Introduction
Appendiceal diseases, including appendicitis, are a
common cause for emergency abdominal surgery, and appendiceal
neoplasms are identified during surgery in 0.7–5% of patients
(1,2).
A relatively recent development has been the incidental detection
of appendiceal lesions during the follow-up of patients for other
conditions, or during routine health screenings. These discoveries
have increased in frequency due to the increased use of abdominal
ultrasonography, computed tomography (CT), Positron emission
tomography (PET/CT) and serum tumor markers for patient follow-up
and screening (3,4). However, surgeons do not always submit
appendectomy specimens for pathological examination, allowing
tumors to go undiagnosed despite surgical treatment (5). Consequently, there has been an increase
in the number of studies describing recurrent neoplasms in the
appendiceal remnant (6). Accordingly,
surgeons are becoming increasingly aware of the importance of
obtaining a pathological confirmation of the diagnosis in patients
treated surgically for appendicitis-like symptoms (7).
In addition to lower birth rates, medical advances
and expanded health screenings have resulted in an aging Japanese
population, and novel therapies have increased the number of
immunocompromised patients (8). There
has also been an increase in international visitors with
Amoeba-associated infectious disease (9,10). These
factors may have contributed to the pattern of appendiceal disease
in Japan; however, during the past 25 years there has been no
single-center investigation of the incidence or types of
appendiceal neoplasms in patients undergoing a total appendectomy
(11). The most recent report was
based on nationwide data from multiple institutions, and thus did
not provide information regarding clinical patterns at a local
level, due to the indications for appendectomy and the resulting
disease statistics varying between institutions (5). To determine whether the pattern of
appendiceal disease is changing, a retrospective single-center
study that assessed the incidence and age distribution of
appendiceal diseases was conducted in the present study, in order
to understand the clinical implications for patients undergoing an
appendectomy.
Patients and methods
Ethical approval
The study was approved by the institutional review
board of Tokai University Hachioji Hospital (Hachioji, Japan;
approval no. R14-242). Informed consent for the appendectomy was
obtained from the patients and/or family members.
Patient selection
Radical appendectomies/ileocecal resections were
performed without attempting conservative therapies, including
antimicrobial agents, if an abdominal CT or ultrasound identified
an appendiceal lesion. By reviewing the pathological database of
the appendectomy specimens from patients who underwent an
appendectomy between March 2002 and September 2014 at Hachioji
hospital, a total of 803 patients were identified, among whom 752
had appendiceal disease. For clinicopathological analysis,
