Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common form of cancer worldwide and the third most frequent cause
of cancer-related death (1,2). Hepatitis B virus (HBV), hepatitis C
virus (HCV), alcohol abuse, and nonalcoholic fatty liver disease
are recognized as the major risk factors for hepatocarcinogenesis
(3). In spite of improving diagnostic
techniques, efficient therapies are limited. Currently, surgical
resection is still the most effective treatment for HCC at an early
stage. However, only 20% of patients with HCC are diagnosed in
early stages, while more than 80% of HCC cases are diagnosed at an
advanced stage with inoperable distant metastases (4). Half of patients present with an advanced
stage of HCC which must receive systemic therapy without surgical
treatment, but the effectiveness of agents such as sorafenib used
in the systematic treatment of advanced HCC is relatively limited
(5). The poor prognosis of HCC
patients is mainly associated with the high rate of intrahepatic
metastasis after treatment. Therefore, it is imperative to develop
new therapeutic strategies for HCC treatment.
Cyclooxygenase (COX)-2, a rate-limiting enzyme in
the synthesis of prostaglandin (PG), has been reported as an
anti-tumor target. A large number of studies have demonstrated that
COX-2 influences many aspects of cancer cells including viability,
motility, survival, invasiveness, and apoptosis resistance
(6–9).
Meloxicam, as a selective inhibitor of cyclooxygenase-2 (COX-2), is
widely used for anti-inflammation. Accumulating evidences have
revealed that the COX-2 inhibitor exerts an anti-proliferative
response in various cancers (10–12). Our
previous studies also revealed that meloxicam inhibited
proliferation and led to apoptosis of HCC cells (13–16).
However, the exact mechanisms of the anti-cancer effects regulated
by meloxicam remain unclear.
The cytoprotective chaperone protein, clusterin, was
first isolated from ram rete testes fluid and is synthesized as a
full-length cluster (60 kDa) in the mitochondria. It encodes two
isoforms with paradoxical activities: Nuclear clusterin (nCLU) and
secretory clusterin (sCLU) which plays a crucial role in regulating
various pathophysiological processes such as tissue remodeling,
reproduction, lipid transport, complement regulation, and apoptosis
(17). sCLU, starting as an
approximately 60 kDa precursor peptide, has been considered as an
anti-apoptotic protein. Recently, several studies have reported
that sCLU is associated with resistance to chemotherapy.
Constitutive over-expression of sCLU has been reported to confer
chemoresistance in cancer therapy, however, down-regulation of sCLU
sensitized pancreatic cancer cells to gemcitabine chemotherapy
(18). Our previous studies also
demonstrated that sCLU contributes to oxaliplatin resistance by
activating the Akt pathway in HCC (19) and down-regulating sCLU could enhance
the sensitivity of HCC cells to gemcitabine by activating the
intrinsic apoptosis pathway (20).
However, the precise mechanisms of sCLU in the resistance of HCC to
chemotherapy are largely unknown. Therefore, we hypothesized that
suppression of sCLU could potentiate the meloxicam-induced
cytotoxicity in HCC cells. In this study, we tried to explore the
role of sCLU in meloxicam-induced cytotoxicity in human HCC
cells.
Materials and methods
Cell lines and culture
Human HCC cell lines, Bel-7402 were obtained from
the American Type Culture Collection (ATCC, Manassas, VA, USA), and
SMMC-7721 cell lines were purchased from the Type Culture
Collection Cell Bank, Chinese Academy of Science, Shanghai, China.
The cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin, and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in 95% air and 5% CO2.
Reagents and antibodies
The meloxicam (Mel) was purchased from EMD Millipore
(Billerica, MA, USA), dissolved in dimethylsulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) at 10 or 50 mM stock. The
concentration of DMSO never exceeded 0.6% (v/v) and equal amounts
of DMSO were added to control cells. MK-2206 (a special inhibitor
of AKT) was obtained from Merck KGaA (Darmstadt, Germany).
Antibodies against MMP-2 (cat. no. 4022), AKT (cat. no. 9272), and
phosphorylated AKT (p-AKT) (Ser473) (cat. no. 9271) were purchased
from Cell Signaling Technology, Inc., (Danvers, MA, USA).
Antibodies against E-cadherin (ab15148) and GAPDH (ab37168) were
obtained from Abcam (Cambridge, UK). The antibody for sCLU
(sc-5289) was purchased from Santa Cruz Biotechnology, Inc.,
(Dallas, TX, USA).
Wound-healing scratch assay
Cell monolayers grown to confluence on 6-well
plastic dishes were wounded by scratching with a pipette tip. The
cells were cultured in the presence or absence of meloxicam (80 µM)
for 24 h. The wounds were photographed (10× objective) at the
indicated time points.
Cell invasion assays
Briefly, the cell invasion assay was performed by
adding Matrigel basement matrix to the upper chamber of transwells.
1×105 cells, in 300 µl of RPMI-1640 medium (with 1% FBS)
containing meloxicam were seeded to the upper chamber of Transwells
(Corning, New York, USA). The bottom wells of the chambers were
filled with 500 µl RPMI-1640 medium (with 10% FBS). After 24 h of
incubation, the chambers were fixed with 95% ethanol and then
stained with 1% crystal violet.
Reverse-transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the cells using Trizol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was
synthesized by using a cDNA synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.). The primers targeting MMP-2 were
(5′-TGACGGTAAGGACGGACTC-3′; 5′-ATACTTCACACGGACCACTTG-3′),
E-cadherin (5′-TGCCCAGAAAATGAAAAAGG-3′; 5′-GGATGACAGCGTGAGAGA-3′),
and GAPDH (5′-TTACTCCTTGGAGGCCATGTGGGC-3′;
5′-ACTGCCACCCAGAAGACTGTGGATGG-3′). Expression levels were
normalized to GAPDH. All protocols were carried out according to
the manufacturer's instructions. Real-time PCR was performed using
MX3000P Real-time PCR systems (Agilent Technologies, Inc., Santa
Clara, CA, USA). Experiments were performed in triplicate, and the
data were calculated by ΔΔCq methods.
