Introduction
Bladder cancer (BC) is one of the most common types
of cancer globally, with high morbidity and mortality rates
(1). The incidence of BC is
increasing in much of the world (1);
however, few novel approaches for the treatment of this disease
have been developed in the past two decades (2). Only programmed cell death (PD)
1/PD-ligand 1 based immunotherapy has displayed promising results
for the management of BC in recent years (3). Cisplatin-based combination chemotherapy
remains the preferred first-line treatment for advanced or
metastatic BC (4). Limited progress
in improving outcomes has created a major incentive for the
analysis of molecular alterations in BC, the identification of
novel potential targets and to accelerate the identification of
novel treatments for this disease (5).
Macrophage stimulating 1 receptor (MST1R, also known
as RON) is a membrane tyrosine kinase receptor that has been
considered to be a valuable target in cancer therapy (6). Elevated RON expression has been detected
in a number of malignant tumor types, including those of the colon,
breast and pancreas (7–9). RON overexpression has been reported to
have prognostic value in predicting patient survival and clinical
outcome in these types of cancer (7–9). The roles
served by RON in BC have been studied extensively in vitro
and in vivo, and results have demonstrated that RON and
other tyrosine kinase receptors, including epidermal growth factor
receptor and MET, exerted their effects collaboratively in BC
carcinogenesis (10,11). Furthermore, macrophage-stimulating
protein, which is the only known ligand of RON, was also detected
in human urine samples (11). These
results indicated that RON serves a key role in the development and
progression of BC.
The results of the previous study indicated that the
inhibition of RON by its specific monoclonal antibody (mAb) zt/g4
in human BC cell lines lead to reduced cell growth and motility,
which provided evidence that RON is a potential target in BC
(11). In the present study, the
expression of RON tyrosine kinase receptor was further assessed via
immunohistochemistry (IHC) in human BC tissues and adjacent
noncancerous tissues. Furthermore, the effect of RON on BC cell
proliferation, apoptosis and migration was also analyzed via the
knockdown of RON expression with specific small interfering RNAs
(siRNAs).
Materials and methods
Main reagents
Cell Counting Kit-8 (CCK-8) was purchased from
Dojindo Molecular Technologies, Inc. (Dojindo laboratories, Inc.,
Kumamoto, Japan). Mouse mAb zt/f2 specific to RON immunoglobulin,
plexins and transcriptional factor domain and rabbit antibody R5029
(specific to the RON C-terminal peptide) were provided by Professor
Yao (Laboratory of Cancer Biology and Therapeutics, First
Affiliated Hospital, Zhejiang University School of Medicine,
Hangzhou, China). The primary antibodies rabbit anti-β-actin (cat.
no. 4970; dilution, 1:1,000), anti-p38 (cat. no. 8690; dilution,
1:1,000), anti-phospho-p38 (cat. no. 4511; dilution, 1:1,000),
anti-extracellular signal-regulated kinase 1/2 (ERK1/2) (cat. no.
9102s; dilution, 1:1,000), anti-phospho-ERK1/2 (cat. no. 4370s;
dilution, 1:1,000), anti-protein kinase B (Akt) (cat. no. 4685s;
dilution, 1:1,000), anti-phospho-Akt (cat. no. 4060s; dilution,
1:1,000), anti-cyclin D1 (cat. no. 2978; dilution, 1:1,000),
anti-cyclin D3 (cat. no. 2936; dilution, 1:2,000),
anti-cyclin-dependent kinase 4 (CDK4; cat. no. 12790; dilution,
1:1,000), anti-cyclin dependent kinase inhibitor 1A (p21) (cat. no.
2947; dilution, 1:1,000) and anti-cyclin dependent kinase inhibitor
1B (p27) (cat. no. 3686; dilution, 1:1,000) were all purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Fetal bovine
serum (FBS), RPMI-1640, L-glutamine and penicillin were purchased
from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Tissue collection and ethics
statement
Specimens from patients with BC (n=106) were
obtained during surgical tumor resection in the Ningbo First
Hospital (Ningbo, China) and with pathological identification in
the Ningbo Diagnostic Pathology Center (Ningbo, China) between
March 2011 and July 2014. Of the total patients, there were 87
males and 19 females, aged between 35 and 84 years old (mean age,
68 years old). The available adjacent non-cancerous samples were
also obtained 1.5 cm away from the cancer tissues and confirmed by
pathologists. None of the patients had undergone chemotherapy or
radiotherapy prior to surgery. The present study was reviewed and
approved by the Ethical Committee of Ningbo First Hospital (Ningbo,
China) and all patients provided written informed consent prior to
sample collection.
IHC and scoring
The tissue samples were fixed with 4%
paraformaldehyde for 24 h at room temperature, embedded in paraffin
and 4-µm thick sections were prepared. Following deparaffnization
with xylene and ethanol (100% ethanol for 5 min, 95% ethanol for 3
min, 85% ethanol for 3 min and 70% ethanol (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) for 3 min, the tissue sections
were incubated for 30 min at room temperature in 0.3%
H2O2 to block endogenous peroxidase
activities. For antigen retrieval, samples were incubated with 0.1
M citrate buffer (pH 6.0) (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) in boiling water for 10 min.
Following rinsing in PBS, non-specific antigens were blocked by 3%
normal bovine serum (Beijing Solarbio Science & Technology Co.,
Ltd.,) for 15 min at room temperature, the slides were incubated
with the anti-RON antibody zt/f2 used at a 1:200 dilution at 4°C
overnight and subsequently incubated with anti-mouse IgG (H+L)
antibody (cat. no. ab6789, dilution, 1:500; Abcam, Cambridge, MA,
USA) for 1 h at room temperature. Detection was performed using an
EnVision system according to the manufacturers protocol (Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) and visualized
with diaminobenzidine as substrate.
Six views were observed per slide and 100 cells were
examined per view at ×400 magnification under a light microscope
(Nikon Corporation, Tokyo, Japan). The stain of RON was scored
depending on the staining proportion (0, 0–4%; 1, 5–24%; 2, 25–49%;
3, 50–74%; and 4, 75–100%) and staining intensity (the intensity of
RON membrane staining: No staining, 0; weak staining, 1; moderate
staining, 2; and strong staining, 3). Finally, a cumulative
evaluation of scores based on the addition of the two results
ranging from 0–7 was conducted. The total expression of RON was
considered as overexpression with a score ≥4 or normal expression
with a score <4.
