Introduction
Incidence of hepatocellular carcinoma (HCC) ranks
fifth among all malignant tumors. HCC is the second leading cause
of cancer-associated mortality in the world. The overall 5-year
survival rate of liver cancer is <5% (1). HCC is often accompanied by various
degrees of cirrhosis, which accounted for between 70–90% of all
cases in China in 2003 (2). As a
result of cirrhosis, the patients with HCC have poor tolerance to
drug therapy and have a high risk of surgical resection (3). Although tumor recurrence may be
prevented by systemic and local chemotherapy to increase the
postoperative survival rate, to a certain extent, following
hepatectomy or liver transplantation, current chemotherapeutics
have poor sensitivity and poor targeting and therapeutic effects
(3). The severe systemic toxic
effects and side effects result in functional damage of important
organs, including the heart, liver and kidney (4). The causes limit the extensive
application of current chemotherapeutics. HCC is drug resistant to
the majority of chemotherapeutics, and the therapeutic effect is
~15% following systemic administration (2). Drug resistance may be overcome by
increasing the effective drug concentration of tumor tissue, and
the therapeutic effect can be increased (3,4). Brucine
is a weak indole alkaloid with poor water solubility. It is one of
the antitumor drugs that has been studied in recent years. The
proliferation of human hepatoma SMMC-7721 cells was inhibited
following the administration of brucine in vitro, which
indicated that the inhibition rate increased as the drug
concentration of brucine increased (5). At a brucine concentration of 320 µg/ml,
the inhibition rate of cell proliferation was close to 100%
(5). Deng et al (6–8) revealed
that brucine was able to induce programmed cell death, caspase-9
proteolysis and mitochondrial membrane depolarization of HepG2
cells to kill liver cancer cells. Brucine was able to inhibit the
tumor growth of mice with solid tumors, to a certain extent, and
stimulate and facilitate the hematopoietic system and immune
system, and restore the damage of liver and kidney function caused
by Heps tumor inoculation (7). The
results demonstrated that brucine was beneficial to the
hematopoietic and immune systems of mice with solid tumors, and may
be a novel and promising antitumor drug.
Brucine is limited in its clinical application for
malignant tumors owing to its high toxicity, poor water solubility,
narrow therapeutic window, and similar toxic and therapeutic
doses.
Nanoparticles (NPs) may be engineered to carry
insoluble or highly toxic drugs using nanotechnology. When
nano-drugs are applied in vivo, the antitumor drugs may be
carried to the tumor tissues selectively, which results in an
increased regional drug concentration and slow release of drugs,
and maintains drug effects for a long time, whereas the drug
concentration is lower than that of the non-tumorous tissues and
other organs (9,10). The research and development of
nano-drugs expand the clinical application of highly toxic
anticancer drugs (11).
In our previous study (12), brucine immuno-nanoparticles were
successfully created by nanotechnology, and the results revealed
that the brucine immuno-nanoparticles were stable, and exhibited
even size distribution and a slow-releasing effect. The brucine
immuno-nanoparticles were able to specifically combine with liver
cancer cells, target the liver cancer cell membrane and exert its
antitumor effects following in vitro application for the
liver cancer SMMC-7721 cells. Brucine immuno-nanoparticles were
able to inhibit the proliferation of liver cancer SMMC-7721 cells
in a time- and dose-dependent manner. Compared with brucine and
brucine nanoparticles, the brucine immuno-nanoparticles exhibited a
more specific targeting for tumor cells, increased local drug
concentration and effectively inhibited cancer cell proliferation,
matrix adhesion, invasion and metastasis (12). Therefore, the present study
investigated the distribution and antitumor effects of brucine
immuno-nanoparticles in vivo by establishing an in
situ liver cancer model in nude mice.
Materials and methods
Materials
Brucine (batch no., 110706-200 505; purity, >99%;
Chengdu Must Bio-Technology Co., Ltd., Chengdu, China),
5-fluorouracil (5-FU; Shanghai Xudong Haipu Pharmaceutical Co.,
Ltd., China; batch no., 090315), carboxylated poly(ethylene glycol)
(PEG)-poly(lactic acid) (PLA) block copolymer (PLA-PEG-COOH; cat.
no., PA20100302; molecular mass, 40 kDa; Jiangsu PegBio Co., Ltd.,
Jiangsu, China), mouse anti-human α-fetoprotein (AFP) monoclonal
antibody (MAb) (molecular mass, 70 kDa; Hangzhou HuaAn
Biotechnology Co., Ltd., Hangzhou, China), brucine nanoparticles
and brucine immuno-nanoparticles (The brucine immuno-nanoparticles
were prepared by the National Pharmaceutical Engineering Research
Center, Shanghai Institute of Pharmaceutical Industry and
Department of Physical Chemistry, Shanghai Normal University), mass
spectrometer (3200 Q Trap tandem mass spectrometer; Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA), the
liquid chromatography system (SIL-HTC, LC-20AD and DGU-20A3;
Shimadzu Corporation, Kyoto, Japan), automatic biochemical analyzer
(Bayer AG, Leverkusen, Germany), abdominal 9.0 MHZ B-type
ultrasonography (Prosound F75; Hitachi, Ltd., Tokyo, Japan), mouse
anti-human Ki-67 MAb (cat. no., P6834; Sigma-Aldrich; Merck KgaA,
Darmstadt, Germany), mouse anti-human CD34 Mab (cat. no., ab187282;
Abcam, Cambridge, UK), citrate antigen retrieval buffer and
diaminobenzidine (DAB) chromogenic kit (Fuzhou Maixin Biotech Co.,
Ltd., Fuzhou, China) for immunohistochemistry, terminal
deoxynucleotidyl transferase dUTP nick-end labeling apoptosis kits
(Boehringer Mannheim GmbH, Mannheim, Germany), fluorescein
isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Shanghai
Unitech Bio-Technology Co., Ltd.), human AFP ELISA kits (IBL
International GmbH, Hamburg, Germany), human hepatoma SMMC-7721
cell line (Shanghai Institutes for Biological Sciences Cell
Institute of the Chinese Academy of Sciences, Shanghai, China) and
300 BALB/c nu/nu male nude mice, weighing 16–20 g, from Shanghai
B&K Universal Group Limited [production license no. SCXK
(Shanghai, China) 2008-0016] were from the suppliers specified. All
nude mice were quarantined for 1 week before the start of the
experiment. Mice were housed in an animal facility maintained on a
12/12 h light/dark cycle, at a constant temperature of 23±1°C and
relative humidity of 44±5%, and were given free access to tap water
and food.
Establishment of an in situ
transplanted liver cancer model in nude mice
Human hepatoma SMMC-7721 cells in the exponential
phase were collected and prepared for cell suspension. Cell
suspension (0.2 ml; 5×106 cells) was subcutaneously
injected into the axilla of nude mice. Tumors formed following
inoculation for 2 weeks. The tumor-bearing mice were sacrificed by
exsanguination under deep isoflurane anesthesia (1.5%) when the
tumor diameter was >1 cm. Tumors were then dissected under
sterile conditions and placed into RPMI-1640 culture medium (cat.
no., C22400500BT; Gibco; Thermo Fisher Scientific, Inc.). The
connective tissue was removed and the tumor tissue was cut into
2×2×2 mm pieces. BALB/c nu/nu male nude mice were anesthetized with
an intraperitoneal injection of 60 mg/kg pentobarbital sodium.
Following abdomen disinfection, an abdominal midline incision 1 cm
below the xiphoid was made, and the left lobe of the liver was
exposed. A tunnel under the liver capsule was made with curved
forceps, the tumor mass was seeded in the tunnel and the abdominal
incision was then closed. When the tumor diameter was >1 cm
determined using ultrasound detection, the mice were randomly
selected for use in the experiments. The experimental protocols
were reviewed and approved by the Committee of Ethics on Animal
Experiments of Shanghai University of Traditional Chinese Medicine
(Shanghai, China); all the animal work procedures were approved by
the Institutional Animal Care and Use Committee of Shanghai
University of Traditional Chinese Medicine (Shanghai, China).
