Introduction
Cervical cancer (CC) is the third most common type
of cancer among females and the second leading cause of
cancer-associated mortality worldwide (1). The incidence and mortality for CC are
relatively low in developed countries compared with that in
developing or undeveloped countries (2). Although the methods for detection and
treatment for CC have been significantly improved, the mortality
rate of patients with malignant CC remains high (3,4).
Additionally, the molecular mechanisms underlying the development
of CC remain poorly understood. Previous studies have demonstrated
that aberrant expression of several proteins is associated with the
proliferation and apoptosis of CC cell lines (5,6).
Therefore, the identification of genetic alterations that may be
associated with the progression of CC may provide insights for the
development of novel therapeutic strategies.
The homeobox (HOX) gene family, which contains a
total of 39 members, encode homeodomain-containing transcription
factors that serve essential functions in cellular development and
differentiation (7,8). Aberrant expression of HOX genes,
including HOXA9, HOXB4, and HOXC10 in CC has been reported in
previous studies (9–11), suggesting that HOX genes may serve an
important function in tumorigenesis. HOXC8 is one of the 39 members
of the HOX family proteins and is overexpressed in several types of
human cancer, including colon (12),
lung (13), prostate (14), and breast cancer (15). Several studies investigated the
molecular mechanisms by which HOXC8 contributes to tumorigenesis.
HOXC8 may exert its oncogenic function through regulating the
expression of other cancer-associated genes. For example, HOXC8 may
promote tumorigenesis by regulating the expression of cadherin-11
in breast cancer (15). Additionally,
the expression of HOXC8 may be regulated by microRNA (miR)-196a
(16). To the best of our knowledge,
the association between the expression of HOXC8 and the progression
of CC has not yet been investigated. Therefore, the present study
aimed to examine the expression of HOXC8 and the clinical
significance of HOXC8 expression in CC.
In the present study, the expression of HOXC8 was
upregulated in CC tissues and cell lines. The association between
the expression of HOXC8 and clinicopathological features was
examined. Furthermore, HOXC8 expression may be used as an
independent predictor for the overall survival of patients with CC.
Additionally, downregulation of HOX8 decreased the proliferation
rate of CC cells. Therefore, HOXC8 may be used as a potential
therapeutic target for CC.
Materials and methods
Patients
A total of 36 patients (mean age, 56.7 years; range,
39–71 years) diagnosed with CC who underwent resection in the
Department of Gynecology, the First Affiliated Hospital of Fujian
Medical University (Fujian, China) were recruited between February
2006 and November 2011. None of the patients received anticancer
treatment including radiotherapy or immunotherapy. Tumor tissues
were obtained from patients with CC. Adjacent normal epithelial
tissues were used as controls. The tissues were frozen in liquid
nitrogen following surgery and stored at −80°C. The
clinicopathological features of patients with CC are presented in
Table I. The study was conducted
according to the declaration of Helsinki. The study was approved by
the Ethics Committee of the First Affiliated Hospital of Fujian
Medical University (Fujian, China). Written informed consent was
obtained from all patients. Overall survival was defined as the
period of time between surgery and mortality.
 | Table I.Association between HOXC8 expression
and clinicopathological features of patients with CC. |
Table I.
Association between HOXC8 expression
and clinicopathological features of patients with CC.
|
|
| HOXC8 expression
level |
|
|---|
|
|
|
|
|
|---|
| Variable | Cases | High | Low | P-value |
|---|
| Age, years |
|
|
|
|
| ≥50 | 21 | 15 | 6 | 0.121 |
|
<50 | 15 | 9 | 6 |
|
| Lymph node
metastasis |
|
|
|
|
|
Negative | 16 | 9 | 7 | 0.074 |
|
Positive | 20 | 15 | 5 |
|
| Tumor
differentiation |
|
|
|
|
| Poor | 23 | 16 | 7 | 0.033 |
|
Well/moderate | 13 | 8 | 8 |
|
| Tumor stage |
|
|
|
|
| I–II | 17 | 13 | 4 | 0.038 |
| III | 19 | 11 | 8 |
|
Cell lines and culture
Human CC cell lines SiHa and HT-3 and normal
cervical cell line Crl-2614 were purchased from the American Type
Culture Collection (Manassas, VA, USA). Cells were maintained in
Dulbecco's modified Eagle's medium (DMEM; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal bovine serum (FBS; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin/streptomycin (Thermo Fisher Scientific, Inc.). All cell
lines were incubated at 37°C in a humidified atmosphere containing
of 5% CO2.
