Introduction
Diallyl disulfide (DADS) is a major sulfuric
compound of garlic, and exerts anti-inflammatory,
immune-modulatory, and enhanced sympathetic activity effects. In
the past few years, an increasing number of studies have indicated
that DADS has antitumor activity in numerous types of tumors,
including in neuroblastoma, breast cancer, colon cancer, lung
cancer and stomach cancer (1–8). The proteomic analysis of 18 differing
types of protein in HL-60 cells treated with DADS confirmed that
parkinsonism associated deglycase (DJ-1) protein expression was
significantly downregulated (9). DJ-1
protein, a 20 kDa size protein, belongs to the Thi/PfpI protein
superfamily and is expressed in lung, prostate and breast cancer
that appears in the serum of patients meaning it may be used as a
biomarker for breast cancer and melanoma (10). The carcinogenicity of DJ-1 is
attributed to a number of factors. Firstly, the cancer may survive
the antioxidant effect of the DJ-1 protein; and secondly, DJ-1 has
the additional ability to resist apoptosis by the chelation of
tumor protein p53, thus reducing the expression of BCL2 associated
X apoptosis regulator and preventing caspase activation to inhibit
target cell death, or by adjusting phosphatase and tensin homologue
(PTEN) activity (11). DJ-1 protein
is a particularly attractive target for cancer treatment as it is
associated with proliferation, invasion, migration,
chemotherapeutic resistance and apoptosis (12). In the human laryngeal carcinoma cell
line snu-46, the enhanced expression of the DJ-1 gene resulted in
invasion and migration capability enhancement (13), which has also been observed in
nasopharyngeal carcinoma (14).
The extensive local invasion and early systemic
spread, and DJ-1 has been identified to regulate Src
phosphorylation in the extracellular signaling pathway to promote
cell migration, and invasion (15).
The Src signaling pathway is associated with tumor invasion and
migration, and an experimental study on the transplantation of a
pancreatic tumor model indicated that the migration and invasion of
pancreatic cancer cells was associated with the Src signaling
pathway (16). Human non-small cell
lung cancer cell line A549 may inhibit Src signaling pathways in
order to weaken the cell invasion and migration (17). The activation of the Src signaling
pathway was revealed to regulate the migration of cells in breast
cancer tissue (18). In the present
study, HL-60 cells with a high expression of DJ-1 in the nucleus
(HHDN) were used to analyze the anti-invasion and anti-migration
effects resulting from DADS treatment, and its mechanism. Western
blot analysis, Transwell migration and invasion chamber assays were
used to examine the mechanism of how DADS impacts the invasion, and
migration ability in HHDN. Thus, providing novel prospects for the
intervention and treatment of leukemia, and a theoretical basis for
the optimization of DADS treatment for its antitumor effects.
Materials and methods
Cell culture and treatment
HL-60 cells, obtained from the Cancer Research
Institute, Xiangya Medical College, Central South University of
China, were cultured in RPMI-1640 medium (Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) with 10% fetal calf serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a 5%
CO2 incubator. Cell culture was replaced with fresh
medium every 2–3 days. The Cancer Research Institute of Central
South University of China had previous successfully established
stable HHDN, as well as an empty vector cell line of HL-60.
Reagents
DADS (purity 80%, with the remaining 20% composed of
diallyl trisulfide and diallyl sulfide), which was purchased from
Honeywell Fluka™ (Thermo Fisher Scientific, Inc.), was
dissolved in Tween-80 at a concentration of 8 mg/ml and stored at
−20°C. DJ-1 monoclonal antibodies were purchased from Upstate
Biotechnology, Inc., (Lake Placid, NY, USA; cat no. 05-828; 1:2,000
dilution). Fak and Src monoclonal antibodies were purchased from
Abgent, Inc. (San Diego, CA, USA; cat no. A01386a; 1:1,000 dilution
and cat no. AM7718a; 1:500 dilution, respectively). Phosphorylated
Src (p-Src) was purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA; cat. no. 2101; 1:1,000 dilution) and
phosphorylated Fak (p-Fak) was purchased from Abgent, Inc. (cat no.
AJ1285e; 1:1,000 dilution). β-actin monoclonal antibodies were
purchased from Abgent, Inc. (cat. no. AM1021B-ev; 1:2,000
dilution).
Determination of cell viability
Cells were plated at a density of 1×104
cells/well in 96-well plates, and the cell viability was determined
using a conventional MTT reduction assay according to the
manufacturer's protocol. MTT assays rely primarily on the
mitochondrial metabolic capacity of viable cells and reflect the
intracellular redox state. Cells were treated with 0.00, 1.25,
2.50, 5.00, 10.00 or 20.00 mg/l of Src inhibitor for 48 h at 37°C.
The medium was removed and 100 µl of DMSO was added to each well to
dissolve the formed formazan dye crystals for 15 min, subsequently
the absorbance at 570 nm was measured using a microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA). Results were
expressed as the percentage of the MTT reduction, assuming that the
absorbance of the control was 100%.
Western blot analysis
Cultures of HL-60 cells in the logarithmic growth
phase at a density of 2×105 cells/ml were harvested,
washed with ice-cold PBS, and suspended in 0.5 ml lysis buffer (10
mmol/l Tris-HCl pH 7.6, 100 mmol/l NaCl, 1.0 mmol/l
ethylenediaminetetraacetic acid and 100 mg/l phenylmethylsulfonyl
fluoride) containing protease inhibitor aprotinin (1 mg/l). Lysates
were centrifuged at 8,000 × g for 10 min at 37°C, and the protein
concentrations in extracts were quantified using a bicinchoninic
acid protein quantified kit according to the manufacturer's
protocol. (Pierce; Thermo Fisher Scientific, Inc.). Total protein
(20–25 µg) was separated using 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and transferred to a
polyvinylidene fluoride membrane. The membrane was blocked using 5%
nonfat milk in Tris-buffered saline (TBS) containing 0.1% Tween-20
for 2 h at room temperature and incubated for 2 h at room
temperature with a 1:1,000 dilution of DJ-1 monoclonal antibodies.
The antibody treated membrane was washed 3 times for 5 min in TBS
containing 0.1% Tween-20 and then incubated with a 1:1,000 dilution
of horseradish peroxidase (HRP)-conjugated secondary antibody
(Sheep anti-rabbit immunoglobulin-HRP; cat no. KGAA35; 1:1,000
dilution; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) for 1 h
at room temperature. The membrane was washed again 3 times for 10
min with TBS containing 0.1% Tween and developed by an enhanced
Chemiluminescence Plus kit according to the manufacturer's protocol
(cat no. CW0049M; Beijing ComWin Biotech Co., Ltd., Beijing,
China). Experimental data were analyzed using GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA). Where indicated, the
blots were stripped and reprobed with antibodies directed against
β-actin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Transwell cell migration assay
Logarithmic growth phase cells were collected and
centrifuged at 200 × g for 5 min. Subsequently, the supernatant was
discarded and serum-free medium (RPMI-1640 medium; Hyclone; GE
Healthcare Life Sciences) was added to form the cell suspension,
adjusting the cell concentration to 2×105 cells/ml. A
total of 500 µl culture medium containing a 0.1 volume fraction of
fetal bovine serum was added to 24-well plates, which were placed
in the Transwell chamber, and 200 µl cell suspension was added and
incubated at 37°C in a volume fraction of 0.05 CO2 for
24 h. Seeded in 96-well plates, each well had 20 µl MTT solution
added and incubation continued for 4 h. Centrifugation was
performed at 400 × g for 5 min at 37°C, and DMSO (150 µl) was added
to each well, agitated for 20 min in a flat shaker, Subsequently,
the absorbance of each well was measured using ELISA at a 450 nm
wavelength (cat no. ab100578; Abcam, Cambridge, UK) according to
the manufacturer's protocol.