Grouping
According to the experimental requirements, five
groups were set up: Normal saline group (NS), 5-FU group, brucine
group, brucine nanoparticle group (Bru-NP) and brucine
immuno-nanoparticle group (Bru-NP-MAb).
Drug doses, blood and tissue specimen
management
Normal saline, 5-FU, brucine, brucine nanoparticles
or brucine immuno-nanoparticles were respectively used to treat the
tumor-bearing mice of various groups via the tail vein. The dose of
brucine in the brucine, Bru-NP and Bru-NP-MAb groups was 3.23
mg/kg. The dose of 5-FU was 20 mg/kg in the 5-FU group. Mice in the
normal saline group received an equal volume of normal saline.
Drugs were administered three times a week in each group. Following
treatment of the tumor-bearing mice for 1, 3, 7, 14, 21 or 30 days,
10 mice from each group were sacrificed by exsanguination under
deep isoflurane anesthesia (1.5%). Tumor volume and weight were
measured, 1 ml peripheral blood was drawn through the inferior vena
cava, and tissues from the heart, lung, spleen, stomach, intestine,
brain, muscle, fat, kidney, liver cancer and para-carcinoma tissues
were preserved at −80°C. The tumor tissues and para-carcinoma
tissues were cut into 1×1×0.5 cm sections, and fixed in anhydrous
methanol at 25°C for 24 h, dehydrated in graduated concentrations
of ethanol (70, 80, 90, 95, 100%) and embedded in paraffin for 4 µm
sections.
Measurement of the liver and kidney
functions and AFP levels in peripheral blood
In each group, 100 µl serum was taken. Using an
automatic biochemical analyzer, liver and kidney function indices,
including albumin, alanine aminotransferase (ALT), aspartate
aminotransferase, alkaline phosphatase, blood urea nitrogen (BUN)
and creatinine (Cr), were determined. In total, 10 samples at the
indicated time were analyzed in each group. Anti-human AFP ELISA
kits were used to determine the serum AFP level in each group.
Distribution of brucine
immuno-nanoparticles in different tissues
On the third day of drug application, 0.2 g heart,
lung, spleen, stomach, intestine, brain, muscle, fat, kidney and
liver cancer tissue, and adjacent cancer tissue from tumor-bearing
animals in each group were separately taken and homogenized.
Homogenate (100 µl) with 20 µl marker (1 µg/ml) and 300 µl methanol
was agitated, and then centrifuged at 11,739 × g at 4°C for 3 min.
A 100 µl volume of supernatant was used to determine the brucine
content using liquid chromatography-mass spectrometry.
Determination of the degree of tumor
necrosis
Paraffin sections of tumor tissue in each group at
different time points were deparaffinized with xylene, rehydrated
in a graded series of ethanol, stained with hematoxylin and eosin
and mounted using neutral gum, and the pathological changes were
then observed under a light microscope (magnification, ×40). The
mean necrotic area in each group was calculated according by 10
tissue slices
The degree of necrosis was evaluated as follows:
Mild necrosis (≤30%), moderate necrosis (30–70%) and severe
necrosis (>70%).
Determination of CD34 and Ki-67
expression in tumor tissues
Paraffin sections of tumor tissue at different time
points in each group were deparaffinized with xylene and rehydrated
in a graded series of ethanol. The slices were then added to
citrate antigen retrieval buffer and boiled in a microwave for 10
min to retrieve tissue antigens. Sections were then rinsed three
times with 0.01 M PBS for 5 min, and mixed with 50 µl mouse
anti-human CD34 Mab (cat. no. ab187282; dilution 1:1,000; Abcam) or
50 µl mouse anti-human Ki-67 MAb (cat. no. P6834; dilution 1:1,000;
Sigma-Aldrich; Merck KGaA). Sections were then incubated at 4°C in
wet conditions for 12 h and at room temperature for 30 min. The
slices were then rinsed three times with 0.01 M PBS for 5 min. DAB
solution was added for staining at room temperature for 3 min, and
0.01 M PBS was used to wash the slices. Hematoxylin was used to
re-stain at room temperature for 10 sec, graduating dilutions of
ethanol were used to dehydrate (70, 80, 90, 95 and 100%), pure
xylene was used for clearing and neutral gum was used to seal the
slices. Finally, the slices were observed under a light microscope
(magnification, ×100).
Tumor cells with brown particles in nuclei were
identified as Ki-67+ cells and were observed under ×100
magnification. The microvessels (<8 red blood cell diameter) and
single endothelial cell were enumerated under a light microscope
(magnification, ×100).
Determination of tumor cell
apoptosis
Paraffin sections of tumor tissue at different time
points in each group were deparaffinized in xylene, rehydrated in a
graded series of ethanol and submerged in 3% hydrogen peroxide
solution at room temperature for 10 min, and were then washed three
times with 0.01 M PBS for 5 min. Proteinase K solution (50 µl; 20
µg/ml) was added to the sample tissue at 37°C in a humidified
chamber for 20 min. Tissues were then washed three times with
distilled water for 5 min. Subsequently, 20 µl labeling buffer was
added to tissue samples at room temperature for 15 min. Next, 20 µl
labeling buffer containing terminal deoxynucleotidyl transferase
and biotin-11-dUTP (1:8) was added to the sample tissue at 37°C in
a humidified chamber for 60 min. The 20X saline sodium citrate
(SSC) solution was diluted 10 times, the labeled tissue samples
were immersed in 2X SSC at room temperature for 15 min, and the
samples were washed three times with 0.01 M PBS for 3 min.
Confining liquid (50 µl) was added to sample tissues at room
temperature for 30 min. The excess liquid was then removed, and 50
µl confining liquid containing avidin-horseradish peroxidase
(dilution, 1:50) was added to sample tissues, which were placed in
a humidified chamber at 37°C for 60 min. Subsequently, the samples
were washed three times with 0.01 M PBS for 3 min. DAB was added
for coloration for 3 min, 0.01 M PBS was used to rinse, serial
dilutions of ethanol were used to dehydrate (70, 80, 90, 95 and
100%), pure xylene was used for clearing and neutral gum was used
to seal the slices. Finally, the samples were observed under a
light microscope (magnification, ×200).
Determination of tumor inhibition
rate
The liver was completely removed and the tumor was
completely dissected. The tumor size was determined using a Vernier
caliper, and the longest and shortest tumor diameters were
respectively recorded by millimeter unit.
Tumor size V = ab2/2 (a, length of tumor;
b, width of tumor)
Tumor inhibition rate (%) = [1 - (Vtreatment
groups/Vcontrol group)] × 100
Effect of brucine immuno-nanoparticles
on the survival time in nude mice bearing tumors
A total of 20 tumor-bearing mice in each group were
observed and the survival time following treatment was recorded.
The dose of brucine in the brucine, Bru-NP and Bru-NP-MAb groups
was 3.23 mg/kg. The dose of 5-FU was 20 mg/kg in the 5-FU group.
Mice in the normal saline group received an equal volume of normal
saline. Drugs were administered via the tail vein three times per
week in each group. The diet, drinking and movement of all nude
tumor-bearing mice in each group was observed twice a day during
treatment. Survival time was defined as the interval between
starting drug administration and final mortality as a direct result
of the tumors.
Life-prolonging rate (%) = (mean survival time in
experimental groups/mean survival time in the control group-1) ×
100
Statistical analysis
Statistical analysis was performed using SPSS
statistics software (version 17.0; SPSS, Inc., Chicago, IL, USA).
Data are expressed as the mean ± standard deviation. A one-way
analysis of variance was used to compare differences between groups
at the same time point. The Levene test was used for variance
homogeneity; the level of Levene test was a=0.05. The least
significant difference test was adopted when variances were
homoscedastic. The approximate F-test Welch method was employed and
Dunnett's T3 method was used between groups when variances were not
homoscedastic. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification of nude mice in an in
situ transplanted liver cancer model
After 4 weeks of in situ tumor inoculation on
the left lobe of the liver in nude mice, abdominal 9.0 MHZ B-type
ultrasonography was used to detect the size and blood supply of the
tumor. When the maximum tumor diameter was >1 cm, the
tumor-bearing mice were randomly grouped for experiments.
