Introduction
Breast cancer is a significant health problem in
women globally, and breast cancer incidence rates are higher in
North America and Western Europe, compared with those in other
parts of the world. In the United States in 2016, there were
~249,260 new cases of breast cancer and 40,890 cancer-related
deaths (1). In China, breast cancer
has become one of the most frequently diagnosed cancers in women
aged 30–59 years old (2), and a high
proportion of the patients are diagnosed at the advanced stages of
disease (2); for those patients,
treatment responses, even with post-surgical chemotherapy and
radiotherapy, remain poor (2). Thus,
novel molecular biomarkers are required for the early detection and
treatment response prediction in patients with breast cancer.
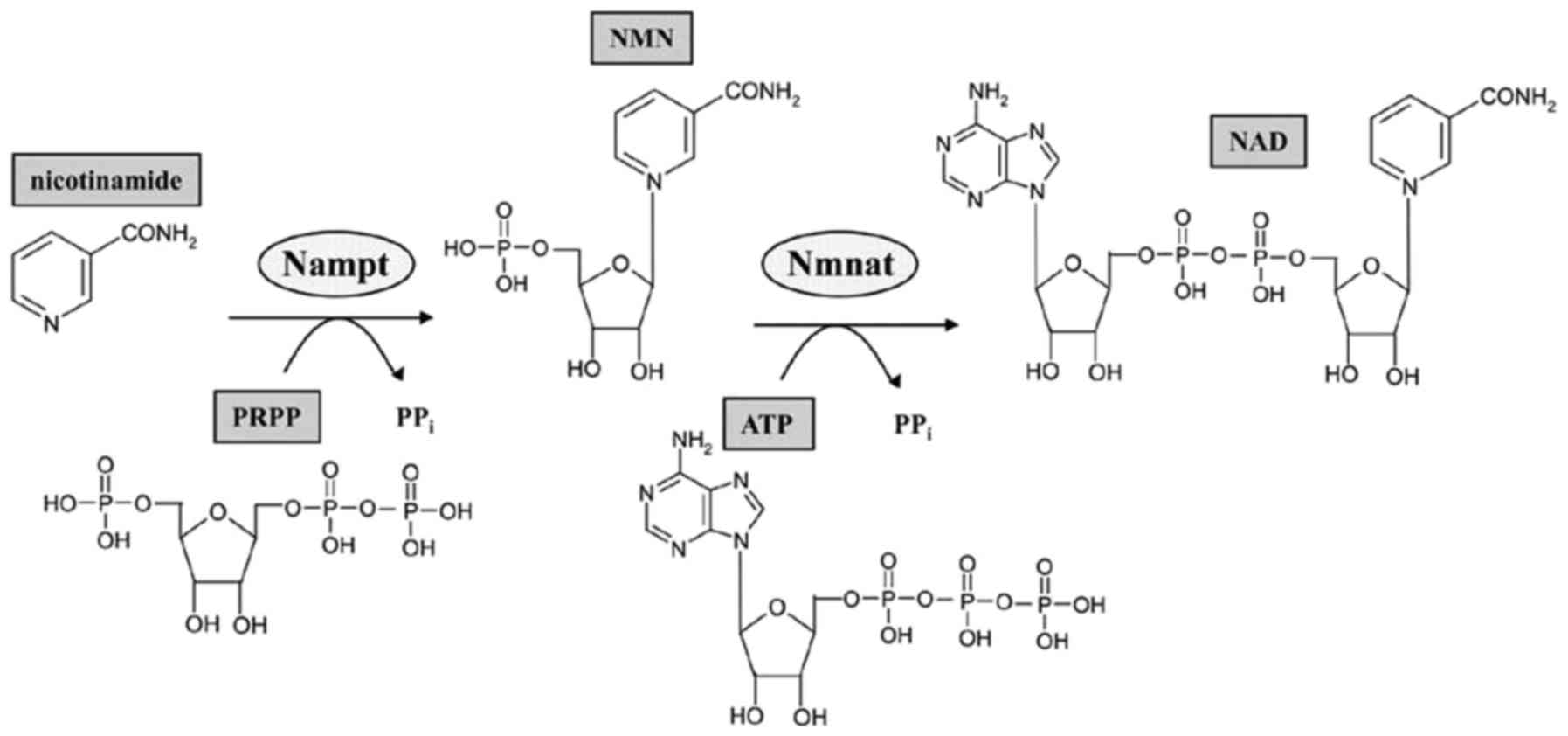
Mammalian cell nicotinamide
phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme in
the biosynthesis of nicotinamide adenine dinucleotide (NAD) that is
responsible for transferring a phosphoribosyl group from
5-phosphoribosyl-1-pyrophosphate to nicotinamide, resulting in the
production of nicotinamide mononucleotide (NMN) and pyrophosphate.
NMN is then converted to NAD by NMN adenylyltransferase (Fig. 1) (3).