Transient transfection
In order to downregulate the expression of HOXC8 in
CC cell lines, a siRNA targeting HOX8 was used in the present
study, which were synthesized by Shanghai GeneChem Co., Ltd.
(Shanghai, China). The sequences used were as follows: HOXC8-siRNA,
5′-CAACACTAACAGTAGCGAA-3′; negative control-siRNA:
5′-UGCAACAUCACGGAAUCAUTT-3′. For transfection, a total of
1×105 cells/well were seeded into six-well plates and
2.5 nmol siRNAs were added to each well. Transfection was conducted
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
48 h of culture, cells were used for subsequent experiments.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the tissues and cell
lines using TRIzol reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA was quantified
using the Nanodrop ND-2000 (Thermo Fisher Scientific, Inc.). RNA
was reverse-transcribed into cDNA using the First Strand cDNA
Synthesis kit (Beyotime Institute of Biotechnology, Haimen, China)
according to the manufacturer's protocol. The primer sequences used
in the present study were as follows: HOXC8 forward,
5′-CACGTCCAAGACTTCTTCCACCACGGC-3′ and reverse,
5′-CACTTCATCCTTCGATTCTGGAACC-3′; GAPDH forward,
5′-TGATGACATCAAGAAGGTGGTGAAG-3′ and reverse,
5′-TCCTTGGAGGCCATGTGGGCCAT-3′. RT-qPCR was performed using the
StepOne Plus Real-Time PCR system (Thermo Fisher Scientific, Inc.)
with SYBR Premix Ex Taq™ II (Takara Biotechnology Co.,
Ltd., Dalian, China) according to the manufacturer's protocol. The
thermocycling conditions were as follows: Initial denaturing step
at 94°C for 5 min, followed by 30 cycles at 94°C for 45 sec and
final extension at 56°C for 45 sec. The expression of HOXC8 was
normalized to GAPDH and evaluated using the 2−ΔΔCq
method (17). The 75th percentile of
HOXC8 expression level was used as cut-off point (1.14) to stratify
patients into high or low HOXC8 expression group.
Western blot analysis
Total protein samples were extracted from the
tissues and cell lines using radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Total protein was quantified using a
bicinchoninic acid assay. Equal amount of protein (50 µg) was
separated using SDS-PAGE (10% gel) and transferred onto
polyvinylidine diflouride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were then blocked with 5% fat-free milk in
Tris-buffered saline containing 0.05% Tween-20 (TBST) at room
temperature for 1 h and incubated with the following primary
antibodies: mouse Anti-HOXC8 monoclonal antibody (1:1,000; cat. no.
sc-517007; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
mouse anti-GAPDH monoclonal antibody (1:1,000; cat. no. sc-47724;
Santa Cruz Biotechnology, Inc.) at 4°C overnight. Membranes were
washed three times with TBST. Following the primary incubation,
membranes were incubated with goat anti-mouse horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no.
sc-2005; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature, according to the manufacturer's protocol. Protein
bands were visualized using enhanced chemiluminescence reagent
(Beyotime Institute of Biotechnology). Densiometric analysis of the
bands was performed using ImageJ software (version 1.43; National
Institutes of Health, Bethesda, MD, USA).
