Introduction
Primary liver cancer, particularly the most
diagnosed subtype of hepatocellular carcinoma (HCC), is a major
health problem. It is the fifth most common malignancy and the
third leading cause of cancer-associated mortality worldwide
(1–3).
The incidence of liver cancer has significant global variations and
is particularly prominent in Eastern Asia (2,3). Liver
cancer has had the second highest mortality rate of
cancer-associated death in China and 17.4% of cancer deaths in
Chinese adults in 2012 were from liver cancer (4). Although numerous studies have conducted
research to improve the prognosis of liver cancer, run clinical
studies and have achieved significant progress over the past few
decades, the overall outcome of liver cancer management remains
unsatisfactory, with the overall survival rate <5 years
(1,5).
To date, curative resection remains the only major therapeutic
method for liver cancer (1); however,
there is a high frequency of postoperative recurrence (3,6). Patients
with liver cancer may not be eligible for resection due to
complexities, including multifocal diseases, the presence of
multiple tumor metastases, insufficient functional hepatic reserve
or severe co-existent cirrhosis (1,2). One of
the major obstacles of liver cancer prognosis is metastasis, which
is the leading cause of tumor mortality (7–9);
therefore, for the majority of patients with primary or metastatic
hepatic malignancies who are not eligible for surgical resection,
the development of novel treatments is required to manage tumor
growth and prevent progression.
Radiofrequency ablation (RFA) is a technology for
the curative treatment of local liver cancer that has evolved
during the past few decades (10,11). RFA
is a safe, minimally invasive, effective and repeatable modality
with fewer complications than resection particularly for smaller
tumors (12–14), and it has become a first-line therapy
for a number of patients with non-resectable malignant cancer
(15–18). The types of RFA include percutaneous
RFA using ultrasound, computed tomography-guided laparoscopic RFA
and laparotomy RFA (19).
Ultrasound-guided laparoscopic RFA therapy, which identifies tumors
and guides the placement of the RFA needle electrode via
ultrasonography, have gained widespread availability and use over
the past five years due to its precise targeting of the tumor
(20). RFA used on hepatic tumors
induces thermal coagulation necrosis of soft tissues, including
partial or complete ablation of non-resectable liver lesions
(19); however, the mechanism
underlying the production of thermal energy used to kill the tumor
cells, and whether the RFA treatment triggers any specific
antitumor effects has not been fully investigated (21).
The innate and adaptive immune cells actively
prevent tumorigenesis in a process termed cancer immunosurveillance
(22). The innate immune system,
including monocytes, macrophages, dendritic cells (DC) and natural
killer (NK) cells, can directly lyse tumor cells (23). NK cells are recognized by their strong
cytolytic activity against tumors and virus infections (24,25). NK
cells also regulate the innate and adaptive immune responses
through cell-to-cell contact and secretion of immunoregulatory
cytokines (26,27). Activated NK cells promote DC cell
maturation and the release of cytokines via the production of
interferon (IFN)-I and tumor necrosis factor (TNF)-α, subsequently
leading to a strong stimulation of immune responses (24). In addition, mature NK cells express
high levels of the NK group 2D (NKG2D) protein (28). Through recognizing ligands expressed
on infected or tumor cells, NKG2D modulates lymphocyte activation
and promotes the immune response, which kills the ligand-expressing
cells (29–31).
RFA local to tumors has been associated with
enhanced systemic antitumor T-cell immune responses (32); however, whether NK cells are involved
with RFA-treated tumor cells as well as the underlying mechanism is
not fully understood (33). In the
present study, the effects of NK cells function and the immune
state in animal models following laparoscopic RFA were
investigated. The activity of NK cells from animal models following
RFA administration was tested on a hepatoblastoma cell line (HepG2)
and it was verified that the cellular killing ability of NK cells
was able to be modulated by RFA. It was additionally demonstrated
that RFA therapy directly enhances NK cell cytotoxicity via
increasing NKG2D expression; therefore, the results provided novel
molecular insights into the tumor suppression of immune cells
during RFA-associated tumor treatment.