Cell viability and western blotting
assays
These methods have been previously described
(14,15,21). Cell
viability assays were performed by using the Cell Counting Kit-8
(CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
After different treatments, protein concentrations in cell extracts
were determined (Bio-Rad Laboratories, Inc., Hercules, CA, USA),
equal amounts of each sample were resolved in SDS-PAGE gels, then
transferred to a polyvinylidene fluoride (PVDF) membrane (EMD
Millipore), and probed with the primary antibodies specific for
sCLU, MMP-2, E-cadherin, AKT, and p-AKT.
sCLU-shRNA and pCDNA3.1-sCLU
Transfection
Four potentially effective targets of sCLU were
designed, synthesized, and inserted into the pMAGic 7.1 vector to
produce four shRNA vectors: CLU-1, CLU-2, CLU-3, and CLU-4
(19). Transfection of cells was
performed using GenJet DNA in vitro transfection reagent
(SignaGen Laboratories, Rockville, MD, USA). pCDNA3.1-sCLU and its
control pCDNA3.1 plasmid were transfected into Bel-7402 and
SMMC-7721 cells as previously reported (22).
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) and were analyzed by one-way ANOVA followed by the Dunnett's
test with SPSS software (v17.0; SPSS, Inc., Chicago, IL, USA), with
values of P<0.05 considered to indicate a statistically
significant difference.
Results