appendiceal neoplasms were primarily classified according to the
2010 World Health Organization (WHO) classification (12). A total of 55 patients were excluded,
including 11 with metastatic carcinoma or tumor invasion from
adjacent organs, and 44 patients with a normal appendix who
underwent an incidental or prophylactic appendectomy during surgery
for other diseases, such as hemicolectomy/ileocecal resection for
cecal cancer, diverticular disease or at the patient's request.
Also, single center cases of material from The Tokai University
Hachioji Hospital (Tokyo, Japan) were investigated.
Pathological examination
The resected appendiceal lesions were routinely
fixed with 10% neutral buffered formalin, embedded in paraffin, and
cut into 3-µm thick sections that were stained with hematoxylin and
eosin. For the present study, 2 expert pathologists and a clinician
reviewed the slides. Appendiceal neoplasms (1–22 slides per case)
were classified as benign (adenoma, dysplasia or mesenchymal tumor)
or malignant [G1-G3 neuroendocrine tumor (NET), adenocarcinoma,
mucinous adenocarcinoma, mixed adenoneuroendocrine carcinoma
(MANEC) arising from pre-existing goblet cell carcinoid (13,14) or
low-grade appendiceal mucinous neoplasm (LAMN)]. As mucinous
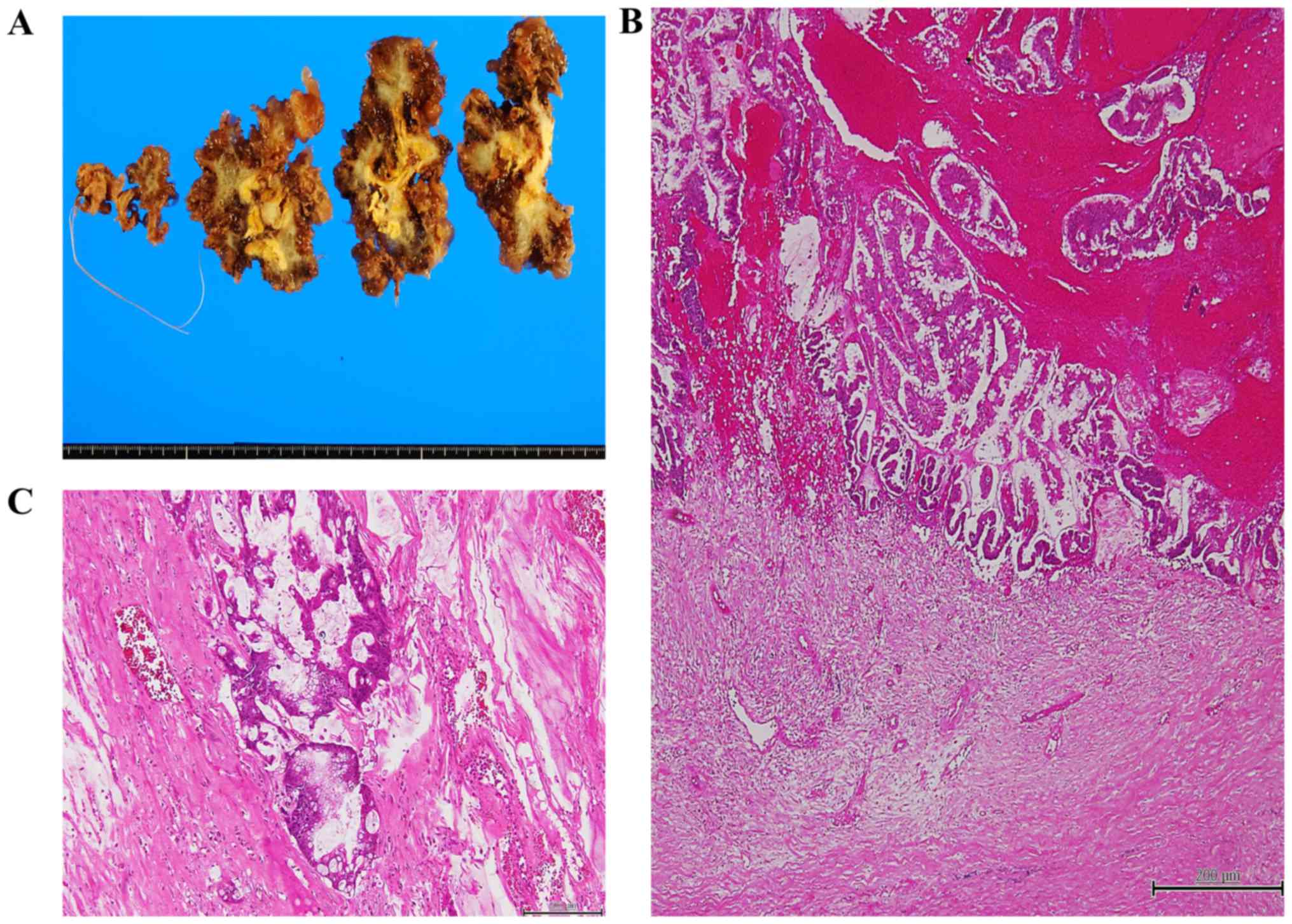
adenocarcinomas exhibit infiltration or invasion with fibrous
desmoplasia, tumors that featured complex epithelial proliferation
(complex papillary fronds and cribriform glandular spaces) or
high-grade cytological atypia (full-thickness nuclear
stratification, vesicular/prominent nuclei, and mitotic activity)
and infiltration with fibrous desmoplasia were classified as
mucinous adenocarcinomas (Fig.
1).
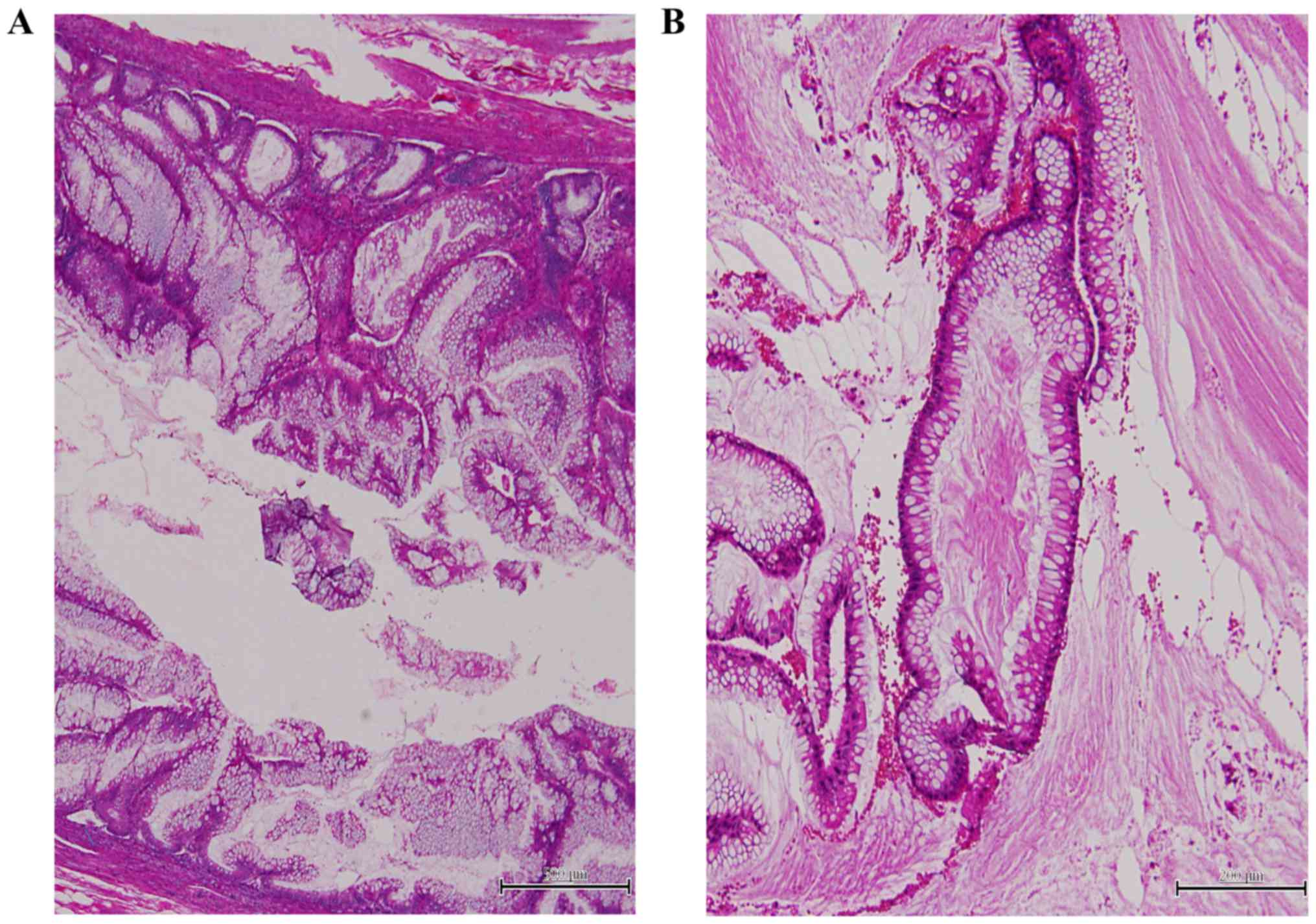
Conversely, tumors that exhibited minimal
architectural complexity (villiform, flat epithelial proliferation
and focal papillary excrescences) and low-grade cytologic atypia
(mild nuclear enlargement, nuclear stratification, rare mitotic
figures and single cell necrosis) were classified as LAMN (Fig. 2A). LAMN may be a precursor to
low-grade pseudomyxoma peritonei (12), although its biological behavior is
ambiguous; therefore, these tumors were classified separately
according to the Japanese Society for Cancer of the Colon and
Rectum classification, although they were also included as
adenocarcinomas based on the 2010 WHO classification (12,13,15).
Pseudomyxoma peritonei accompanying mucinous
adenocarcinoma or LAMN were also identified. Peritoneal tumors
composed of atypical glands in desmoplastic stroma or tumors with
high cellularity and cytological atypia containing pools of mucin
were classified as mucinous adenocarcinoma-associated pseudomyxoma
peritonei (high-grade; Fig. 1C)
(12), whereas tumors composed of
mucinous epithelium with low-grade atypia and containing mucin
pools were classified as LAMN-associated pseudomyxoma peritonei
(low-grade; Fig. 2B) (12). Acellular mucinosis were not