Materials and methods
Animal model of tumorigenesis
A total of 5 New Zealand white rabbits (3 males and
2 females, supplied by Hainan Veterans General Hospital, Haikou,
China), aged between two to three months, weighing 2.5–3.0 kg were
randomly allocated and housed with free access to water and food,
with a 12:12-h day/night cycle and at a constant room temperature.
Rabbits were inoculated with VX2 cells (from the Chongqing Medical
University, Chongqing, China) in their hind limbs, and served as
donors for liver tumor implantation and strain propagation.
Briefly, lateral aspects of the hind limb of rabbits were locally
shaved and disinfected using alcohol spray, following
anesthetization by intravenous injection of sodium pentobarbital
(30 mg/kg). A 0.5–1.0 ml VX2 tumor cell suspension, containing
1×106 VX2 cells, was injected into the gluteal muscle of
the hind limb of the rabbits. At two weeks after the implantation,
the substantial mass was palpable in the tumor-bearing rabbits. All
tumor-bearing animals were sacrificed with minimal pain and
distress, and the hind limb tumors were harvested.
A total of seven New Zealand white rabbits were
inoculated with VX2 tumor cells from the donor rabbits. The
implantation was conducted as described previously (34). The tumor sections of 1–4
mm3, were implanted into the liver parenchyma of
anesthetic recipient rabbits. The liver incision was sealed and the
abdominal wall was closed, creating two layers. The animal
experiments in the present study were evaluated and approved by the
Institutional Animal Care and Use Committee of Research Center for
Drug Safety Evaluation of Hainan (HNYWAPZX201607011).
Radiofrequency ablation
At 2 weeks following the establishment of the rabbit
hepatic tumor model, RFA was applied onto prominent tumor cells of
the rabbits. The liver lobe containing the VX2 tumor was explored
and a single needle or a needle cluster (for larger tumors, which
were >3 cm) with an internally cooled electrode was positioned
to the tumor under ultrasonographic guidance. The frequency for RFA
was 500 kHz and not pulse modulated. An RF current was emitted for
12 or 15 min (longer time for larger tumors >3 cm) using a 200 W
generator that delivered continuous RF energy using an automatic
impedance controller. Tumor cells were heated above 50°C and a
post-operative ultrasound was performed to identify complications,
including bleeding.
Histological studies
Tumor specimens were fixed using 10% formaldehyde at
4°C overnight and embedded in optimal cutting temperature compound.
Hematoxylin and eosin (H&E) staining was performed at room
temperature to assess the morphology of tissue sections, as
described previously (35).
Flow cytometric sorting of NK
cells
NK cells from the rabbit peripheral blood were
blocked with 1% BSA-PBS on ice for 10 min, stained with anti-rabbit
CD56 (cat. no. 3606; 1:200; Cell Signaling Technology, Inc., MA,
USA), NKG2D (cat. no. ab203353; Abcam) or CD69 antibody (cat. no.
ab13168; Abcam) at 4°C for 15 min. The cells were then washed by 1X
PBS three times at 4°C prior to being re-suspended with secondary
antibody fluorescein isothiocyanate (1:500 diluted in 5% BSA-PBS;
cat. no. F2765; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The different cellular subsets were sorted using
a FACSAria™ (BD Biosciences, Franklin Lakes, NJ, USA)
cell sorter. Data was analyzed by using BD FACSDiva™
software (version 6.0; BD Biosciences).
IFN-γ and TNF-α analysis
The IFN-γ and TNF-α concentrations in the cell
culture supernatants were determined using aduo-set ELISA kit (cat.
no. H052), according to the manufacturer's instructions (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China).