Increasing evidence suggests that NAMPT is a multifunctional enzyme
with crucial roles in in metabolism and immune response, and
altered NAMPT expression is associated with human tumorigenesis
(4). Silencing of NAMPT expression in
pancreatic cancer cells has been demonstrated to induce tumor cell
metabolic collapse and cell death both in vitro and in
vivo (5), whereas NAMPT
overexpression is associated with poor treatment response of breast
cancer patients to doxorubicin-based chemotherapy (6). Previous studies from our group have
demonstrated that NAMPT is highly expressed in gastric cancer and
is associated with malignant behaviors of cancer cells as well as
resistance to chemotherapy (7,8). The aim
of the present study was to detect NAMPT expression in normal and
cancerous breast tissues using immunohistochemistry and to examine
its association with clinicopathological and survival data from
breast cancer patients.
Materials and methods
Patient samples
Paired cancerous and adjacent noncancerous breast
tissues were collected from 83 newly diagnosed and surgically
treated breast cancer patients at Weihai Municipal Hospital
(Weihai, China) between January and December 2008. The adjacent
normal tissues were >5 cm away from the tumor lesions. All
patients were histologically diagnosed with invasive ductal
carcinoma and aged 29–66 years old, with an average age of 47
years. None of the patients received presurgical radiotherapy or
chemotherapy. The present study was approved by the Ethics
Committee of Weihai Municipal Hospital, and informed consent was
obtained from each patient. The histologic types and grades of the
primary tumors were determined according to the modifications of
the World Health Organization classification (9), whereas the staging of breast cancer was
defined according to the tumor-node-metastasis (TNM) system
(10).
Immunohistochemistry
All tissue samples were fixed in 10% buffered
formalin at room temperature for 24 h and subsequently embedded
into paraffin. Tissue sections 4-µm thick were then prepared from
these paraffin blocks and immunohistochemically stained using the
streptavidin peroxidase (SP) technique. In brief, tissue sections
were deparaffinized in xylene, rehydrated in a series of ethanol
solutions, and then submerged in tap water. The tissue sections
were subjected to antigen retrieval in a pressure cooker containing
0.01 M citrate buffer and blocking of the peroxidase activity in 3%
H2O2 for 30 min at room temperature. Next,
they were incubated with 20% normal goat serum (Abcam, Cambridge,
MA, USA) diluted in PBS for 30 min and then with an anti-NAMPT
antibody (cat. no., ab45890; 1:50; Abcam) at 4°C overnight. The
next day, the tissues sections were washed with PBS briefly three
times, and then incubated with a goat anti-mouse immunoglobulin G
(cat. no., sc-2039; 1:200; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) conjugated with SP for 30 min at room temperature. After
washing with PBS, the tissue sections were subjected to a
colorimetric reaction using 3,3′-diaminobenzidine solution, then
counterstained with hematoxylin briefly, mounted with mounting
medium, and covered with a coverslip. The immunostained tissue
sections were reviewed and photographed under a light microscope.
Image acquisition and analysis were then performed. Positively
stained cells appeared brown or displayed brown cytoplasmic
granules in the cytoplasm. NAMPT immunostaining scores were based
on the intensity of the immunostaining and the % of positively
stained cells. Immunostaining intensity was scored as follows: 0,
no staining; 1, weak staining; 2, moderate staining; and 3, strong
staining. Percentage of positive staining was scored as follows; 1,
≤25% positive cells; 2, 26–50% positive cells; 3, 51–75% positive
cells; and 4, ≥76% positive cells. The sum of these two scores
resulted in a final score for each case to determine high vs. low
expression of NAMPT protein (a score of ≥3 was termed high NAMPT
expression, whereas a score of 1–2 was termed low NAMPT
expression). The staining of each tissue section was scored
separately by two independent experts simultaneously, and
discordant scores were re-evaluated and scored with their
consensual opinion.
Statistical analysis
All statistical analyses were performed using the
SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA).
Comparisons of NAMPT expression with clinicopathological
parameters, including tumor stage, tumor grade, age at diagnosis,
body mass index, tumor size, lymph node status, recurrence,
estrogen receptor (ER) status, progesterone receptor (PR) status,
and human epidermal growth factor receptor 2 (HER2) status, were
analyzed using the χ2 or Fisher's exact test (when a
cell in a 2×2 table had an expected frequency of ≤5). Survival
curves were generated using Kaplan-Meier curves and statistically
analyzed using the log-rank test. Multivariate analysis was
performed using Cox's proportional hazard model. P<0.05 was
considered to indicate a statistically significant difference.
