (Fig. 3A-C). The median expression of CYT2 was
increased significantly between T2-3 and T4 (5.36, vs. 39.48,
respectively), and the expression was significantly increased in
lymph node-positive cases, compared with that in lymph
node-negative cases (5.36, vs. 50.59, P=0.015). The expression of
CYT2 did not differ significantly between stages I and II, however,
it was significantly higher in stage III (median=50.59), compared
with that in stage I (median=5.9) and stage II (median =3.34;
P<0.05).
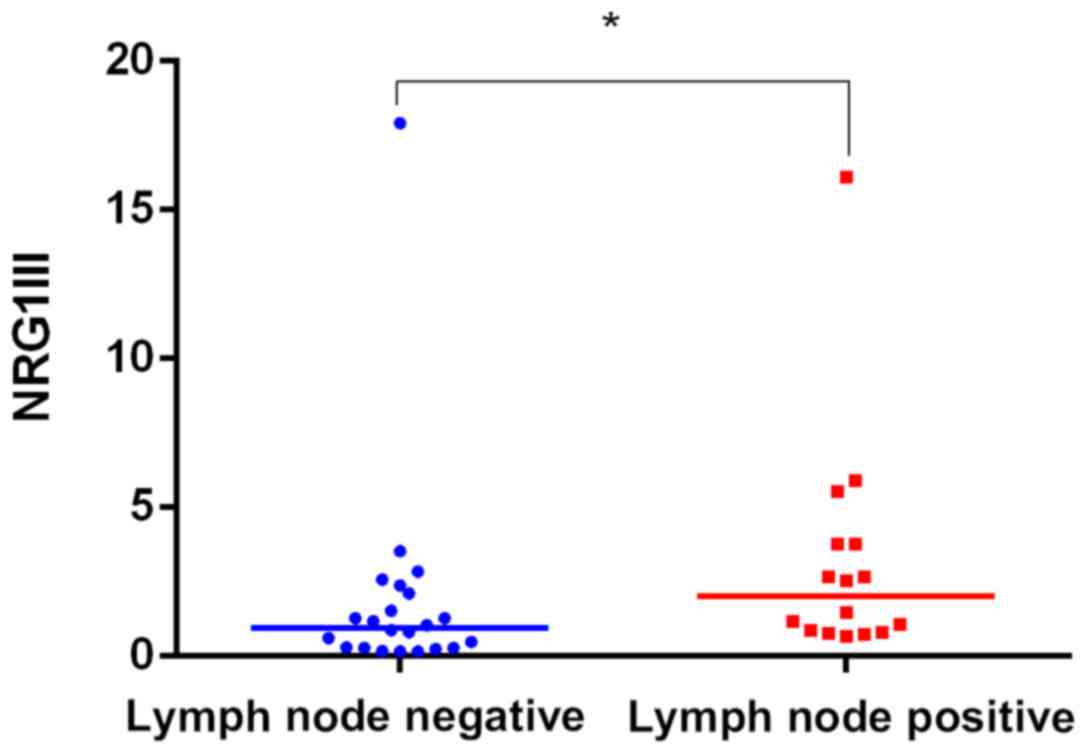
The expression of NRG1 III was correlated with lymph
node metastasis. The median expression was higher in the lymph
node-positive cases than in the lymph node-negative cases (0.96 vs.
2.00; P=0.015; Fig. 4). There was no
correlation between the expression of CYT-1, CYT-2 or NRG1 III with
age, gender, tumor size, tumor grade and CEA levels (P>0.05,
Table II).
 | Table II.Association between human epidermal
growth factor receptor 4 and NRG1 expression with
clinicopathological parameters of colorectal cancer. |
Table II.