Cell Counting Kit-8 (CCK-8)
Cell proliferation was evaluated using a CCK-8
assay. Cells transfected with HOXC8-siRNA or negative control-siRNA
were seeded into 96-well plates at a density of ~2×103
cells/well in a volume of 100 µl for 0, 24, 48, and 72 h and
cultured in DMEM medium supplemented with 10% fetal bovine serum
and 1% penicillin/streptomycin at 37°C in a humidified atmosphere
containing of 5% CO2. At indicated timepoints, 10 µl
CCK-8 solution (Beyotime Institute of Biotechnology) was added to
each well and incubated for 1 h. The absorbance of each well was
measured using a microplate reader at 450 nm. Three individual
experiments were performed.
Statistical analysis
Data were analyzed using SPSS software (version
18.0; SPSS, Inc., Chicago, IL, USA. The relevant data are expressed
as the mean ± standard deviation. Statistical significance between
two groups was determined using a Student's t-test. One-way
analysis of variance followed by post hoc analysis with Tukey's
test was used to examine differences among multiple groups. The
chi-square test was used to analyze the associations between HOXC8
expression and clinicopathological features. Overall survival of
patients with CC was evaluated using Kaplan-Meier estimator
analysis and log-rank test. Univariate and multivariate Cox
regression models were used to identify independent prognostic
factors associated with the overall survival of patients with CC.
Three individual experiments were performed. P<0.05 was
considered to indicate a statistically significant difference.
Results
HOXC8 is highly expressed in CC
tissues and cell lines
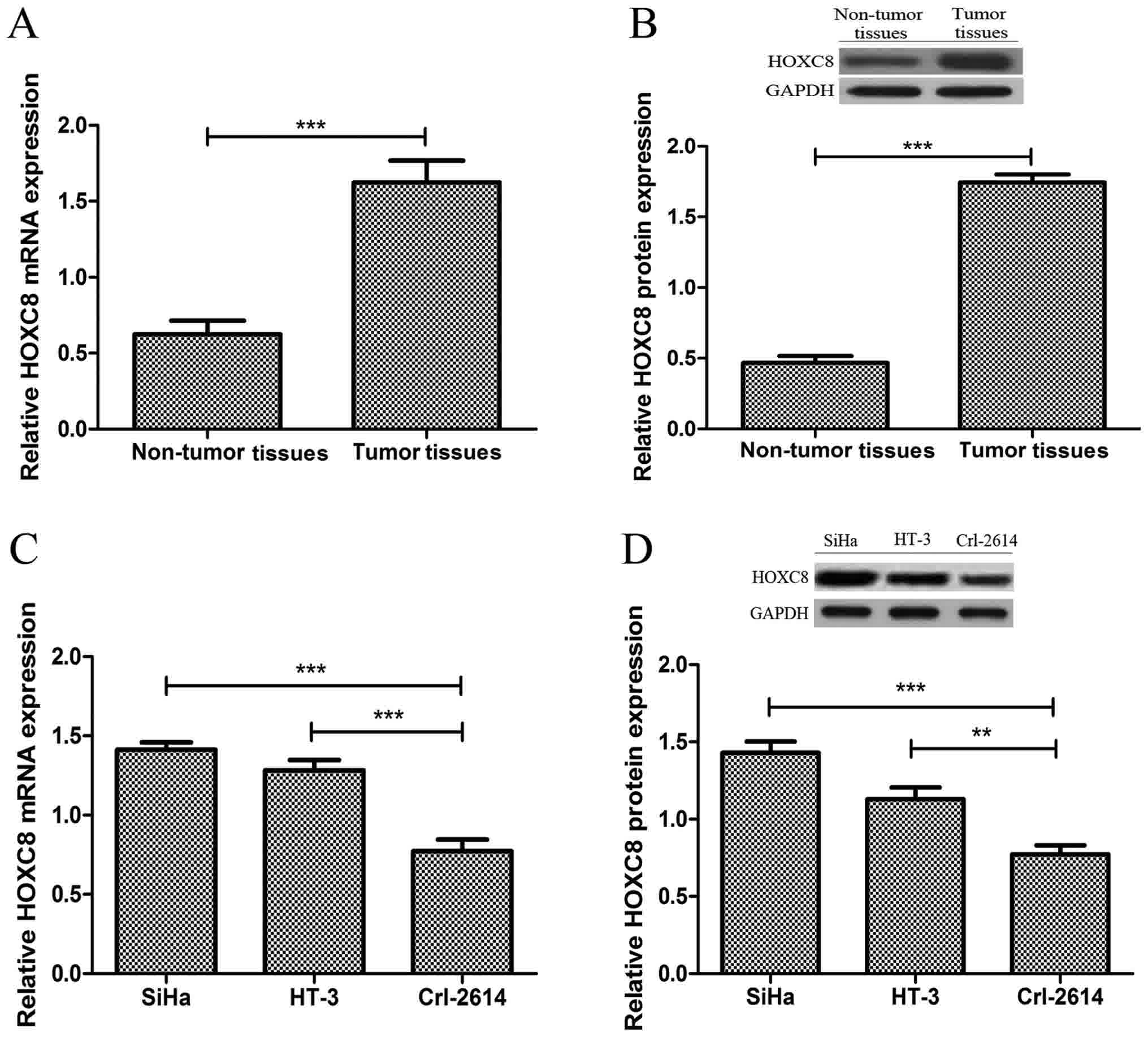
The expression of HOXC8 in CC tissues and adjacent
non-tumor tissues was evaluated using RT-qPCR, and western blot
analysis. As presented in Fig. 1A,
mRNA expression levels of HOXC8 was significantly increased in CC
tissues compared with that in adjacent non-tumor tissues
(P<0.001). Western blot analysis confirmed that the expression
