Introduction
Breast cancer is a common malignant tumor that
presents a serious threat to female health. The incidence of breast
cancer has increased in China in recent decades, and the age of
onset has decreased (1,2). The development of breast cancer is
complex, and the specific underlying mechanisms remain to be
elucidated. Previous studies have indicated that the inhibition of
cellular apoptosis is closely associated with the occurrence and
development of breast cancer (3,4). The
cysteine proteinase (Caspase) family serves an important role in
the signaling pathways of cellular apoptosis (5). Interleukin (IL)-1β transferase (ICE;
Caspase-1) is one of the Caspase family members. Previous studies
have demonstrated that Caspase-1 primarily activates IL-1β, with
little effect on cellular apoptosis (6). However, other studies have identified
that the overexpression of Caspase-1 may induce cellular apoptosis,
while the silencing of Caspase-1 may confer a tumor growth
advantage (7,8). Clinical studies have indicated that the
expression of Caspase-1 is decreased in a number of tumor tissues,
including ovarian, prostate and colon cancer (9,10).
However, the function of Caspase-1 in the development of breast
cancer remains unclear. In the present study, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
used to detect the expression of Caspase-1 mRNA in breast cancer
and tumor-adjacent tissues, while the effects of the Caspase-1
small molecule inhibitor Ac-YVAD-CMK on the proliferation,
apoptosis and invasion of breast cancer cells were detected, in
order to investigate correlations between the expression and the
occurrence and development of breast cancer.
Subjects and methods
Clinical data
All patients were treated in Department of
Galactophore, the First People's Hospital of Xinxiang (Xinxiang,
China) between January 2015 and January 2016. All tissue specimens
were obtained from female patients who underwent the surgical
removal of tumors and were pathologically diagnosed with primary
breast cancer under a fluorescence microscope (magnification, ×40;
Olympus AX80; Olympus Corporation, Tokyo, Japan). The breast cancer
tissues and corresponding tumor-adjacent tissues were removed and
stored at −80°C for later use. A total of 30 cases were selected,
with ages ranging from 36 to 65 years (median ages, 50.18±8.42
years). All cases had not undergone chemo- or radiotherapy prior to
surgery. The present study was conducted in accordance with the
Declaration of Helsinki (2013 version), and with approval from the
Ethics Committee of The First People's Hospital of Xinxiang.
Written informed consent was obtained from all participants.
Extraction of total RNA and RT-qPCR
detection
A total of 100 g tissues were obtained from each
specimen; 1 ml TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was added and mixed with tissue
homogenizer (PRO-200; Proscientific, Inc., Oxford, CT, USA),
following which the total RNA was extracted using TRIzol reagent,
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). The total RNA concentration and purity was
detected using a UV spectrophotometer (Thermo Fisher Scientific,
Inc.), and reverse transcription was conducted using a PrimeScript
RT reagent kit (Invitrogen; Thermo Fisher Scientific, Inc.); the
synthesized cDNA product was stored at −20°C for later use. The
primer sequences for RT-qPCR (Yingjun Co., Shanghai, China) are
listed in Table I. GAPDH was used as
a reference gene. An RT-qPCR system (ABI PRISM® 7300;
Applied Biosystems, Foster City, CA, USA) was used to detect the
expression of Caspase-1 mRNA. The total RT-qPCR amplification
reaction volume was 25 µl and the thermocycler conditions were as
follows: 50°C for 2 min, then 95°C Taq enzyme activation for 10
min, followed by 95°C for 15 sec and then 60°C for 1 min, for 40
cycles. The 2−ΔΔCq method was utilized to demonstrate
relative expression (11).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| Caspase-1 |