Liver and kidney functions and AFP
level in peripheral blood
On the 30th day of drug application, serum ALT level
in the brucine group was increased compared with that of the
Bru-NP-MAb group (F=3.996; P<0.05; n=10). On the 14th day, the
serum BUN level was increased in the Bru-NP group when compared
with the Bru-NP-MAb group (F=1.898; P<0.05; n=10). On the 21st
day, the serum Cr level was increased in the Bru-NP group when
compared with the Bru-NP-MAb group (F=1.587; P<0.05; n=10).
Between the first day and the 14th day, AFP levels
in the control group remained relatively stable and were increased
slightly. On the seventh day, AFP levels in the Bru-NP-MAb group
were significantly decreased compared with those of the brucine and
5-FU groups (F=17.027; P<0.05; n=10). On the 14th, 21st and 30th
days, AFP levels were decreased significantly in the Bru-NP-MAb
group when compared with the other treatment groups (F=16.977,
16.425 and 12.574, respectively; P<0.05; n=10; Table I).
 | Table I.Serum α-fetoprotein level of various
groups at the indicated times. |
Table I.
Serum α-fetoprotein level of various
groups at the indicated times.
| Group | 1 day | 3 days | 7 days | 14 days | 21 days | 30 days |
|---|
| NS |
13.13±1.63 |
13.08±1.69 |
13.31±1.35 |
13.41±1.96 |
15.59±2.39 |
16.32±3.21 |
| 5-FU |
14.06±2.05 |
10.40±2.18a,b |
11.48±2.56b |
10.74±1.38a,b |
8.65±2.83a,b |
10.02±4.23a,b |
| Brucine |
12.71±1.09 |
8.96±2.71a,b |
9.49±2.41a,b |
13.44±1.96b |
13.78±1.96b |
15.33±2.11b |
| Bru-NP |
13.67±1.36 |
6.41±1.23a |
7.10±0.95a |
9.10±1.16a,b |
11.23±2.53a,b |
11.73±1.85a,b |
| Bru-NP-MAb |
12.26±1.33 |
6.33±0.91a |
6.33±0.95a |
5.90±0.94a |
5.47±0.80b |
6.12±0.89a |
Tissue distribution of brucine
immuno-nanoparticles
After 3 days of drug application, no brucine was
detected in the heart, lung, spleen, stomach, intestine, muscle,
fat, kidney, tumors and adjacent tumor liver tissues in the brucine
group. In the Bru-NP group, brucine was detected in the spleen,
lung, kidney, muscle, tumor and adjacent tumor liver tissues. In
the Bru-NP-MAb group, brucine was only detected in the spleen,
tumor and adjacent tumor liver tissues. The brucine concentration
of tumor tissues in the Bru-NP-MAb group was significantly
increased compared with that of the Bru-NP group, and the brucine
concentration of liver tissues near the tumor was significantly
decreased compared with that of the Bru-NP group (P<0.05). The
brucine concentration of lung, kidney and muscle tissues in the
Bru-NP-MAb group was significantly lower than that of the Bru-NP
group (F=520.792 and 445.846, respectively; P<0.05; n=10). No
significant difference in the brucine concentration of spleen
tissues between the Bru-NP-MAb and the Bru-NP groups was identified
(F=0.004; P>0.05; n=10) (Fig.
1).
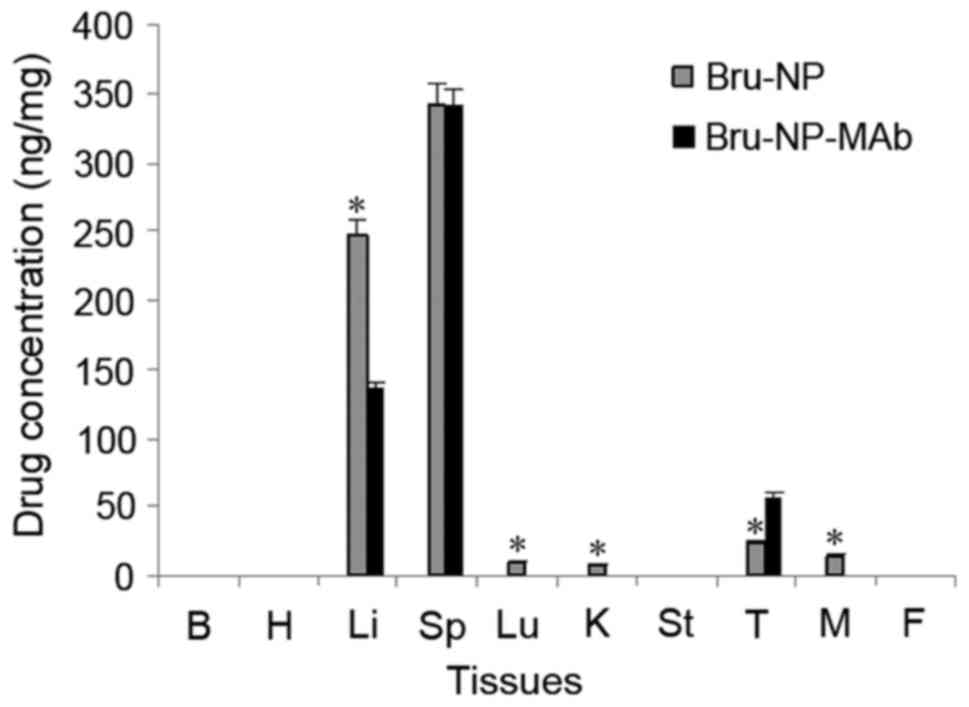 | Figure 1.Tissue distribution of brucine
following in vivo application for 3 days. *P<0.05 vs.
Bru-NP-MAb. B, brain; H, heart; Li, liver; Sp, spleen; Lu, lung; K,
kidney; St, stomach; T, tumor; M, muscle; F, fat; Bru-NP, brucine
nanoparticle; MAb, monoclonal antibody. |
Degree of tumor necrosis
After 14 days of drug application, the degree of
tumor necrosis increased in the brucine, 5-FU and Bru-NP-MAb groups
when compared with the normal saline group (F=6.299; P<0.05;
n=10). After 30 days of drug application, the degree of tumor
necrosis in the Bru-NP-MAb and 5-FU groups was more marked compared
with that of the brucine and Bru-NP groups (F=17.769; P<0.05;
n=10). On the 30th day, the degree of tumor necrosis in the
Bru-NP-MAb and 5-FU groups reached the peak, but there was no
marked difference between the Bru-NP-MAb and 5-FU groups (Fig. 2).