sCLU knockdown decreases HCC cells
invasion
In our previous studies, our results showed that
meloxicam suppressed HCC cell survival and its cytotoxicity
increased in a concentration-dependent manner. Moreover, we found
that HCC cells expressed different levels of COX-2 and sCLU
protein, and Bel-7402 and SMMC-7721 cells expressed higher levels
of COX-2 and sCLU than other HCC cells (14,19,20).
Therefore, in the present study, Bel-7402 and SMMC-7721 cells were
chosen for the following experiments. CLU has been reported to be
associated with invasion and metastasis (23,24). In
this study, we first used the shRNA approach to investigate the
role of sCLU in HCC cell invasion. In our previous study, we
designed four pMAGic7.1-based shRNA vectors (CLU1, CLU2, CLU3, and
CLU4) to down-regulate expression of sCLU in HCC cell lines. We
found that CLU4 shRNA displayed the strongest gene-silencing
ability (19). Therefore, CLU4 shRNA
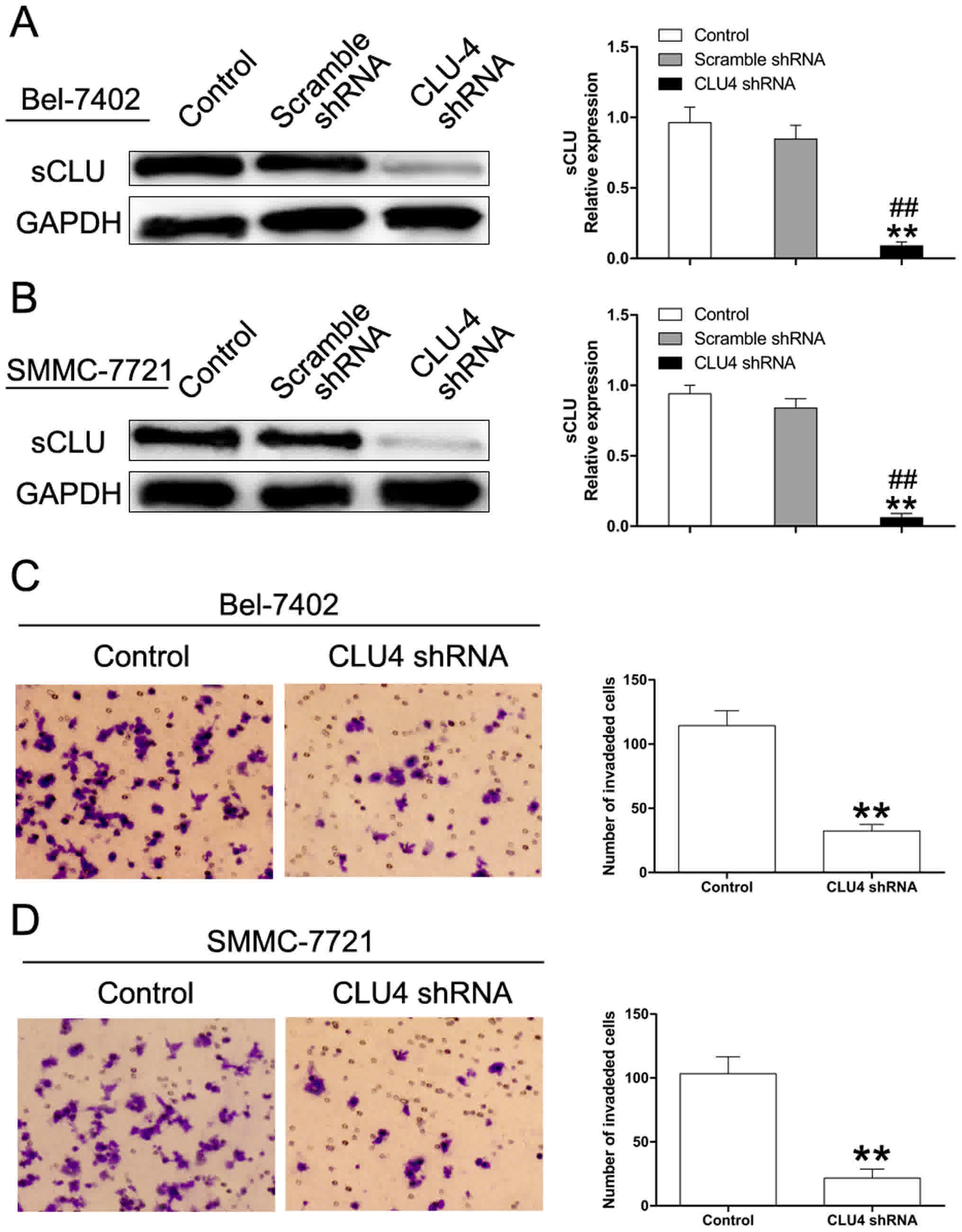
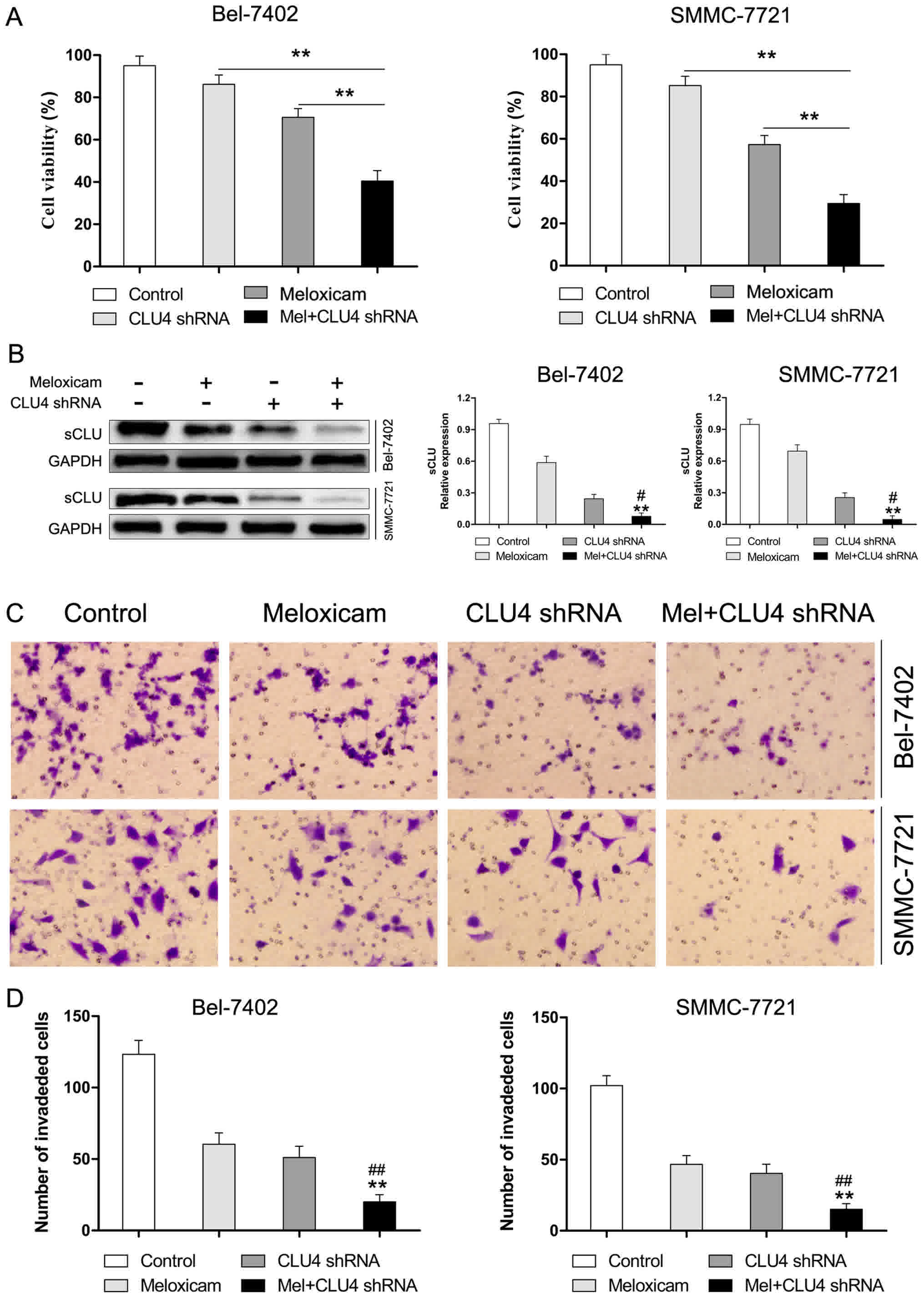
was used in the current work. As depicted in Fig. 1A and B, CLU4 shRNA significantly
decreased expression of sCLU. Matrigel invasion assays showed that
knockdown of sCLU by CLU4 shRNA notably impaired invasive abilities
of both Bel-7402 and SMMC-7721 cells suggesting the essential role
of sCLU in conferring invasive properties to HCC cells (Fig. 1C and D).