|
|
Forward |
TTTCCGCAAGGTTCGATTTTCA |
|
Reverse |
GGCATCTGCGCTCTACCATC |
| GAPDH |
|
|
Forward |
GGAGCGAGATCCCTCCAAAAT |
|
Reverse |
GGCTGTTGTCATACTTCTCATGG |
Western blotting
MDA-MB-231 cells (Cell Bank of Chinese Academy of
Sciences, Shanghai, China) were cultured in RPMI-1640 medium
(Hyclone; GE Healthcare, Chicago, IL, USA) supplemented with 10%
fetal bovine serum (FBS; Si Ji Qing, Hangzhou, China) and incubated
at 37°C in 5% CO2, and with Ac-YVAD-CMK at different
final concentrations (0, 5, 10, 20 and 30 µmol/l; Bachem AG,
Bubendorf, Switzerland) for 48 h; following trypsinization, the
cells were collected; total protein samples were extracted using
radioimmunoprecipitation assay lysis buffer and the protein
concentration was detected using a BCA protein quantitative reagent
kit (Thermo Fisher Scientific, Inc.). The proteins (100 ng protein
per lane) were separated via 10% SDS-PAGE, transferred to a
polyvinylidene fluoride membrane using a wet chemistry method
(12), and agitated with 5% dried
skimmed milk at room temperature and blocked for 2 h; the membrane
was washed thrice with TBS-T and then incubated with a Caspase-1
antibody (catalog no. 2225S; dilution, 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) or a β-actin antibody (catalog
no. BM0627; dilution, 1:300; Wuhan Boster Biological Technology,
Ltd., Wuhan, China) at 4°C overnight. Subsequent to washing with
TBS-T, the membranes were incubated with a horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (catalog
no. GA1014; dilution, 1:5,000; Wuhan Boster Biological Technology,
Ltd.) at room temperature for 1 h; following this, ECL solution
(EMD Millipore, Billerica, MA, USA) was used for chemical
luminescence, and the EC3 Imaging System (UVP, Inc., Upland, CA,
USA) was used to visualize the protein bands, quantified by grey
level analysis using Image J software (National Institutes of
Health, Bethesda, MD, USA). The experiment was repeated three
times.
MTT assay
MDA-MB-231 cells were cultured in L-15 medium
(Hyclone; GE Healthcare, Chicago, IL, USA) (containing 10% fetal
bovine serum, 100 U/ml penicillin and 100 U/ml streptomycin); the
MDA-MB-231 cells at the logarithmic growth phase were digested, and
then inoculated into 96-well plate at 100 µl/well (containing
~1×104 cells) for 24 h. A total of 6 parallel wells were
set for the Ac-YVAD-CMK group (final concentration, 10 µmol/l) and
the control group [dimethyl sulfoxide (DMSO)]. After 24, 48 and 72
h of culturing, 50 µl 1X MTT solution was added into each well and
cultured for 4 h in an incubator at 37°C. The supernatant was
removed, and 150 µl DMSO was added into each well, following which
the contents were agitated using a plate shaker. The absorbance
[optical density (OD)] value of each well was detected at a
wavelength of 490 nm using a microplate reader (CliniBio,
Eugendorf, Austria); the OD values represent the relative
proliferation level of the cells. The experiment was repeated three
times.
Cell apoptosis experiment
MDA-MB-231 cells were inoculated into 6-well plates
(1×106 cells/well). When its confluence was 60%, the
Ac-YVAD-CMK (final concentration, 10 µmol/l) and control (DMSO)
were added. Three parallel wells were selected for each group.
After 24 h of culturing, the cells were digested using 0.25%
trypsin (Gibco; Thermo Fisher Scientific, Inc.) and collected,
following the protocol of the Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection kit (Kaiji Biotech
Co., Nanjing, China). The cells were suspended in 500 µl Binding
Buffer prior to the addition of 5 µl Annexin V-FITC. Subsequently,
5 µl PI was added and the mixture was incubated for 10 min in the
dark, following which the apoptotic cells were detected using flow
cytometry (FACSCalibur™; BD Biosciences, Franklin Lakes, NJ, USA),
The apoptotic rate was calculated according to the proportions of
cells in the upper and lower right-hand quadrants. The experiment
was repeated three times.