Results
Differential expression of NAMPT in
normal breast and cancerous tissues
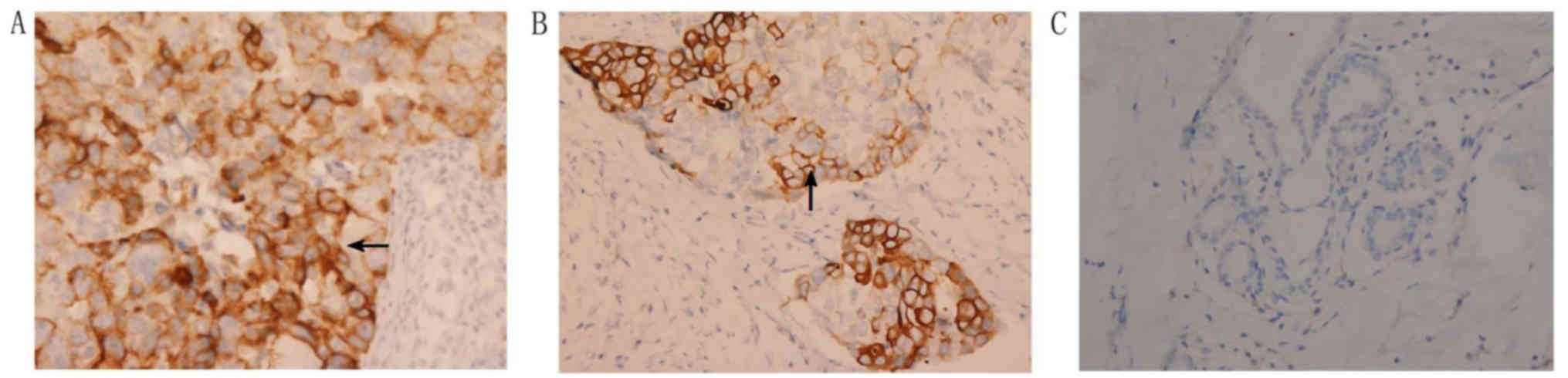
NAMPT protein expression was localized in the
cytoplasm and cell membrane of positive tumor or normal cells.
Specifically, NAMPT protein was mostly expressed in breast invasive
ductal carcinoma as well as in a few adjacent normal mammary glands
(Fig. 2). There was no single tumor
with completely negative NAMPT expression (Fig. 2). Expression of NAMPT protein was
significantly upregulated in breast cancer tissues compared with
normal breast tissues (P<0.001; Table
I). Then, the association of NAMPT expression with the
clinicopathological data from the breast cancer patients was
analyzed. The results demonstrated that upregulated NAMPT protein
expression was associated with a larger tumor size, lymph node
metastasis, advanced clinical TNM stages, and ER and PR expression
(Table II).
 | Table I.NAMPT expression in breast cancer
tissue samples. |
Table I.
NAMPT expression in breast cancer
tissue samples.
| Group | High NAMPT
expression | Low or no NAMPT
expression | P-value |
|---|
| Breast cancer | 40 | 43 |
|
| Adjacent normal
tissues | 0 | 83 | 0.001 |