Cell line and cell culture
The human 5637 BC cell line was obtained from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2 in RPMI-1640 medium
containing 10% FBS, 2 mM L-glutamine and 100 U/ml penicillin.
Western blot analysis
Western blot analysis was performed as previously
described (11). Cellular proteins
(100 µg/sample) were separated by 8% or 12% SDS-PAGE under reduced
conditions. Individual proteins were detected using the
aforementioned primary antibodies followed by horseradish
peroxidase-coupled secondary antibodies (cat. no. ab205718,
dilution, 1:5,000; Abcam, Cambridge, MA, USA). Densitometry was
analyzed using Quantity One software (version 4.4.02; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Transfection of BC cells
Gene silencing was performed using scrambled siRNA
(cat. no. sc-37007, nominated as si-Scr) and human RON
sequence-specific duplex siRNA (cat. no. sc-36434, nominated as
si-RON), which were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The 5637 cells were seeded into a plate when
they were 50–60% confluent at the time of transfection. The 5637
cells were transfected with 75 pmol si-RON and si-Scr using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
following the manufacturer's protocol. At 48 h after transfection,
the expression of RON was analyzed via western blot analysis as
previously stated.
Cell viability assay
Sensitivity of the cells to RON knockdown by si-RON
was analyzed using the CCK-8. Briefly, a total of
1×104/well cells were seeded in 96-well plates in 100 µl
culture medium (RPMI-1640 with 10% FBS) for 24 h at 37°C and then
transfected with si-RON and si-Scr as previously described.
Following transfection, cells were cultured for 0, 24, 48, 72 h
individually. The cells were then treated with 10 ul/well of CCK-8
solution and incubated for another 2 h at 37°C. The absorbance
values of each well were measured using a Multiskan Go plate reader
(Thermo Fisher Scientific, Inc.) at a wavelength of 450 nm. The
experiment was performed in triplicate.
Flow cytometric assays for cell cycle
distribution and apoptosis
Transfected cells (2×105) were harvested
and fixed with 75% ethanol overnight at −20°C. The fixed cells were
treated with 1 mg/ml RNase A (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) in darkness at 37°C for 30 min and with 50 µg/ml propidium
iodide (PI; Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. The
cells were analyzed using a flow cytometer (FACScan; BD
Biosciences, Franklin Lakes, NJ, USA) to investigate cell cycle
distribution. Quadrant analysis was performed using the CellQuest
Pro software (version 5.1; BD Biosciences, Franklin Lanes, NJ,
USA)
The cell apoptosis assay was performed using an
Annexin-V/Fluorescein Isothiocyanate Apoptosis Detection kit (BD
Biosciences, Franklin Lakes, NJ, USA). Transfected cells were
collected and washed twice with cold PBS prior to staining with
Annexin V and PI solution for 15 min in darkness at room
temperature. The ratios of apoptotic cells were determined using a
flow cytometer (FACScan; BD Biosciences).
Wound healing assay
Cells from the si-RON and the si-Scr groups were
incubated in RPMI-1640 with 10% FBS in 6-well plates. Following
transfection with si-RON or si-Scr, a small wound area was produced
in the 90% confluent monolayer using a 200-µl pipette tip in a
lengthwise stripe. Following incubation at 37°C for 24 h, the area
covered by migrated cells was examined under a light microscope
(Nikon Corporation) and images were captured at ×100 magnification.
Experiments were repeated in triplicate.
Statistical analysis
Statistical analyses were performed using the SPSS
software package (version 20; IBM Corp., Armonk, NY, USA). Data are
presented as the mean ± standard deviation. Statistical analysis
was conducted using the Student's t-test for paired samples and the
χ2-test was used for univariate analysis of RON
proteins. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of RON protein in BC and
paraneoplastic tissues
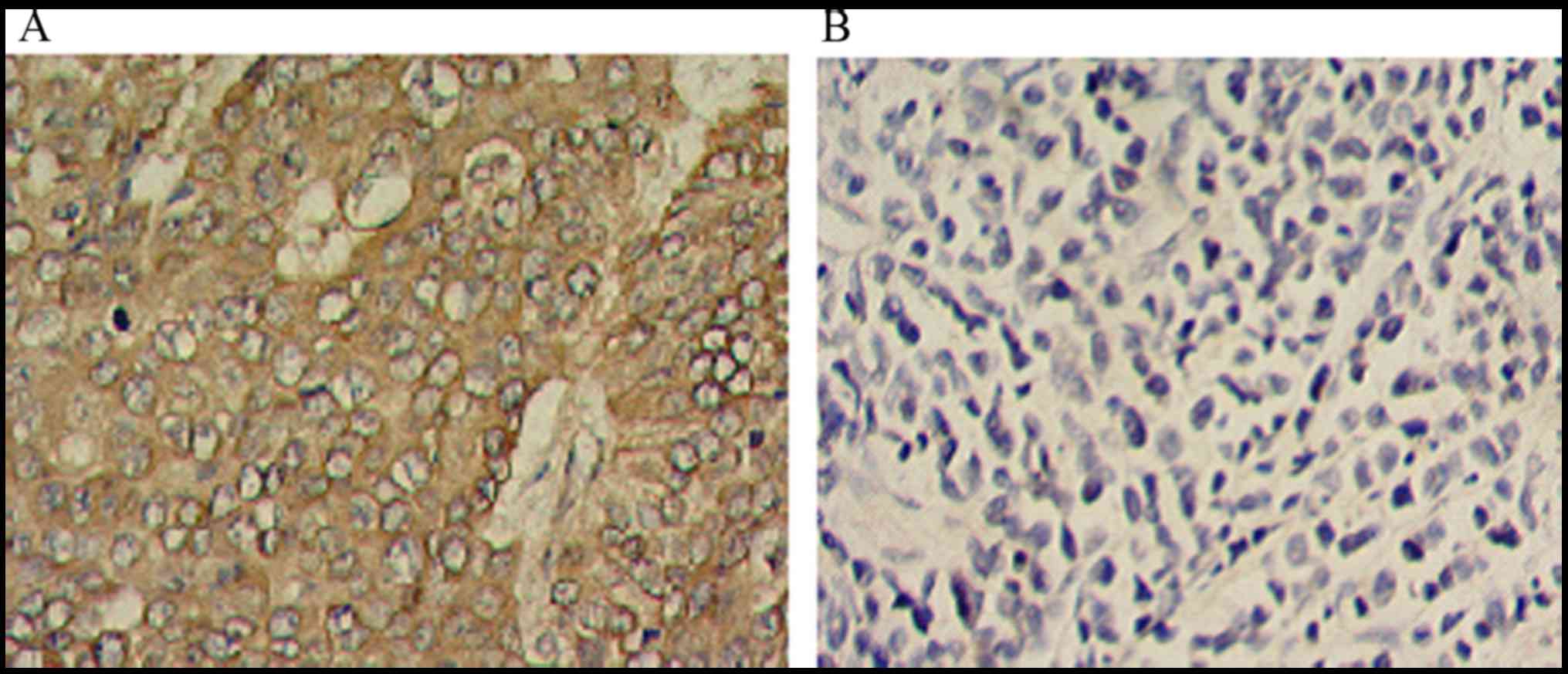
The presence of RON protein was observed in the
plasma membrane of BC cells, whereas the paraneoplastic tissues
exhibited negative or low staining (Fig.