Transwell cell invasion assay
Solid Matrigel was placed in the refrigerator
overnight at 4°C and then dissolved into liquid. A 24-well culture
plate was placed into the Transwell chamber, and diluted Matrigel
(50 µl) was added to each chamber under 15°C. Subsequently, it was
placed into a cell incubator overnight Logarithmic growth phase
cells were collected, the serum removed and RPMI-1640 medium
(Hyclone; GE Healthcare, Chicago, IL, USA) prepared as the cell
suspension, which adjusted the cell concentration to
2×105 cells/ml. In the 24-well plates placed in the
lower chamber, a 0.1 volume fraction of fetal bovine serum medium
(500 µl) was added, in addition to 200 µl cell suspension and
cultured for 24 h. The chamber was immersed in 4% paraformaldehyde
for 30 min at 37°C and rinsed two times with PBS. Then, when
completely dry, staining was performed using a pre-diluted 1:9
Giemsa dye liquor at room temperature for 20–30 min. Subsequently
it was rinsed with deionized water several times, and several
sections were selected at random to be visualized under the light
microscope (magnification, ×20). The number of cells were then
calculated.
Statistical analysis
Data are expressed as the mean ± the standard
deviation. The significance of inter-group differences was
evaluated using a one-way analysis of variance with Scheffe's test
used for post hoc comparisons. Transwell and western blot data were
analyzed using SPSS statistical software (version 18.0; SPSS,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
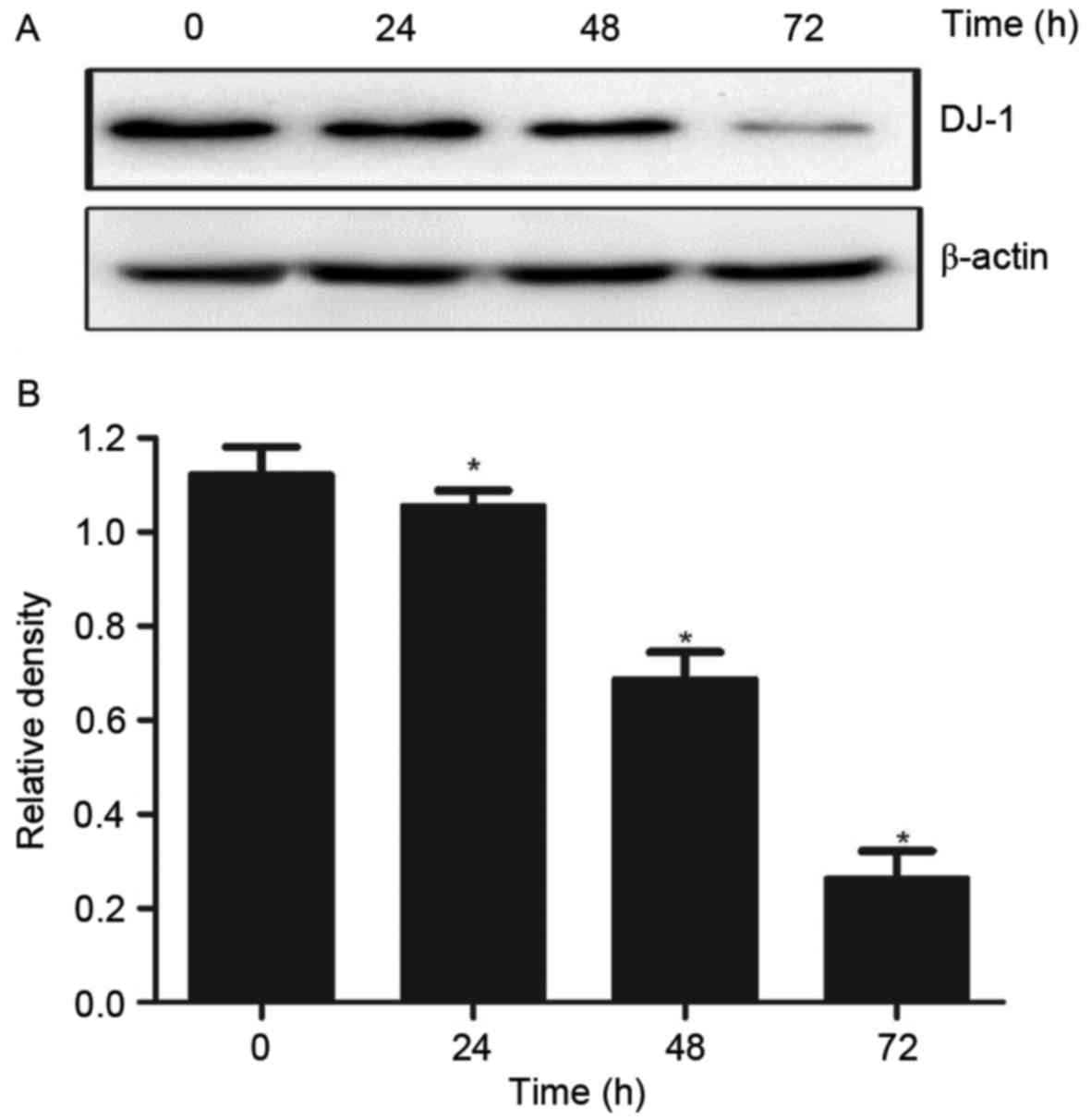
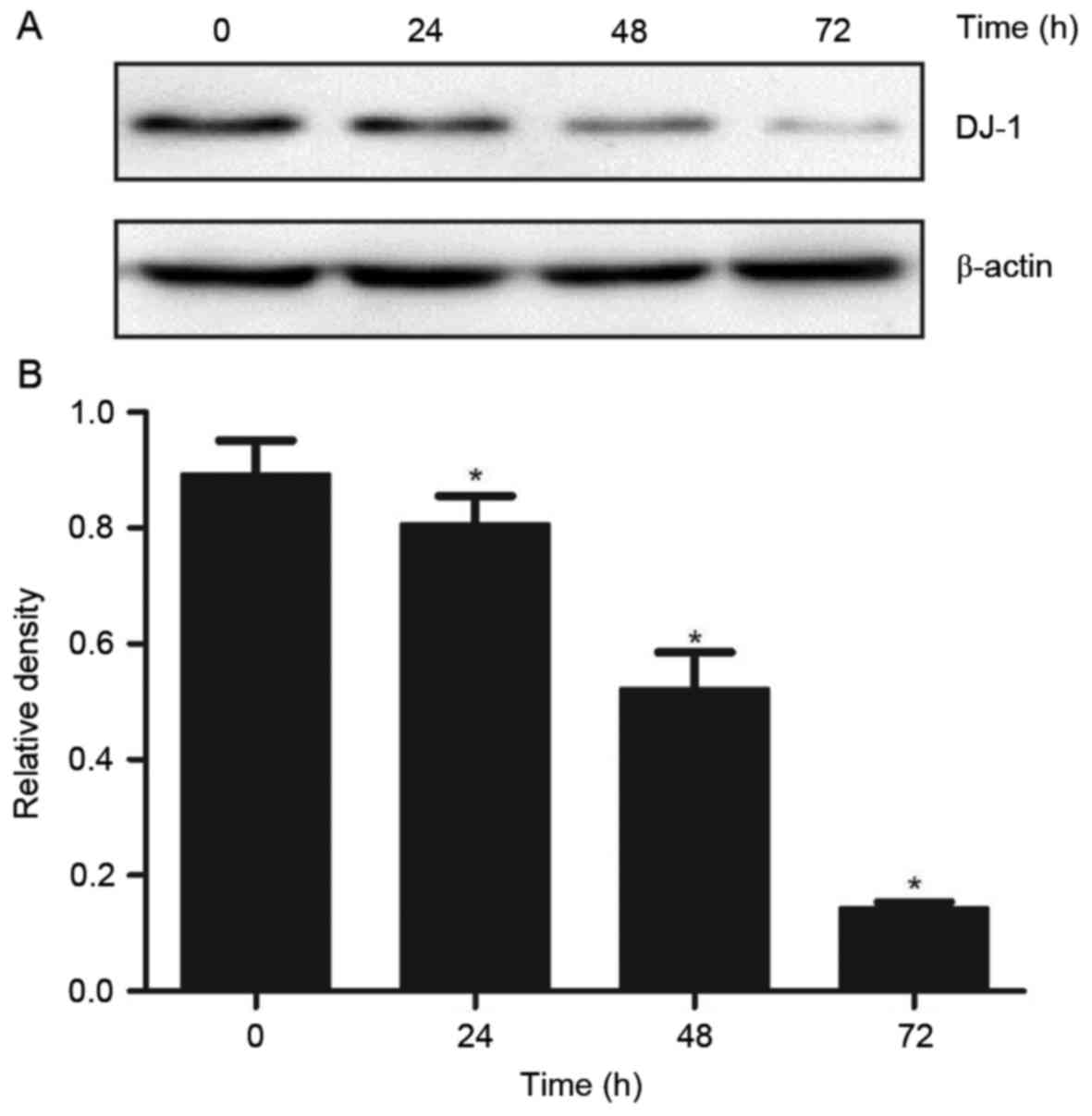
DADS treatment affects the expression
levels of DJ-1 protein in HL-60 cells
In order to test the effect of DADS on the
expression levels of DJ-1 protein in HL-60 cells, the DJ-1 protein
expression levels were measured following DADS treatment in HL-60.