 | Table II.Association of NAMPT expression with
clinicopathological characteristics in breast cancer patients. |
Table II.
Association of NAMPT expression with
clinicopathological characteristics in breast cancer patients.
|
| Level of NAMPT
expression |
|
|---|
|
|
|
|
|---|
| Variables | Low (n) | High (n) | P-value |
|---|
| Age (years) |
|
|
|
| ≤50 | 25 | 19 |
|
|
>50 | 18 | 21 | 0.330 |
| Tumor size (cm) |
|
|
|
|
<2 | 25 | 12 |
|
| ≥2 | 18 | 28 | 0.010 |
| LN metastasis |
|
|
|
| No | 36 | 24 |
|
| Yes | 7 | 16 | 0.016 |
| TNM stage |
|
|
|
| I/II | 37 | 26 |
|
|
III/IV | 6 | 14 | 0.030 |
| Grade |
|
|
|
| I/II | 34 | 28 |
|
| III | 9 | 12 | 0.340 |
| ER expression |
|
|
|
|
Negative | 6 | 19 |
|
|
Positive | 37 | 21 | 0.001 |
| PR expression |
|
|
|
|
Negative | 11 | 23 |
|
|
Positive | 32 | 17 | 0.003 |
| HER2 expression |
|
|
|
|
Negative | 24 | 27 |
|
|
Positive | 19 | 13 | 0.274 |
Association of NAMPT protein
expression with survival of breast cancer patients
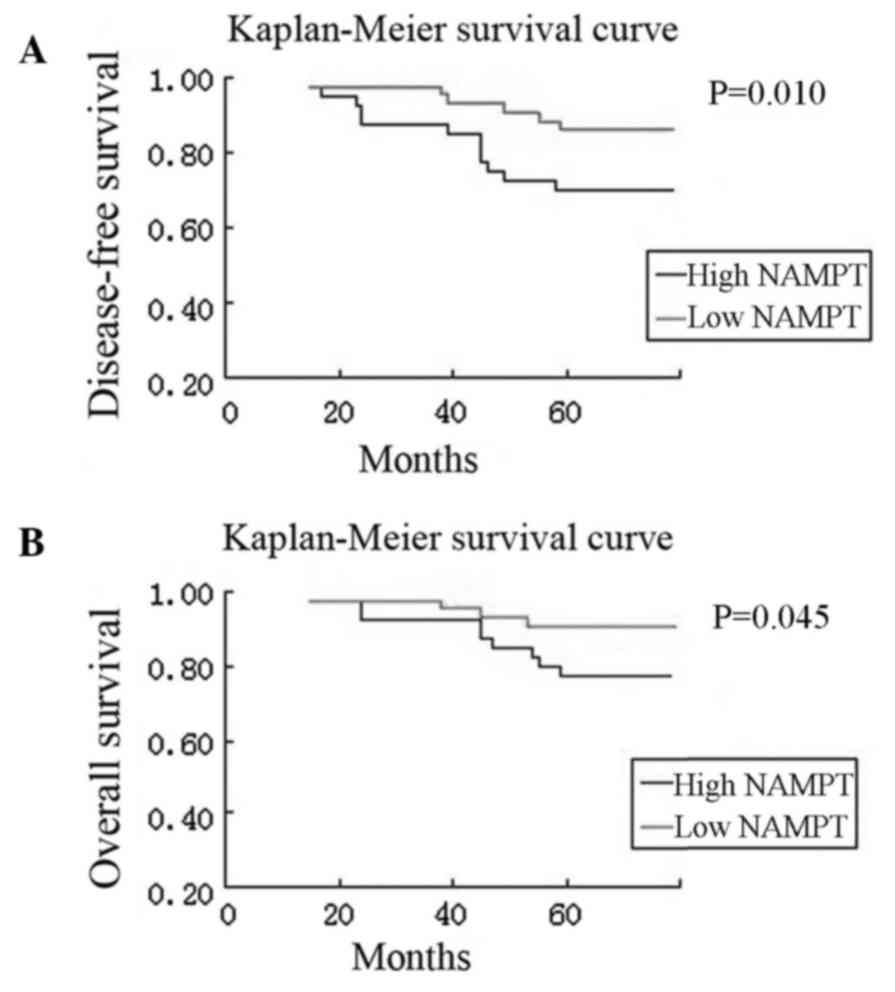
Next, the association of NAMPT expression with
survival of the breast cancer patients was examined. Kaplan-Meier
curves, stratified by high vs. low NAMPT expression, revealed that