In vitro killing assay
The killing activity of NK cells was tested via flow
cytometry. Briefly, NK cells were separated using a CD56 positive
selection kit (EasySep™ Human CD56 Positive Selection
kit; Stemcell Technologies, Inc., Beijing, China), according to the
manufacturer's protocol. The cytotoxicity assay was performed as
described by the study of Hoppner et al (36). Briefly, peripheral blood mononuclear
cells from VX2 tumor rabbits were incubated with selection kit and
separated with magnet. NK cells remained in the tube while unwanted
cells were poured off. The selected NK cells together with
macrophages from VX2 tumor rabbits treated with/without RFA were
used as effector cells. HepG2 human hepatoblastoma cells purchased
from the Cell Bank of Shanghai Institute of Biochemistry and Cell
Biology Chinese Academy of Sciences, were cultured in in RPMI-1640
medium supplemented with 10% (v/v) fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin glutamine (all Gibco; Thermo
Fisher Scientific, Inc.) and used as target cells. The effector
cells were co-cultured with HepG2 cells for 4 h at 37°C in an
atmosphere containing 5% CO2, following which the cell
mixture was stained with 7-aminoactinomycin D (7-AAD; Beckman
Coulter, Inc., Brea, CA, USA) in the dark for 15 min. Flow
cytometry data were resolved using a FACSAria flow cytometer (BD
Biosciences) and analyzed using FlowJo 7.2.5 software (Tree Star
Inc., Ashland, OR, USA). NK cytotoxicity (%) was calculated as the
proportion of cells positive for 7-AAD.
Quantitative polymerase (qPCR)
Reverse transcription and followed by quantitative
PCR was used to examine the expression level of FasL and perforin.
Briefly, total RNA from rabbit blood was extracted by
TRIzol® reagent (Life Technologies; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Total
RNA (500 ng) was reversed transcribed with Superscript IV
Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol and using random primers.
qPCR analyses were performed with primers for FasL and perforin
with the SsoFast SYBR-Green qPCR mix (cat. no. 1725201; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's protocol on an Eppendorf MasterCycler Realplex with
the thermal cycling conditions were composed of an initial
denaturation step at 98°C for 5 min, then 45 cycles at 95°C for 30
sec and 60°C for 30 sec. The experiments were carried out in
duplicate for each data point. The relative quantification in gene
expression was determined using the 2−ΔΔCq method
(37).
Statistical analysis
The results are expressed as the mean ± standard
deviation from tumor cells in the control or experimental animals.
For calculations using one-way analysis of variance (with Tukey's
honest significant difference post hoc test), SPSS 18.0 (SPSS,
Inc., Chicago, IL, USA) was used. Graphs were produced using
GraphPad Prism software v6.01 (GraphPad Software, Inc., La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment and evaluation of the
VX2 liver tumor model in rabbits
The VX2 is a fast-growing adenocarcinoma cell line,
which has been extensively used to study various aspects of tumor
behavior (38). To establish the
rabbit liver tumor model, a VX2 cell suspension was inoculated into
the sub-capsule of the left anterior lobe of the rabbit liver.