Association between human epidermal
growth factor receptor 4 and NRG1 expression with
clinicopathological parameters of colorectal cancer.
|
|
| CYT1 | CYT2 | NRG1 III |
|---|
|
|
|
|
|
|
|---|
| Variable | n | Median | IQP | P-value | Median | IQP | P-value | Median | IQP | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
|
|
| ≤62 | 19 | 6.25 | 16.63 | 0.15 | 13.95 | 76.93 | 0.71 | 1.18 | 0.82 | 0.77 |
|
>62 | 19 | 3.18 | 12.73 |
| 10.33 | 56.71 |
| 1.17 | 0.25 |
|
| Gender |
|
|
|
|
|
|
|
|
|
|
|
Male | 23 | 3.60 | 13.25 | 0.10 | 8.83 | 47.79 | 0.39 | 1.17 | 0.76 | 0.56 |
|
Female | 15 | 6.56 | 31.34 |
| 50.59 | 154.29 |
| 1.18 | 0.39 |
|
| Tumor size |
|
|
|
|
|
|
|
|
|
|
| <4
cm | 17 | 4.92 | 14.12 | 0.69 | 8.83 | 56.21 | 0.73 | 1.27 | 0.89 | 0.50 |
| >4
cm | 21 | 3.60 | 15.10 |
| 13.95 | 132.45 |
| 1.78 | 0.17 |
|
|
Differentiation |
|
|
|
|
|
|
|
|
|
|
|
High | 24 | 4.16 | 10.04 | 0.32 | 18.11 | 7.040 | 0.80 | 1.11 | 0.29 | 0.73 |
|
Poor | 14 | 6.41 | 6.012 |
| 5.26 | 192.93 |
| 1.78 | 0.9 |
|
| TNM stage |
|
|
|
|
|
|
|
|
|
|
| I | 6 | 0.42 | 2.19 | 0.03a | 5.90 | 7.71 | 0.038a | 0.84 | 0.67 | 0.08 |
| II | 16 | 10.25 | 29.44 |
| 5.34 | 102.96 |
| 1.22 | 0.28 |
|
|
III | 16 | 4.06 | 8.90 |
| 50.59 | 166.36 |
| 2.00 | 0.94 |
|
| T stage |
|
|
|
|
|
|
|
|
|
|
|
T2/T3 | 14 | 0.62 | 8.05 | 0.03a | 5.36 | 11.86 | 0.018a | 0.96 | 0.06 | 0.20 |
| T4 | 24 | 5.24 | 20.76 |
| 39.48 | 189.12 |
| 1.49 | 0.61 |
|
| Lymph node |
|
|
|
|
|
|
|
|
|
|
|
Positive | 22 | 4.83 | 19.90 | 0.87 | 5.36 | 35.16 | 0.015a | 0.96 | 0.90 | 0.03a |
|
Negative | 16 | 4.06 | 8.90 |
| 50.59 | 66.36 |
| 2.00 | 0.94 |
|
| CEA |
|
|
|
|
|
|
|
|
|
|
|
Normal | 31 | 4.93 | 13.49 | 0.71 | 7.33 | 77.21 | 0.42 | 1.17 | 0.97 | 0.46 |
|
High | 7 | 4.73 | 14.72 |
| 22.70 | 69.93 |
| 1.52 | 0.11 |
|
Correlation analysis between the
expression of CYT1 and CYT2 HER4 isoforms and NRF1 III
As shown in Table
III, the expression of CYT1 and CYT-2 were associated (r=0.481,
P<0.05) and the expression of CYT2 and NRG1 were also associated
(r=0.691, P<0.01).
 | Table III.Correlation between the expression of
human epidermal growth factor receptor 4 isoforms CYT1, CYT2 and
NRG1 III. |
Table III.
Correlation between the expression of
human epidermal growth factor receptor 4 isoforms CYT1, CYT2 and
NRG1 III.
|
| CYT1 | CYT2 | NRG1III |
|---|
|
|
|
|
|
|---|
| Isoform | r | P-value | r | P-value | r | P-value |
|---|
| CYT1 | – | – | 0.481 | <0.05 | 0.373 | >0.05 |
| CYT2 | 0.481 | <0.05 | – | – | 0.691 | <0.01 |
| NRG1III | 0.373 | >0.05 | 0.691 | <0.01 | – | – |
Analysis of variables associated with
lymph node metastasis of CRC
As shown in Table IV,
the univariate analysis showed that the expression of CYT2 was
significantly associated with lymph node metastasis of CRC
(P=0.047), whereas no significant associations were found between
lymph node metastasis and age (P=0.372), gender (P=0.213), tumor
differentiation (P=0.396), CEA level (P=0.641), KRAS mutation
(P=0.333), tumor size (P=0.521), or the expression of CYT1
(P=0.654), NRG1I (P=0.671), NRG1II (P=0.490), NRG1III (P=0.192),
JM-a (P=0.317) or JM-b (P=0.192).
 | Table IV.Univariate analysis and multivariate
regression of variables associated with lymph node metastasis. |
Table IV.