level of HOXC8 was also significantly increased in CC tissues
compared with that adjacent non-tumor tissues (P<0.001; Fig. 1B). Patients were divided into two
groups based on the expression levels of HOXC8: HOX8 high (n=24)
and low (n=12) groups. The expression of HOXC8 was also evaluated
in CC cell lines, HiHa and HT-3, and normal cervical cell line
Crl-2614. As presented Fig. 1C and D,
the mRNA and protein expression of HOXC8 was increased
significantly in both CC cell lines compared with that in normal
cervical cell line. These results suggest that HOX8 may serve an
important function in CC.
Effects of HOXC8 expression on cell
proliferation
In order to assess the effect of the expression of
HOXC8 on cell proliferation, SiHa and HT-3 cells were transfected
with HOXC8-specific and negative control-siRNAs. The cell
proliferation rate was evaluated using a CCK-8 assay.
Downregulation of HOXC8 (achieved by transfection with
HOXC8-specific siRNA) in CC cell lines was confirmed using RT-qPCR
(Fig. 2A) and western blot analysis
(Fig. 2B and C). The results
demonstrated that the proliferation rates of SiHa and HT-3 cells
were significantly increased compared with that in the normal
cervical cell line at the time points investigated (Fig. 2D). Additionally, transfection using
HOXC8-specific siRNA significantly downregulated the proliferation
rate of CC cell lines at the time points investigated (Fig. 2E and F). These results suggest that
HOXC8 may be involved in the progression of CC by regulating cell
proliferation.
Association between the expression of
HOXC8 and clinicopathological features of patients with CC
The clinicopathological features of all patients are
summarized in Table I. The results
demonstrated that increased expression of HOXC8 in CC was
significantly associated with tumor differentiation (P=0.033) and
tumor stage (P=0.038; Table I).
However, no significant association was identified between
increased expression of HOXC8 and other parameters, including age
and lymph node metastasis (P>0.05; Table I).