Then, seven rabbits that exhibited considerable tumor growth at two
weeks following VX2 cell implantation were used in the present
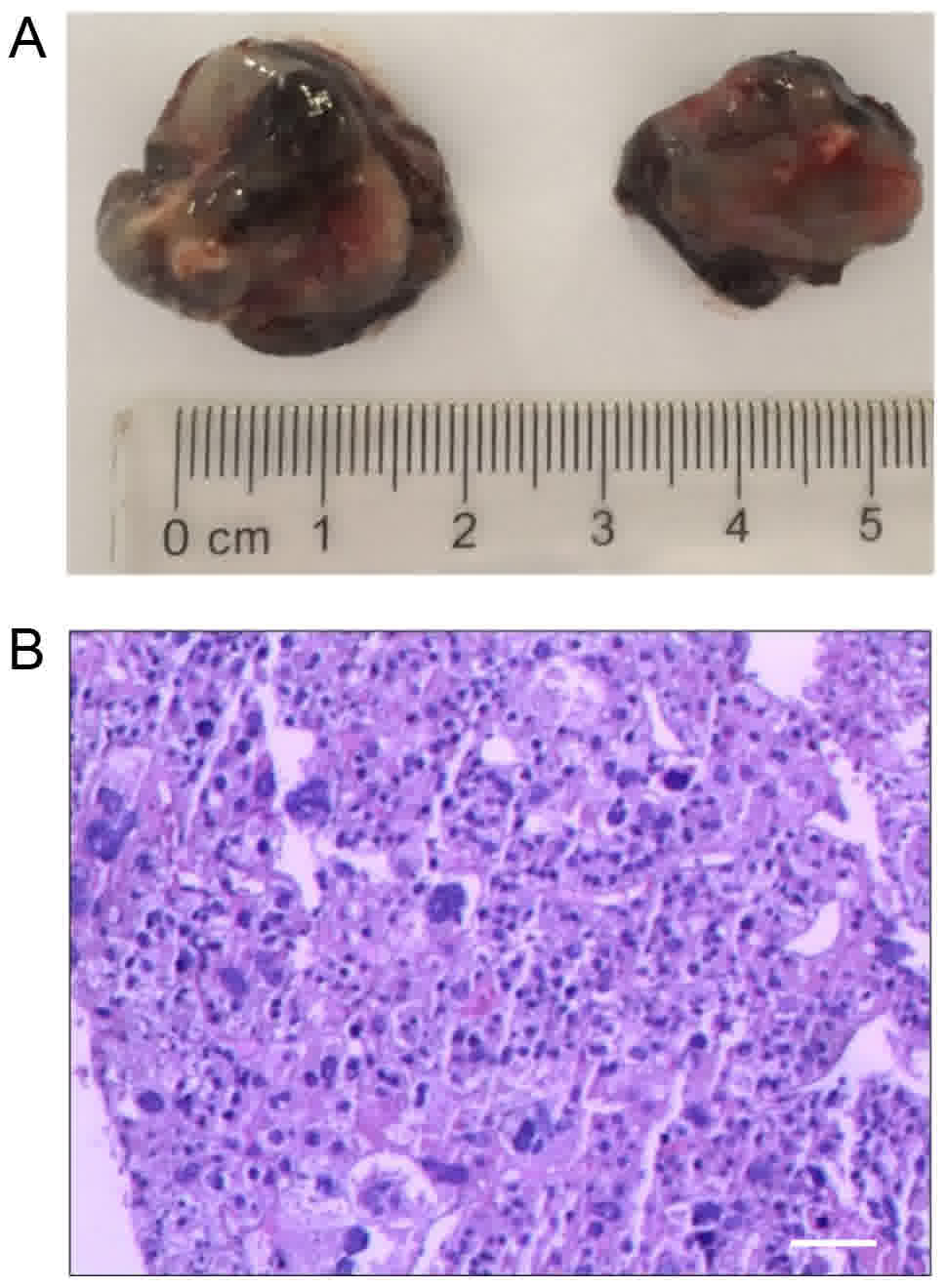
study. The tumors were round in shape and were as large as 2 cm in
diameter, with a total weight of 5.2±2.0 g (Fig. 1A). Microscopic examination via H&E
staining revealed that the tumors had notable necrosis in their
centers and that the cells were irregularly arranged, indicating an
invasive growth capability (Fig.
1B).