1). The association between total RON expression and clinical
parameters was analyzed. As summarized in Table I, the positive expression rates of the
RON was 54.7% (58/106) in BC cases and 17.6% (6/34) in
paraneoplastic tissues. RON overexpression was positively
associated with the number of tumors the patient had, histological
grading, pathological stage and distant metastasis. No statistical
differences were observed between RON overexpression and
characteristics of age and sex.
 | Table I.Association between the RON expression
and the clinical pathological features in bladder cancer. |
Table I.
Association between the RON expression
and the clinical pathological features in bladder cancer.
|
|
| RON expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | Patients, n | Low | High |
χ2-value | P-value |
|---|
| Tissue |
|
|
| 15.54 | 0.000 |
| All
tumor | 106 | 48 | 58 |
|
|
|
Paracancerous | 34 | 28 | 6 |
|
|
| Sex |
|
|
| 2.749 | 0.097 |
|
Male | 87 | 41 | 46 |
|
|
|
Female | 19 | 5 | 14 |
|
|
| Age, years |
|
|
| 0.197 | 0.657 |
|
<70 | 51 | 21 | 30 |
|
|
|
≥70 | 55 | 25 | 30 |
|
|
| Number of tumor
nodules |
|
|
| 9.348 | 0.002 |
|
Single | 63 | 35 | 28 |
|
|
|
Multiple | 43 | 11 | 32 |
|
|
| Distant
metastasis |
|
|
| 7.323 | 0.007 |
| M0 | 90 | 44 | 46 |
|
|
| M1 | 16 | 2 | 14 |
|
|
| Pathological
stage |
|
|
| 4.652 | 0.031 |
|
Tis-T1 | 42 | 28 | 14 |
|
|
|
T2-T4 | 64 | 29 | 35 |
|
|
| Histological
grading |
|
|
| 9.667 | 0.008 |
|
Urothelial carcinoma, grade
I | 10 | 8 | 2 |
|
|
|