categorized as an appendiceal neoplasms, but rather as a secondary
lesion as mucocele is a descriptive term, according to the 2010 WHO
classification (12).
Variables assessed
The variables for evaluation included the age and
sex of the 752 patients, the initial surgical findings (neoplasm or
inflammation), and the age and sex of the patients grouped
according to the 2 basic lesion types. The present study focused on
the pathological characteristics of the appendiceal neoplasms and
the clinical characteristics of the patients with these neoplasms.
In this group of patients, the presenting symptoms, imaging
diagnosis, preoperative diagnosis, and the types and incidence of
appendiceal lesions determined by postoperative pathological
examination were also reviewed. Special note was made of the
intramucosal lesion types.
Statistical analysis
Patients with appendiceal neoplasms were divided
into two groups aged <60 and ≥60 years, and the difference in
the incidence of appendiceal neoplasms was analyzed by the
Mann-Whitney U and χ2 tests. All statistical analyses
were performed with SPSS 21.1 (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient data
The 752 patients ranged in age from 2 to 89 years,
with a mean age of 34.2 years. There were 431 males and 321
females. The surgical diagnosis was inflammation (appendicitis) in
735 patients (97.7%), and appendiceal neoplasm in 17 patients
(2.3%).
Appendiceal neoplasms
The profiles of the 17 patients with appendiceal
neoplasms are included in Table I,
whereas the age-specific distribution of the histologic diagnoses
are included in Table II. The 17
patients ranged in age from 36 to 86 years, with a mean age of 77.0
years. There were 10 men and 7 women. The majority of the
appendiceal neoplasms were identified in patients aged ≥60 years
(14/17, 82.3%), and there was a significant difference in incidence
between patients aged ≥60 years and those aged <60 years
(P<0.001). The chief presenting symptom of the majority of
patients with an appendiceal neoplasm was right lower quadrant pain
(n=11, 64.7%). A total of 2 patients (11.7%) presented with an
abdominal mass, 1 patient (5.88%) had generalized abdominal
discomfort, and 3 patients (17.6%) had no symptoms. In these 3
patients, the appendiceal neoplasm was identified with the
investigation of elevated serum tumor markers, such as
carcinoembryonic antigen, or incidentally by PET/CT or preoperative
workup for gastric cancer. The tumor was benign in 3 patients
(17.6%) and malignant in 14 patients (82.3%). In the 3 patients
with benign disease, the preoperative clinical diagnosis was
appendiceal tumor, appendiceal cancer, and secondary appendicitis
caused by ileus strangulation, respectively. In the 14 patients
with malignancy, the preoperative clinical diagnosis was
appendicitis (n=8, 57.1%), perforated appendiceal diverticulitis
(n=1, 5.88%) or appendiceal neoplasm (n=5, 35.3%).
 | Table I.Clinicopathological data of the
patients with appendiceal neoplasms. |
Table I.