Western blot analysis results indicated that the expression levels
of DJ-1 protein in HHDN cells treated with DADS for 24, 48 and 72 h
was significantly reduced, demonstrating a time-dependent decrease
(P<0.05; Fig. 1).
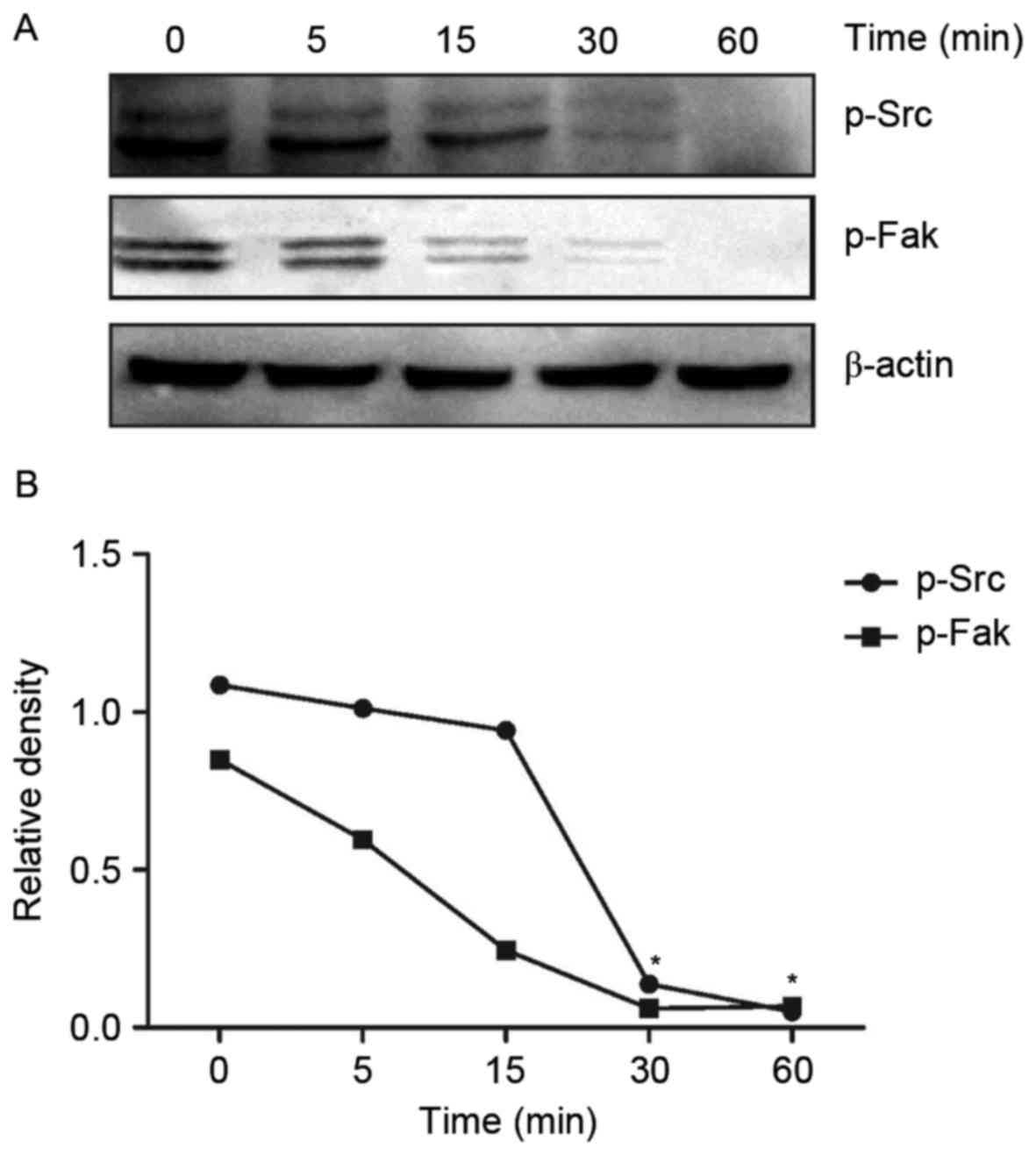
DADS affects the Src signaling pathway
in HHDN cells
In order to test the effect of DADS treatment on the
Src signaling pathway, the protein expression levels of
DADS-treated HHDN cells at time intervals of 0, 5, 15, 30 and 60
min was examined. Compared with untreated cells, after HL-60 cells
were treated with DADS for 30 min, the expression of p-Src and
p-Fak was gradually inhibited (Fig.
2A). It also demonstrated a direct decline in protein
expression levels with an increase in DADS treatment time (Fig. 2B). The protein expression levels of
Src and Fak almost reached zero after 60 min of treatment, when the
expression of Src and Fak was absolutely inhibited.