Urothelial carcinoma, grade
II | 41 | 25 | 16 |
|
|
|
Urothelial carcinoma, grade
III | 55 | 20 | 35 |
|
|
Knockdown of RON inhibits cell
proliferation in the 5637 cells
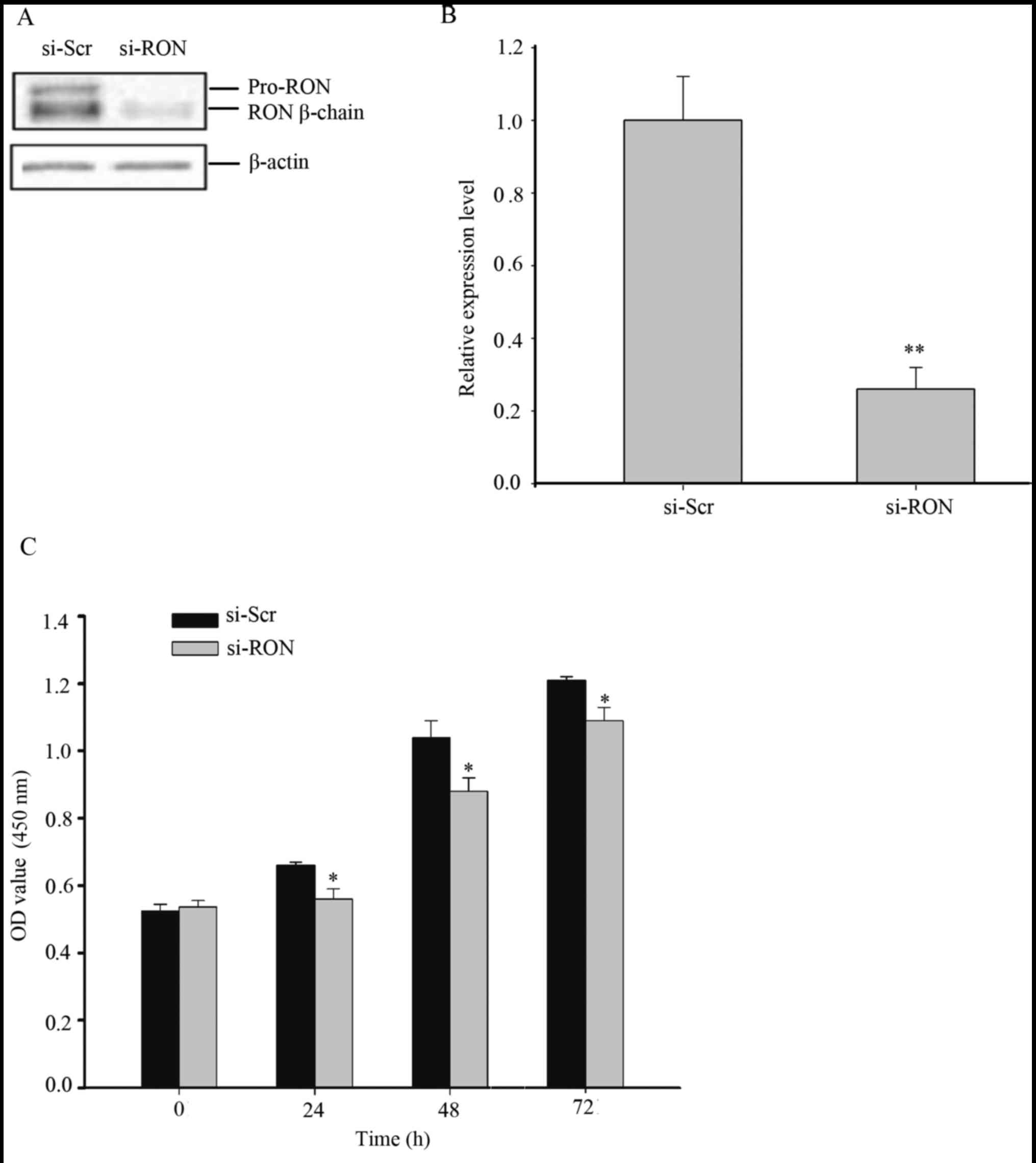
It was previously determined that the level of RON
expression in 5637 cells was high (11), therefore 5637 cells were selected for
future studies. To confirm the knockdown efficiency of si-RON,
si-RON was transiently transfected into the 5637 cells. As depicted
in Fig. 2A and B, at 48 h following
transfection, the protein expression level of RON was significantly
downregulated by transfection with si-RON, compared with si-Scr
(P<0.01). A CCK-8 assay was performed to analyze the
proliferation rate of the 5637 cells following silencing of RON.
The results of the CCK-8 assay demonstrated that si-RON notably
inhibited cell proliferation, compared with cells treated with
si-Scr (Fig. 2C, P<0.05).
Downregulation of RON expression
inhibits 5637 cell migration
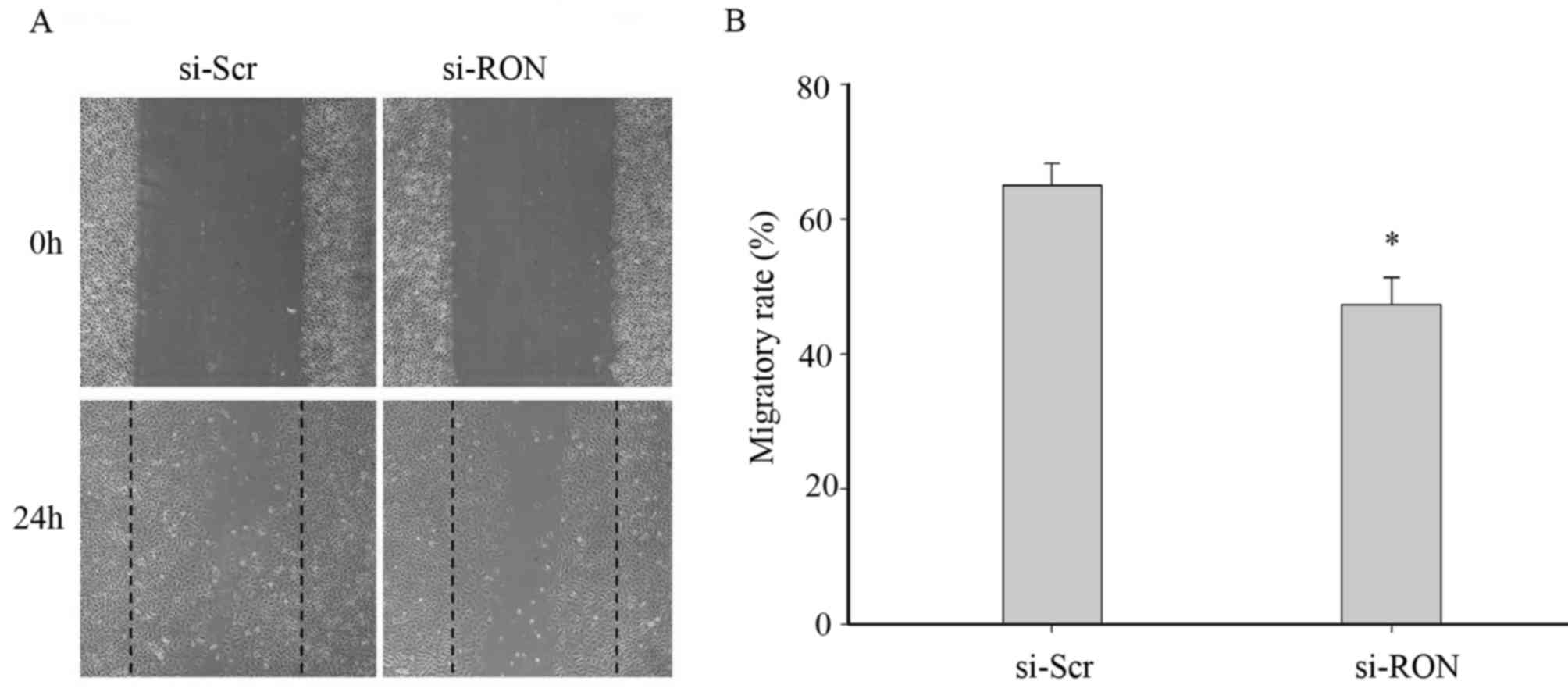
To assess whether the downregulation of RON affected
the cellular migration, a wound-healing assay was conducted on the
5637 cells in the si-RON and si-Scr groups. As depicted in Fig. 3, the number of migrated cells in the
si-RON group (47.37±7.02%) was significantly lower than that in the
si-Scr group (65.00±5.57%) at 24 h (P<0.05) once the wound was
produced on the cell monolayer, which indicated that the
downregulation of RON expression could significantly decrease the
migration of 5637 cells.