Transwell cell invasion
experiment
The Transwell inserts were coated with 100 µl
Matrigel and irradiated with UV at 20 Gy for 2 h. The MDA-MB-231
cells pretreated with Ac-YVAD-CMK (10 µmol/l) were trypsinized, and
the cell density was calculated. The cells were seeded into the
upper Transwell chambers at 100 µl/well (~1×105 cells),
and 500 µl L-15 medium containing 10% fetal bovine serum was added
into the lower chambers. After 24 h of culturing, the Transwell
insert was removed and washed with PBS; the cells in the upper
layer was removed with a cotton bud and fixed with 95% ethanol at
37°C for 1 h, prior to staining with 4 g/l Trypan Blue solution.
Using an inverted microscope (magnification, ×40; TS100; Nikon,
Tokyo, Japan), the cells penetrating the membrane in 10 randomly
selected fields of view were counted. The experiment was repeated
three times.
Statistical analysis
SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. The observation data were normally
distributed. Comparisons between two groups were performed using a
paired t-test, and comparisons between more than two groups were
conducted using a one-way analysis of variance and a pair-group
Least Significant Difference test. P<0.05 was considered to
indicate a statistically significant difference
Results
Comparison of Caspase-1 mRNA
expression in breast cancer and tumor-adjacent tissues
RT-qPCR detected the expression of Caspase-1 mRNA in
breast cancer and tumor-adjacent tissues obtained from patients.
Compared with the corresponding tumor-adjacent tissues, the breast