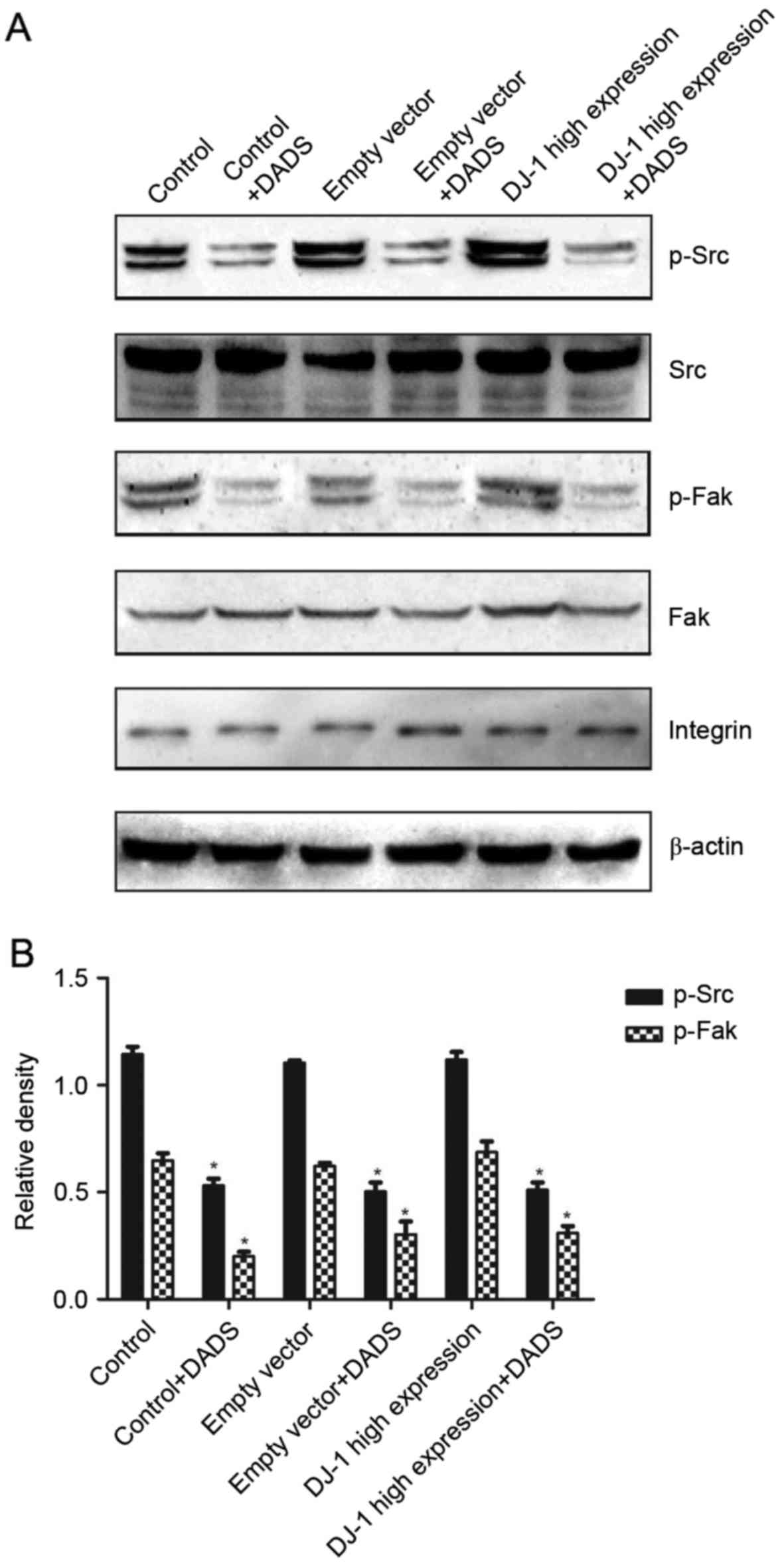
After 30 min of treatment with DADS, in a control
group, empty vector group and high expression group, the expression
levels of p-Frk and p-Src compared with the untreated groups was
significantly reduced, with a statistically significant difference
(P<0.05; Fig. 3). No significant
difference was identified in the high expression group compared
with the control group and empty vector group (P>0.05),
indicating that DADS may inhibit the Src signaling pathway.
Additionally, in the three groups of cells without DADS treatment,
Src and Fak were highly expressed compared with treated cells. In
leukemia cells, the Src signaling pathway is in an activated
state.
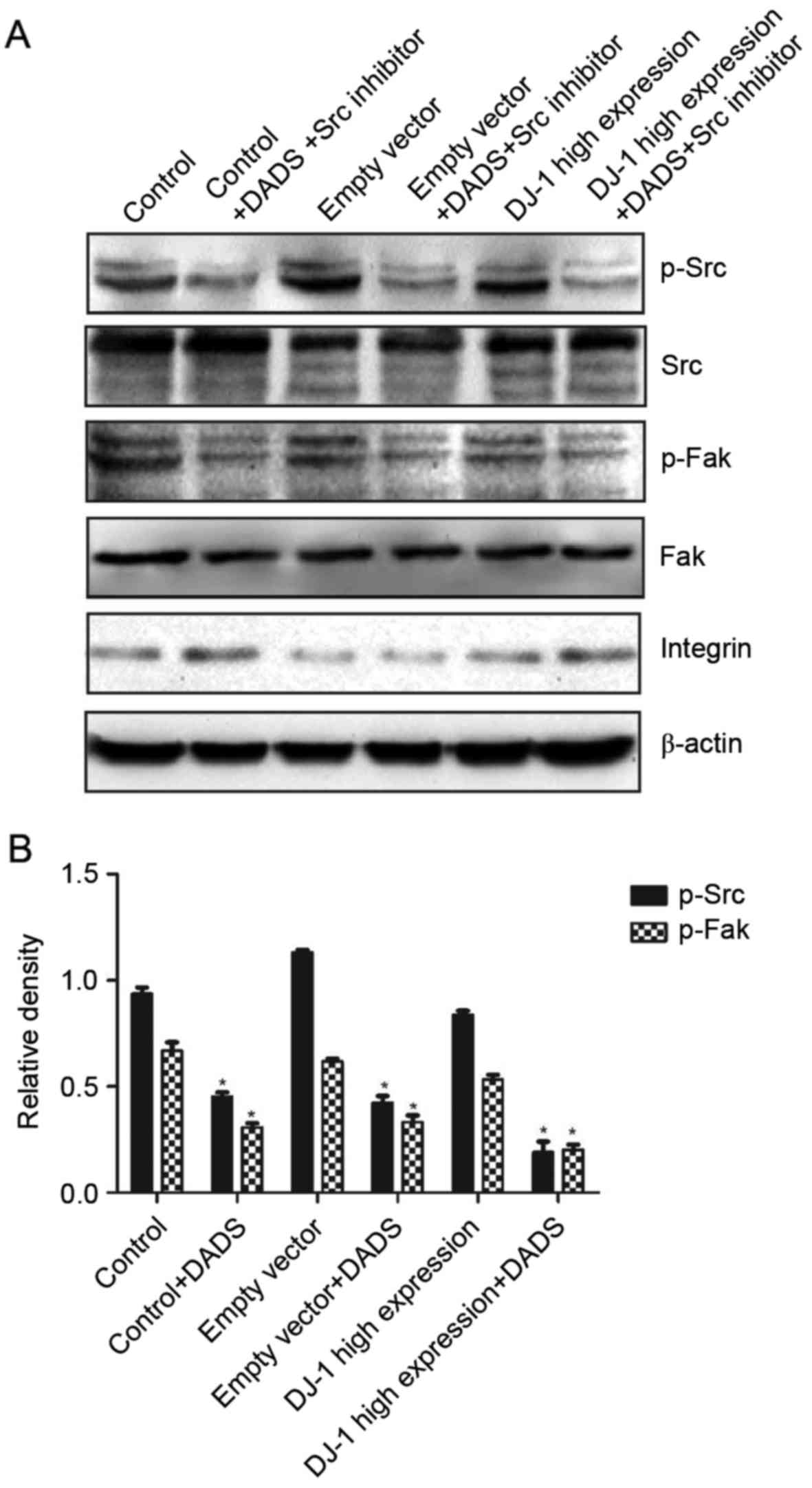
Effect of DADS and Src inhibitor on
the Src signaling pathway of HHDN cells
Following treatment with DADS and Src inhibitor, the
expression levels of p-Src and p-Fak compared with the untreated
group were significantly inhibited, and the difference was
statistically significant (P<0.05; Fig. 4). However, there was no significant
difference between the high expression group and the control group
and empty vector group (P>0.05). The results demonstrated that
DADS and Src inhibitors may be combined to inhibit the Src
signaling pathway.