Knockdown of RON promotes apoptosis in
the 5637 cells
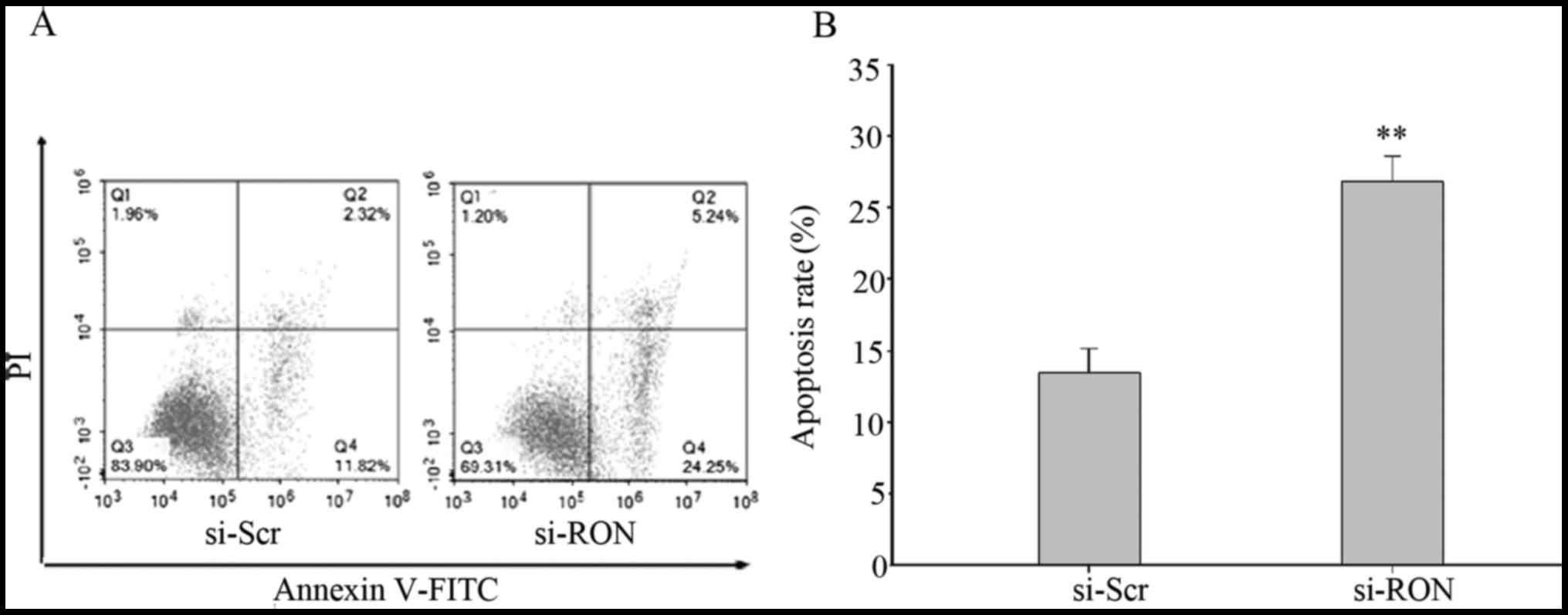
Flow cytometry was performed to investigate whether
the knockdown of RON increased the proportion of apoptotic 5637
cells. Flow cytometric analysis indicated that the proportion of
early and late apoptotic cells were significantly increased in
cells in the si-RON group, compared with those in si-Scr group
(Fig. 4A and B; P<0.01).
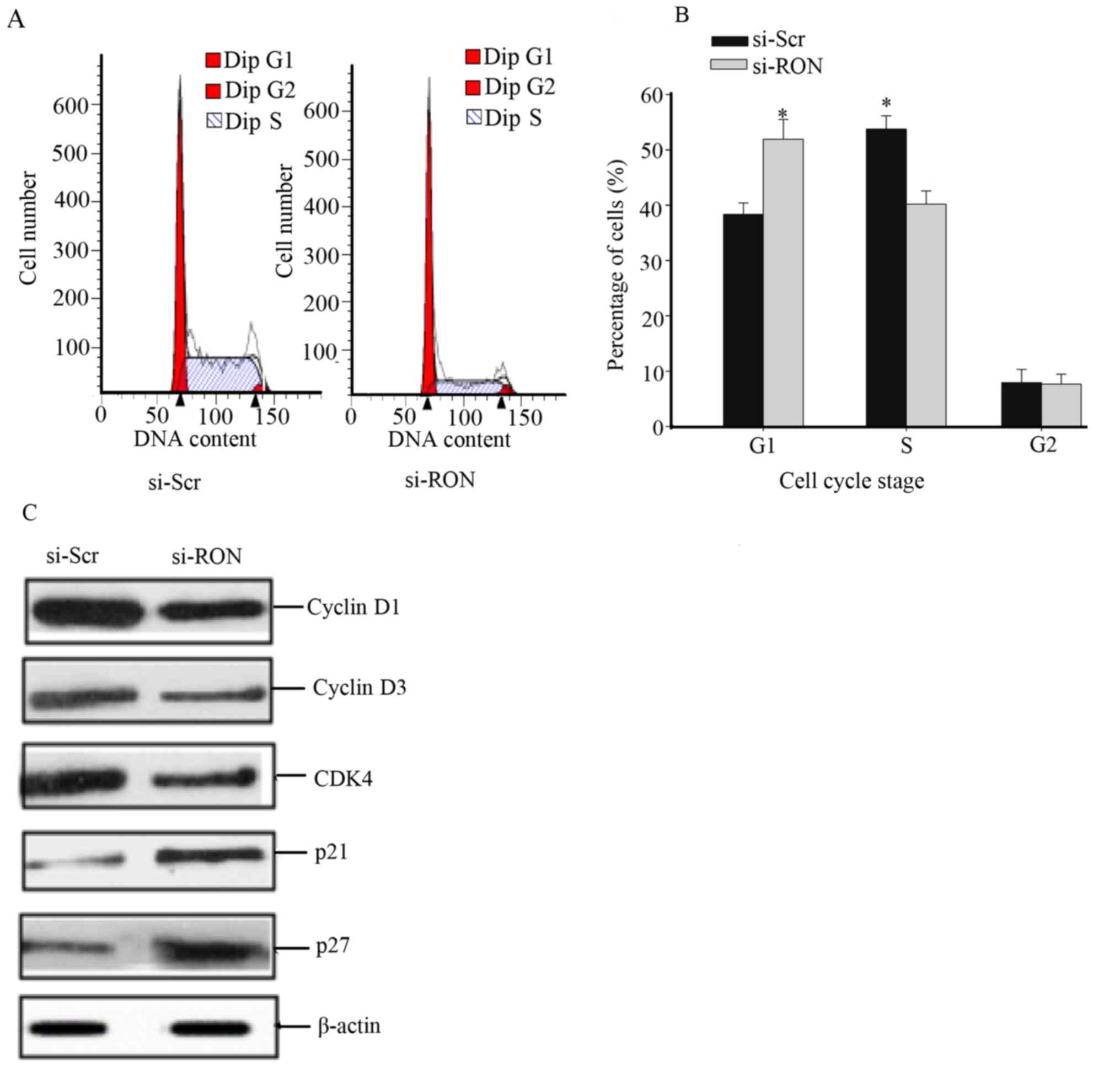
Knockdown of RON induces cell cycle
arrest at the G1/S phase in the 5637 cells
Owing to the growth inhibitory response of si-RON
treatment in 5637 cells, the effect of this treatment on the cell
cycle distribution was studied. The 5637 cells were incubated with
si-RON or si-Scr for 48 h, following which cell cycle analysis was
performed. The proportions of si-RON group cells were significantly
reduced in S phase and increased in G1 phase, compared with those
of si-Scr cells (Fig. 5A and B;
P<0.05). Furthermore, the effects of RON on CDKs and CDK