cancer tissues had significantly decreased expression of Caspase-1
mRNA, and the difference was statistically significant (P<0.05),
which indicated that Caspase-1 may serve a function during the
development of breast cancer (Table
II).
 | Table II.mRNA expression of miRNA-204 in breast
cancer and tumor-adjacent tissues. |
Table II.
mRNA expression of miRNA-204 in breast
cancer and tumor-adjacent tissues.
| Group | Caspase-1
(2−ΔΔCq) mRNA of | t | P-value |
|---|
| Breast cancer
tissues |
2.14±0.93 | 9.842 | <0.000001 |
| Tumor-adjacent
tissues |
4.62±1.02 |
|
|
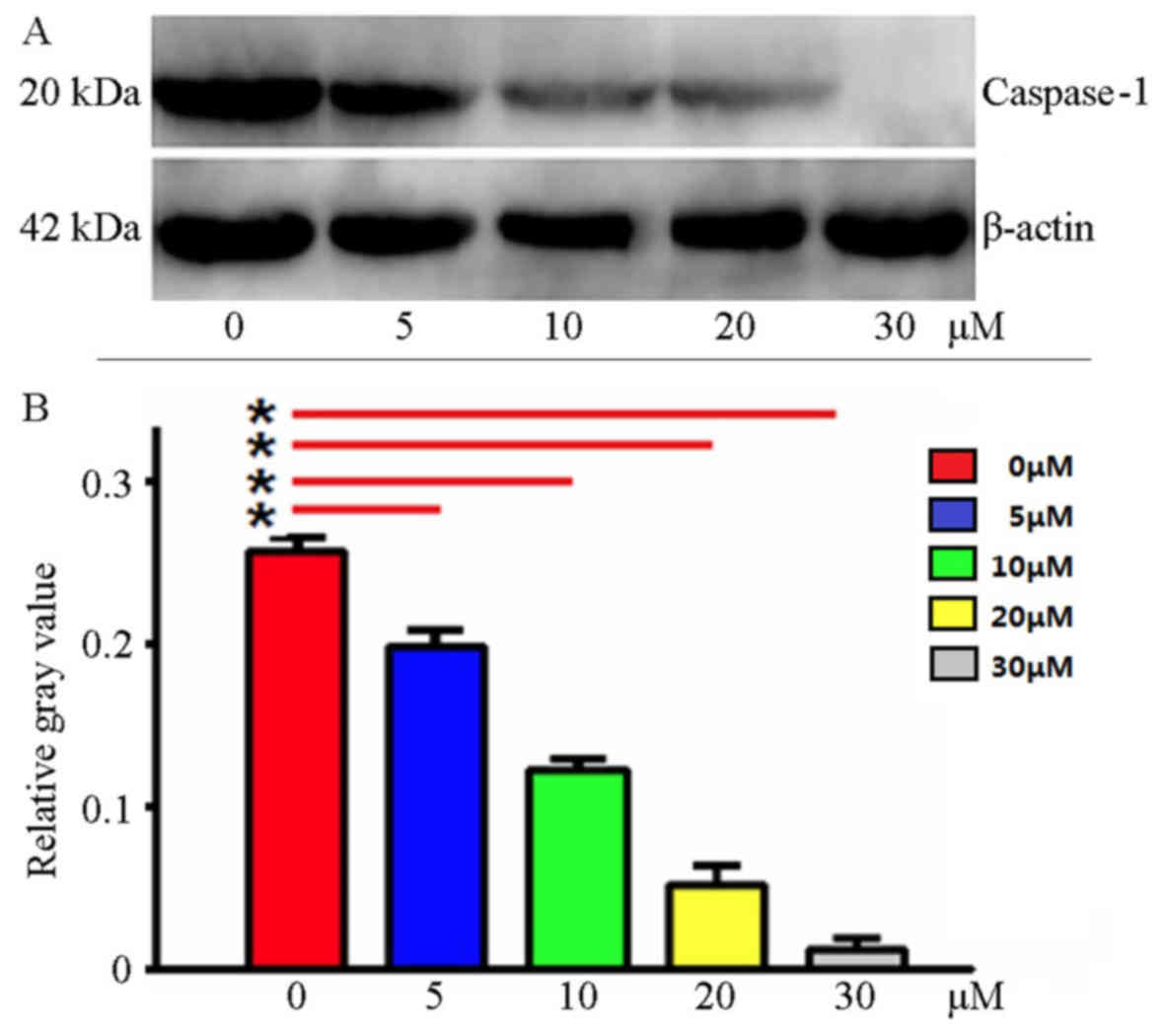
Ac-YVAD-CMK inhibits Caspase-1 protein
expression
Using western blotting, Caspase-1 protein expression
was detected in the MDA-MB-231 cells following treatment with
Ac-YVAD-CMK at various final concentrations. The results indicated
that Ac-YVAD-CMK may inhibit Caspase-1 protein expression; as the
Ac-YVAD-CMK concentration increased, the expression of Caspase-1
decreased; the gray level analysis indicated statistical
significance (P<0.05; Fig. 1).
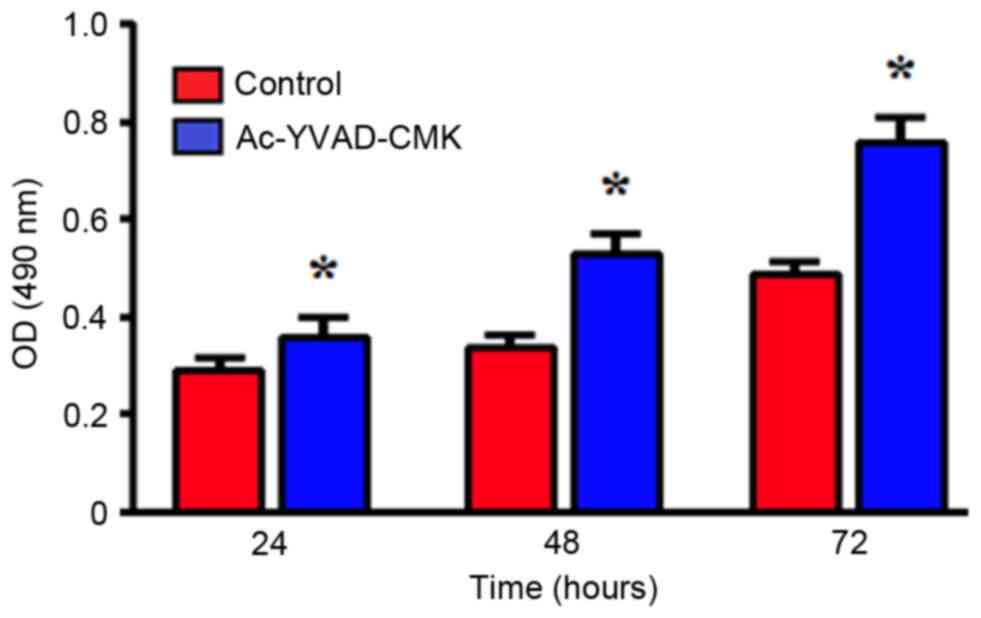
Effects of the inhibition of Caspase-1
protein expression on proliferation ability
An MTT assay was used to detect the changes in
MDA-MB-231 cell proliferation following treatment with 10 µmol/l
Ac-YVAD-CMK for 24, 48 and 72 h. Compared with the control group,
the MDA-MB-231 cells treated with Ac-YVAD-CMK exhibited
significantly increased proliferation ability, in a time-dependent
manner (P<0.05; Fig. 2).