Univariate analysis and multivariate
regression of variables associated with lymph node metastasis.
|
| Lymph node
metastasis |
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
|
| Yes | No | Univariate
analysis | Multivariate
regression |
|---|
|
|
|
|
|
|
|---|
| Variable | n | % | n | % | χ2 | P-value | OR | 95%CI | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
|
|
|
≤62 | 9 | 56.2 | 10 | 45.5 | 0.432 | 0.372 | 0.124 | 0.009 | 1.732 | 0.121 |
|
>62 | 7 | 43.8 | 12 | 54.5 |
|
|
|
|
|
|
| Gender |
|
|
|
|
|
|
|
|
|
|
|
Male | 8 | 50 | 15 | 68.2 | 1.282 | 0.213 | 4.212 | 0.495 | 35.851 | 0.188 |
|
Female | 8 | 50 | 7 | 31.8 |
|
|
|
|
|
|
|
Differentiation |
|
|
|
|
|
|
|
|
|
|
|
Poor | 11 | 68.8 | 13 | 59.1 | 0.371 | 0.369 | 3.826 | 0.417 | 35.109 | 0.235 |
|
High | 5 | 31.2 | 9 | 40.9 |
|
|
|
|
|
|
| CEA |
|
|
|
|
|
|
|
|
|
|
|
Normal | 13 | 81.2 | 18 | 81.8 | 0.002 | 0.641 | 2.428 | 0.066 | 89.515 | 0.630 |
|
High | 3 | 18.8 | 4 | 18.2 |
|
|
|
|
|
|
| KRAS |
|
|
|
|
|
|
|
|
|
|
|
Wild | 6 | 37.5 | 13 | 59.1 | 2.197 | 0.333 | 0.388 | 0.082 | 1.825 | 0.231 |
|
Mutation | 4 | 25 | 5 | 22.7 |
|
|
|
|
|
|
|
Indefinite | 6 | 37.5 | 4 | 18.2 |
|
|
|
|
|
|
| Tumor size |
|
|
|
|
|
|
|
|
|
|
| <4
cm | 6 | 37.5 | 11 | 50 | 0.585 | 0.521 | 9.183 | 0.302 | 279.614 | 0.203 |
| >4
cm | 10 | 62.5 | 11 | 50 |
|
|
|
|
|
|
| CYT1 |
|
|
|
|
|
|
|
|
|
|
|
≤50 | 14 | 87.5 | 19 | 86.4 | 0.10 | 0.654 | 0.001 | 0.001 | 1.821 | 0.071 |
|
>50 | 2 | 12.5 | 3 | 13.6 |
|
|
|
|
|
|
| CYT2 |
|
|
|
|
|
|
|
|
|
|
|
≤50 | 7 | 43.8 | 17 | 77.3 | 4.474 | 0.047a | 23.255 | 1.187 | 455.481 | 0.038a |
|
>50 | 9 | 56.3 | 5 | 22.7 |
|
|
|
|
|
|
| NRG1I |
|
|
|
|
|
|
|
|
|
|
| ≤5 | 15 | 93.8 | 21 | 95.5 | 0.054 | 0.671 | 0.470 | 0.002 | 105.457 | 0.785 |
|
>5 | 1 | 6.2 | 1 | 4.5 |
|
|
|
|
|
|
| NRG1II |
|
|
|
|
|
|
|
|
|
|
| ≤5 | 10 | 62.5 | 15 | 68.2 | 0.133 | 0.490 | 7.478 | 0.087 | 644.66 | 0.376 |
|
>5 | 6 | 37.5 | 7 | 31.8 |
|
|
|
|
|
|
| NRG1III |
|
|
|
|
|
|
|
|
|
|
| ≤5 | 13 | 81.3 | 21 | 95.5 | 1.984 | 0.192 | 5,292 | 0.236 | 1,186,341 | 0.093 |
|
>5 | 3 | 18.8 | 1 | 4.5 |
|
|
|
|
|
|
| JMa |
|
|
|
|
|
|
|
|
|
|
|
≤10 | 12 | 75 | 19 | 86.4 | 0.796 | 0.317 | 0.274 | 0.001 | 52.022 | 0.628 |
|
>10 | 4 | 25 | 3 | 13.6 |
|
|
|
|
|
|
| JMb |
|
|
|
|
|
|
|
|
|
|
|
≤10 | 13 | 81.2 | 21 | 95.5 | 1.984 | 0.192 | 1.455 | 0.017 | 123.84 | 0.869 |
|
>10 | 3 | 18.8 | 1 | 4.5 |
|
|
|
|
|
|
Logistic regression revealed that the expression of
CYT2 was significantly associated with lymph node metastasis of
CRC. In terms of the odds ratios (ORs), the variable of the
expression of CYT2 had the most marked effect on lymph node
metastasis; the OR of lymph node metastasis in cancer with CYT2
expression >50 was 23.255 times higher than that with CYT2
expression ≤50 (P=0.038; Table