inhibitors (CDKIs) were evaluated, which are involved in regulation
of cell cycle arrest in BC cells (11). The 5637 cells treated with si-RON
exhibited an increased expression of p27 and p21, whereas the
expression of cyclin D1, cyclin D3 and CDK4 were decreased compared
with cells transfected with si-Scr (Fig.
5C).
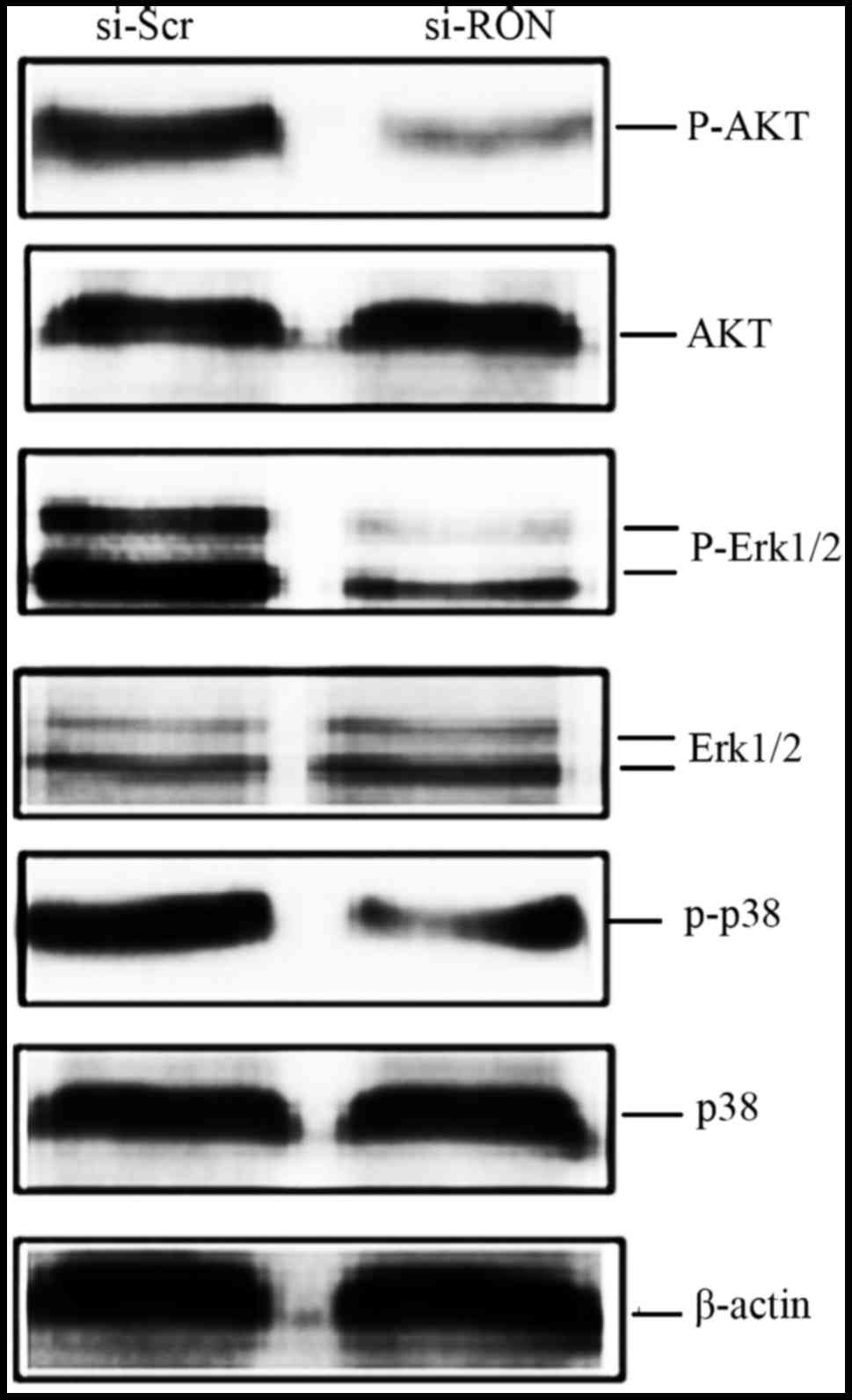
Knockdown of RON decreases the
phosphorylation of Akt and mitogen-activated protein kinases
(MAPKs) in 5637 cells
To investigate the potential signaling pathways
involved in RON-mediated cellular migration, apoptosis and cell
cycle arrest, the phosphoinositide 3-kinase (PI3K)-Akt and MAPK
pathways were selected for further study as the two pathways are
responsible for cell proliferation, migration, apoptosis and cell
cycle arrest (12). As depicted in
Fig. 6, the phosphorylation levels of
Akt, ERK1/2 and p38 in the si-RON group of 5637 cells were lower,
than those in the si-Scr group at 48 h. The levels of total Akt,
ERK1/2 and p38 appeared unchanged.