high NAMPT expression was associated with a poor overall and
disease-free survival in patients, while breast cancer tissues
without or with low NAMPT expression had better disease-free and
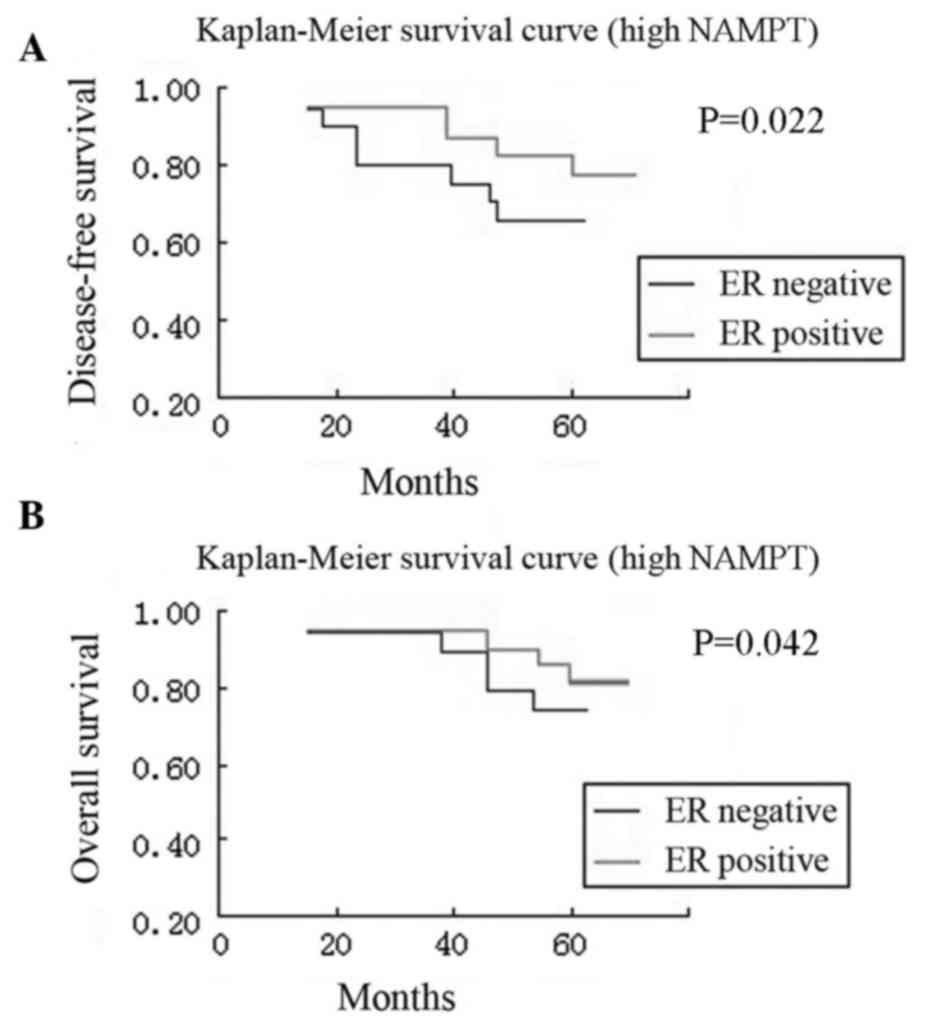
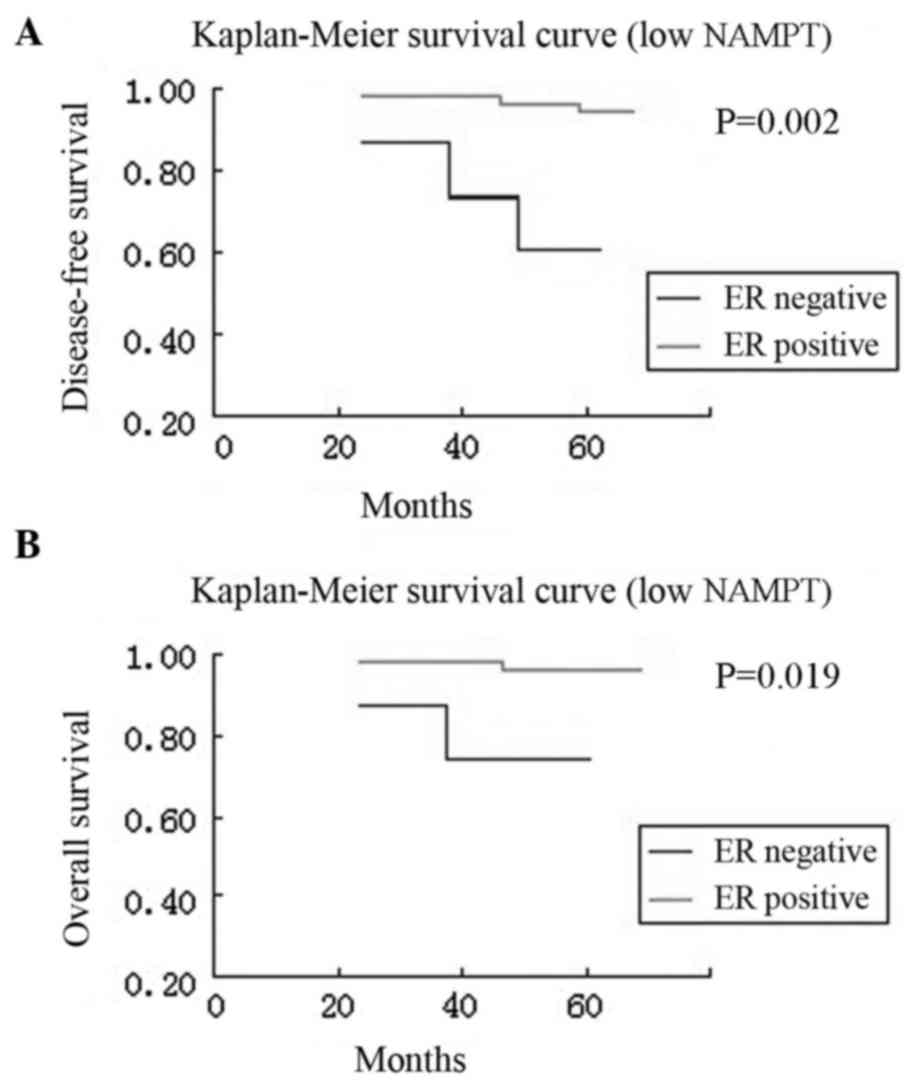
overall survival rates (P=0.010 and P=0.045; Fig. 3). Furthermore, compared with patients
with ER-positive tumors, patients with ER-negative tumors had a
significantly worse disease-free survival both in the high NAMPT
group and in the low NAMPT group (P=0.022 and P=0.002,
respectively) and overall survival both in the high NAMPT group and
in the low NAMPT group (P=0.042 and P=0.019, respectively)
(Figs. 4 and 5).
To evaluate the factors associated with NAMPT
expression in these patients, hazard ratios (HRs) were estimated by
univariate and multivariate Cox regression analyses (Tables III and IV). For the univariate analysis, the
significant factors associated with disease-free survival included
tumor stage (HR=52.39, 95% CI=11.90–230.65, P<0.001), tumor
grade (HR=2.66, 95% CI=1.01–7.01, P=0.047), ER status (HR=0.29, 95%
CI=0.12–0.72, P=0.007) and NAMPT expression (HR=3.13, 95%
CI=1.27–7.73, P=0.013; Table III).