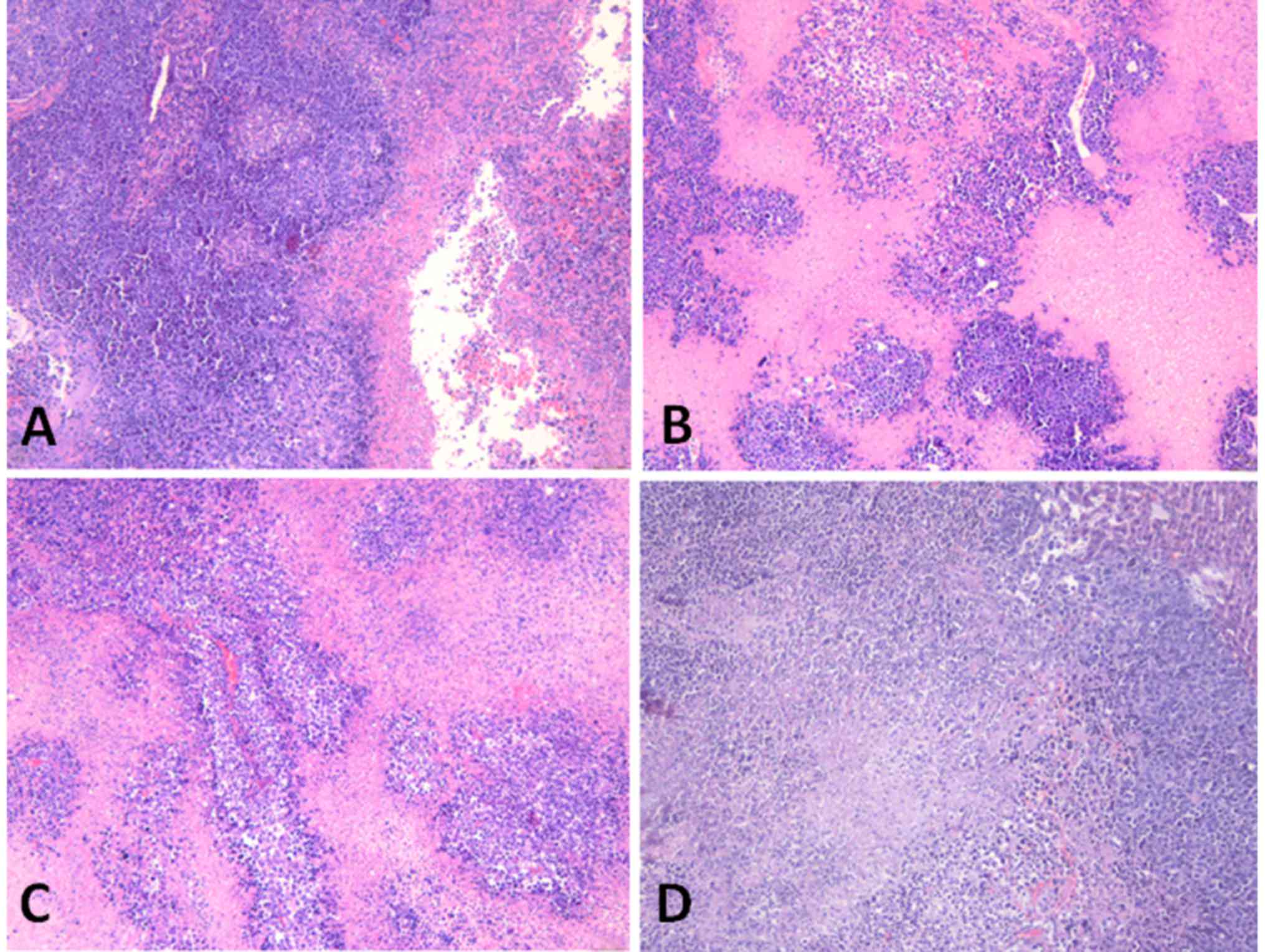 | Figure 2.Tumor necrosis in various groups
following in vivo application for 30 days (hematoxylin and
eosin staining; magnification, ×40). (A) Brucine group,
46.17±12.58% tumor necrosis. (B) Bru-NP group, 63.50±15.08% tumor
necrosis. (C) Bru-NP-MAb group, 80.67±10.91% tumor necrosis. (D)
5-FU group, 70.50±10.33% tumor necrosis. The degree of tumor
necrosis was more severe in treated groups compared with that of NS
group; the degree of tumor necrosis in the Bru-NP-MAb and 5-FU
groups was severe necrosis, but there was no significant difference
between the Bru-NP-MAb and 5-FU groups (P>0.05). Bru-NP, brucine
nanoparticle; MAb, monoclonal antibody; 5-FU, 5-fluorouracil; NS,
normal saline. |
Expression of CD34 and Ki-67 in tumor
tissues
The microvascular density (MVD) of tumor tissue was
counted in 5 randomly selected visual fields using light microscopy
(magnification, ×200), and the vascular endothelial cells stained
brown were considered positive for expression of CD34 or Ki-67
respectively. The percentage of MVD = the number of
microvascular/visual field) × 100%. The percentage of Ki-67
expression (%) = (Ki-67 positive cell count/total tumor cells) ×
100%. The mean of the MVD or Ki-67 expression in each group was
calculated according to 10 tissue slices.
After 14 and 30 days of drug application, CD34
expression of the tumor tissues in the 5-FU group, the Bru-NP and
Bru-NP-MAb group significantly decreased when compared with the
normal saline group (F=3.230 and 19.257; P<0.05; n=10). The CD34
expression of the tumor tissue in the Bru-NP-MAb and 5-FU groups
markedly decreased (15.17±5.81 cells/field vs. 16.50±6.38
cells/field, Bru-NP-MAb group vs. 5-FU group). The CD34 expression
of the tumor tissue among the 5-FU, Bru-NP and Bru-NP-MAb group was
significantly different (F=9.257; P<0.05; n=10; Fig. 3).
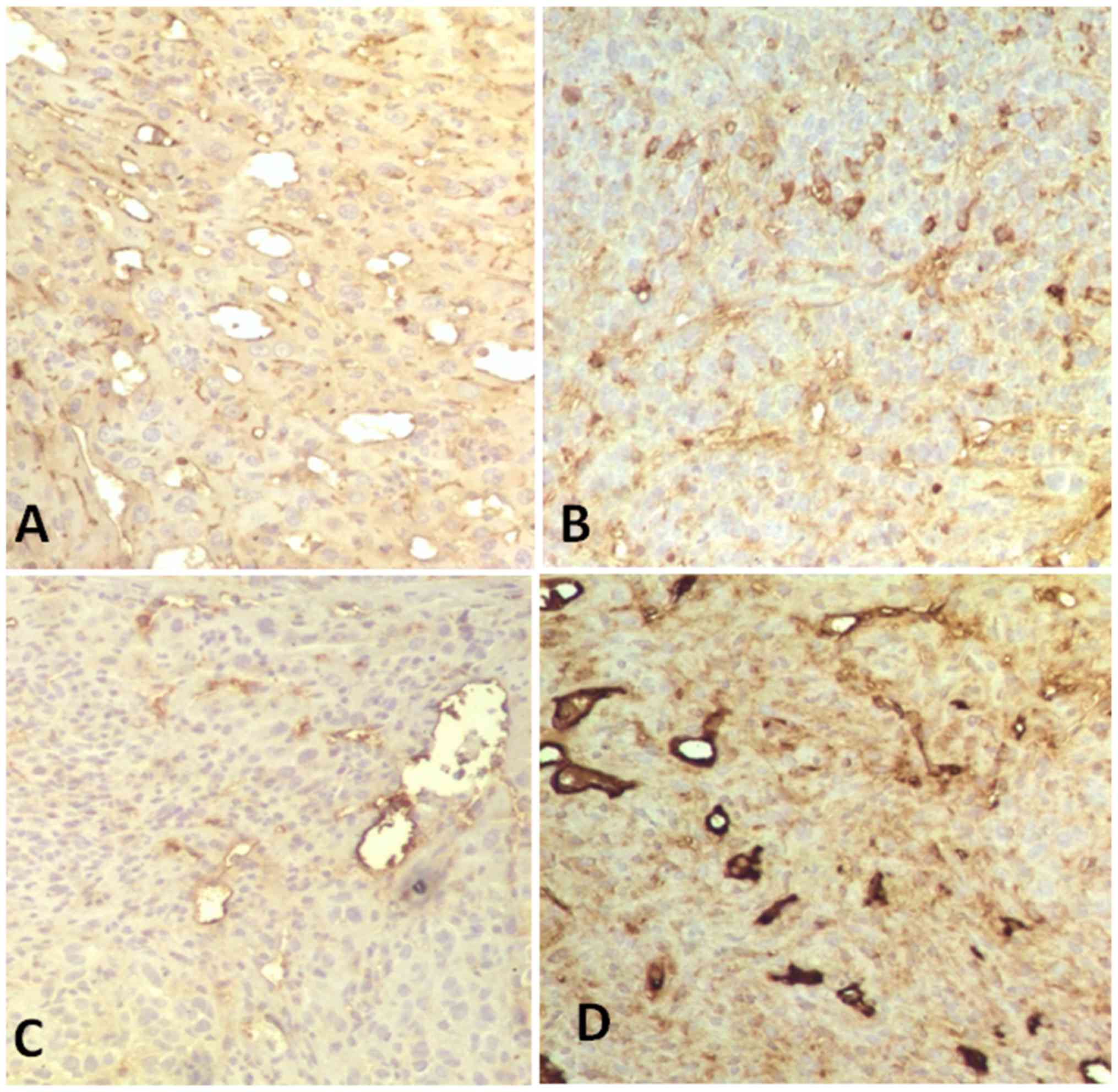 | Figure 3.CD34 expression in tumor tissues in
various groups following in vivo application for 30 days
(immunohistochemical staining; magnification, ×200). (A) Brucine
group, 60.83±18.96% of CD34+ tumor cells of tumor
tissues. (B) Bru-NP group, 32.17±12.12% of CD34+ tumor
cells of tumor tissues. (C) Bru-NP-MAb group, 15.17±5.81% of
CD34+ tumor cells of tumor tissues. (D) 5-FU group,
16.50±6.38% of CD34+ tumor cells of tumor tissues. The
number of CD34+ tumor cells in the Bru-NP-MAb and 5-FU
groups was lower than that of the other groups, but there was no
significant difference between the Bru-NP-MAb and 5-FU groups
(P>0.05). Bru-NP, brucine nanoparticle; MAb, monoclonal
antibody; 5-FU, 5-fluorouracil; CD34, cluster of differentiation