Clinicopathological data of the
patients with appendiceal neoplasms.
| Patient | Sex | Age, years | Chief
symptom/sign | Imaging diagnosis
(CT/US) | Preoperative
diagnosis | Final pathological
diagnosis (depth) |
|---|
| 1 | F | 79 | RLQ pain | Mucinous
adenocarcinoma | Appendiceal
tumor | Tubular
adenomaa |
| 2 | M | 83 | RLQ pain | Secondary
appendicitis caused by ileus strangulation | Secondary
appendicitis caused by ileus strangulation | Tubulovillous
adenomaa |
| 3 | M | 77 | CT detection | Mucinous | Appendiceal
cancer | (Mesenchymal)
schwannoma |
| 4 | M | 61 | RLQ pain | Acute
appendicitis | Acute
appendicitis | Adenocarcinoma
(se) |
| 5 | M | 67 | RLQ pain | Acute
appendicitis | Acute
appendicitis | Adenocarcinoma
(mp) |
| 6 | F | 59 | CEA elevation | Mucinous tumor | Appendiceal
tumor | Mucinous
adenocarcinoma (se) |
| 7 | M | 78 | PET detection | Appendiceal
tumor | Appendiceal
cancer | Mucinous
adenocarcinoma (m)a |
| 8 | F | 79 | Right lower abdominal
mass | Mucinous adenoma | Mucinous adenoma | Mucinous
adenocarcinoma (m)a |
| 9 | F | 85 | Abdominal mass,
fever | PMP | PMP | Mucinous
adenocarcinoma (PMP) |
| 10 | F | 60 | RLQ pain | Acute appendicitis
(abscess) | Acute appendicitis
(perforated) | LAMN |
| 11 | M | 77 | RLQ pain | Acute appendicitis
(abscess) | Acute appendicitis
(perforated, abscess) | LAMN |
| 12 | F | 71 | Epigastralgia | Mucinous adenoma | Mucinous adenoma | LAMN |
| 13 | F | 71 | RLQ pain | Acute
appendicitis | Acute
appendicitis | LAMN |
| 14 | M | 80 | RLQ pain | Perforated
appendiceal diverticulitis (free air) | Perforated
appendiceal diverticulitis | LAMN (PMP) |
| 15 | M | 86 | RLQ pain | Acute
appendicitis | Acute
appendicitis | LAMN |
| 16 | M | 36 | RLQ pain | Acute appendicitis
(phlegmon) | Acute
appendicitis | G1 NET |
| 17 | M | 43 | RLQ pain | Acute
appendicitis | Acute
appendicitis | MANEC (ss) |
 | Table II.Age distribution of patients with
appendiceal neoplasms (n=17). |
Table II.
Age distribution of patients with
appendiceal neoplasms (n=17).
|
|
| Age range,
years |
|
|---|
| Diagnosis | Code | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | Incidence, % |
|---|
| Benign |
| Tubular
adenoma | 8211/0 |
|
|
|
|
|
| 5.9 |
|