Effect of DADS and Src inhibitor on
the DJ-1 protein expression levels of HHDN cells
Compared with the untreated group, DJ-1 protein
expression levels demonstrated a time-dependent decrease with DADS
and Src inhibitor treated HHDN at 24, 48 and 72 h, with a
statistically significant difference (P<0.05), indicating that
the DJ-1 gene may be a potential therapeutic target (Fig. 5).
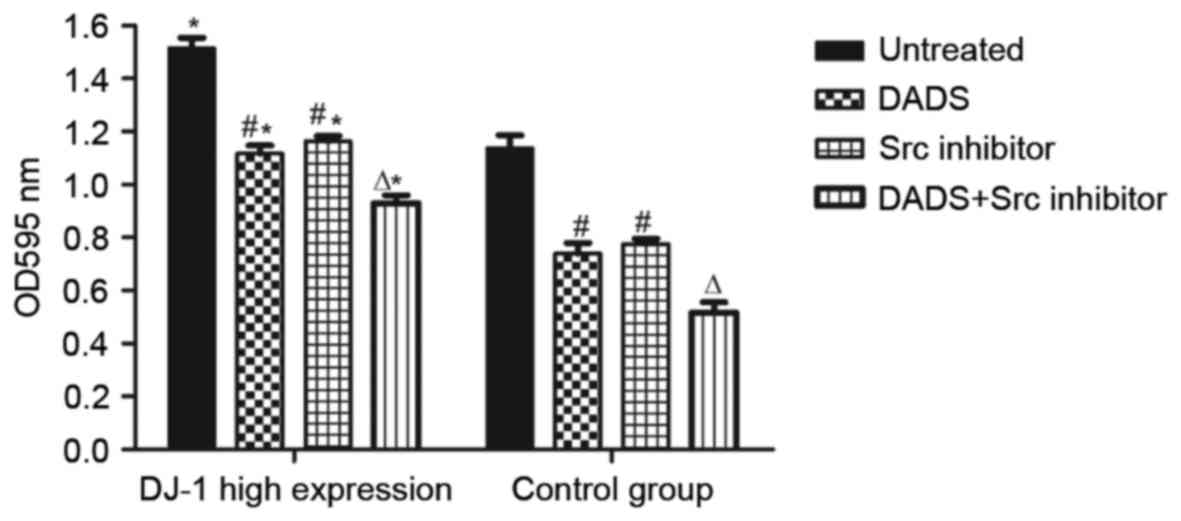
Effect of DADS and Src inhibitor
treatment on the invasion and migration ability of HHDN cells
It was revealed that in the high expression cells of
each group compared with the control group, the high expression
group presented a significantly higher migration rate (P<0.05),
which demonstrated a positive association between the high
expression of DJ-1 and the migration of HL-60 cells (Table I, Fig.
6). Following treatment with DADS for 24 h, the cell migration
rate of the two groups decreased significantly compared with the
control (P<0.05), while the groups treated with Src inhibitor
for 24 h presented no significant difference compared with the DADS
groups (P>0.05). Groups treated with combined DADS and Src
inhibitor treatment presented a significantly lower cell migration
rate compared with groups treated with either DADS or Src inhibitor
alone (P<0.05). All results indicate that DADS and Src inhibitor
treatments individually inhibit leukemia cell migration, and
demonstrate synergistic effects on the inhibition of leukemia cell
migration.
 | Table I.Effect of DADS and Src inhibitor
treatments on the migration ability of HHDN cells. |
Table I.
Effect of DADS and Src inhibitor
treatments on the migration ability of HHDN cells.
| Group | No treatment
group | DADS-treated
group | Src
inhibitor-treated group | Combined treatment
group |
|---|
| HHDN |
1.514±0.04a |
1.117±0.03b,a |
1.163±0.02b,a |
0.929±0.04c,a |
| HL-60 |
1.136±0.05 |
0.739±0.04b |
0.775±0.02b |
0.516±0.03c |
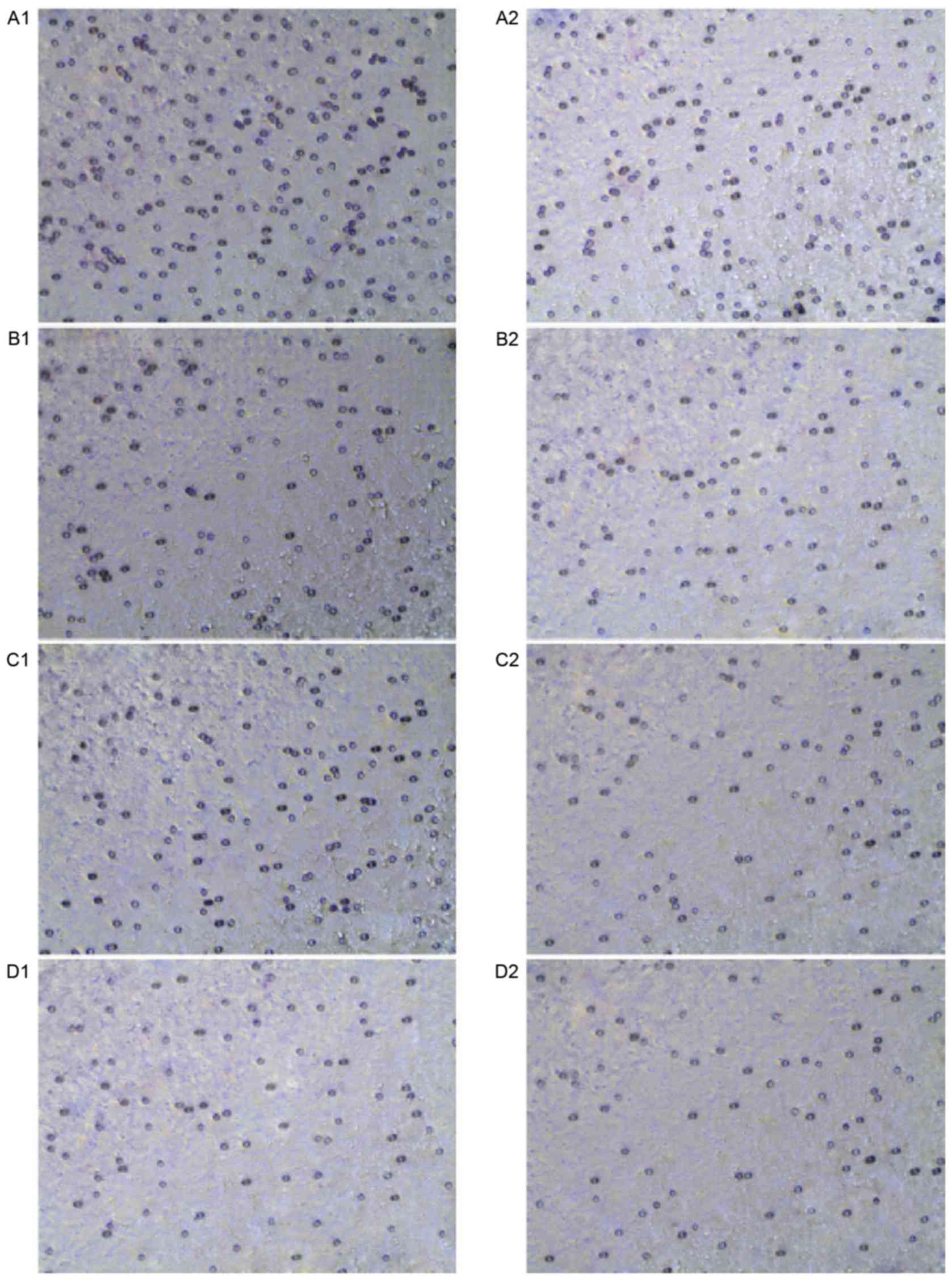
The invasion cell numbers through the matrigel in
the high expression groups were greater compared with the numbers
in the control groups, which indicated a positive association
between the high expression of DJ-1 and the invasion ability of
HL-60 cells. Following treatment with DADS for 24 h, the number of
cells counted that had invaded through the matrigel in the DADS
treated group had decreased significantly compared to the untreated
group, however no difference was apparent compared with the groups
treated with Src inhibitor treatment for 24 h (P>0.05).