sCLU over-expression increases HCC
cancer cell invasion
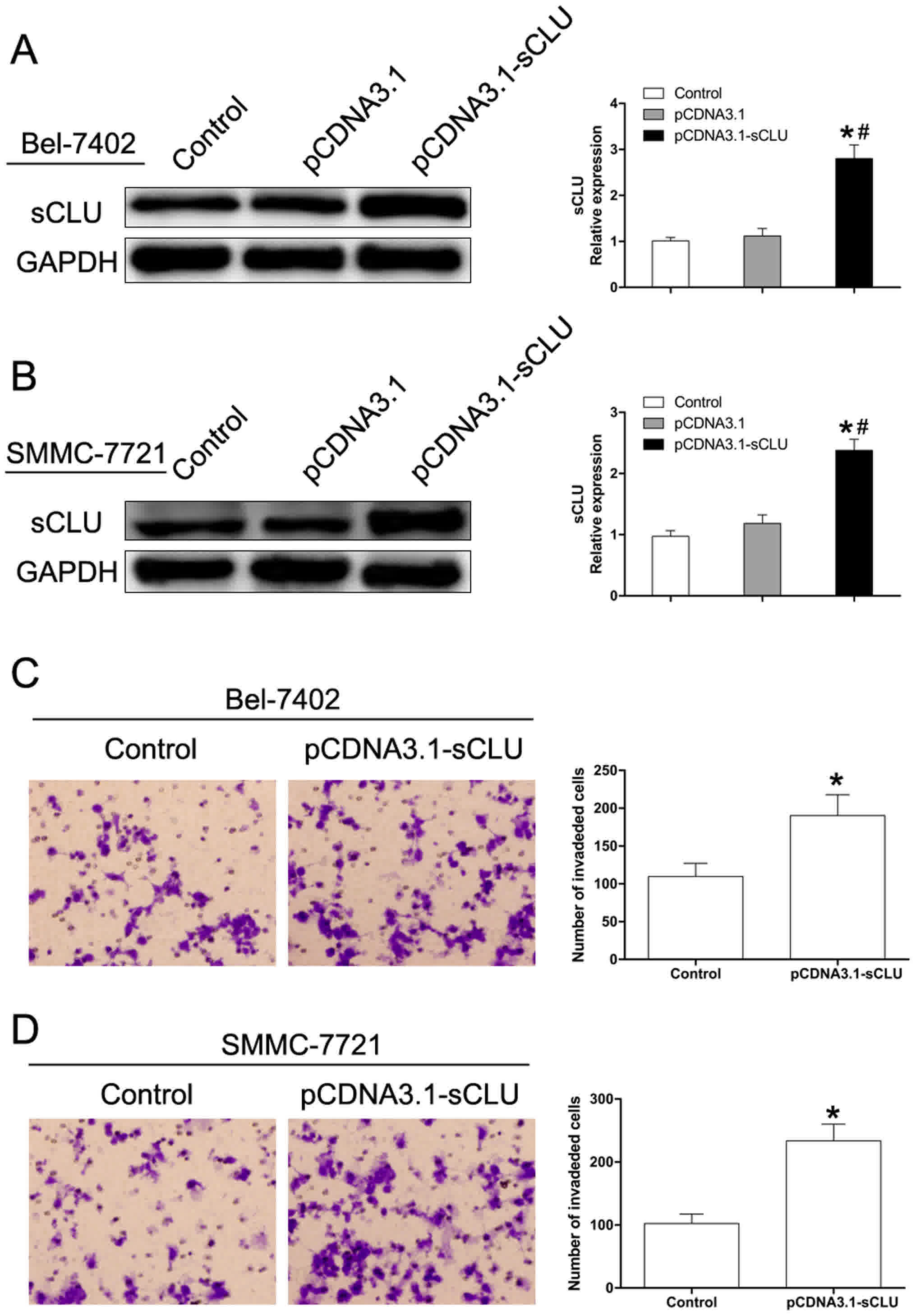
To further investigate the effect of sCLU in
regulating Bel-7402 and SMMC-7721 cell invasion, sCLU was
over-expressed (Fig. 2A). As shown in
Fig. 2, over-expression of sCLU
significantly enhanced invasive abilities of both Bel-7402 and
SMMC-7721 cells. These results supported our hypothesis that sCLU
confers invasive characteristics to Bel-7402 and SMMC-7721
cells.
sCLU regulates expression of MMP-2 and
E-cadherin in HCC cells in vitro
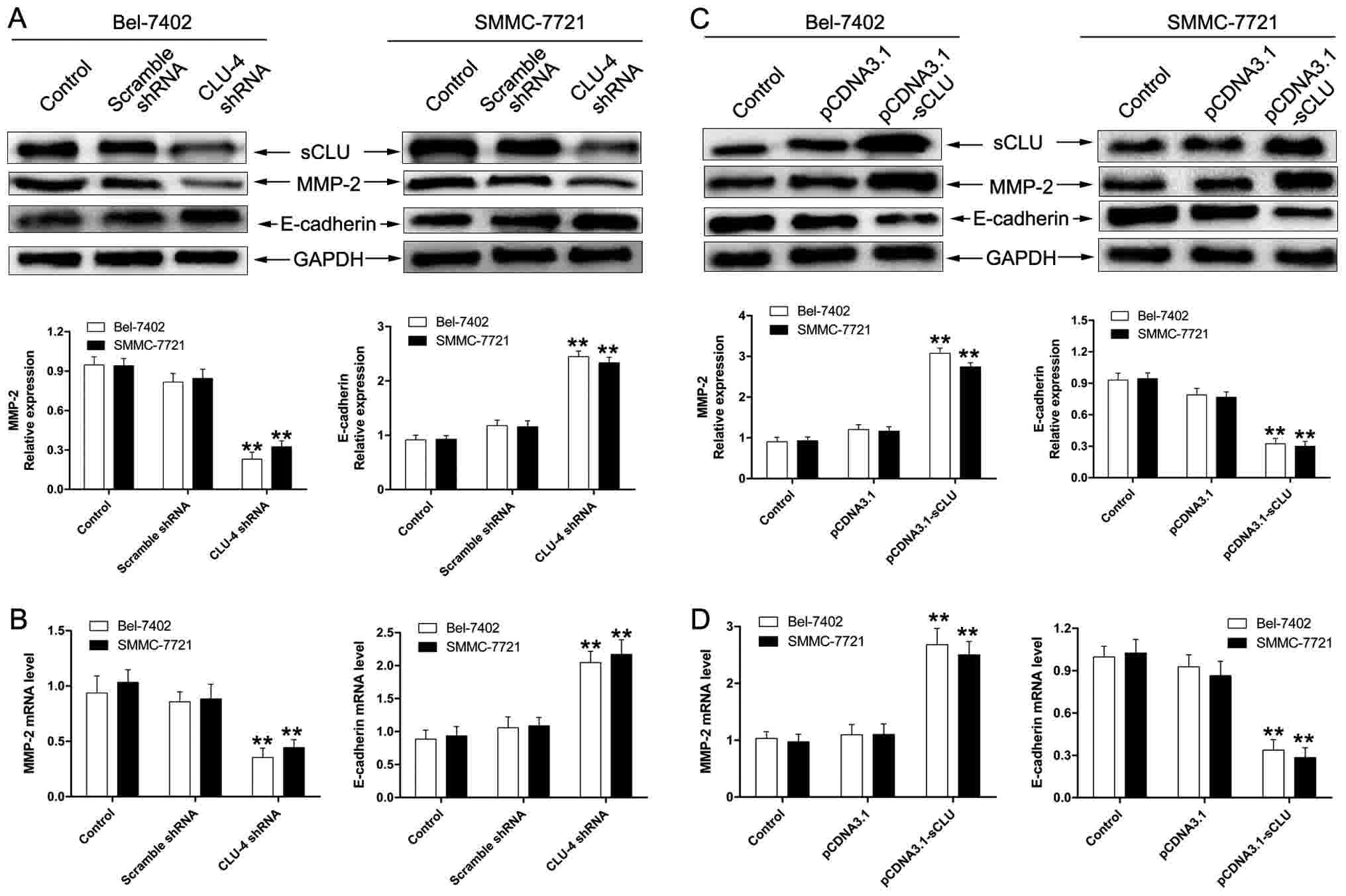
As matrix metallo-proteinase (MMP)-2 and E-cadherin
activity has been considered to exert a crucial role in tumor
invasion, we first examined whether sCLU could lead to MMP-2 and
E-cadherin activity in Bel-7402 and SMMC-7721 cells. As shown in
Fig. 3A and B, cells transfected with
CLU4 shRNA significantly suppressed expression of MMP-2 and
enhanced the extent of E-cadherin. Furthermore, we examined the
effect of sCLU over-expression on expression of MMP-2 and
E-cadherin. As expected, Bel-7402 and SMMC-7721 cells transfected
with pCDNA3. 1-sCLU notably up-regulated expression of MMP-2 and
down-regulated expression of E-cadherin (Fig. 3C and D). These results demonstrated
the involvement of sCLU in the regulation of MMP-2 and E-cadherin
in HCC cells in vitro.