Tubulovillous adenoma | 8263/0 |
|
|
|
|
| 1 | 5.9 |
|
Mesenchymal schwannoma | 9570/0 |
|
|
|
| 1 |
| 5.9 |
| Malignant |
|
Adenocarcinoma (MOD) | 8140/3 |
|
|
| 1 |
|
| 5.9 |
|
Adenocarcinoma (papillary and
MOD) | 8140/3 |
|
|
| 1 |
|
| 5.9 |
|
Mucinous adenocarcinoma (with
PMP) | 8480/3 |
|
| 1 |
| 2 | 1 | 17.7 |
| Low
grade appendiceal neoplasm (with PMP) | 8480/1 |
|
|
| 1 | 3 | 2 | 41.2 |
| Grade 1
neuroendocrine tumor (carcinoid) | 8240/3 | 1 |
|
|
|
|
| 5.9 |
| Mixed
adenoneuroendocrine carcinoma | 8244/3 |
| 1 | | | | | 5.9 |
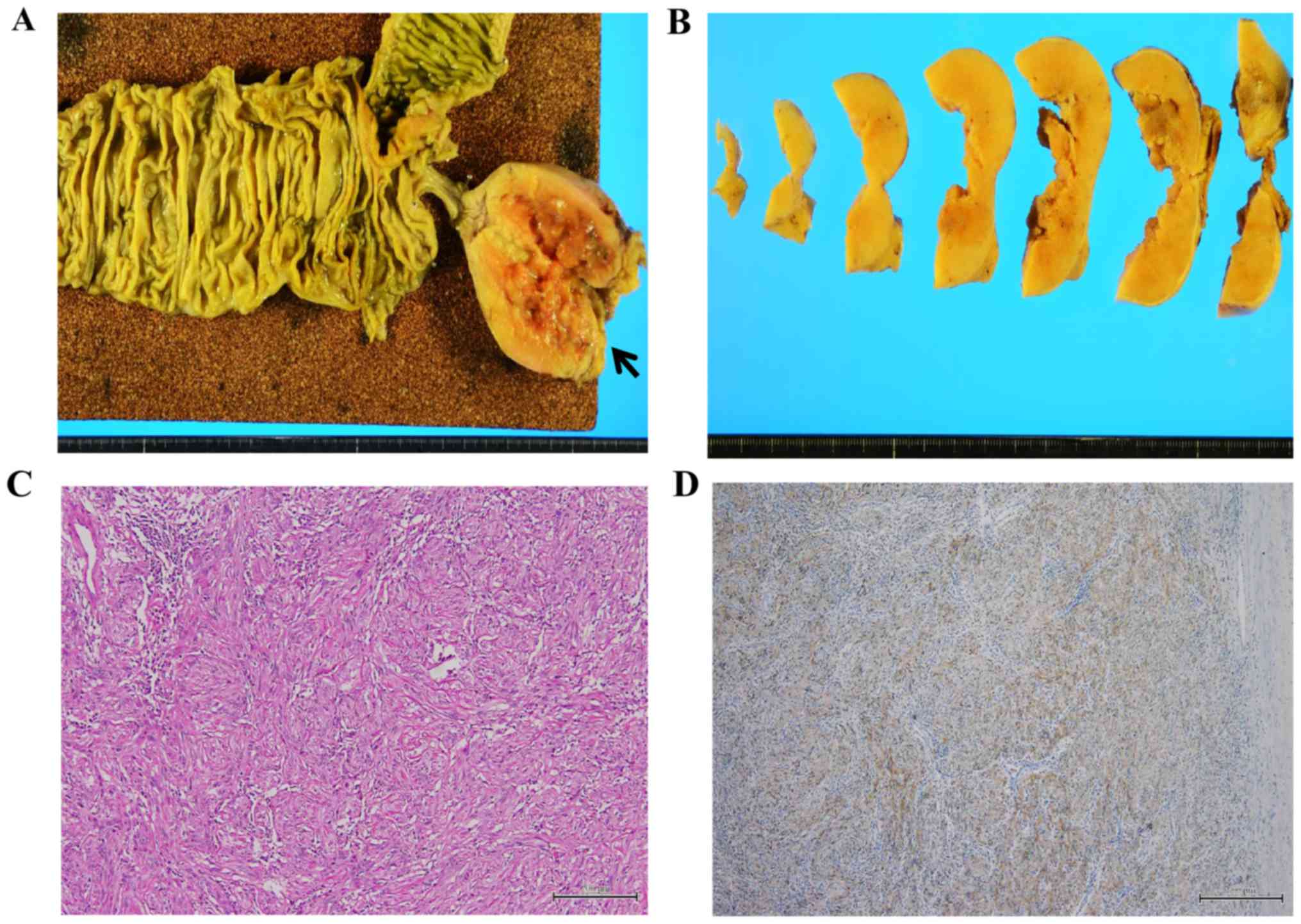
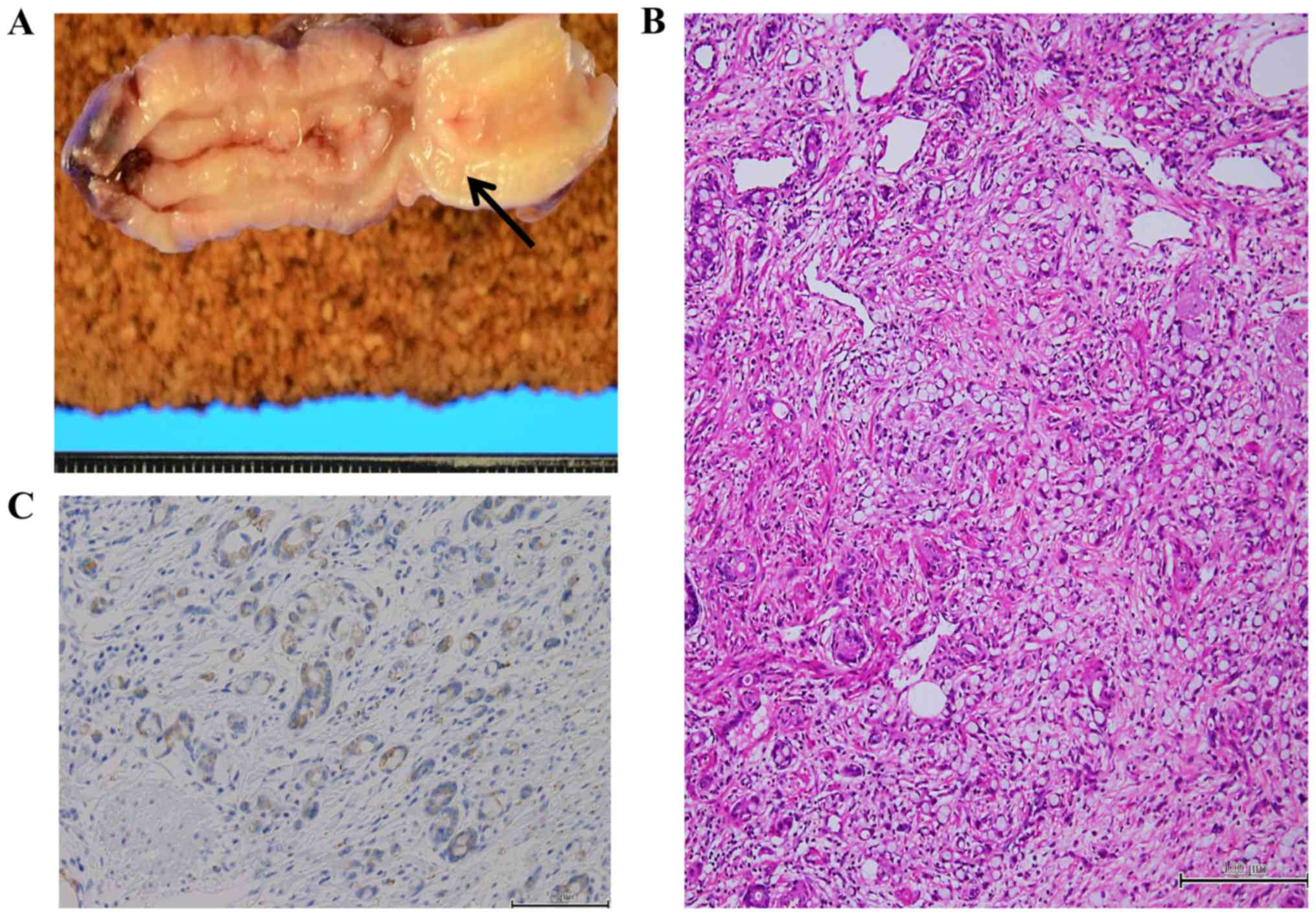
The 3 benign neoplasms included 1 tubular adenoma, 1
tubulovillous adenoma, and 1 mesenchymal schwannoma (Fig. 3). The 14 malignant neoplasms included
6 adenocarcinomas (4 mucinous adenocarcinomas, 1 papillary
adenocarcinoma and 1 moderately differentiated adenocarcinoma), 1
MANEC (Fig. 4), 1 neuroendocrine
tumor (G1), and 6 LAMNs (1 with low-grade pseudomyxoma peritonei).
One malignant tumor was associated with high-grade pseudomyxoma
peritonei. None of the cases were considered discordant, i.e.,
there were no low-grade appendiceal tumors combined with high-grade
peritoneal tumors. A total of 4 (23.5%) of the lesions were
intramucosal neoplasms, including adenomas and in situ
carcinomas (patients 1, 2, 7, and 8).
Inflammation (appendicitis)
The 735 patients with appendicitis ranged in age
from 2 to 89 years, with a mean age of 28.0 years. They included
421 males and 314 females. Appendicitis was catarrhal in 80
patients (10.9%), phlegmonous in 352 patients (47.9%), and
gangrenous in 268 patients (36.5%). A total of 26 patients (3.5%)
had chronic inflammatory appendicitis that had not resolved with
antibiotic treatment, 8 patients (1.1%) had appendiceal
diverticulitis and 1 patient (0.1%) had an underlying Entamoeba
histolytica infection. Almost half (43.1%) of the patients with
appendicitis were <10 years old. Seven of these young patients
had gangrenous appendicitis, and 6 (85.5%) of those 7 patients were
under 4 years of age. More than half (63.7%) of the patients with
gangrenous appendicitis were over 60 years old.
Discussion
Recent trends in appendiceal pathology in patients