IV).
Discussion
In the present study, it was demonstrated that HER4
isoforms CYT1 and CYT2, and their ligand NRG1 type III were
upregulated in human CRC tissues. However, there was no significant
difference in the expression of the other two HER4 isoforms (JM-a
and JM-b) or the NRG1 type I and type II isoforms between CRC and
normal tissues. The expression levels of CYT2 and CYT1 were closely
associated with the TNM stage and tumor invasion depth of CRC, and
the expression of CYT2 was associated with lymph node metastasis in
CRC. However, only NRG1 type III was associated with lymph node
metastasis in CRC.
In contrast to other members of the HER family, a
single HER4 gene has four isoforms: JM-a, JM-b, CYT1 and CYT2,
which are produced by alternative splicing. CYT1 and CYT2 differ by
16 amino acids present in the cytoplasmic tail of CYT1, which are
not present in CYT2. This difference in the structure of CYT1 and
CYT2 leads to their different cell location, resulting in different
and even opposite roles in cell regulation. In the present study,
it was found that the expression of CYT1 in CRC tissues was
positively correlated with the depth of tumor invasion and TNM
stage. Previous studies have indicated that CYT-1 is an independent
prognostic factor of ovarian cancer, and that CYT-1 may promote the
progression of ovarian cancer and malignant melanoma (18,19). In
malignant melanoma, the expression of CYT1 suggested a short
progression-free survival rate (19).
Another study revealed that ERBB4 CYT1 has a novel oncogenic role
in breast cancer (20). The mechanism
by which CYT1 promotes tumor progression may be through activating
the phosphatidylinositol-3 kinase/Akt signaling pathway to induce
tumor cells to evade apoptosis.
Compared with CYT1, the role of CYT2 in cancer,
particularly in the colon, remains to be fully elucidated. In
bladder cancer, the expression of JM-a/CYT2 and estrogen receptor
may be indicative of improved prognosis of bladder cancer (21). A previous study found that the CYT2
variant, but not the CYT1 variant, protected EGFR from
ligand-induced degradation by competing with EGFR for binding to a
complex containing the E3 ubiquitin ligase c-Cbl and the adaptor
Grb2 (22). In addition, another
study showed that the ErbB4 CYT2 isoform promoted the transition
from colon adenoma to carcinoma following adenomatous polyposis
coli loss (23). However, another
study demonstrated that the CYT2 isoform had an inhibitory effect
on cancer cell growth (24). These
inconsistent results may be due to the different cell types and the
different expression levels of HER family members. The specific
mechanism by which CYT2 promotes the occurrence and development of
CRC requires further investigation.
NRG1 is important in the tumor microenvironment.