34. |
After 1 day and 14 days, Ki-67 expression in tumor
tissues decreased significantly in the 5-FU, Bru-NP and Bru-NP-MAb
groups when compared with the normal saline group (F=4.546;
F=5.039; P<0.05; n=10). Ki-67 expression of tumor tissues in the
Bru-NP-MAb group was decreased compared with that of the brucine
group after 14 days (F=2.321; P<0.05; n=10). After 30 days,
Ki-67 expression of tumor tissues in the brucine and Bru-NP groups
was significantly increased compared with that of the Bru-NP-MAb
group (F=18.937; P<0.05; n=10). As the time of drug application
increased, 5-FU, brucine, brucine nanoparticles and brucine
immuno-nanoparticles all noticeably inhibited the Ki-67 expression
in the tumor tissues. The inhibitory effect on the Ki-67 expression
was most noticeable in the Bru-NP-MAb group (Fig. 4).
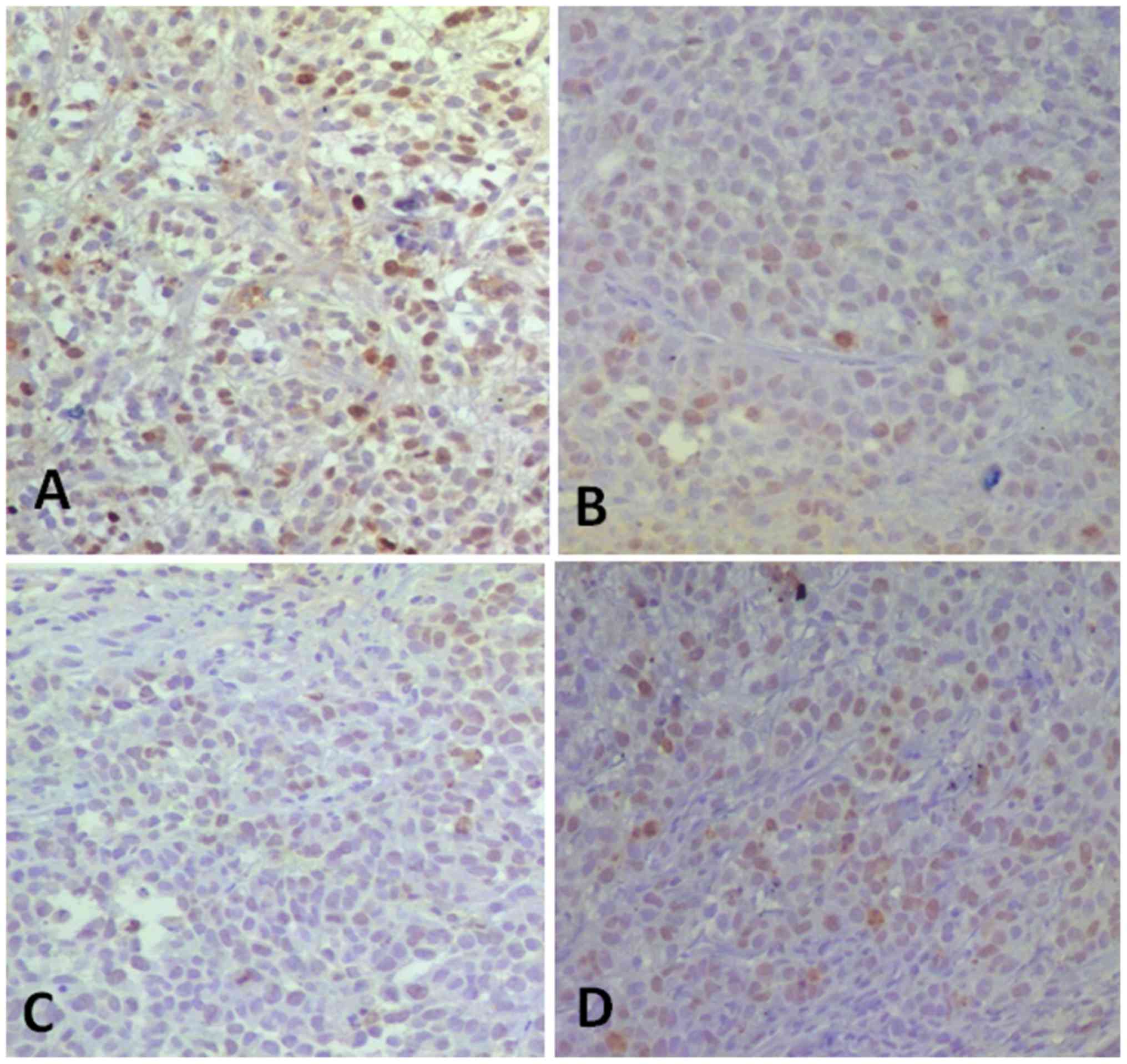 | Figure 4.Ki-67 expression in tumor tissues in
various groups following in vivo application for 30 days
(immunohistochemical staining; magnification, ×200). (A) Brucine
group, 46.67±11.25% of CD34+ tumor cells of tumor
tissues. (B) Bru-NP group, 35.50±7.18% of CD34+ tumor
cells of tumor tissues. (C) Bru-NP-MAb group, 16.67±8.16% of
CD34+ tumor cells of tumor tissues. (D) 5-FU group,
25.00±7.07% of CD34+ tumor cells of tumor tissues. The
number of Ki-67+ tumor cells in the treated groups was
less than that of the NS group, the number of Ki-67+
tumor cells in the Bru-NP-MAb and 5-FU groups was lower than that
of the other groups, but there was no significant difference
between the Bru-NP-MAb and 5-FU groups (P>0.05). Bru-NP, brucine
nanoparticle; MAb, monoclonal antibody; 5-FU, 5-fluorouracil; CD34,
cluster of differentiation 34; NS, normal saline. |
Tumor cell apoptosis
Cells were counted in 5 randomly selected visual
fields using light microscopy (magnification, ×200), and the cells
stained brown were considered apoptotic cells. The apoptotic index
of tumor cells = apoptotic cell count/total cell count × 100%. The
mean of the apoptotic index in each group was calculated according
to 10 tissue slices.
After 1, 14 and 30 days of drug application, the
apoptotic indices of tumor cells in the brucine group, 5-FU, Bru-NP
and Bru-NP-MAb groups were increased compared with the saline group
(F=233.731, F=133.667 and F=92.441, respectively; all P<0.05;
n=10). The apoptotic index of tumor cells in the Bru-NP-MAb group
was increased compared with that of the Bru-NP group (P<0.05;
n=10). On the first, 14th and 30th day, the apoptotic indices of
tumor cells in the Bru-NP-MAb group revealed the greatest increase,
and were 53.22±3.23, 40.97±3.18 and 39.93±3.78%, respectively
(Fig. 5).