Meloxicam treatment attenuates HCC
cancer cell proliferation and invasion
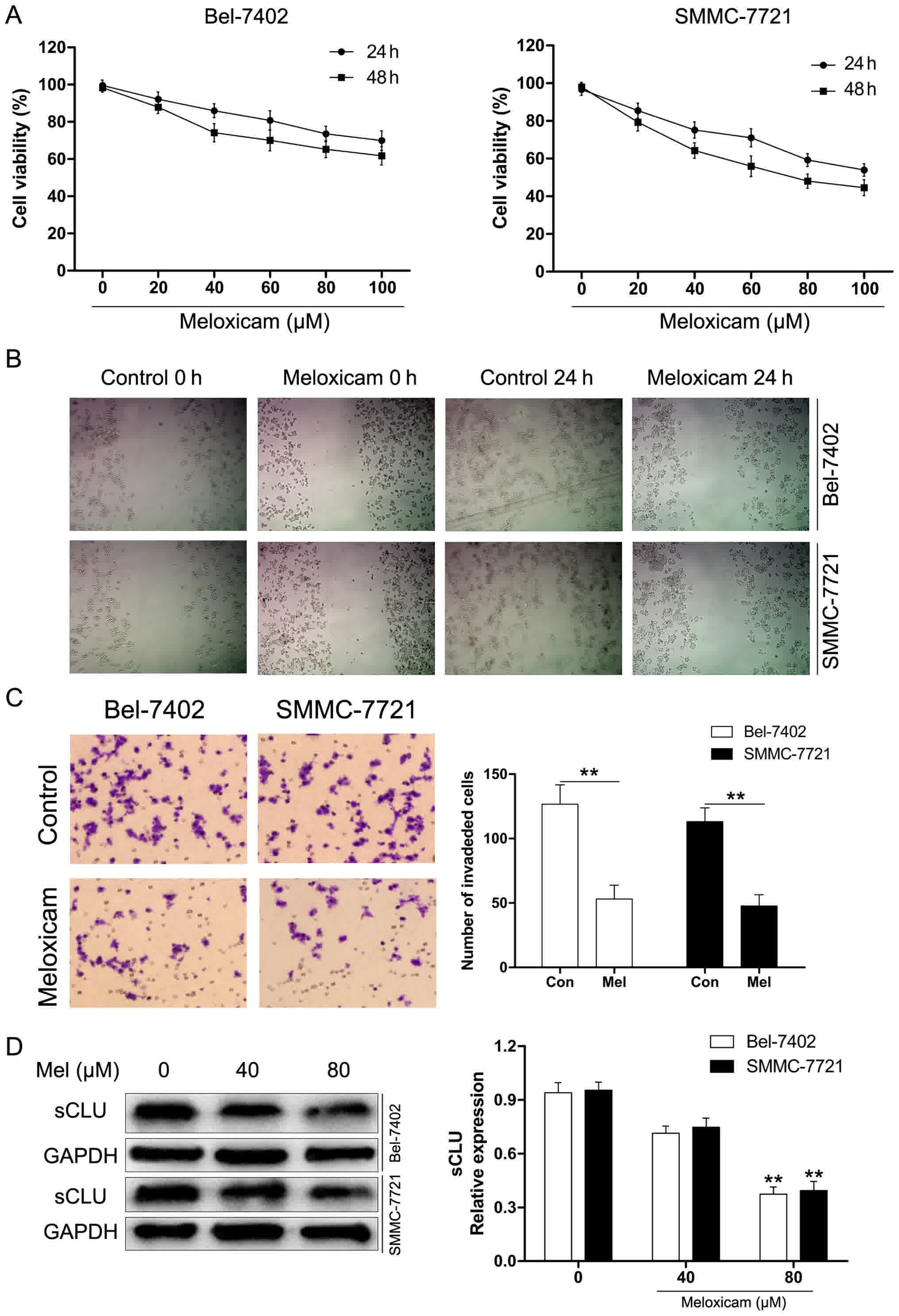
Our results for the CCK-8 assay presented in
Fig. 4A showed that meloxicam notably
inhibited proliferation of Bel-7402 and SMMC-7721 cells in a time-
and concentration-dependent manner. To further explore the role of
meloxicam on invasion of HCC cancer cells, Bel-7402 and SMMC-7721
cells were treated with meloxicam (80 µM) for 24 h. As determined
by scratch motility assay, meloxicam treatment significantly
suppressed the migratory potential of Bel-7402 and SMMC-7721 cells
(Fig. 4B). Furthermore, we used
Transwell assays to examine invasion. As shown in Fig. 4C, the results were consistent with
those of the scratch assay. Considering the crucial role of sCLU in
invasion, we hypothesized whether meloxicam could target sCLU for
its anti-tumor effect. To clarify this issue, we examined
expression of sCLU in meloxicam treatment in Bel-7402 and SMMC-7721
cells. As shown in Fig. 4D, meloxicam
treatment significantly decreased expression of sCLU in a
dose-dependent manner in Bel-7402 and SMMC-7721 cells. These data
suggested that meloxicam is an effective inhibitor against invasion
and regulates sCLU in HCC cells.
sCLU is responsible for
meloxicam-regulated suppression of proliferation and invasion in
HCC cells
To investigate whether sCLU contributes to the
function of meloxicam in mediating proliferation and invasion in
Bel-7402 and SMMC-7721 cells, expression of sCLU was knocked down
by CLU4 shRNA. As shown in Fig. 5A,
exposure to meloxicam after inhibition of sCLU induced a
significant suppression of cell proliferation. Moreover, we found
that suppressed expression of sCLU (Fig.