undergoing appendectomy at our institution in Japan were
investigated, particularly whether there was an increase in the
incidence of appendiceal neoplasms over time. During the period
from March 2002 through September 2014, the incidence of
appendiceal neoplasms among patients undergoing appendectomy was
2.3%. This was 3 times higher than the incidence of 0.7% reported
~50 years ago by Collins et al (16) and was ~5 times higher than the
incidence reported at other Japanese institutions (0.05%) (14). The majority (23.5%) of the appendiceal
neoplasms identified in the patients were intramucosal neoplasms,
including adenoma or noninvasive carcinoma (15). Historically, these appendiceal lesions
have been difficult to detect by diagnostic imaging
(radiography/ultrasonography) alone (16–18). Until
the 1990s, the decision to perform appendectomy was based on the
patient's symptoms, findings on physical examination (especially
palpation) and evidence of infection (elevation of the white blood
cell count or C-reactive protein) (19–21). An
appendectomy was not typically performed in the absence of
symptoms, as abdominal imaging was not widely practiced at that
time.
The results of the present study suggest that the
relatively recent increase in the application of newer imaging
modalities for the diagnosis and health screening of appendiceal
diseases has led to the increased identification of asymptomatic
appendiceal neoplasms. The sensitivity of these diagnostic methods
has caused the detection of appendiceal neoplasms to increase
(22).
The majority (64.7%) of the patients in the present
study with mucosal appendiceal neoplasms were aged ≥60 years, which
is similar to the age to patients with colon cancer (6,23). It is
generally accepted that the incidence of colon cancer has increased
in Japan due to the aging of the population and the adoption of an
increasingly westernized diet (24,25). By
analyzing the age-adjusted mortality and diagnosis rates for colon
cancer in Japan. It has been confirmed that the incidence rate of
colon cancer approximately doubled over the 40-year period from
1960 to 1999 (from 22–53 per 100.000 people) (23). Since 1999, the incidence rate of colon
cancer has remained the same (23).