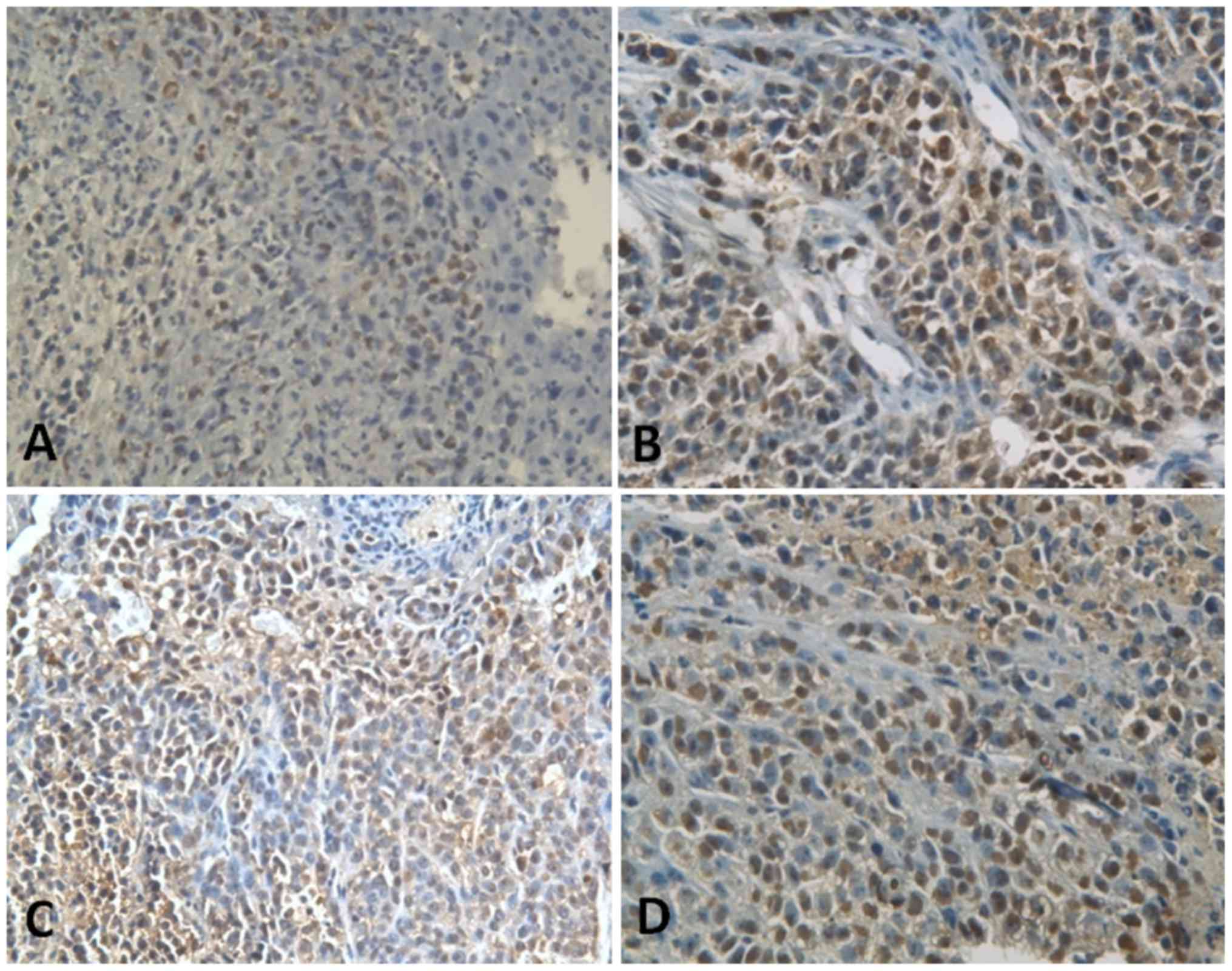 | Figure 5.Apoptotic index of tumor cells in
various groups after in vivo application for 1 day
(immunohistochemical staining; magnification, ×200). (A) Brucine
group, 24.08±2.33% of apoptotic tumor cells of tumor tissues. (B)
Bru-NP group, 33.59±1.48% of apoptotic tumor cells of tumor
tissues. (C) Bru-NP-MAb group, 53.22±3.23% of apoptotic tumor cells
of tumor tissues. (D) 5-FU group, 27.87±3.13% of apoptotic tumor
cells of tumor tissues. The apoptotic index of tumor cells in the
treated groups was increased compared with that of the NS group,
the apoptotic index of tumor cells was highest in the Bru-NP-MAb
group, and there was a significant difference between the
Bru-NP-MAb and other treatment groups (P<0.05). Bru-NP, brucine
nanoparticle; MAb, monoclonal antibody; 5-FU, 5-fluorouracil; NS,
normal saline. |
Tumor inhibition rate
After 3, 7, 14, 21 and 30 days of drug application,
the tumor size in the brucine group, 5-FU, Bru-NP and Bru-NP-MAb
groups decreased significantly when compared with the normal saline
group (F=7.715, 35.252, 59.825, 389.271 and 360.477, respectively;
all P<0.05; n=10). After 7, 14, 21 and 30 days of drug
application, tumor size decreased significantly in the Bru-NP-MAb
group when compared with the brucine, 5-FU and Bru-NP groups
(F=35.252, 59.825, 389.271 and 360.477, respectively; all
P<0.05; n=10; Table II). After 7,
14, 21 and 30 days of drug application, the tumor inhibition rates
in the Bru-NP-MAb group were 36.73, 54.77, 69.48 and 75.96%,
respectively, and were significantly increased compared with that
of the brucine, 5-FU and Bru-NP groups at the same time point
(F=12.517, 15.265, 19.126 and 26.524; P<0.05; n=10). After 30
days of drug application, the tumor inhibition rate in the
Bru-NP-MAb group was 75.96%, and was significantly increased
compared with that of the 5-FU, brucine and Bru-NP groups (58.87,
47.08 and 51.49%; F=26.524; P<0.05; n=10).
 | Table II.Tumor volumes of various groups at
the indicated times. |
Table II.
Tumor volumes of various groups at
the indicated times.
| Group | 1 day | 3 days | 7 days | 14 days | 21 days | 30 days |
|---|
| NS |
224.17±18.15 |
306.75±13.98 |
564.08±42.94 |
1,026.17±88.30 |
1,688.50±79.08 |
2,214.67±146.26 |
| 5-FU |
219.33±15.10 |
248.33±21.06a,b |
414.17±20.73a,b |
651.25±44.92a,b |
758.5±53.91a,b |
910.92±37.00a,b |
| Brucine |
227.17±13.96 |
275.17±25.74a |
514.92±25.70a,b |
833.75±73.89a,b |
980.92±55.50a,b |
1,171.92±59.57a,b |
| Bru-NP |
208.90±14.90 |
298.00±17.54 |
447.30±40.82a,b |
768.00±61.06a,b |
888.10±36.33a,b |
1,074.40±64.21a,b |
| Bru-NP-MAb |
212.25±10.44 |
275.75±10.47a |
356.92±21.20a |
464.17±21.45a |
515.33±32.50a |
532.50±38.21a |
Survival time and life-prolonging rate
of tumor-bearing animals
The survival time of tumor-bearing mice in the 5-FU,
Bru-NP and Bru-NP-MAb groups was increased compared with that of
the saline group (F=9.010; P<0.05; n=20). The survival time in
the Bru-NP-MAb group was increased compared with that of the 5-FU,
brucine and Bru-NP groups (F=11.210; P<0.05; n=20). Compared
with the brucine, Bru-NP and 5-FU groups, the life-prolonging rate
was the highest in the Bru-NP-MAb group (60.43 vs. 1.38, 19.09 and
25.2%; F=12.160; P<0.05; n=20; Table
III).
 | Table III.Survival times of animals with
bearing tumor in various groups. |
Table III.