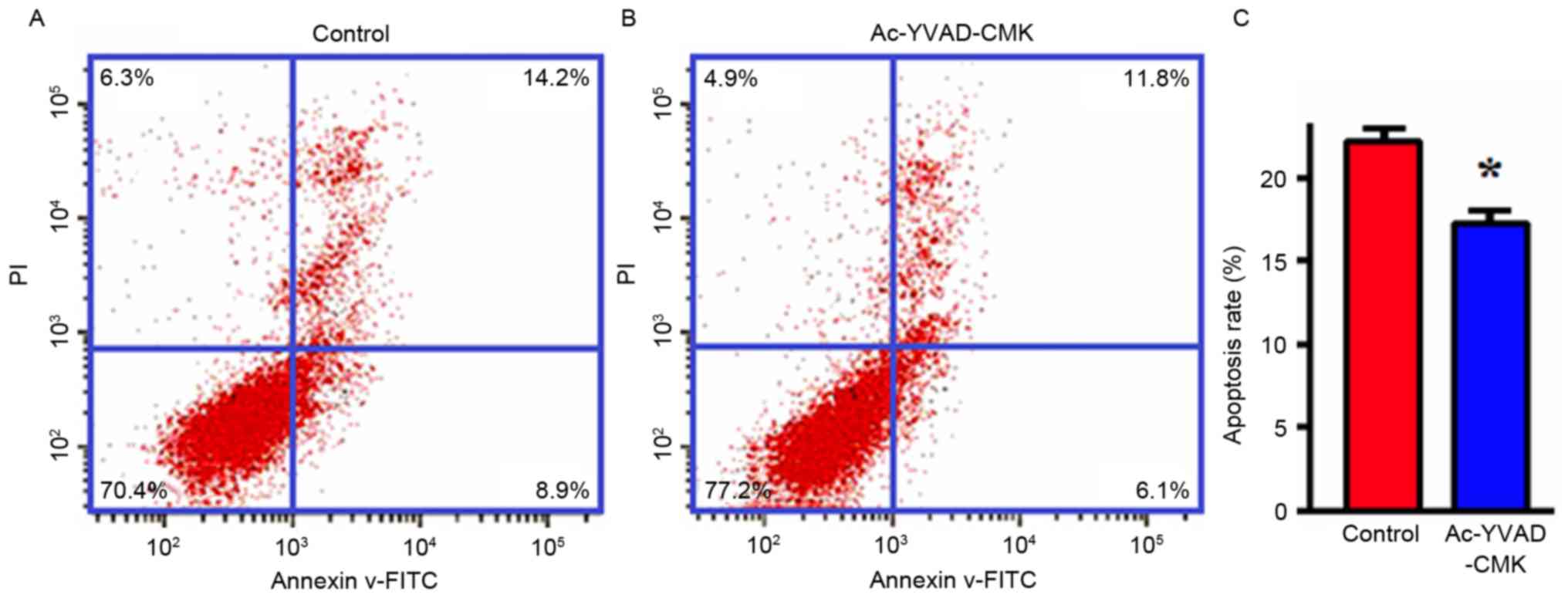
Effects of the inhibition of Caspase-1
protein expression on apoptosis
MDA-MB-231 cells were treated with Ac-YVAD-CMK
(final concentration, 10 µmol/l) for 24 h, and then the apoptotic
levels were detected with flow cytometry. The results indicated
that the apoptotic proportion of MDA-MB-231 cells in the
Ac-YVAD-CMK group was markedly decreased compared with that of the
control group, and that this difference was statistically
significant (P<0.05; Fig. 3).