5B) dramatically reduced invasion in Bel-7402 and SMMC-7721
cells treated with meloxicam (Fig. 5C and
D). These data revealed that sCLU is an important mediator for
meloxicam in mediating cell proliferation and invasion in Bel-7402
and SMMC-7721 cells.
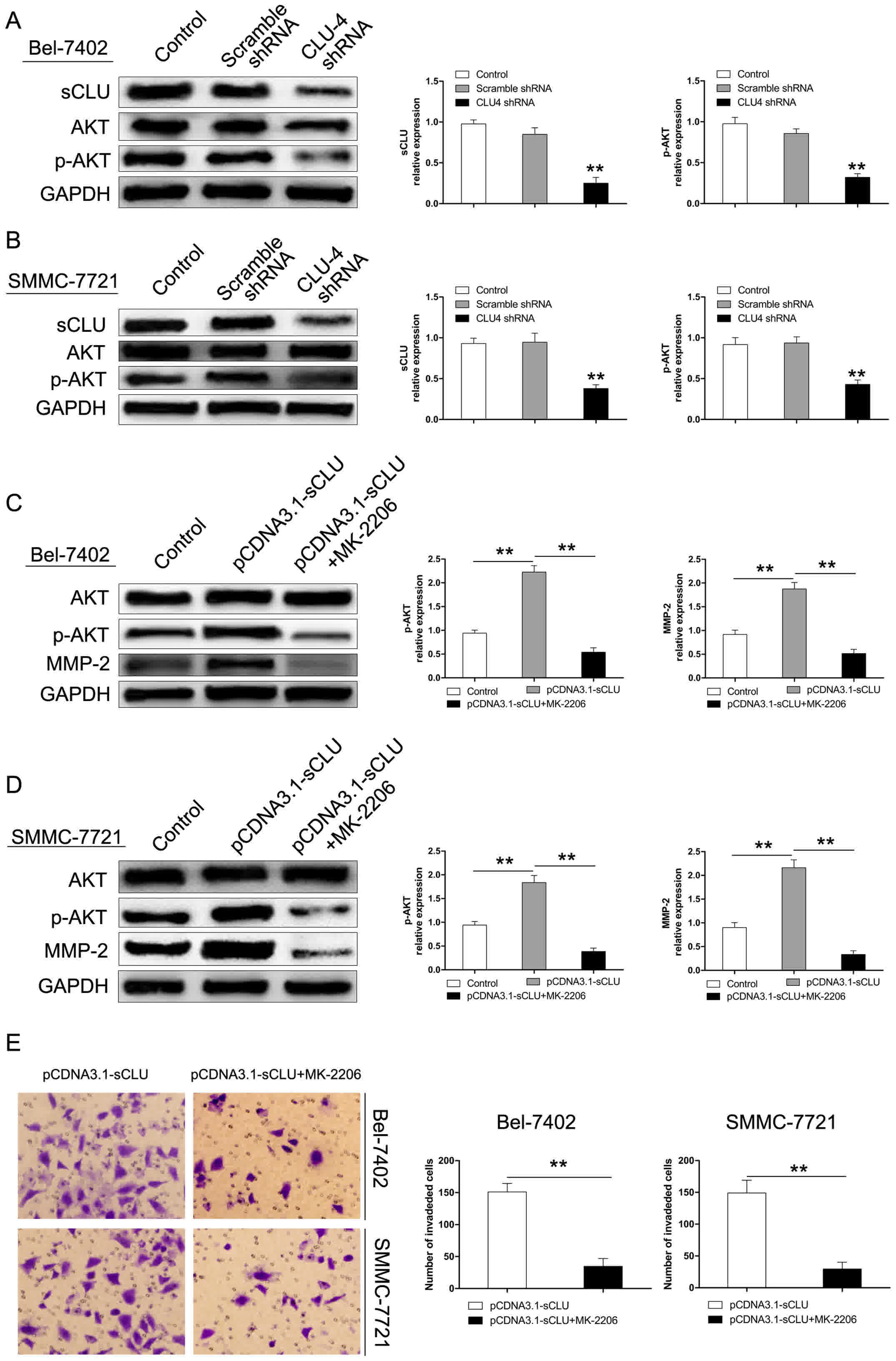
Up-regulation of MMP-2 by sCLU is
dependent on AKT activation in HCC cells in vitro
The AKT pathway has been considered to exert a
crucial role in regulating cell growth, proliferation, survival,
and motility (25–28). Our previous studies also revealed that
sCLU suppressed HCC cell apoptosis induced by AKT inhibition
(19). In this study, to investigate
whether the AKT signaling pathway mediates the regulatory effect of
sCLU on MMP-2 expression, Bel-7402 and SMMC-7721 cells were
pre-incubated with MK-2206, an AKT specific inhibitor. As shown in
Fig. 6A and B, the extent of p-AKT
was decreased in both cells after transfection with CLU4 shRNA.
However, over-expression of sCLU significantly enhanced the level
of p-AKT in Bel-7402 and SMMC-7721 cells. Furthermore, expression
of MMP-2 was significantly inhibited by MK-2206 (Fig. 6C and D). In addition, suppression of
AKT by MK-2206 significantly reduced invasion of Bel-7402 and
SMMC-7721 cells transfected by pCDNA3.1-sCLU (Fig. 6E). These results suggested that the
AKT signaling pathway may exert an important role in mediating
MMP-2 expression and sCLU-induced invasiveness in Bel-7402 and
SMMC-7721 cells.