By contrast, the only significant factor associated with overall
survival was tumor stage (HR=55.02, 95% CI=7.12–425.20, P<0.001;
Table IV). However, after adjusting
for the patient age at diagnosis, tumor stage, tumor grade, ER
status, HER2 status, and NAMPT expression by multivariate Cox
regression analysis, only tumor stage (HR=63.42, 95%
CI=12.91–311.48, P<0.001 and HR=115.26, 95% CI=13.24–1003.63,
P<0.001, respectively) and NAMPT expression (HR=0.53, 95%
CI=0.17–1.59, P=0.255 and HR=0.20, 95% CI=0.04–0.89, P=0.034,
respectively) were significant independent predictors of
disease-free and overall survival in the breast cancer patients
(Tables III and IV).
 | Table III.Univariate and multivariate analyses
of disease-free survival in breast cancer cases. |
Table III.
Univariate and multivariate analyses
of disease-free survival in breast cancer cases.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
| >50 | 0.80 | 0.32–1.98 | 0.622 | 0.91 | 0.32–2.59 | 0.861 |
|
≤50 | 1.00 |
|
| 1.00 |
|
|
| Stage |
|
|
|
|
|
|
|
III/IV | 52.39 | 11.90–230.65 | <0.001 | 63.42 | 12.91–311.48 | <0.001 |
|
I/II | 1.00 |
|
| 1.00 |
|
|
| Grade |
|
|
|
|
|
|
|
III/IV | 2.66 | 1.01–7.01 | 0.047 | 0.98 | 0.33–2.95 | 0.967 |
|
I/II | 1.00 |
|
| 1.00 |
|
|
| ER |
|
|
|
|
|
|
|
Positive | 0.29 | 0.12–0.72 | 0.007 | 0.49 | 0.16–1.54 | 0.225 |
|
Negative | 1.00 |
|
| 1.00 |
|
|
| HER2 |
|
|
|
|
|
|
|
Positive | 0.97 | 0.38–2.45 | 0.942 | 0.81 | 0.28–2.32 | 0.687 |
|
Negative | 1.00 |
|
| 1.00 |
|
|
| NAMPT |
|
|
|
|
|
|
|
High | 3.13 | 1.27–7.73 | 0.013 | 0.53 | 0.17–1.59 | 0.255 |
|
Low | 1.00 |
|
| 1.00 |
|
|
 | Table IV.Univariate and multivariate analyses
of overall survival in breast cancer cases. |
Table IV.
Univariate and multivariate analyses
of overall survival in breast cancer cases.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
>50 | 0.96 | 0.32–2.86 | 0.941 | 0.82 | 0.24–2.81 | 0.754 |
|
≤50 | 1.00 |
|
| 1.00 |
|
|
| Stage |
|
|
|
|
|
|
|
III/IV | 55.02 | 7.12–425.20 | <0.001 | 115.26 | 13.24–1003.63 | <0.001 |
|
I/II | 1.00 |
|
| 1.00 |
|
|
| Grade |
|
|
|
|
|
|
|
III/IV | 2.56 | 0.79–8.31 | 0.118 | 0.90 | 0.24–3.33 | 0.872 |
|
I/II | 1.00 |
|
| 1.00 |
|
|
| ER |
|
|
|
|
|
|
|
Positive | 0.59 | 0.19–1.81 | 0.359 | 0.80 | 0.19–3.39 | 0.766 |
|
Negative | 1.00 |
|
| 1.00 |
|
|
| HER2 |
|
|
|
|
|
|
|
Positive | 0.72 | 0.22–2.35 | 0.591 | 0.80 | 0.22–2.91 | 0.731 |
|
Negative | 1.00 |
|
| 1.00 |
|
|
| NAMPT |
|
|
|
|
|
|
|
High | 1.34 | 0.41–4.35 | 0.628 | 0.20 | 0.04–0.89 | 0.034 |
|
Low | 1.00 |
|
| 1.00 |
|
|
Discussion
Although early detection and treatment of breast
cancer have significantly advanced compared with decades ago, more
research is needed for the molecular diagnosis and prognosis
prediction of breast cancer. In the current study, NAMPT expression
was analyzed and associated with breast cancer development and
progression in order to potentially identify it as a novel
biomarker for breast cancer. The present data demonstrated that
NAMPT expression was significantly higher in breast cancer tissues
compared with normal mammary gland tissues, and that upregulated
NAMPT protein expression was associated with a larger tumor size,
advanced clinical TNM stages, lymph node metastasis, and ER and PR
expression. Furthermore, NAMPT expression was associated with poor
overall and disease-free survival in patients, whereas breast
cancer without or with low NAMPT expression had a better
disease-free and overall survival. In conclusion, the present
findings suggest that NAMPT upregulation may contribute to breast
cancer development and progression and that detection of NAMPT
protein levels may serve as a biomarker for the early detection and
prognosis prediction of breast cancer.