Discussion
The aberrant expression of RON has been verified to
serve a notable role in the development and progression of colon,
breast, bladder and pancreatic cancer, and other types of
epithelial cancer (7–14). Overexpression of RON is associated
with poor pathological characteristics in these cancer types
(15). In the present study, 106 BC
clinical specimens were examined for RON expression. The majority
of patient specimens (54.7%) were determined to be positive for RON
expression, and the level of RON expression in the cancer tissues
was significantly higher than that in the adjacent tissues
(Fig. 1). Subsequently, analysis of
the association between the expression of RON and
clinicopathological features revealed that the positive expression
rate of RON protein was closely associated with the number of
tumors, distant metastasis, histological grading and pathological
stage (Table I). Cheng et al
(9) previously reported that RON was
overexpressed in only 32.8% of analyzed BC samples. The disparity
in results between the study by Cheng et al (9) and the present study could be attributed
to the difference in antibodies used to detect RON expression, and
different criteria used to interpret the results. In the present
study, the level of RON expression was evaluated in a standardized
and semi-quantitative manner using the mAb zt/f2, which is
frequently regarded as an effective tool in immunochemistry for its
high reactivity, specificity and minimal background in human tissue
sections (15).
Overexpression of RON is associated with oncogenic
properties, including the promotion of cellular proliferation,
migration, invasion and survival in several human cancer cell lines
(16–19). To verify the potential role of RON in
BC cells, its expression level in BC cell lines was analyzed and
the 5637 cells were selected for further study as they have a
relatively high RON expression (11).
Subsequently, si-RON was used to knockdown RON expression in the
5637 cells (Fig. 2A). The results
indicated that knockdown of RON could notably inhibit cellular
proliferation (Fig. 2B) and decrease
migration (Fig. 3).
The potential functions of RON in the regulation of
cell apoptosis and cell cycle progression have been investigated in
several cancer cell lines (20–22).
However, there is limited information regarding the influence of
RON on apoptosis and cell cycle progression in human BC. In the
present study, RON knockdown promoted apoptosis and induced
G1/S arrest in the 5637 cells (Figs. 4 and 5).
CDKs and cyclins serve a crucial role in the regulation of cell
cycle progression (23,24). The G1 cyclin-CDK complex
cyclin D-CDK4/6 induces a transition from G1 to S phase
(25). CDKIs have been regarded as
putative tumor suppressors (26). p21
and p27 are CDKIs that bind to cyclin-CDK complexes to inactivate
them, inhibiting cell cycle progression (26). The results indicated that RON
knockdown promoted G1/S arrest via the downregulation of
cyclin D1, cyclin D3 and CDK4, and the upregulation of p21 and p27,
indicating that RON serves a vital role in regulating cell
cycle-associated proteins in BC (Fig.
5C).
RON signaling is conventionally transmitted by the
RAS-MAPK cascade and the PI3K-Akt pathway (12). Activation of the PI3K-Akt pathway is
involved in RON-mediated cell apoptosis, proliferation, migration
and cell matrix invasion (12,27,28).
Furthermore, RON-mediated MAPK signaling cascade directs various
cellular programs, including cell growth, migration, survival and
differentiation (12,19,28,29). To
observe whether si-RON inhibited downstream signal transduction,
si-RON and si-Src were transfected into the 5637 cells. Data
indicated that the Ser473 phosphorylation levels on Akt and the
phosphorylation of ERK1/2 and p38 were significantly decreased by
RON knockdown in these cells (Fig.
6). Considering these results, it appears likely that si-RON
induced the inhibition of Akt and MAPK phosphorylation; these
processes are involved in phenotypic changes to 5637 cells,
including inhibition of cell proliferation and motility, induction
of G1/S cell cycle arrest and increased apoptosis.
Knockdown of RON by siRNA affected the phosphorylation level of
Akt, ERK1/2 and p38; however, the more complicated mechanisms
underlying the Akt and MAPK signaling pathways following RON
knockdown require further investigation in future studies.
In summary, the data produced in the present study
indicated that RON was overexpressed in BC and was associated with
poor pathological features. Knockdown of RON regulates BC cell
behaviors through the modulation of Akt and MAPK signaling in human
BC cells. These results indicate that RON is a potential target for
therapeutic intervention in BC and have provided an experimental
basis for future investigations.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272828 to Dr QM),
Zhejiang Provincial Foundation for Medical and Health Sciences
(grant nos. 2016KYB263 and 2014KYB355 to Dr QM, and grant no.
2017KY576 to Dr JFC) and Ningbo Natural Science Foundation (grant
no. 2015A610224 to Dr JFC, and grant no. 2016A610159 to Dr
XYL).
Availability of data and materials
The authors declare that the datasets generated are
all included in the current study and are available from the
corresponding author on reasonable request.
Authors' contributions
JFC participated to the experimental design,
interpreted the results and wrote the manuscript; BXY, LM and XYL
performed experiments and analyzed the results; JHJ coordinated the
experimental work, interpreted the results and contributed to the
critical revision; QM designed the research plan, interpreted the
results and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin-Doyle W and Kwiatkowski DJ:
Molecular biology of bladder cancer. Hematol Oncol Clin North Am.