Morphological changes following
RFA
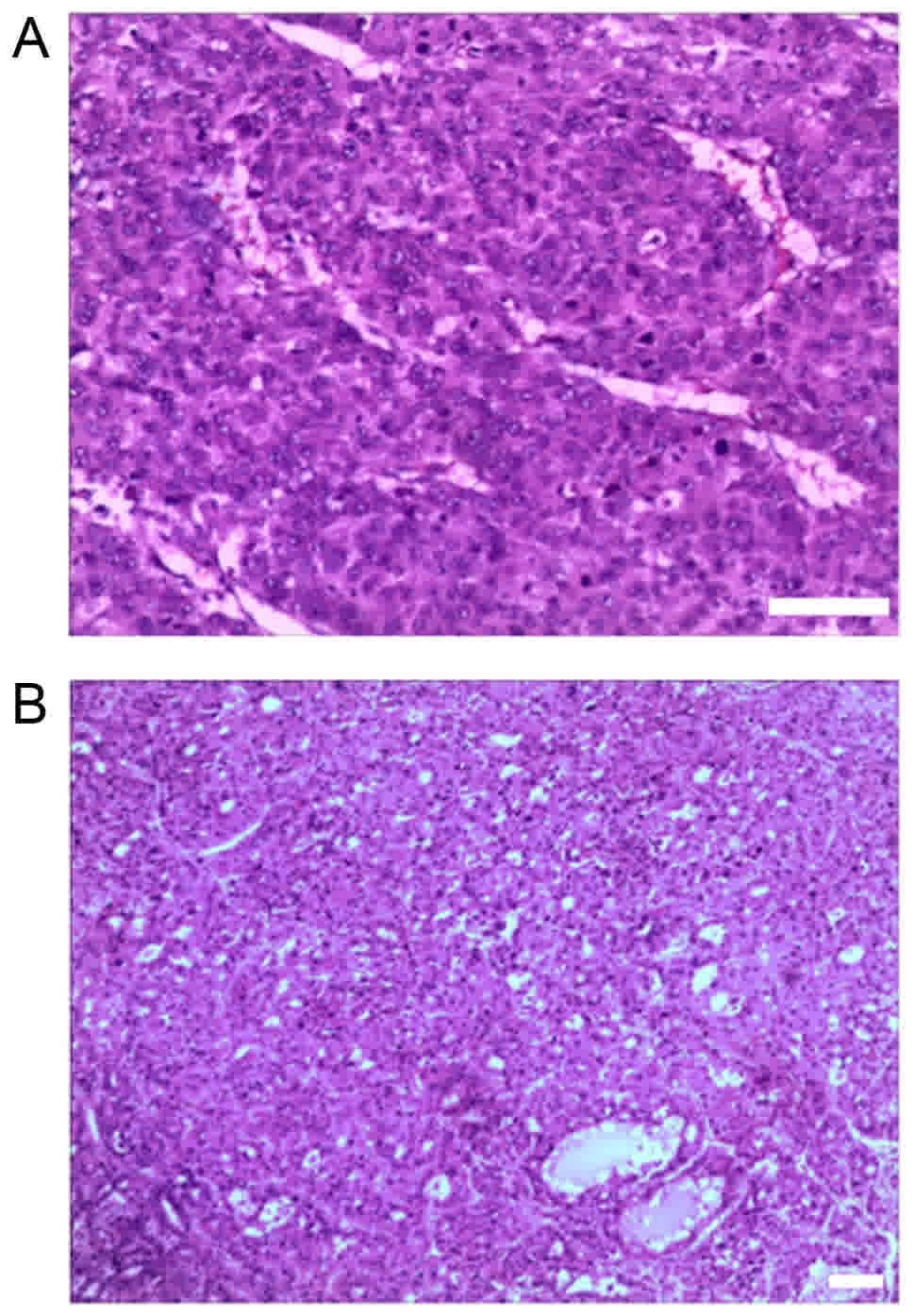
Next, the morphological alterations following RFA
administration were further examined. Representative venous
thromboses were identified in the portal or hepatic vein branches
central to RFA zones (Fig. 2A). At
four weeks after thermal ablation, the coagulation on the tumor
gradually became white. The boundaries between the tumor cells and
surrounding non-tumorous cells were less clear, indicating a
clearance of tumor cells. Furthermore, inflammatory cell
infiltration was observed in parts of the remaining tumor cells
(Fig. 2B).