Indeed, NAMPT has been reported to be overexpressed
in several types of human cancer and to induce resistance to
therapy (3), suggesting that NAMPT
could be used as a chemotherapeutic target (3). Translationally, NAMPT might be a useful
biomarker for carcinogenesis and tumor progression (4). For example, upregulated NAMPT expression
has been associated with an increase in melanoma volume, early
metastases, and tumor cell de-differentiation (11). In addition, NAMPT overexpression has
been observed in hematological malignancies, such as lymphomas, and
is associated with aggressive phenotypes of malignant lymphoma
(12). In solid tumors, NAMPT is
upregulated in prostate, gastric, and colorectal cancers (13–15), and
NAMPT inhibition reduces the growth and invasiveness of prostate
cancer cells in vitro (15). A
previous study has reported that detection of NAMPT, vascular
endothelial growth factor, and HER2 could be useful as a biomarker
panel for the diagnosis and prognosis of human breast cancer
(16). The current study further
confirmed the overexpression of NAMPT protein in invasive breast
cancer and it association with breast cancer progression and
prognosis. Of note, a previous in vitro study has
demonstrated that NAMPT expression influences breast cancer cell
metastatic activity and adhesion by inhibition of integrin function
(17).
Breast cancer exhibits many molecular alterations;
for example, BRCA mutations are the main hereditary factor
in the development of breast cancer, while the expression of poly
(ADP-ribose) polymerase 1 (PARP1) contributes to the BRCA1
phenotype in basal-like and triple-negative breast cancers
(18). In addition, BRCA1-mediated
NAD synthesis is largely responsible for PARP1 activity in breast
cancer cells, while NAMPT regulates NAD levels towards aberrant
PARP1 activity (19). Indeed, Bajrami
et al (20) have reported that
an NAMPT inhibitor in combination with a PARP inhibitor has a
significant therapeutic potency in nude mouse triple-negative
breast cancer cell xenografts. NAMPT was originally cloned as a
putative cytokine to enhance the maturation of B lymphocyte
precursors (21). Later, it was
reported that NAMPT is able to promote both B lymphocyte and
vascular smooth muscle cell maturation as well as inhibit
neutrophil apoptosis (22). A
previous study has demonstrated that NAMPT expression can predict
recurrence-free survival of lung and breast cancer patients
(23). Another recent study has
revealed a novel mechanism by which breast cancer cells are able to
protect themselves from glucose deprivation-induced oxidative
stress through NAMPT to maintain NADPH levels (24). Together, these findings indicate that
NAMPT overexpression can alter human immune responses and promote
breast cancer progression.
FK866 (also known as APO866) is a small-molecule
inhibitor of NAMPT with potency and selectivity; in vitro,
it is able to suppress the growth of cancer cells and induce tumor
cells to undergo apoptosis through NAD depletion (25). Notably, FK866 can selectively inhibit
various types of cancer cells, but not normal cells (25). The current data indicate that breast
cancer may be another organ site where NAMPT inhibitors may be
useful in the clinic.
To fully understand the physiological relevance of
NAMPT expression in normal and cancerous mammary gland cells,
further research will be necessary. For example, future studies may
include information on subtypes of breast cancer and expression of
other molecules to examine the associations with NAMPT expression.
In addition, the data from the current study need to be verified
using a larger sample size before NAMPT is used clinically as a
biomarker. Further studies will also assess NAMPT in mediating
breast cancer progression and the potential of targeting NAMPT as a
therapeutic strategy in breast cancer.
Acknowledgements
The authors would like to thank the staff of the
Genetic Disease Research Institution, Xi'an Jiaotong University
(Xi'an, China) for their technical assistance.
Funding
This study was supported in part by a grant from
Weihai Municipal Hospital Research Fund (grant no.
WHSLYYZBB2016-107).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author upon reasonable
request.
Author's contributions
SJZ and TQB generated and analyzed data for all
figures; CXQ generated and analyzed data for Tables I and II; XQY generated data for Tables III and IV; KP verified statistical analysis; TQB
wrote the manuscript, which was approved by all authors; SJZ
supervised the project and secured funding.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Weihai Municipal Hospital (Weihai, China), and informed consent was
obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shackelford RE, Mayhall K, Maxwell NM,
Kandil E and Coppola D: Nicotinamide phosphoribosyltransferase in
malignancy: A review. Genes Cancer. 4:447–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bi TQ and Che XM: Nampt/PBEF/visfatin and
cancer. Cancer Biol Ther. 10:119–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chini CC, Guerrico AM, Nin V,
Camacho-Pereira J, Escande C, Barbosa MT and Chini EN: Targeting of
NAD metabolism in pancreatic cancer cells: Potential novel therapy
for pancreatic tumors. Clin Cancer Res. 20:120–130. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Folgueira MA, Carraro DM, Brentani H,
Patrão DF, Barbosa EM, Netto MM, Caldeira JR, Katayama ML, Soares
FA, Oliveira CT, et al: Gene expression profile associated with
response to doxorubicin-based therapy in breast cancer. Clin Cancer
Res. 11:7434–7443. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bi TQ, Che XM, Liao XH, Zhang DJ, Long HL,
Li HJ and Zhao W: Overexpression of Nampt in gastric cancer and
chemopotentiating effects of the Nampt inhibitor FK866 in
combination with fluorouracil. Oncol Rep. 26:1251–1257.