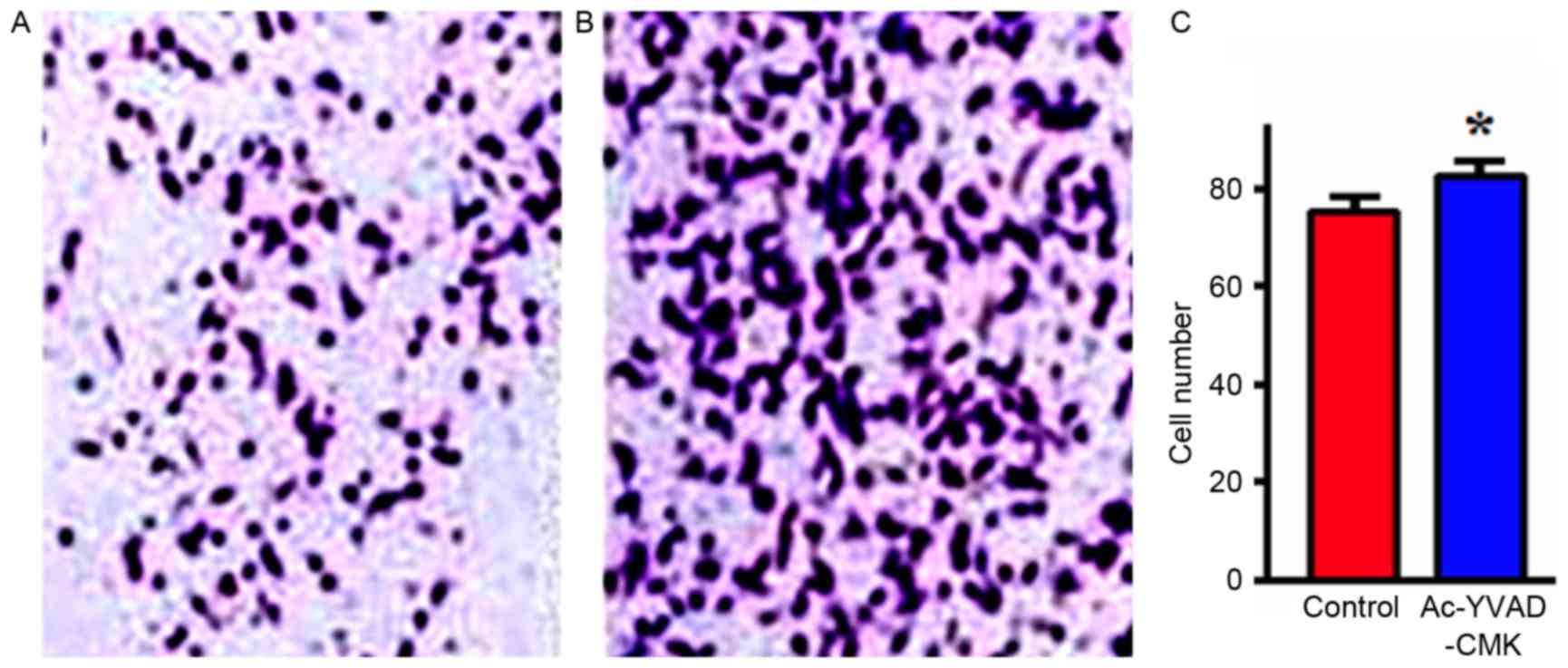
Effects of the inhibition of Caspase-1
protein expression on cell invasion
MDA-MB-231 cells were treated with Ac-YVAD-CMK
(final concentration, 10 µmol/l) for 24 h, and then the invasion
ability in vitro was detected with a Transwell assay. The
results indicated that the number of MDA-MB-231 cells that
penetrated the upper chamber layer in the Ac-YVAD-CMK group was
significantly increased, compared with in the control group, a
difference that was statistically significant (P<0.05; Fig. 4).