Discussion
Recurrence and metastasis are recognized as the
major leading causes of poor prognosis of HCC patients (23). In spite of great progress in exploring
the molecular mechanism of invasion and metastasis of HCC, it has
fallen well short of its goals due to the poor prognosis of HCC
patients. Therefore, it is worthwhile to study the molecular
mechanism of HCC invasiveness.
sCLU has been considered as a stress-induced
chaperone that confers proliferative and survival advantages to
many cancers, including retinal (29), breast (30), lung (22), ovarian (24), and cervical (31) cancer. Our previous studies also
demonstrated that sCLU is over-expressed in HCC cells (19,20).
Recently, several studies reported that sCLU over-expression plays
an important role in regulating invasion and migration (23,32).
However, the role of sCLU in HCC cell invasion has yet to be
elucidated. In this study, we observed that down-regulation of sCLU
by CLU4 shRNA significantly alleviated invasiveness whereas
over-expression sCLU notably enhanced the number of invasive cells
in Bel-7402 and SMMC-7721 cells. Furthermore, we found that sCLU
exerted a crucial role in regulating invasiveness of Bel-7402 and
SMMC-7721 cells via mediating the levels of MMP-2 and E-cadherin.
These results suggested that sCLU confers invasive characteristics
to HCC cells through regulating expression of MMP-2 and E-cadherin
protein in HCC cells.
The selective COX-2 inhibitor meloxicam has been
reported to exert anti-invasion responses in various tumors
(33–35). Our previous data also showed that
meloxicam inhibited migration and invasion in HCC cells (14,15).
However, it remains unknown whether sCLU participates in the
anti-invasion effects of HCC to meloxicam. In this current work, we
found that the extent of sCLU was decreased after treatment with 80
µM meloxicam. These results might contribute to the observation
that meloxicam could suppress the proliferation and invasion of
Bel-7402 and SMMC-7721 cells. Moreover, the combinations of
meloxicam and CLU4 shRNA significantly decreased invasion in
Bel-7402 and SMMC-7721 cells. These finding suggested that as an
important mediator of invasiveness, sCLU may be responsible for
meloxicam regulated suppression of proliferation and invasion in
HCC cells.
In the present study, we also investigated whether
the AKT signaling pathway was involved in regulation of MMP-2
expression by treating Bel-7402 and SMMC-7721 cells with the AKT
specific inhibitor, MK-2206. We found that inhibition of AKT by
MK-2206 dramatically decreased the level of MMP-2 in
over-expressing sCLU HCC cells. Several studies reported that CLU
confers proliferative and survival advantages through the AKT
signaling pathway (23,36). In the present study, we found that
over-expression of sCLU significantly potentiated expression of
p-AKT and MMP-2. However, down-regulation of sCLU by CLU4 shRNA
alleviated the extent of p-AKT. These data revealed that sCLU may
promote HCC invasion via the AKT signaling pathway.
In conclusion, we found that the inhibitory effect
of meloxicam on invasion in HCC cells was through down-regulation
of sCLU expression. Furthermore, our data promote a novel mechanism
that sCLU activates the AKT signaling pathway, which promotes
expression of MMP-2 and induces invasion of HCC cells. The
targeting of sCLU suggests a novel therapeutic strategy against
invasion in HCC.
Acknowledgements
Thanks to Dr. Edward C. Mignot, Shandong University,
for linguistic advice.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Said A and Wells J: Management of
hepatocellular carcinoma. Minerva Med. 100:51–68. 2009.PubMed/NCBI
|
|
5
|
Zhuang L, Xu L, Wang P, Jiang Y, Yong P,
Zhang C, Zhang H, Meng Z and Yang P: Na+/K+-ATPase α1 subunit, a
novel therapeutic target for hepatocellular carcinoma. Oncotarget.
6:28183–28193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu SM and Kim SJ: DNA-hypomethylating
agent, 5′-azacytidine, induces cyclooxygenase-2 expression via the
PI3-kinase/Akt and extracellular signal-regulated kinase-1/2
pathways in human HT1080 fibrosarcoma cells. Int J Oncol.
47:1469–1475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diab S, Fidanzi C, Léger DY, Ghezali L,
Millot M, Martin F, Azar R, Esseily F, Saab A, Sol V, et al:
Berberis libanotica extract targets NF-κB/COX-2, PI3K/Akt and
mitochondrial/caspase signalling to induce human erythroleukemia
cell apoptosis. Int J Oncol. 47:220–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao J, Guo T, Dong Q, Zhang J and Li Y:
miR-26b is downregulated in human tongue squamous cell carcinoma
and regulates cell proliferation and metastasis through a
COX-2-dependent mechanism. Oncol Rep. 33:974–980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Wang Z, Li J, Zhang W, Ren F and
Yue W: NFATc1 activation promotes the invasion of U251 human
glioblastoma multiforme cells through COX-2. Int J Mol Med.
35:1333–1340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q,
Zhang HL and Zhou YN: Celecoxib regulates apoptosis and autophagy
via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer
cells. Int J Mol Med. 33:1451–1458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yusup G, Akutsu Y, Mutallip M, Qin W, Hu
X, Komatsu-Akimoto A, Hoshino I, Hanari N, Mori M, Akanuma N, et
al: A COX-2 inhibitor enhances the antitumor effects of
chemotherapy and radiotherapy for esophageal squamous cell
carcinoma. Int J Oncol. 44:1146–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qian M, Qian D, Jing H, Li Y, Ma C and
Zhou Y: Combined cetuximab and celecoxib treatment exhibits a
synergistic anticancer effect on human oral squamous cell carcinoma
in vitro and in vivo. Oncol Rep. 32:1681–1688. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Chen X, Dong X, Xu Z, Jiang H and
Sun X: Specific COX-2 inhibitor, meloxicam, suppresses
proliferation and induces apoptosis in human HepG2 hepatocellular
carcinoma cells. J Gastroenterol Hepatol. 21:1814–1820. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong X, Li R, Xiu P, Dong X, Xu Z, Zhai B,
Liu F, Jiang H, Sun X, Li J and Qiao H: Meloxicam executes its
antitumor effects against hepatocellular carcinoma in
COX-2-dependent and -independent pathways. PLoS One. 9:e928642014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong J, Xiu P, Dong X, Wang F, Wei H,
Wang X, Xu Z, Liu F, Li T, Wang Y and Li J: Meloxicam combined with
sorafenib synergistically inhibits tumor growth of human
hepatocellular carcinoma cells via ER stress-related apoptosis.