Bone marrow stromal cells, cancer-associated fibroblasts and cancer
cells can secrete NRG1 (25). NRG1
can be secreted by endothelial cells through autocrine or paracrine
mechanisms of angiogenesis in ischemic tissues, in order to meet
the needs of the rapid growth of tumor (26). NRG1 can be divided into at least three
subsets, namely NRG1I, NRG1II and NRG1III. The expression of these
isoforms shows tissue specificity and have different biological
roles. Among the three subtypes of NRG1, the present study found
that only the expression of NRG1III was increased in CRC. In
addition, it was found that the expression of CYT2 was positively
correlated with the expression of NRG1III, and the two were
associated with lymph node metastasis in CRC. Therefore, the NRG1
III/CYT2 pathway may be important in the invasion and lymph node
metastasis of CRC. However, in the present study, only the mRNA
expression levels of the NRG1 and CYT2 isoforms were detected by
RT-qPCR analysis, and additional experiments are required to detect
protein expression levels of NRG1 and CYT2 isoforms via western
blot or immunohistochemical analyses to confirm the conclusions.
This is a major limitation of the present study.
In conclusion, the study is the first, to the best
of our knowledge, to demonstrate upregulation in the expression
levels of CYT1, CYT2 and NRG1 III in CRC. It was also found that
CYT-2 expression >50 is a risk factor for lymph node metastasis
in CRC. Therefore, CY-2 and NRG1III may be involved in the
progression of CRC.
Acknowledgements
This study was supported by the Natural Science
Foundation of Hebei Province of China (grant no. H2016307010).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Panoilia E, Schindler E, Samantas E,
Aravantinos G, Kalofonos HP, Christodoulou C, Patrinos GP, Friberg
LE and Sivolapenko G: A pharmacokinetic binding model for
bevacizumab and VEGF165 in colorectal cancer patients. Cancer
Chemother Pharmacol. 75:791–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Santoro V, Jia R, Thompson H, Nijhuis A,
Jeffery R, Kiakos K, Silver AR, Hartley JA and Hochhauser D: Role
of reactive oxygen species in the abrogation of oxaliplatin
activity by cetuximab in colorectal cancer. J Natl Cancer Inst.
108:djv3942015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dawson H and Lugli A: Molecular and
pathogenetic aspects of tumor budding in colorectal cancer. Front
Med (Lausanne). 2:112015.PubMed/NCBI
|
|
5
|
Roskoski R Jr: The ErbB/HER family of
protein-tyrosine kinases and cancer. Pharmacol Res. 79:34–74. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Veikkolainen V, Vaparanta K, Halkilahti K,
Iljin K, Sundvall M and Elenius K: Function of ERBB4 is determined
by alternative splicing. Cell Cycle. 10:2647–2657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muraoka-Cook RS, Sandahl MA, Strunk KE,
Miraglia LC, Husted C, Hunter DM, Elenius K, Chodosh LA and Earp HS
III: ErbB4 splice variants Cyt1 and Cyt2 differ by 16 amino acids
and exert opposing effects on the mammary epithelium in vivo. Mol
Cell Biol. 29:4935–4948. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lédel F, Stenstedt K, Hallström M,
Ragnhammar P and Edler D: HER3 expression in primary colorectal
cancer including corresponding metastases in lymph node and liver.
Acta Oncol. 54:480–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurppa KJ, Denessiouk K, Johnson MS and
Elenius K: Activating ERBB4 mutations in non-small cell lung
cancer. Oncogene. 35:1283–1291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williams CS, Bernard JK, Demory Beckler M,
Almohazey D, Washington MK, Smith JJ and Frey MR: ERBB4 is
over-expressed in human colon cancer and enhances cellular
transformation. Carcinogenesis. 36:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohd Nafi SN, Generali D, Kramer-Marek G,
Gijsen M, Strina C, Cappelletti M, Andreis D, Haider S, Li JL,
Bridges E, et al: Nuclear HER4 mediates acquired resistance to
trastuzumab and is associated with poor outcome in HER2 positive
breast cancer. Oncotarget. 5:5934–5949. 2014.PubMed/NCBI
|
|
12
|
Machleidt A, Buchholz S, Diermeier-Daucher
S, Zeman F, Ortmann O and Brockhoff G: The prognostic value of Her4
receptor isoform expression in triple-negative and Her2 positive
breast cancer patients. BMC Cancer. 13:4372013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JY, Jung HH, Do IG, Bae S, Lee SK, Kim
SW, Lee JE, Nam SJ, Ahn JS, Park YH and Im YH: Prognostic value of
ERBB4 expression in patients with triple negative breast cancer.