Furthermore, the groups treated with combined DADS and Src
inhibitor demonstrated a lower cell count compared with the groups
treated with either DADS or Src inhibitor alone (P<0.05;
Table II and Fig. 7). The results revealed that DADS and
Src inhibitor treatments individually inhibit leukemia cell
invasion, and demonstrated synergistic effects on the inhibition of
leukemia cell invasion.
 | Table II.Effect of DADS and Src inhibitor
treatments on the invasion ability of HHDN. |
Table II.
Effect of DADS and Src inhibitor
treatments on the invasion ability of HHDN.
| Group | No treatment
group | DADS-treated
group | Src
inhibitor-treated group | Combined treatment
group |
|---|
| HHDN |
221.7±5.897a |
155.3±6.173a,b |
162.7±3.844a,b |
89.67±4.910a,c |
| HL-60 cells |
175.3±6.642 |
117.3±5.783b |
122.3±3.930b |
73.67±4.410c |
Discussion
DADS, an organic sulfuric compound present in
garlic, is a promising anticancer candidate that inhibits a variety
of types of tumor (19–23). In tumor cell differentiation, cell
cycle regulation and apoptosis, it may inhibit the expression of
certain oncogenes (24). In addition,
one study has demonstrated that DADS exhibits stronger antitumor
effects compared with cisplatin on certain tumors, and presents
fewer side effects (25).
In clinical practice, >90% of patients with
cancer succumb due to tumor invasion and metastasis (26). A previous study has demonstrated that
DADS significantly inhibited the proliferation of HL-60 cells
cultured in vitro in a dose-responsive manner. Moderate
doses (>1.25 mg/l) may induce apoptosis in HL-60 cells, whereas
low-dose DADS (<1.25 mg/l) induced the differentiation of HL-60
cells (27). It was preliminarily
identified that DADS is able to induce the expression of 18
differing types of protein in human leukemia HL-60 cells, of which
DJ-1 protein may be downregulated, which belongs to a
cancer-causing protein family associated with oncogenesis and
development (28).
DJ-1 is an oncogenic protein that regulates the
interaction between proteins and RNA, and previous studies have
revealed that DJ-1 is highly expressed in lung, esophageal,
pancreatic, liver, breast and laryngeal cancer, as well as other
malignant tumors (29–32). Upregulated expression of the DJ-1 gene
may promote oncogenesis, and inhibit the reduce proliferation of
chemotherapeutic drugs against cancer cells, which is associated
with chemotherapeutic resistance. These studies suggest that the
cancer-promoting gene DJ-1 may be used to diagnose and predict
prognosis in patients with cancer, and has potential value in
clinical practice (33).
DJ-1, expressed in the cytoplasm, nucleus and
mitochondria, is a regulatory molecule of gene transcription. In
the S phase, it is transferred from the cytoplasm to the nucleus,
and DJ-1 expressed in different subcellular locations regulates
different physiological and pathological features. If expression is
localized to the mitochondria, then it is involved in oxidative
stress process (33,34), whilst nuclear localized expression
inhibits apoptosis (35,36). DJ-1 highly expressed in the nucleus
promotes HL-60 cell proliferation and migration, and enhances
invasion capability, but an interfering DJ-1 gene is able to
enhance proliferation inhibition against DADS and induce the
differentiation of HL-60 cells (37).
HHDN are a highly invasive cell line, as the
migration and invasion ability of tumor cells appears to be
associated with highly-expressed DJ-1; however, its mechanism
remains unclear (14,30,38–40). In
the present study, western blot analysis was used to examine how
DADS affects the expression of the DJ-1 protein in HHND cells, and
the DJ-1 protein expression levels revealed a time-dependent
decrease with DADS-treatment. Thus, it was posited that DADS may
downregulate the expression of DJ-1, and inhibit the migration and
invasion ability of HHND cells. However, the specific mechanism
remains unknown and is yet to be confirmed.
DJ-1 promotes tumor cell division, proliferation,
migration and invasion, and is likely to be involved in several
coordinated intracellular molecular pathways. Li et al
(41) reported that the DJ-1 protein
is one of the major negative regulator proteins of the PTEN tumor
suppressor gene. DJ-1 protein promotes tumor cell proliferation and
growth by inhibiting PTEN activity, and stimulating the
phosphoinositide 3-kinase/protein kinase B signaling pathway
(42). DJ-1 promotes nuclear
translocation of nuclear factor-κβ, regulates cell differentiation
and inhibits apoptosis (43). DJ-1
regulates the transcription factor nuclear factor erythroid
2-related factor 2 signaling pathway and promotes cytoprotective
gene expression (44). It is also a
target of regulation of Src and extracellular signal-regulated
kinase signaling pathways, promoting tumor cell proliferation,
migration and invasion (45).
It was revealed that integrins are associated with
tumor cell adhesion, migration and invasion in a transplanted tumor
model of breast cancer in zebrafish, and mice (46,47). The
extracellular matrix-integrin signal transduction pathway is
involved with the Src signaling pathway, where Fak is a key factor.
Under the action of integrin, Fak phosphorylates and interacts with
Src (48). An experimental study has
confirmed that interfering PTEN may inhibit the phosphorylation of
Fak so as to inhibit the proliferation, migration and invasion of
liver cancer, and leukemia cell (42). Thus, it was hypothesized that DJ-1 and
Src signaling pathways may be potentially associated in the
invasion, and migration of leukemic cells (49). Scr promotes cell proliferation,
adhesion, migration and invasion in tumorigenesis (50). Src activation is required for
induction of integrin (51). In the
present study, a western blot analysis was used to examine how DADS
influences the Src signaling pathways in HHND cells, and further
confirmed that DADS may inhibit the invasion of and migration of
HHND cells through negative regulation of the Src signaling
pathway. The results revealed that DADS may inhibit the Src
signaling pathway.
Src kinases in targeted therapy is an important
topic (52). Inhibiting Src activity
may inhibit the proliferation of a variety of types of tumor,
including the invasive migratory effect (53,54). In
the present study, it was revealed that Src treatment may inhibit
the migration and invasion ability of leukemia cells, whereas the
combination of DADS and Src treatments demonstrated more
significant inhibitory effects. Therefore, it may be considered
that antagonizing the Src signaling pathway inhibits the invasion
and migration ability of leukemia cells.
In conclusion, DADS suppresses the invasion and
migration of HHND cells by antagonizing the Src signaling pathway
and downregulating its expression. It was demonstrated that DJ-1
may be a potential target for gene therapy, and may improve the
DADS antitumor effect on leukemia. Finally, Src inhibitor combined
with DADS treatment may be used as a type of therapy to achieve
more improved antitumor effects, providing a more safe and
effective drug for patients with leukemia.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Construct
Program of the Key Discipline in the Hunan Province of China [grant
no. (2011)76], the National Natural Science Foundation of China
(grant nos. 81100375; 81400117) and the Platform Open Innovation
Fund Project for Hunan Province Universities (grant no. 1
1K057).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
RL and YY conducted the majority of the experiments
and analyzed data, and wrote the manuscript. JQ, QL, WW, JW and YT
performed experiments. LY contributed reagents and performed
experiments. HT designed the project and led the team to accomplish
it. All authors reviewed the manuscript.