Association between the expression of
HOXC8 and overall survival rate of patients with CC
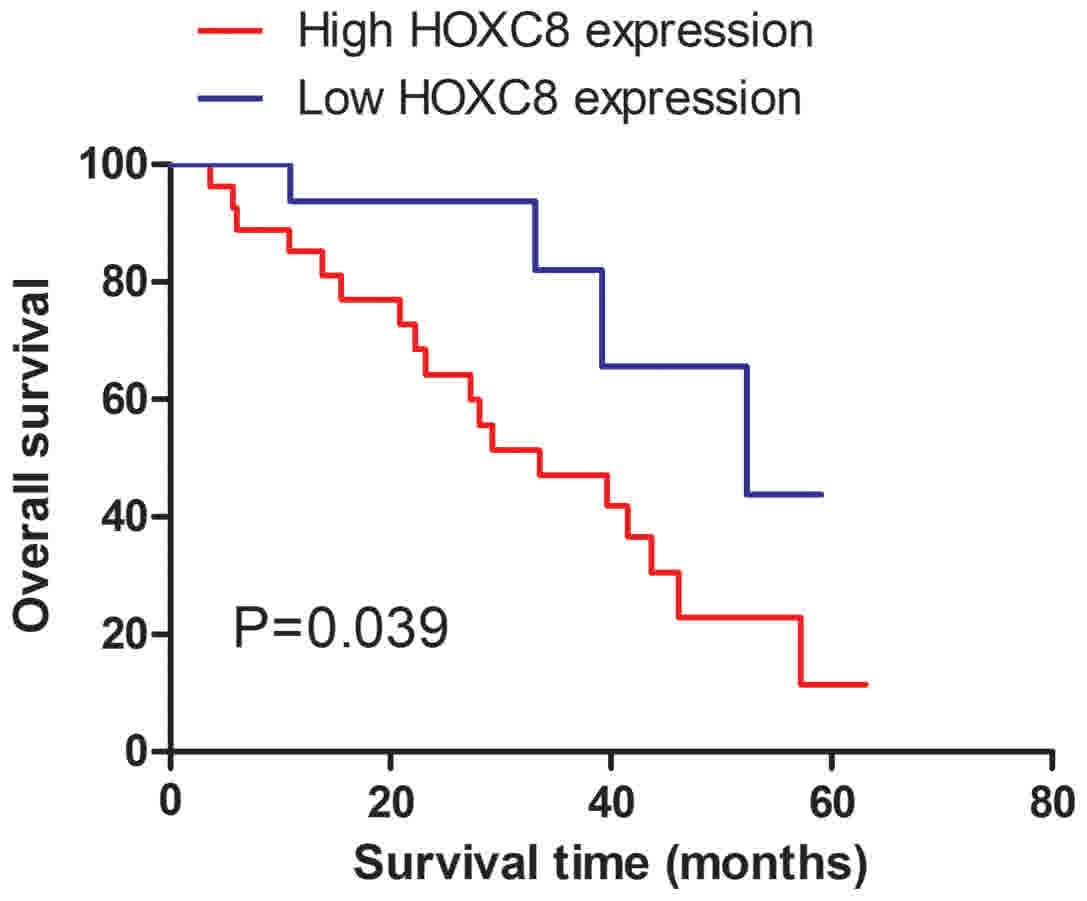
The association between the expression of HOXC8 and
overall survival of patients with CC was evaluated using
Kaplan-Meier survival curves, and the log-rank test. The expression
of HOXC8 was significantly associated with overall survival of
patients with CC (P=0.039; Fig. 3),
suggesting that patients with CC with increased expression of HOXC8
exhibited a poor 5-year overall survival compared with those with
low expression of HOXC8. Additionally, independent predictor
factors for the overall survival of CC patients with CC were
examined using univariate and multivariate Cox regression models.
The results demonstrated that the expression of HOXC8 (P=0.028),
tumor differentiation (P=0.038) and tumor stage (P=0.022) were
identified as independent prognostic factors for the overall
survival of patients with CC (Table
II).
 | Table II.Univariate and multivariate analyses
of overall survival rate. |
Table II.