BMC Cancer. 16:1382016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Canfield K, Li J, Wilkins OM, Morrison MM,
Ung M, Wells W, Williams CR, Liby KT, Vullhorst D, Buonanno A, et
al: Receptor tyrosine kinase ERBB4 mediates acquired resistance to
ERBB2 inhibitors in breast cancer cells. Cell Cycle. 14:648–655.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao WJ: The expression and localization
of neuregulin-1 (Nrg1) in the gastrointestinal system of the rhesus
monkey. Folia Histochem Cytobiol. 51:38–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Papaleo F, Yang F, Paterson C, Palumbo S,
Carr GV, Wang Y, Floyd K, Huang W, Thomas CJ, Chen J, et al:
Behavioral, neurophysiological, and synaptic impairment in a
transgenic neuregulin1 (NRG1-IV) murine schizophrenia model. J
Neurosci. 36:4859–4875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paatero I, Lassus H, Junttila TT, Kaskinen
M, Bützow R and Elenius K: CYT-1 isoform of ErbB4 is an independent
prognostic factor in serous ovarian cancer and selectively promotes
ovarian cancer cell growth in vitro. Gynecol Oncol. 129:179–187.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nielsen TO, Poulsen SS, Journe F, Ghanem G
and Sorensen BS: HER4 and its cytoplasmic isoforms are associated
with progression-free survival of malignant melanoma. Melanoma Res.
24:88–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wali VB, Gilmore-Hebert M, Mamillapalli R,
Haskins JW, Kurppa KJ, Elenius K, Booth CJ and Stern DF:
Overexpression of ERBB4 JM-a CYT-1 and CYT-2 isoforms in transgenic
mice reveals isoform-specific roles in mammary gland development
and carcinogenesis. Breast Cancer Res. 16:5012014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Munk M, Memon A, Poulsen SS, Borre M, Nexo
E and Sorensen BS: The HER4 isoform JM-a/CYT2 relates to improved
survival in bladder cancer patients but only if the estrogen
receptor α is not expressed. Scand J Clin Lab Invest. 73:503–513.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kiuchi T, Ortiz-Zapater E, Monypenny J,
Matthews DR, Nguyen LK, Barbeau J, Coban O, Lawler K, Burford B,
Rolfe DJ, et al: The ErbB4 CYT2 variant protects EGFR from
ligand-induced degradation to enhance cancer cell motility. Sci
Signal. 7:ra782014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bae JA, Kho DH, Sun EG, Ko YS, Yoon S, Lee
KH, Ahn KY, Lee JH, Joo YE, Chung IJ, et al: Elevated Coexpression
of KITENIN and the ErbB4 CYT-2 isoform promotes the transition from
colon adenoma to carcinoma following APC loss. Clin Cancer Res.
22:1284–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nielsen TO, Sorensen S, Dagnæs-Hansen F,
Kjems J and Sorensen BS: Directing HER4 mRNA expression towards the
CYT2 isoform by antisense oligonucleotide decreases growth of
breast cancer cells in vitro and in vivo. Br J Cancer.
108:2291–2298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han ME, Kim HJ, Shin DH, Hwang SH, Kang CD
and Oh SO: Overexpression of NRG1 promotes progression of gastric
cancer by regulating the self-renewal of cancer stem cells. J
Gastroenterol. 50:645–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park J, Sarode VR, Euhus D, Kittler R and
Scherer PE: Neuregulin 1-HER axis as a key mediator of
hyperglycemic memory effects in breast cancer. Proc Natl Acad Sci
USA. 109:pp. 21058–21063. 2012; View Article : Google Scholar : PubMed/NCBI
|