Oncol Rep. 34:2142–2150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong J, Dong X, Xiu P, Wang F, Liu J, Wei
H, Xu Z, Liu F, Li T and Li J: Blocking autophagy enhances
meloxicam lethality to hepatocellular carcinoma by promotion of
endoplasmic reticulum stress. Cell Prolif. 48:691–704. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shannan B, Seifert M, Leskov K, Willis J,
Boothman D, Tilgen W and Reichrath J: Challenge and promise: Roles
for clusterin in pathogenesis, progression and therapy of cancer.
Cell Death Differ. 13:12–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Q, Wang Z, Zhang K, Liu X, Cao W,
Zhang L, Zhang S, Yan B, Wang Y and Xia C: Clusterin confers
gemcitabine resistance in pancreatic cancer. World J Surg Oncol.
9:592011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiu P, Dong X, Dong X, Xu Z, Zhu H, Liu F,
Wei Z, Zhai B, Kanwar JR, Jiang H, et al: Secretory clusterin
contributes to oxaliplatin resistance by activating Akt pathway in
hepatocellular carcinoma. Cancer Sci. 104:375–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiu P, Xu Z, Liu F, Li Z, Li T, Zou F, Sun
X and Li J: Downregulating sCLU enhances the sensitivity of
hepatocellular carcinoma cells to gemcitabine by activating the
intrinsic apoptosis pathway. Dig Dis Sci. 59:1798–1809. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang F, Dong X, Xiu P, Zhong J, Wei H, Xu
Z, Li T, Liu F, Sun X and Li J: T7 peptide inhibits angiogenesis
via downregulation of angiopoietin-2 and autophagy. Oncol Rep.
33:675–684. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Zhang K, Liu Z, Hao F, Wang M, Li
X, Yin Z and Liang H: Secreted clusterin gene silencing enhances
chemosensitivity of a549 cells to cisplatin through AKT and ERK1/2
pathways in vitro. Cell Physiol Biochem. 33:1162–1175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Jin G, Jin H, Wang N, Luo Q, Zhang
Y, Gao D, Jiang K, Gu D, Shen Q, et al: Clusterin facilitates
metastasis by EIF3I/Akt/MMP13 signaling in hepatocellular
carcinoma. Oncotarget. 6:2903–2916. 2015.PubMed/NCBI
|
|
24
|
Fu Y, Lai Y, Liu J, Liu X, You Z and Yang
G: Lentivirus-mediated shRNA interference of clusterin blocks
proliferation, motility, invasion and cell cycle in the ovarian
cancer cells. J Ovarian Res. 8:592015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He J, Zhu G, Gao L, Chen P, Long Y, Liao
S, Yi H, Yi W, Pei Z, Wu M, et al: Fra-1 is upregulated in gastric
cancer tissues and affects the PI3K/Akt and p53 signaling pathway
in gastric cancer. Int J Oncol. 47:1725–1734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Raha S, Yumnam S, Hong GE, Lee HJ,
Saralamma VV, Park HS, Heo JD, Lee SJ, Kim EH, Kim JA and Kim GS:
Naringin induces autophagy-mediated growth inhibition by
downregulating the PI3K/Akt/mTOR cascade via activation of MAPK
pathways in AGS cancer cells. Int J Oncol. 47:1061–1069. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin H, Qiao F, Wang Y, Xu Y and Shang Y:
Curcumin inhibits cell proliferation and induces apoptosis of human
non-small cell lung cancer cells through the upregulation of
miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol
Rep. 34:2782–2789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lim HS, Kang YJ, Sung B, Kim SH, Kim MJ,
Kim HR, Kim SJ, Choi YH, Moon HR, Chung HY and Kim ND: Novel
dihydrobenzofuro[4,5-b][1,8]naphthyridin-6-one derivative, MHY-449,
induces cell cycle arrest and apoptosis via the downregulation of
Akt in human lung cancer cells. Oncol Rep. 34:2431–2438. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song HB, Jun HO and Kim JH, Yu YS, Kim KW,
Min BH and Kim JH: Anti-apoptotic effect of clusterin on
cisplatin-induced cell death of retinoblastoma cells. Oncol Rep.
30:2713–2718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Wang X, Zhao H, Liang B and Du Q:
Clusterin confers resistance to TNF-alpha-induced apoptosis in
breast cancer cells through NF-kappaB activation and Bcl-2
overexpression. J Chemother. 24:348–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JH, Lee JY, Rho SB, Choi JS, Lee DG,
An S, Oh T, Choi DC and Lee SH: PACAP inhibits tumor growth and
interferes with clusterin in cervical carcinomas. FEBS Lett.
588:4730–4739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwang S, Lee DH, Lee IK, Park YM and Jo I:
Far-infrared radiation inhibits proliferation, migration, and
angiogenesis of human umbilical vein endothelial cells by
suppressing secretory clusterin levels. Cancer Lett. 346:74–83.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qiu X, Cheng JC, Chang HM and Leung PC:
COX2 and PGE2 mediate EGF-induced E-cadherin-independent human
ovarian cancer cell invasion. Endocr Relat Cancer. 21:533–543.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhattacharya A, Li Y, Shi Y and Zhang Y:
Enhanced inhibition of urinary bladder cancer growth and muscle
invasion by allyl isothiocyanate and celecoxib in combination.
Carcinogenesis. 34:2593–2599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Z, Liu M, Liu X, Huang S, Li L, Song
B, Li H, Ren Q, Hu Z, Zhou Y and Qiao L: COX-2 regulates E-cadherin
expression through the NF-κB/Snail signaling pathway in gastric
cancer. Int J Mol Med. 32:93–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ammar H and Closset JL: Clusterin
activates survival through the phosphatidylinositol 3-kinase/Akt
pathway. J Biol Chem. 283:12851–12861. 2008. View Article : Google Scholar : PubMed/NCBI
|