Discussion
Among the female-specific cancer types, breast
cancer highest associated cause of mortality in developing
countries (13). In China, breast
cancer accounts for ~12.2% of global cases per year, while the
associated mortalities account for ~9.6% (14). Breast cancer is a complex
heterogeneous tumor, involving numerous factors during its
occurrence and development, but the specific mechanisms remain
unclear. Previous studies have hypothesized that the occurrence and
development of breast cancer is closely correlated with the
inhibition of apoptosis (3).
Apoptosis is the process of programmed cell death,
controlled by genes, so as to maintain a stable internal
environment, which involves the activation, expression and
regulation of a series of genes (15). The Caspase family is the major
executor of apoptotic cytoclasis, and serves important functions
during the apoptotic process (16,17).
Caspase-1 is a member of the Caspase family, and was the first to
be identified (18). As the typical
inflammation-associated Caspase, Caspase-1 is a key effector
molecule during the inflammatory response, regulating the
inflammatory reaction in the tumor microenvironment and the
anti-tumor immunity of organisms (19,20).
Recent studies demonstrated that Caspase-1 functions in apoptosis
(21,22). Hu et al (23) identified that the formation of tumors
in Caspase-1-deficient (Casp1 (−/-) mice was increased, and that
Caspase-1 affects tumorigenesis not by regulating colon
inflammation, but by regulating the colon epithelial cell
proliferation and apoptosis. Chen et al (24) injected 4T1 cells into the mammary fat
pads of mice to establish a xenograft model of breast cancer; on
this basis, they introduced the Caspase-1 specific-inhibitor
Ac-YVAD-CMK via intraperitoneal injection into tumor-bearing mice,
and identified that Ac-YVAD-CMK may increase the tumor weight and
splenomegaly, demonstrating that Caspase-1 may inhibit tumor
development by regulating the development of MDSCs in peripheral
tissues (peripheral blood, spleen) and in the tumor
microenvironment.
Based on these previous studies, the present study
analyzed whether Caspase-1 is involved in the occurrence and
development of breast cancer by regulating the proliferation and
apoptosis of breast cancer cells. Firstly, the expression of
Caspase-1 mRNA in breast cancer tissues and tumor-adjacent tissues
was detected. It was identified that the expression of Caspase-1 in
breast cancer tissues was significantly decreased compared with in
tumor-adjacent tissues, which is consistent data from a study
examining prostatic cancer by Veeranki (25). It suggests that low expression levels
of Caspase-1 may, to a certain degree, function in promoting the
occurrence and development of breast cancer. Following this, the
Caspase-1 small molecule inhibitor Ac-YVAD-CMK was used to treat
MDA-MB-231 cells in order to decrease the expression of Caspase-1,
and then the cell biological function of MDA-MB-231 was detected.
The experimental results demonstrated that the proliferation and
invasion abilities of cells were markedly increased, while the
apoptotic levels were significantly decreased compared with the
controls, which may potentially promote the development of breast
cancer.