Ethics and consent to participate
Medical Ethical Committee of University of South
China approved, and written informed consent was gained from all
participants.
Consent for publication
The study participants provided consent for the data
to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pratheeshkumar P, Thejass P and Kutan G:
Diallyl disulfide induces caspase-dependent apoptosis via
mitochondria-mediated intrinsic pathway in B16F-10 melanoma cells
by up-regulating p53, caspase-3 and down-regulating
pro-inflammatory cytokines and nuclear factor-κβ-mediated Bcl-2
activation. J Environ Pathol Toxicol Oncol. 29:113–125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altonsy MO, Habib TN and Andrews SC:
Diallyl disulfide-induced apoptosis in a breast-cancer cell line
(MCF-7) may be caused by inhibition of histone deacetylation. Nutr
Cancer. 64:1251–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang H, Kong Y, Guo J, Tang Y and Xie X,
Yang L, Su Q and Xie X: Diallyl disulfide suppresses proliferation
and induces apoptosis in human gastric cancer through Wnt-1
signaling pathway by up-regulation of miR-200b and miR-22. Cancer
Lett. 340:72–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao X, Chen B, Liu X, Liu P, Zheng G, Ye
F, Tang H and Xie X: Diallyl disulfide suppresses SRC/Ras/ERK
signaling-mediated proliferation and metastasis in human breast
cancer by up-regulating miR-34a. PLoS One. 9:e1127202014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Truong D, Hindmarsh W and O'Brien PJ: The
molecular mechanisms of diallyl disulfide and diallyl sulfide
induced hepatocyte cytotoxicity. Chem Biol Interact. 180:79–88.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee IC, Kim SH, Baek HS, Moon C, Kim SH,
Kim YB, Yun WK, Kim HC and Kim JC: Protective effects of diallyl
disulfide on carbon tetrachloride-induced hepatotoxicity through
activation of Nrf2. Environ Toxicol. 30:538–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin X, Zhang J, Li X, Liu D, Feng C, Liang
R, Zhuang K, Cai C, Xue X, Jing F, et al: DADS suppresses human
esophageal xenograft tumors through RAF/MEK/ERK and
mitochondria-dependent pathways. Int J Mol Sci. 15:12422–12441.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsubura A, Lai YC, Kuwata M, Uehara N and
Yoshizawa K: Anticancer effects of garlic and garlic-derived
compounds for breast cancer control. Anticancer Agents Med Chem.
11:249–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jie HE WY and Huang WG: Analysis of
protein expression of cell differentiation induced by HL-60 cells
by two dimensional gel electrophoresis. J Nan Uni (Med Edi).
32:143–6. 2004.
|
|
10
|
Chien CH, Lee MJ, Liou HC, Liou HH and Fu
WM: Local immunosuppressive microenvironment enhances migration of
melanoma cells to lungs in DJ-1 knockout mice. PLoS One.
10:e01158272015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan J, Ren H, Jia N, Fei E, Zhou T, Jiang
P, Wu M and Wang G: DJ-1 decreases Bax expression through
repressing p53 transcriptional activity. J Biol Chem.
283:4022–4030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pei XJ, Wu TT, Li B, Tian XY, Li Z and
Yang QX: Increased expression of macrophage migration inhibitory
factor and DJ-1 contribute to cell invasion and metastasis of
nasopharyngeal carcinoma. Int J Med Sci. 11:106–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang B, Qin H, Wang Y, Chen W, Luo J, Zhu
X, Wen W and Lei W: Effect of DJ-1 overexpression on the
proliferation, apoptosis, invasion and migration of laryngeal
squamous cell carcinoma SNU-46 cells through PI3K/AKT/mTOR. Oncol
Rep. 32:1108–1116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Devine MJ, Plun-Favreau H and Wood NW:
Parkinson's disease and cancer: Two wars, one front. Nat Rev
Cancer. 11:812–823. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
He X, Zheng Z, Li J, Ben Q, Liu J, Zhang
J, Ji J, Yu B, Chen X, Su L, et al: DJ-1 promotes invasion and
metastasis of pancreatic cancer cells by activating SRC/ERK/uPA.
Carcinogenesis. 33:555–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Che P, Yang Y, Han X, Hu M, Sellers JC,
Londono-Joshi AI, Cai GQ, Buchsbaum DJ, Christein JD, Tang Q, et
al: S100A4 promotes pancreatic cancer progression through a dual
signaling pathway mediated by Src and focal adhesion kinase. Sci
Rep. 5:84532015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ku MJ, Kim JH, Lee J, Cho JY, Chun T and
Lee SY: Maclurin suppresses migration and invasion of human
non-small-cell lung cancer cells via anti-oxidative activity and
inhibition of the Src/FAK-ERK-β-catenin pathway. Mol Cell Biochem.
402:243–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang XQ, Liu XF, Yao L, Chen CQ, Lin JF,
Gu ZD, Ni PH, Zheng XM and Fan QS: Focal adhesion kinase regulates
the phosphorylation protein tyrosine phosphatase-α at Tyr789 in
breast cancer cells. Mol Med Rep. 11:4303–4308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altonsy MO, Habib TN and Andrews SC:
Diallyl disulfide-induced apoptosis in a breast-cancer cell line
(MCF-7) may be caused by inhibition of histone deacetylation. Nutr
Cancer. 64:1251–1260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwon KB, Yoo SJ, Ryu DG, Yang JY, Rho HW,
Kim JS, Park JW, Kim HR and Park BH: Induction of apoptosis by
diallyl disulfide through activation of caspase-3 in human leukemia
HL-60 cells. Biochem Pharmacol. 63:41–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jakubíková J and Sedlák J: Garlic-derived
organosulfides induce cytotoxicity, apoptosis, cell cycle arrest
and oxidative stress in human colon carcinoma cell lines.
Neoplasma. 53:191–199. 2006.PubMed/NCBI
|
|
22
|
Wu XJ, Kassie F and Mersch-Sundermann V:
The role of reactive oxygen species (ROS) production on diallyl
disulfide (DADS) induced apoptosis and cell cycle arrest in human
A549 lung carcinoma cells. Mutat Res. 579:115–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakagawa H, Tsuta K, Kiuchi K, Senzaki H,
Tanaka K, Hioki K and Tsubura A: Growth inhibition effects of
diallyl disulfide on human breast cancer cell lines.
Carcinogenesis. 22:891–897. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin DY, Kim GY, Lee JH, Choi BT, Yoo YH
and Choi YH: Apoptosis induction of human prostate carcinoma DU145
cells by diallyl disulfide via modulation of JNK and PI3K/AKT
signaling pathways. Int J Mol Sci. 13:14158–14171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsubura A, Lai YC, Kuwata M, Uehara N and
Yoshizawa K: Anticancer effects of garlic and garlic-derived
compounds for breast cancer control. Anticancer Agents Med Chem.
11:249–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carpinteiro A, Becker KA, Japtok L,
Hessler G, Keitsch S, Požgajovà M, Schmid KW, Adams C, Müller S,
Kleuser B, et al: Regulation of hematogenous tumor metastasis by
acid sphingomyelinase. EMBO Mol Med. 7:714–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu MH TL, Li LP, Huang WG and Su Q: Effect
of growth inhibition and differentiation of HL-60 cell induced by
diallyl disulfide. Zhonghua Xue Ye Xue Za Zhi. 25:300–2. 2004.
|
|
28
|
He J SQ, Huang WG, Xie HL, Liang SP, Song
Y and Xie JY: Proteomic initial Analysis of differentiation of
human myeloid leukemia cells induced by diallyl disulfide. FEBS J.