Univariate and multivariate analyses
of overall survival rate.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| HOXC8 | 2.453 | 1.053–5.176 | 0.038 | 2.534 | 1.107–5.796 | 0.028 |
| Age | 2.119 | 0.836–5.370 | 0.114 | – | – | – |
| Lymph node
metastasis | 2.213 | 0.894–5.478 | 0.086 | – | – | – |
| Tumor
differentiation | 2.249 | 1.015–4.985 | 0.046 | 2.446 | 1.050–5.701 | 0.038 |
| Tumor stage | 2.313 | 1.059–5.052 | 0.035 | 2.578 | 1.144–5.807 | 0.022 |
Discussion
Previous studies have reported the upregulation of
HOXC8 in various types of cancer suggesting a possible function in
tumor progression (12–15). Additionally, the molecular mechanisms
underlying HOXC8-mediated tumor progression have been investigated
over the last decades (18–20). Emerging evidence suggested that
silencing of HOXC8 in cancer cells may inhibit cell proliferation
and migration in vitro (19,21). These
findings suggest that HOXC8 may serve an essential function in the
development and progression of cancer. A previous study has
investigated the expression of HOXC8 in CC cell lines, but the
clinical significance of HOXC8 in CC remains unknown (22).
In the present study, the expression of HOXC8 was
examined in CC tissues and cell lines using RT-qPCR, and western
blot analysis. The results demonstrated that the mRNA expression
level of HOXC8 was significantly upregulated in CC tissues and cell
lines compared with non-tumor tissue, and the normal cervical cell
line. Additionally, the protein expression level of HOXC8 was
examined using western blot analysis and was also identified to be
significantly increased in CC tissues and cell lines. Therefore,
the results demonstrated that HOXC8 may serve an important function
in the development of CC. Additionally, overall survival of all
enrolled patients with CC was examined using Kaplan-Meier method
and log-rank test. The results demonstrated that patients with
increased expression of HOXC8 exhibited significantly shorter
overall survival compared with those with low expression of HOXC8.
Additionally, the expression of HOXC8 was associated with
clinicopathological features of patients with CC, including tumor
differentiation and tumor stage, suggesting that HOXC8 may be
involved in the progression of CC. Univariate and multivariate
analyses were performed using Cox's proportional hazards model and
revealed that HOXC8 may be an independent predictor for the overall
survival of patients with CC, thus suggesting an important function
of HOXC8 in CC.
The ability of HOXC8 to regulate the proliferation
of CC cells was also examined. The results demonstrated that CC
cell lines exhibited significantly increased proliferation rates
compared with that in the normal cervical cell line. Additionally,
downregulation of HOXC8, which was achieved by HOXC8-specific
siRNA, significantly decreased the proliferation rate of CC cell
lines. Therefore, these results indicated that HOXC8 may contribute
to tumor progression by promoting cell proliferation. However, it
would also be interesting to investigate whether the ectopic
expression of HOXC8 serves a role in normal cervical cell lines, to
examine whether or not HOXC8 can promote tumor initiation.
The results of the present study demonstrated that
HOXC8 was significantly overexpressed in CC tissues. In addition,
the overexpression of HOXC8 was identified to be associated with
tumor differentiation and tumor stage, and indicated poor prognosis
for CC. Therefore, HOXC8 may be used as a potential therapeutic
target in CC. However, a limitation of the present study was the
small cohort size used. Therefore, further large-scale studies are
required to confirm these conclusions and investigate the
underlying molecular mechanisms of HOXC8 in CC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH designed the study. YH, LC and AG performed all
experiments. YH and AG collected and interpreted the data. LC and
AG wrote the manuscript, and YH revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Fujian Medical University (Fujian,
China), and written informed consent was obtained from all
patients.
Consent for publication
This manuscript does not contain any identifying
information for the enrolled patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma C, Nusri Qel-A, Begum S, Javed E,
Rizvi TA and Hussain A: (-)-Epigallocatechin-3-gallate induces
apoptosis and inhibits invasion and migration of human cervical
cancer cells. Asian Pac J Cancer Prev. 13:4815–4822. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye HN, Zhang YY, Geng L and Li ZJ: Cdc42
expression in cervical cancer and its effects on cervical tumor
invasion and migration. Int J Oncol. 46:757–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Zhang R, Zhou SJ and Ji YQ:
Overexpression of Notch1 is associated with the progression of
cervical cancer. Oncol Lett. 9:2750–2756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cong L, Zhang F and Shang H: Notch1
targeted regulation of mir-224/LRIG2 signaling for the
proliferation and apoptosis of cervical cancer cells. Oncol Lett.