As the appendix and colon develop from the midgut (26), it seems reasonable that an increased
incidence of colon cancer would coincide with an increase in
appendiceal neoplasms (20). This may
explain the increased incidence of appendiceal neoplasms among
patients undergoing appendectomy at our hospital.
Although Collins et al (16) and Connor et al (27) reported that over half (51–57%) of the
appendiceal neoplasms detected at appendectomy were carcinoid
tumors (G1 NET), intramucosal neoplasms accounted for 23.5% of the
appendiceal neoplasms in the patients included in the present
study, and this is similar to previous Asian reports, including
from Korea and Taiwan (2,18). Therefore, the major types of
appendiceal lesions may be influenced by geographic and ethnic
factors, culminating in epidemiological differences between Asian
and Western populations.
It can be difficult to precisely diagnose an
appendiceal neoplasm from imaging findings prior to surgery.
Therefore, pathological examination of the resected appendix is
important to determine whether a lesion is benign or malignant, in
addition to the depth of invasion and vascular invasion (19). Therefore, a sufficiently detailed
examination should be conducted, including a consideration of
whether additional resection is necessary (19). The relatively recent increased
application of newer imaging modalities to diagnosis and health
screening may have led to the increased identification of
asymptomatic appendiceal neoplasms (22). A number of studies have indicated that
the incidence of necrotic and penetrating appendicitis is higher in
pediatric and elderly populations (28–30).
In conclusion, the findings of the present study
indicate that the incidence of appendiceal neoplasms is increasing.
Accordingly, surgeons should be aware of the possibility of these
entities, particularly in patients aged ≥60 years, due to the
frequent occurrence of malignant and potentially malignant
neoplasms in appendectomy materials (2.3%, ~3–5 times higher than
previously reported). In addition, the frequent preoperative
diagnosis of appendicitis in patients with appendiceal neoplasms
(57.1%) and characteristic age distribution of patients with
gangrenous appendicitis (≤4, 85.5%; ≥60, 63.7%) as demonstrated in
the present study.
Acknowledgements
The authors would like to thank the staff at the
Department of Diagnostic Pathology of The Tokai University Hachioji
Hospital for their technical assistance and insightful
comments.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TakaT designed and performed the research for the
present study, contributed to the analysis and wrote the present
manuscript. TakuT designed the research for the present study,
provided pathological advice and supervised the present study. TS
contributed to analysis and interpretation of data in the
pathological field. SH, SY, HS and SS contributed to analysis and
interpretation of data in the surgical field. MM and HM contributed
to analysis and interpretation of data and assisted in the
preparation of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
review board of Tokai University Hachioji Hospital (approval no.
R14-242). Written informed consent for the appendectomy was
obtained from the patients and/or family members.
Consent for publication
Written informed consent for publication of the
present study was obtained from the patients and/or family
members.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Deans GT and Spence RA: Neoplastic lesions
of the appendix. Br J Surg. 82:299–306. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee WS, Choi ST, Lee JN, Kim KK, Park YH
and Baek JH: A retrospective clinicopathological analysis of
appendiceal tumors from 3,744 appendectomies: A single-institution
study. Int J Colorectal Dis. 26:617–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rao PM, Rhea JT, Rattner DW, Venus LG and
Novelline RA: Introduction of appendiceal CT: Impact on negative
appendectomy and appendiceal perforation rates. Ann Surg.
229:344–349. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung DS, Kang S and Park JB: Incidental
finding of appendiceal adenocarcinoma in F-18 FDG PET/CT for health
screening. Nucl Med Mol Imaging. 46:308–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arai T, Kanazawa K, Sakurai U, Sawabe M,
Tei S, Honma N, et al: Pathology of appendiceal disease.
Gastrointestinal pathology ll. - lower intestine. Pathol Clin Med.
29:1105–1113. 2011.(In Japanese).
|
|
6
|
Ishihara H, Kataoka M, Hasegawa K,
Nakayama H, Takeda S and Kondo K: A case of appendiceal mucinous
cystadenoma in persistent appendix. J Jpn Surg Assoc. 74:3073–3076.
2013.(In Japanese). View Article : Google Scholar
|
|
7
|
Aida N, Jingu K, Uematsu T and Kitabayashi
H: A case report of mucinous cystadenocarcinoma arising from the
incision scar of appendectomy for appendicitis 46 years after
surgery. J Jpn Surg Assoc. 74:3077–3081. 2013.(In Japanese).
View Article : Google Scholar
|
|
8
|
Tamura J, Kitagawa K, Ura K and Baba N: A
case of multiple colon perforation caused by fulminant amoebic
colitis associated with HIV infection. Rinshogeka. 64:993–997.
2009.(In Japanese).
|
|
9
|
Okamoto M, Oohata K, Sasahira N, Yoshida
H, Hada T, Katamoto T, Yamaji Y, Kawabe T and Omata M: Three cases
of amebic colitis detected by positive fecal occult blood test in
mass screening. Prog Dig Endosc. 61:106–107. 2002.(In Japanese).
View Article : Google Scholar
|
|
10
|
Miyasaka Y, Yasui D, Nakagawa M, Watanabe
M and Doi F: A case of acute amebic appendicitis. Shokakigeka.