2011.PubMed/NCBI
|
|
8
|
Long HL, Che XM, BI TQ, Li HJ, Liu JS and
Li DW: The expression of nicotinamide phosphoribosyl transferase
and vascular endothelial growth factor-A in gastric carcinoma and
their clinical significance. Zhonghua Wai Ke Za Zhi. 50:839–842.
2012.PubMed/NCBI
|
|
9
|
Tavassoli FA: Pathology of the Breast.
McGraw-Hill Medical; New York, NY, USA: pp. 254–397. 1999
|
|
10
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th ed. New
York: Springer; 2010
|
|
11
|
Maldi E, Travelli C, Caldarelli A,
Agazzone N, Cintura S, Galli U, Scatolini M, Ostano P, Miglino B,
Chiorino G, et al: Nicotinamide phosphoribosyltransferase (NAMPT)
is over-expressed in melanoma lesions. Pigment Cell Melanoma Res.
26:144–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Olesen UH, Hastrup N and Sehested M:
Expression patterns of nicotinamide phosphoribosyltransferase and
nicotinic acid phosphoribosyltransferase in human malignant
lymphomas. APMIS. 119:296–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakajima TE, Yamada Y, Hamano T, Furuta K,
Gotoda T, Katai H, Kato K, Hamaguchi T and Shimada Y: Adipocytokine
levels in gastric cancer patients: Resistin and visfatin as
biomarkers of gastric cancer. J Gastroenterol. 44:685–690. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakajima TE, Yamada Y, Hamano T, Furuta K,
Matsuda T, Fujita S, Kato K, Hamaguchi T and Shimada Y:
Adipocytokines as new promising markers of colorectal tumors:
Adiponectin for colorectal adenoma, and resistin and visfatin for
colorectal cancer. Cancer Sci. 101:1286–1291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang B, Hasan MK, Alvarado E, Yuan H, Wu H
and Chen WY: NAMPT overexpression in prostate cancer and its
contribution to tumor cell survival and stress response. Oncogene.
30:907–921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Y, Guo M, Zhang L, Xu T, Wang L and Xu
G: Biomarker triplet NAMPT/VEGF/HER2 as a de novo detection
panel for the diagnosis and prognosis of human breast cancer. Oncol
Rep. 35:454–462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santidrian AF, LeBoeuf SE, Wold ED,
Ritland M, Forsyth JS and Felding BH: Nicotinamide
phosphoribosyltransferase can affect metastatic activity and cell
adhesive functions by regulating integrins in breast cancer. DNA
Repair. 23:79–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Domagala P, Huzarski T, Lubinski J, Gugala
K and Domagala W: PARP-1 expression in breast cancer including
BRCA1-associated, triple negative and basal-like tumors:
Possible implications for PARP-1 inhibitor therapy. Breast Cancer
Res Treat. 127:861–869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li D, Bi FF, Chen NN, Cao JM, Sun WP, Zhou
YM, Li CY and Yang Q: A novel crosstalk between BRCA1 and poly
(ADP-ribose) polymerase 1 in breast cancer. Cell Cycle.
13:3442–3449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bajrami I, Kigozi A, van Weverwijk A,
Brough R, Frankum J, Lord CJ and Ashworth A: Synthetic lethality of
PARP and NAMPT inhibition in triple-negative breast cancer cells.
EMBO Mol Med. 4:1087–1096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Samal B, Sun Y, Stearns G, Xie C, Suggs S
and McNiece I: Cloning and characterization of the cDNA encoding a
novel human pre-B-cell colony-enhancing factor. Mol Cell Biol.
14:1431–1437. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia SH, Li Y, Parodo J, Kapus A, Fan L,
Rotstein OD and Marshall JC: Pre-B cell colony-enhancing factor
inhibits neutrophil apoptosis in experimental inflammation and
clinical sepsis. J Clin Invest. 113:1318–1327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou T, Wang T and Garcia JG: Expression
of nicotinamide phosphoribosyltransferase-influenced genes predicts
recurrence-free survival in lung and breast cancers. Sci Rep.
4:61072014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong SM, Park CW, Kim SW, Nam YJ, Yu JH,
Shin JH, Yun CH, Im SH, Kim KT, Sung YC and Choi KY: NAMPT
suppresses glucose deprivation-induced oxidative stress by
increasing NADPH levels in breast cancer. Oncogene. 35:3544–3554.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zerp SF, Vens C, Floot B, Verheij M and
van Triest B: NAD+ depletion by APO866 in combination
with radiation in a prostate cancer model, results from an in vitro
and in vivo study. Radiother Oncol. 110:348–354. 2014. View Article : Google Scholar : PubMed/NCBI
|