29:191–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bellmunt J, Powles T and Vogelzang NJ: A
review on the evolution of PD-1/PD-L1 immunotherapy for bladder
cancer: The future is now. Cancer Treat Rev. 54:58–67. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pinto IG: Systemic therapy in bladder
cancer. Indian J Urol. 33:118–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohammed AA, El-Tanni H, El-Khatib HM,
Mirza AA, Mirza AA and Alturaifi TH: Urinary bladder cancer:
Biomarkers and target therapy, new era for more attention. Oncol
Rev. 10:3202016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang MH, Padhye SS, Guin S, Ma Q and Zhou
YQ: Potential therapeutics specific to c-MET/RON receptor tyrosine
kinases for molecular targeting in cancer therapy. Acta Pharmacol
Sin. 31:1181–1188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park YL, Lee GH, Kim KY, Myung E, Kim JS,
Myung DS, Park KJ, Cho SB, Lee WS, Jung YD, et al: Expression of
RON in colorectal cancer and its relationships with tumor cell
behavior and prognosis. Tumori. 98:652–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feres KJ, Ischenko I and Hayman MJ: The
RON receptor tyrosine kinase promotes MSP-independent cell
spreading and survival in breast epithelial cells. Oncogene.
28:279–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng HL, Liu HS, Lin YJ, Chen HH, Hsu PY,
Chang TY, Ho CL, Tzai TS and Chow NH: Co-expression of RON and MET
is a prognostic indicator for patients with transitional-cell
carcinoma of the bladder. Br J Cancer. 92:1906–1914. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu PY, Liu HS, Cheng HL, Tzai TS, Guo HR,
Ho CL and Chow NH: Collaboration of RON and epidermal growth factor
receptor in human bladder carcinogenesis. J Urol. 176:2262–2267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen JF, Yu BX, Yu R, Ma L, Lv XY, Cheng Y
and Ma Q: Monoclonal antibody Zt/g4 targeting RON receptor tyrosine
kinase enhances chemosensitivity of bladder cancer cells to
Epirubicin by promoting G1/S arrest and apoptosis. Oncol Rep.
37:721–728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao HP, Zhou YQ, Zhang R and Wang MH:
MSP-RON signaling in cancer: Pathogenesis and therapeutic
potential. Nat Rev Cancer. 13:466–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas RM, Toney K, Fenoglio-Preiser C,
Revelo-Penafiel MP, Hingorani SR, Tuveson DA, Waltz SE and Lowy AM:
The RON receptor tyrosine kinase mediates oncogenic phenotypes in
pancreatic cancer cells and is increasingly expressed during
pancreatic cancer progression. Cancer Res. 67:6075–6082. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zarei O, Benvenuti S, Ustun-Alkan F,
Hamzeh-Mivehroud M and Dastmalchi S: Strategies of targeting the
extracellular domain of RON tyrosine kinase receptor for cancer
therapy and drug delivery. J Cancer Res Clin Oncol. 142:2429–2446.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang MH, Lee W, Luo YL, Weis MT and Yao
HP: Altered expression of the RON receptor tyrosine kinase in
various epithelial cancers and its contribution to tumorigenic
phenotypes in thyroid cancer cells. J Pathol. 213:402–411. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wagh PK, Peace BE and Waltz SE:
Met-related receptor tyrosine kinase Ron in tumor growth and
metastasis. Adv Cancer Res. 100:1–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camp ER, Liu W, Fan F, Yang A, Somcio R
and Ellis LM: RON, a tyrosine kinase receptor involved in tumor
progression and metastasis. Ann Surg Oncol. 12:273–281. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang MH, Wang D and Chen YQ: Oncogenic and
invasive potentials of human macrophage-stimulating protein
receptor, the RON receptor tyrosine kinase. Carcinogenesis.
24:1291–1300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Danilkovitch-Miagkova A: Oncogenic
signaling pathways activated by RON receptor tyrosine kinase. Curr
Cancer Drug Targets. 3:31–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung CY, Park YL, Song YA, Myung E, Kim
KY, Lee GH, Ki HS, Park KJ, Cho SB, Lee WS, et al: Knockdown of RON
inhibits AP-1 activity and induces apoptosis and cell cycle arrest
through the modulation of Akt/FoxO signaling in human colorectal
cancer cells. Dig Dis Sci. 57:371–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song YA, Park YL, Kim KY, Myung E, Chung
CY, Cho SB, Lee WS, Jung YD, Kweon SS and Joo YE: RON is associated
with tumor progression via the inhibition of apoptosis and cell
cycle arrest in human gastric cancer. Pathol Int. 62:127–136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho SB, Park YL, Song YA, Kim KY, Lee GH,
Cho DH, Myung DS, Park KJ, Lee WS, Chung IJ, et al: Small
interfering RNA-directed targeting of RON alters invasive and
oncogenic phenotypes of human hepatocellular carcinoma cells. Oncol
Rep. 26:1581–1586. 2011.PubMed/NCBI
|
|
23
|
Morgan DO: Cyclin-dependent kinases:
Engines, clocks, and microprocessors. Annu Rev Cell Dev Biol.
13:261–291. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murray AW and Marks D: Can sequencing shed
light on cell cycling? Nature. 409:844–846. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Connell-Crowley L, Elledge SJ and Harper
JW: G1 cyclin-dependent kinases are sufficient to initiate DNA
synthesis in quiescent human fibroblasts. Curr Biol. 8:65–68. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vermeulen K, van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Y, Hu J, Zheng J, Li J, Wei T, Zheng
Z and Cheng Y: Down-regulation of the PI3K/Akt signaling pathway
and induction of apoptosis in CA46 Burkitt lymphoma cells by
baicalin. J Exp Clin Cancer Res. 31:482012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang MH, Zhang R, Zhou YQ and Yao HP:
Pathogenesis of RON receptor tyrosine kinase in cancer cells:
Activation mechanism, functional crosstalk, and signaling
addiction. J Biomed Res. 27:345–356. 2013.PubMed/NCBI
|
|
29
|
Lu Y, Yao HP and Wang MH: Multiple
variants of the RON receptor tyrosine kinase: Biochemical
properties, tumorigenic activities, and potential drug targets.
Cancer Lett. 257:157–164. 2007. View Article : Google Scholar : PubMed/NCBI
|