Survival times of animals with
bearing tumor in various groups.
| Group | Survival times,
days |
|---|
| NS |
50.80±12.30 |
| 5-FU |
63.60±16.26a,b |
| Brucine |
50.10±11.19 |
| Bru-NP |
60.50±12.54b |
| Bru-NP-MAb |
81.50±14.25a |
Discussion
Chemotherapy is an important treatment for malignant
tumors. A number of antitumor drugs of low molecular mass tend to
spread evenly in the body, which results in relatively uniform
tissue distribution (11). These
drugs cannot distinguish tumor cells from normal cells, leading to
a number of adverse effects, including bone marrow suppression and
gastrointestinal toxicity, which limit their clinical application
(11). In 1906, Enrilich first
proposed the concept of targeted drug delivery, namely to
selectively distribute drugs within the lesions so as to decrease
the toxic side effect on normal tissues, to increase the drug
concentration in the target tissue and to enhance the
bioavailability of drugs. The most notable feature of targeted
drugs is the ability to deliver the therapeutic drug to the target
organ, increase the concentration of drugs in the target tissues,
organs and cells, and prolong the time of drug effect (12). Thereby, targeted drugs are able to
lead to a decrease in the dose, a decrease in the toxic side effect
and an increase in drug efficiency (13). Targeted drug application is
particularly suitable to certain antitumor drugs with an increased
toxic side effect. Currently, the most common carriers are
endogenous macromolecules or macromolecules through chemical
synthesis, such as albumin, antibodies, glycoproteins, dextran or
poly-amino acids, which are based on physical and chemical
properties of carriers and target sites (14). Ideal carriers have high solubility,
high stability, high purity, non-toxicity, biodegradability and low
antigenicity (15).
PLA is a polymer with ideal biocompatibility and
biodegradability, with metabolic end-products in vivo of
carbon dioxide and water (15). PEG
is a safe non-toxic hydrophilic polymer, often used as a protective
agent of various types of microsphere (16). The PLA structure containing a large
number of ester bonds has a decreased water solubility (16). Hydrophilic PEG chains and hydrophobic
PLA chains may be used to prepare the PEG-PLA nanoparticles using
physical and chemical techniques (16). The PEG-PLA nanoparticles have a marked
hydrophilicity and are able to markedly enhance long-circulating
and slow-release effects (16).
PEG-PLA is an amphiphilic block copolymer with favorable
biocompatibility and biosecurity (17). The block copolymers, as an effective
drug carrier, have a long cycle, high bioavailability and low
toxicity, and are able to automatically aggregate in aqueous
solution to form a core-shell structure and bind the insoluble drug
with the internal hydrophobic PLA chains, thereby increasing the
drug solubility (17). The PEG-PLA
block copolymers may be degraded into PEG and PLA, which are
non-toxic and may be completely discharged (17). The PEG-PLA block copolymers are able
to bind specific ligands to possess the active targeting of drug
delivery and be evenly distributed in the body following in
vivo application. Thus, the drugs carried by the PEG-PLA block
copolymers may be delivered to specific targeted organs or tissues
(18). As an effective carrier, the
PEG-PLA block copolymers have clear advantages in targeted drug
delivery, and have been considered as a preferred drug carrier in
recent years (19).
As a drug carrier coupled with MAbs, which confer on
the carrier high specificity and targeting, the drugs may be
specifically delivered to tumor tissues where tumor cells produce a
specific protein following carriage of antitumor drugs in
vivo. The targeted drugs have a maximal antitumor effect and
lower toxic side effect, which is regarded as one of the most
promising drug therapies for malignant tumors in the future
(20–22). Kan et al (23) prepared the human serum albumin (HAS)
adriamycin (ADM) nanoparticles combined with HAb18 MAbs and
obtained HAb18-HSA (ADM) nanoparticles. In vivo studies
demonstrated that HAb18-HSA ADM nanoparticles exhibited an
increased tumor inhibition rate compared with that of HSA ADM
nanoparticles and ADM (P<0.001). Liu and Cai (24) used the dual-functional cross-linker
succinimidyl 3-(2-pyridyldithio) propionate to couple the human
liver cancer MAb HAb18 to prepare mitoxantrone-loaded albumin
microspheres. In vitro study revealed that the immune
microspheres were able to specifically accumulate around the human
liver cancer SMMC-7721 cells and be internalized into the targeted
cells, which results in slow drug release within cells and
destruction of the SMMC-7721 cells.
In recent years, a number of studies (25,26)
indicated that brucine exerted marked inhibitory effects on tumor
cell proliferation. In vitro studies demonstrated that
brucine exerted, to a certain degree, an inhibitory effect on cell
proliferation on stomach cancer SGC7901 and MGC803, lung cancer
A549, colon cancer LoVo, liver cancer SMMC7721 and HepG2 cells.
However, brucine is a highly toxic drug with a narrow therapeutic
window, narrow safe range and short half-life. An excessive dose
will easily cause central toxicity, including dizziness, headache
and epileptic seizures, which limit its clinical application. To
decrease the toxicity of brucine, Qu et al (27) used stearoyl ethanolamine-PEG 2000 to
modify the surface of liposomes and developed brucine stealth
liposomes. The results demonstrated that stealth liposomes were
able to markedly enhance the antitumor effects of brucine. Wu et
al (28) combined
doxorubicin-poly(butyl cyanoacrylate) nanoparticles with the acid
ferritin MAb and prepared liver-specific doxorubicin
immuno-nanoparticles. Following accumulation of doxorubicin in the
liver tumor, it is able to markedly increase the drug
concentration, extend the time at which it is effective, enhance
the efficacy and decrease the drug toxicity to other organs. As
aforementioned, Kan et al (29) also demonstrated that the targeted drug
exhibited more marked antitumor effects compared with single
chemotherapy drug.
AFP is a biomarker to detect hepatocellular
carcinoma; the sensitivity and specificity of early diagnosis for
HCC are 79 and 78%, respectively (30–32). In
order to decrease the toxicity of brucine, in our previous study
(12), the carboxylated PEG-PLA
copolymer was selected as a carrier material. Phacoemulsification
technology was applied to prepare the brucine nanoparticles, and
chemical coupling technology was applied to couple the brucine
nanoparticles to anti-human AFP MAb to successfully prepare the
brucine immuno-nanoparticles. In vitro studies indicated
that the brucine immuno-nanoparticles had a uniform size
distribution and were evenly distributed around the cell membrane
of liver cancer SMMC-7721 cells. The results revealed that the
brucine immuno-nanoparticles specifically accumulated in the cell
membrane in a similar ring shape and inhibited the proliferation of
human hepatoma SMMC-7721 cells (12).
In the present study, a nude mice liver cancer model
was established by in situ inoculation technology. Following
application of brucine immuno-nanoparticles in vivo for 3
days, brucine was not detected in every tissue in the brucine
group. In the Bru-NP group, brucine was detected in the spleen,
lung, kidney, muscle, tumor and liver tissues near the tumor.
Brucine was only detected in the spleen, tumor and liver tissues
near the tumor in the Bru-NP-MAb group. The drug concentration of
brucine in the Bru-NP-MAb group was significantly increased
compared with that of the Bru-NP group. The drug concentration of
brucine in the liver tissues near tumor was significantly decreased
compared that of the Bru-NP group. The results indicated that the
brucine immuno-nanoparticles have promising slow-releasing and
antitumor effects.
The results of the present study demonstrated that
in vivo application of the brucine immuno-nanoparticles
caused temporary liver and kidney function damage and significantly
decreased the secretion of AFP by liver cancer SMMC-7721 cells. The
brucine immuno-nanoparticles have good slow release and tumor
targeting properties, and inhibit the expression of CD34 and
angiogenesis of tumor tissues, induce tumor cell apoptosis and
inhibit tumor growth. Furthermore, the brucine immuno-nanoparticles
significantly prolonged the survival time of tumor-bearing
mice.