28:503–506. 2005.(In Japanese).
|
|
11
|
Iwashita A, Yamada Y, Yao K, Arita M,
Takasaki J and Tsuda S: Clinicopathological study on 2,169
appendices. Ito Cho. 10:1185–1194. 1990.(In Japanese).
|
|
12
|
Carr NJ, Sobin LH, Komminoth P, Arnold R,
Cappela C, Klimstra DS, et al: Tumours of the appendixWHO
Classification of Tumours of the Digestive System. Bosman TF,
Carneiro F, Hruban RH and Theise ND: 4th edition. International
Agency for Research on Cancer (IARC); Lyon: pp. 119–129. 2010
|
|
13
|
Rindi G, Arnold R, Bosman FT, Capella C,
Klimstra DS, Kloppel G, Paul Komminoth and Solcia E: Nomenclature
and classification of neuroendocrine neoplasms of the digestive
systemBosman TF, Carneiro F, Hruban RH and Theise ND: WHO
Classification of Tumours of the Digestive System. 4th ed. Lyon:
International Agency for Research on Cancer (IARC); pp. 13–14.
2010
|
|
14
|
Ozawa H, Moritani K, Wada O, Fujita S and
Kotake K: Statistics of appendiceal malignant tumors: Data from the
JSCCR registry and the Japan autopsy annual database. Stomach and
Intestine (Tokyo). 49:495–499. 2014.(in Japanese).
|
|
15
|
Japanese Society for Cancer of the Colon
and Rectum (JSCCR): General rules for Clinical and Pathological
Studies on Cancer of the Colon, Rectum and Anus. 8th edition.
Kanehara Shuppan, Tokyo: 2013
|
|
16
|
Collins DC: 71,000 human appendix
specimens. A final report, summarizing forty years' study. Am J
Proctol. 14:265–281. 1963.PubMed/NCBI
|
|
17
|
Matsuda K, Tsukamoto M, Fukushima Y,
Akahane T, Horiuchi A, Shimada R, Nakamura K, Tsuchiya T, Hayama T,
Yamada H, et al: Clinical features and treatment principles of
appendiceal cancers. Stomach and Intestine (Tokyo). 49:509–519.
2014.(In Japanese).
|
|
18
|
Pickhardt PJ, Levy AD, Rohrmann CA Jr and
Kende AI: Primary neoplasms of the appendix: Radiologic spectrum of
disease with pathologic correlation. Radiographics. 23:645–662.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HT, Lee YT, Chou AS, Wu YK, Yin WY,
Lee MC and Hsu YH: Primary appendiceal malignancy: A
clinicopathologic study. Kaohsiung J Med Sci. 22:618–625. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lewis FR, Holcroft JW, Boey J and Dunphy
JE: Appendicitis. A critical review of diagnosis and treatment in
1,000 cases. Arch Surg. 110:677–684. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jess P, Bjerregaard B, Brynitz S,
Holst-Christensen J, Kalaja E and Lund-Kristensen J: Acute
appendicitis. Prospective trial concerning diagnostic accuracy and
complications. Am J Surg. 141:232–234. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Izbicki JR, Knoefel WT, Wilker DK,
Mandelkow HK, Müller K, Siebeck M and Schweiberer L: Accurate
diagnosis of acute appendicitis: A retrospective and prospective
analysis of 686 patients. Eur J Surg. 158:227–231. 1992.PubMed/NCBI
|
|
23
|
Health and Welfare Statistics Association:
J Health Welfare Stat Tokyo. 61:63–67. 2014/2015.(In Japanese).
|
|
24
|
Kolonel LN, Hinds MW and Hankin JH: Cancer
patterns among migrant and native-born Japanese in Hawaii in
relation to smoking, drinking and dietary habitsGenetic and
Environmental Factors in Experimental and Human Cancer. Gelboin HV,
Mac Mahon B, Matsushima T, Sugimura T, Takayama S and Takebe H:
Japan Scientific Societies Press; Tokyo: pp. 327–340. 1980
|
|
25
|
Maskarinec G and Noh JJ: The effect of
migration on cancer incidence among Japanese in Hawaii. Ethn Dis.
14:431–439. 2004.PubMed/NCBI
|
|
26
|
Lunniss PJ, Stringer MD and Standring S:
Gray's anatomy: The anatomical basis of medicine and surgery.
Elsevier; pp. 1136–1159. Philadelphia: 2016
|
|
27
|
Connor SJ, Hanna GB and Frizelle FA:
Appendiceal tumors: Retrospective clinicopathologic analysis of
appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum.
41:75–80. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawashima H, Iwanaka T, Arai M, Kudo S,
Fujishiro J and Imaizumi S: Clinical analysis of timing of
laparoscopic appendectomy for appendicitis in children. J Jpn Soc
Emer Ped (JJSEP). 2:31–34. 2003.(In Japanese).
|
|
29
|
Watanabe S, Watanabe A and Fukuda C:
Diagnosis of childhood perforated appendicitis and active
observation of suspected appendicitis. J Jpn Soc Emer Ped (JJSEP).
2:25–29. 2003.(In Japanese).
|
|
30
|
Zbierska K, Kenig J, Lasek A, Rubinkiewicz
M and Wałęga P: Differences in the clinical course of acute
appendicitis in the elderly in comparison to younger population.
Pol Prezegl Chir. 88:142–146. 2016.
|