In summary, the low expression levels of Caspase-1
serve a vital role in the occurrence and development of breast
cancer, and affect the proliferation, apoptosis and invasion of
breast cancer cells. Thus, Caspase-1 may be a novel target molecule
for treating breast cancer, representing a novel avenue for the
exploitation of drugs used to treat these tumors.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Song QK, Wang XL, Zhou XN, Yang HB, Li YC,
Wu JP, Ren J and Lyerly HK: Breast cancer challenges and screening
in China: Lessons from current registry data and population
screening studies. Oncologist. 20:773–779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song QK, Li J, Huang R, Fan JH, Zheng RS,
Zhang BN, Zhang B, Tang ZH, Xie XM, Yang HJ, et al: Age of
diagnosis of breast cancer in China: Almost 10 years earlier than
in the United States and the European union. Asian Pac J Cancer
Prev. 15:10021–10025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jordan VC: The new biology of
estrogen-induced apoptosis applied to treat and prevent breast
cancer. Endocr Relat Cancer. 22:R1–R31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Q, Du X, Zhou B, Li J, Lu W, Chen Q
and Gao J: Mitochondrial dysfunction is responsible for fatty acid
synthase inhibition-induced apoptosis in breast cancer cells by
PdpaMn. Biomed Pharmacother. 96:396–403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galluzzi L, Lopez-Soto A, Kumar S and
Kroemer G: Caspases connect cell-death signaling to organismal
homeostasis. Immunity. 44:221–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winkler S and Rösen-Wolff A: Caspase-1: An
integral regulator of innate immunity. Semin Immunopathol.
37:419–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sagulenko V, Vitak N, Vajjhala PR, Vince
JE and Stacey KJ: Caspase-1 is an apical caspase leading to
caspase-3 cleavage in the AIM2 inflammasome response, independent
of caspase-8. J Mol Biol. 430:238–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mascarenhas DPA, Cerqueira DM, Pereira
MSF, Castanheira FVS, Fernandes TD, Manin GZ, Cunha LD and Zamboni
DS: Inhibition of caspase-1 or gasdermin-D enable caspase-8
activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog.
13:e10065022017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nunes T and de Souza HS: Inflammasome in
intestinal inflammation and cancer. Mediators Inflamm.
2013:6549632013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ericsson C and Nistér M: Protein
extraction from solid tissue. Methods Mol Biol. 675:307–312. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
da Costa Vieira RA, Biller G, Uemura G,
Ruiz CA and Curado MP: Breast cancer screening in developing
countries. Clinics (Sao Paulo). 72:244–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eroglu M and Derry WB: Your neighbours
matter-non-autonomous control of apoptosis in development and
disease. Cell Death Differ. 23:1110–1118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Man SM and Kanneganti TD: Converging roles
of caspases in inflammasome activation, cell death and innate
immunity. Nat Rev Immunol. 16:7–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adamiec-Mroczek J, Zajac-Pytrus H and
Misiuk-Hojlo M: Caspase-dependent apoptosis of retinal ganglion
cells during the development of diabetic retinopathy. Adv Clin Exp
Med. 24:531–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martinon F and Tschopp J: Inflammatory
caspases and inflammasomes: Master switches of inflammation. Cell
Death Differ. 14:10–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guey B and Petrilli V: Assessing Caspase-1
activation. Methods Mol Biol. 1417:197–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Winkler S, Hedrich CM and Rösen-Wolff A:
Caspase-1 als regulates der autoinflammation bei rheumatischen
Erkrankungen. Z Rheumatol. 75:265–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sollberger G, Strittmatter GE, Grossi S,
Garstkiewicz M, Keller Auf dem U, French LE and Beer HD: Caspase-1
activity is required for UVB-induced apoptosis of human
keratinocytes. J Invest Dermatol. 135:1395–1404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha
X, Cheng X, Wang J, Qin X, Yu J, et al: Caspase-1 inflammasome
activation mediates Homocysteine-induced Pyrop-apoptosis in
endothelial cells. Circ Res. 118:1525–1539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu B, Elinav E, Huber S, Booth CJ, Strowig
T, Jin C, Eisenbarth SC and Flavell RA: Inflammation-induced
tumorigenesis in the colon is regulated by caspase-1 and NLRC4.
Proc Natl Acad Sci USA. 107:21635–21640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen YJ, Zheng W, Niu ZY, Wu Z and Shen
PP: Caspase-1modulates breast cancer growth and the development of
myeloid-derived suppressor cells. Chin J Immun. 29:1128–1134.
2013.
|
|
25
|
Veeranki S: Role of inflammasomes and
their regulators in prostate cancer initiation, progression and
metastasis. Cell Mol Biol Lett. 18:355–367. 2013. View Article : Google Scholar : PubMed/NCBI
|