13:2304–2308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Li Y, Liu N, Gao Y and Li L:
MiR-23b controls ALDH1A1 expression in cervical cancer stem cells.
BMC Cancer. 17:2922017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akam M: Hox and HOM: Homologous gene
clusters in insects and vertebrates. Cell. 57:347–349. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhatlekar S, Fields JZ and Boman BM: HOX
genes and their role in the development of human cancers. J Mol Med
(Berl). 92:811–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alvarado-Ruiz L, Martinez-Silva MG,
Torres-Reyes LA, Pina-Sanchez P, Ortiz-Lazareno P, Bravo-Cuellar A,
Aguilar-Lemarroy A and Jave-Suarez LF: HOXA9 is underexpressed in
cervical cancer cells and its restoration decreases proliferation,
migration and expression of epithelial-to-mesenchymal transition
genes. Asian Pac J Cancer Prev. 17:1037–1047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barba-de la Rosa AP, Briones-Cerecero E,
Lugo-Melchor O, De León-Rodríguez A, Santos L, Castelo-Ruelas J,
Valdivia A, Piña P, Chagolla-López A, Hernandez-Cueto D, et al: Hox
B4 as potential marker of non-differentiated cells in human
cervical cancer cells. J Cancer Res Clin Oncol. 138:293–300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhai Y, Kuick R, Nan B, Ota I, Weiss SJ,
Trimble CL, Fearon ER and Cho KR: Gene expression analysis of
preinvasive and invasive cervical squamous cell carcinomas
identifies HOXC10 as a key mediator of invasion. Cancer Res.
67:10163–10172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vider BZ, Zimber A, Chastre E, Gespach C,
Halperin M, Mashiah P, Yaniv A and Gazit A: Deregulated expression
of homeobox-containing genes, HOXB6, B8, C8, C9, and Cdx-1, in
human colon cancer cell lines. Biochem Biophys Res Commun.
272:513–518. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omatu T: Overexpression of human homeobox
gene in lung cancer A549 cells results in enhanced motile and
invasive properties] Hokkaido Igaku Zasshi. 74:367–376. 1999.(In
Japanese).
|
|
14
|
Javed S and Langley SE: Importance of HOX
genes in normal prostate gland formation, prostate cancer
development and its early detection. BJU Int. 113:535–540. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Chao F, Huang B, Liu D, Kim J and
Huang S: HOXC8 promotes breast tumorigenesis by transcriptionally
facilitating cadherin-11 expression. Oncotarget. 5:2596–1607. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mueller DW and Bosserhoff AK: MicroRNA
miR-196a controls melanoma-associated genes by regulating HOX-C8
expression. Int J Cancer. 129:1064–1074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmittgen Td and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruthala K, Gadi J, Lee JY, Yoon H, Chung
HJ and Kim MH: Hoxc8 downregulates Mgl1 tumor suppressor gene
expression and reduces its concomitant function on cell adhesion.
Mol Cells. 32:273–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Axlund SD, Lambert JR and Nordeen SK:
HOXC8 inhibits androgen receptor signaling in human prostate cancer
cells by inhibiting SRC-3 recruitment to direct androgen target
genes. Mol Cancer Res. 8:1643–1655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lei H, Wang H, Juan AH and Ruddle FH: The
identification of Hoxc8 target genes. Proc Natl Acad Sci USA.
102:2420–2424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Zhang M, Chen H, Dong Z, Ganapathy
V, Thangaraju M and Huang S: Ratio of miR-196s to HOXC8 messenger
RNA correlates with breast cancer cell migration and metastasis.
Cancer Res. 70:7894–7904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alami Y, Castronovo V, Belotti D,
Flagiello D and Clausse N: HOXC5 and HOXC8 expression are
selectively turned on in human cervical cancer cells compared to
normal keratinocytes. Biochem Biophys Res Commun. 257:738–745.
1999. View Article : Google Scholar : PubMed/NCBI
|