272 Suppl 1:4402005.
|
|
29
|
Zhang HY, Wang HQ, Liu HM, Guan Y and Du
ZX: Regulation of tumor necrosis factor-related apoptosis-inducing
ligand-induced apoptosis by DJ-1 in thyroid cancer cells. Endocr
Relat Cancer. 15:535–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu F, Liang YQ and Huang ZM: The
expression of DJ-1 gene in human hepatocellular carcinoma and its
relationship with tumor invasion and metastasis. Zhonghua Gan Zang
Bing Za Zhi. 17:203–206. 2009.(In Chinese). PubMed/NCBI
|
|
31
|
Zhu XL, Wen WP, Lei WB, Chai LP, Hou WJ,
Wen YH and Wang XR: DJ-1 expression in laryngeal squamous cell
carcinoma and its relationship with tumor recurrence and
metastasis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
45:497–501. 2010.(In Chinese). PubMed/NCBI
|
|
32
|
Wei W, Tang C, Zhan X, Yi H and Li C:
Effect of DJ-1 siRNA on biological behavior of human lung squamous
carcinoma SK-MES-1 cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
38:7–13. 2013.(In Chinese). PubMed/NCBI
|
|
33
|
Junn E, Jang WH, Zhao X, Jeong BS and
Mouradian MM: Mitochondrial localization of DJ-1 leads to enhanced
neuroprotection. J Neurosci Res. 87:123–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hao LY, Giasson BI and Bonini NM: DJ-1 is
critical for mitochondrial function and rescues PINK1 loss of
function. Proc Natl Acad Sci USA. 107:9747–9752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Junn E, Taniguchi H, Jeong BS, Zhao X,
Ichijo H and Mouradian MM: Interaction of DJ-1 with Daxx inhibits
apoptosis signal-regulating kinase 1 activity and cell death. Proc
Natl Acad Sci USA. 102:9691–9696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hwang S, Song S, Hong YK, Choi G, Suh YS,
Han SY, Lee M, Park SH, Lee JH, Lee S, et al: Drosophila DJ-1
decreases neural sensitivity to stress by negatively regulating
Daxx-like protein through dFOXO. PLoS Genet. 9:e10034122013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
JuanWang, Jing yY, Qing Yuxian, Tang
Qingye, Li Qi and Hui Su Tan: DADS inhibits proliferation and
differentiation of human leukemia HL60 cells by down regulated.
Chinese Pharmacological Bulletin. 416–20. 2015.
|
|
38
|
Fang M, Zhong XY, Du B, Lin CL, Luo F,
Tang LJ and Chen J: Role of DJ-1-induced PTEN down-regulation in
migration and invasion of human glioma cells. Chin J Cancer.
29:988–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ismail IA, Kang HS, Lee HJ, Kim JK and
Hong SH: DJ-1 upregulates breast cancer cell invasion by repressing
KLF17 expression. Br J Cancer. 110:1298–1306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu ZM, Li ZR, Huang Y, Yu HH, Huang XS,
Yan YF, Shao JH and Chen HP: DJ-1 is involved in the peritoneal
metastasis of gastric cancer through activation of the Akt
signaling pathway. Oncol Rep. 31:1489–1497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Cui J, Zhang CH, Yang DJ, Chen JH,
Zan WH, Li B, Li Z and He YL: High-expression of DJ-1 and loss of
PTEN associated with tumor metastasis and correlated with poor
prognosis of gastric carcinoma. Int J Med Sci. 10:1689–1697. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gupta A and Dey CS: PTEN, a widely known
negative regulator of insulin/PI3K signaling, positively regulates
neuronal insulin resistance. Mol Biol Cell. 23:3882–3898. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McNally RS, Davis BK, Clements CM,
Accavitti-Loper MA, Mak TW and Ting JP: DJ-1 enhances cell survival
through the binding of Cezanne, a negative regulator of NF-kappaB.
J Biol Chem. 286:4098–4106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ismail IA, Shakor Abdel AB and Hong SH:
DJ-1 protects breast cancer cells against
2′-benzoyloxycinnamaldehyde-induced oxidative stress independent of
Nrf2. J Cell Physiol. 230:2262–2269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He X, Zheng Z, Li J, Ben Q, Liu J, Zhang
J, Ji J, Yu B, Chen X, Su L, et al: DJ-1 promotes invasion and
metastasis of pancreatic cancer cells by activating SRC/ERK/uPA.
Carcinogenesis. 33:555–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chang AY and Wang M: Molecular mechanisms
of action and potential biomarkers of growth inhibition of
dasatinib (BMS-354825) on hepatocellular carcinoma cells. BMC
Cancer. 13:2672013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Y, Drabsch Y, Pujuguet P, Ren J, van
Laar T, Zhang L, van Dam H, Clément-Lacroix P and Ten Dijke P:
Genetic depletion and pharmacological targeting of αv integrin in
breast cancer cells impairs metastasis in zebrafish and mouse
xenograft models. Breast Cancer Res. 17:282015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim SA, Kwon SM, Kim JA, Kang KW, Yoon JH
and Ahn SG: 5′-Nitro-indirubinoxime, an indirubin derivative,
suppresses metastatic ability of human head and neck cancer cells
through the inhibition of Integrin β1/FAK/Akt signaling. Cancer
Lett. 306:197–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Q, Tang Y, Qin J, Yi L, Yang Y, Wang J,
He J, Su Q and Tan H: Subcellular localization of DJ-1 in human
HL-60 leukemia cells in response to diallyl disulfide treatment.
Mol Med Rep. 14:4666–4672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ceppi P, Papotti M, Monica V, Lo Iacono M,
Saviozzi S, Pautasso M, Novello S, Mussino S, Bracco E, Volante M
and Scagliotti GV: Effects of Src kinase inhibition induced by
dasatinib in non-small cell lung cancer cell lines treated with
cisplatin. Mol Cancer Ther. 8:3066–3074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sánchez-Bailón MP, Calcabrini A,
Gómez-Domínguez D, Morte B, Martin-Forero E, Gómez-López G,
Molinari A, Wagner KU and Martín-Pérez J: Src kinases catalytic
activity regulates proliferation, migration and invasiveness of
MDA-MB-231 breast cancer cells. Cell Signal. 24:1276–1286. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Koppikar P, Choi SH, Egloff AM, Cai Q,
Suzuki S, Freilino M, Nozawa H, Thomas SM, Gooding WE, Siegfried JM
and Grandis JR: Combined inhibition of c-Src and epidermal growth
factor receptor abrogates growth and invasion of head and neck
squamous cell carcinoma. Clin Cancer Res. 14:4284–4291. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang SE, Xiang B, Zent R, Quaranta V,
Pozzi A and Arteaga CL: Transforming growth factor beta induces
clustering of HER2 and integrins by activating Src-focal adhesion
kinase and receptor association to the cytoskeleton. Cancer Res.
69:475–482. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sun B, Meng J, Xiang T, Chen Z, Li Y, Lu
L, Zhang S and Chen X: Jianpijiedu fang improves survival of
hepatocarcinoma mice by affecting phosphatase and tensin homolog,
phosphoinositide 3-kinase, and focal adhesion kinase. J Tradit Chin
Med. 33:479–485. 2013. View Article : Google Scholar : PubMed/NCBI
|