In conclusion, the results of the present study
demonstrated that in vivo application of brucine
immune-nanoparticles is able to achieve delivery of brucine to
tumor tissues and result in a continuous and slow release of the
brucine within tumor tissues. The antitumor effect of the brucine
immune nanoparticles was more marked compared with that of brucine,
brucine nanoparticles and 5-FU. The brucine immune-nanoparticles
are a promising targeted drug for HCC in the future. Further
prospective study will involve preclinical trials to demonstrate
the potential of clinical application of the targeted drug delivery
system towards liver cancer therapy.
Acknowledgements
The present study was supported by the Shanghai
Education Commission (grant no. 07CZ017), the Shanghai Science and
Technology Commission (grant no. 1052nm060000) and the National
Natural Science Foundation of China (grant no. 30873341).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye
QH, Wang L, Zhou J, Qiu SJ, Li Y, et al: A decade's studies on
metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol.
130:187–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu CL and Fan ST: Nonresectional
therapies for hepatocellular carcinoma. Am J Surg. 173:358–365.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang TS, Wang CH, Hsieh RK, Chen JS and
Fung MC: Gemcitabine and doxorubicin for the treatment of patients
with advanced hepatocellular carcinoma: A phase I–II tria1. Ann
Oncol. 13:1771–1778. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qin JM, Xu XJ, Sheng X, Li Q, Yin PH,
Zhang M, Yang L and Sang ZQ: Anti-hepatocellular carcinoma study of
the brucine in rats. Chin J Gen Surg. 26:219–221. 2011.(In
Chinese).
|
|
6
|
Deng X, Yin F, Lu X, Cai B and Yin W: The
apoptotic effect of brucine from the seed of Strychnos
nux-vomica on human hepatoma cells is mediated via Bcl-2 and
Ca2+ involved mitochondrial pathway. Toxicol Sci.
91:59–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deng XK, Cai BC, Yin W, Liu TS, Sun Q and
Li WD: Research of the anti-tumor effect and toxicity of brucine in
Heps tumor-bearing mice. Chin Pharmacol Bulletin. 22:35–39.
2006.(In Chinese).
|
|
8
|
Deng XK, Cai BC, Yin W, Zhang XC, Li WD
and Sun Q: Brucine on mouse tumor inhibition. Chin J Nat Med.
3:392–395. 2005.(In Chinese).
|
|
9
|
Qin JM, Zhang YD, Wang HY and Wu MC:
Nanotechnology usage in diagnosis and treatment of liver diseases.
Chin J Modern Med. 13:49–52. 2003.(In Chinese).
|
|
10
|
Qin JM, Zhang YD, Wang HY and Wu MC: Usage
and progress of nanodrug in hepatic disease. Chin J Hepatobiliary
Surg. 10:646–648. 2004.(In Chinese).
|
|
11
|
Gao Y, Li HL, Lv QZ and Zhai GX:
Application of active transport path in targeting drug delivery
system of liver. Chin J Pharmaceuticals. 39:542–547. 2008.(In
Chinese).
|
|
12
|
Qin JM, Yin PH, Li Q, Sa ZQ, Sheng X, Yang
L, Huang T, Zhang M, Gao KP, Chen QH, et al: Anti-tumor effects of
brucine immuno-nanoparticles on hepatocellular carcinoma. Int J
Nanomedicine. 7:369–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gregoriadis G: Liposome Technology. 3. CRC
Press; Boca Raton, FL: pp. 751984
|
|
14
|
Hou XM, Cui LL, Li GD and Li WH: Advance
on targeted drug delivery in cancer therapy. J Pharm Pract.
25:273–275. 2007.
|
|
15
|
Vasir JK and Labhasetwar V: Targeted drug
delivery in cancer therapy. Technol Cancer Res Treat. 4:363–374.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stefani M, Coudane J and Vert M: In vitro
ageing and degradation of PEG-PLA diblock copolymer-based
nanoparticles. Polymer Degradation Stability. 91:2554–2559. 2006.
View Article : Google Scholar
|
|
17
|
Lu Y, Li J and Wang G: In vitro and in
vivo evaluation of mPEG-PLA modified liposomes loaded
glycyrrhetinie acid. Int J Pharm. 356:274–281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park EK, Lee SB and Lee YM: Preparation
and characterization of methoxy poly(ethylene
glyco1)/poly(epsilon-caprolactone) amphiphilic block copolymeric
nanospheres for tumor-specific folate-mediated targeting of
anticancer drugs. Biomaterials. 26:1053–1061. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei Q, Wei W, Tian R, Wang LY, Su ZG and
Ma GH: Preparation of uniform-sized PELA microspheres with high
encapsulation efficiency of antigen by premix membrane
emulsification. J Colloid Interface Sci. 323:267–273. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van der Heiden PL, Jedema I, Willemze R
and Barge RM: Efficacy and toxicity of gemtuzumab ozogamicin in
patients with acute myeloid leukemia. Eur J Haematol. 76:409–413.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adams GP and Weiner LM: Monoclonal
antibody therapy of cancer. Nat Biotechnol. 23:1147–1157. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu AM and Senter PD: Arming antibodies:
Prospects and challenges for immunoconjugates. Nat Biotechnol.
23:1137–1146. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kan HP, Liu ZG, Tan YF, Lin YX, Li CF and
Zhou J: Preparation of immune nanospheres of human liver cancer and
observation of anti-cancer effect. J South Med Univ. 28:1503–1505.
2008.(In Chinese).
|
|
24
|
Liu XB and Cai MY: Preparation of immune
nanospheres of human liver cancer and identification of
immunological properties in vitro. Chin J Immunol. 16:262–265.
2000.(In Chinese).
|
|
25
|
Li L: Study outline of effect and
attenuation of brucine. Asia-Pacific Trad Med. 5:19–21. 2007.(In
Chinese).
|
|
26
|
Wang L, Cai BC, Yang H, YU ZL, Deng XK and
Zhang ZJ: A Comparative study on pharmacokinetics of vauqueline
solution and vauqueline liposome in rabbits. J Nanjing TCM Univ.
22:165–167. 2006.(In Chinese).
|
|
27
|
Qu YQ, Guo YQ, Ha HX, Chen J and Cai BC:
Comparison of the antitumor effect between brucine stealth liposome
and brucine conventional liposome. J Trad Chin Drug Res Clin
Pharmcol. 19:361–363. 2008.(In Chinese).
|
|
28
|
Wu J, Nantz MH and Zern MA: Targeting
hepatocytes for drug and gene delivery: Emerging novel approaches
and applications. Front Biosci. 7:d717–725. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kan HP, Tan YF, Li KF and Zhou J:
Preparation and anticancer effects of immunonanospheres containing
paclitaxel against human liver cancer. J Mod Dig Interven.
15:227–229. 2010.(In Chinese).
|
|
30
|
Hu KQ, Kyulo NL, Lim N, Elhazin B,
Hillebrand DJ and Bock T: Clinical significance of elevated
alpha-fetoprotein (AFP) in patients with chronic hepatitis C, but
not hepatocellular carcinoma. Am J Gastroenterol. 99:860–865. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Montaser LM, Abbas OM, Saltah AM and Waked
IA: Circulating AFP mRNA as a possible indicator of hematogenous
spread of HCC cells: A possible association with HBV infection. J
Egypt Natl Cancer Inst. 19:48–60. 2007.
|
|
32
|
Kamiyama T, Takahashi M, Nakagawa T,
Nakanishi K, Kamachi H, Suzuki T, Shimamura T, Taniguchi M, Ozaki
M, Matsushita M, et al: AFP mRNA detected in bone marrow by
real-time quantitative RT-PCR analysis predicts survival and
recurrence after curative hepatectomy for hepatocellu1ar carcinoma.
Ann Surg. 244:451–463. 2006.PubMed/NCBI
|



