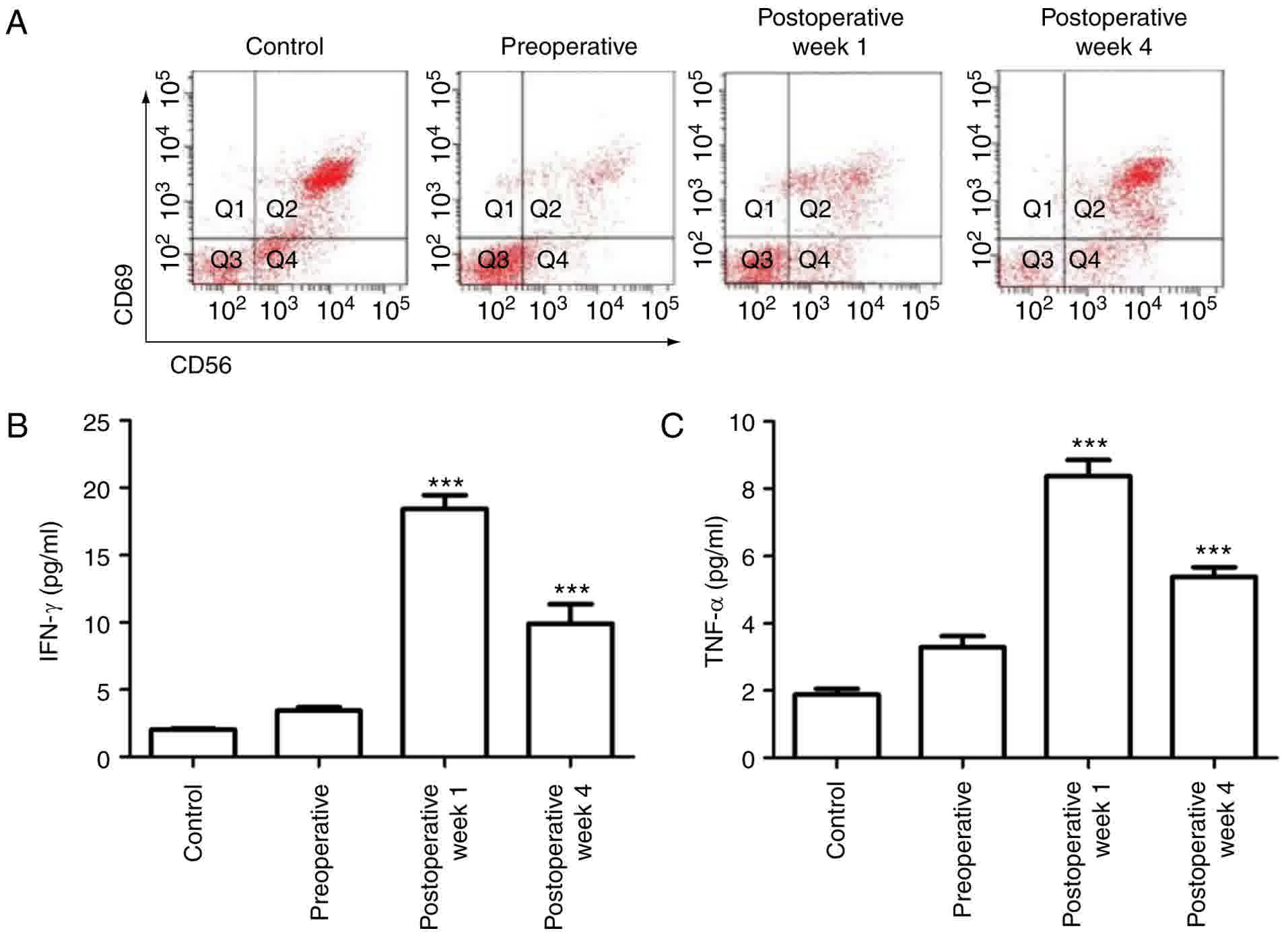
RFA activates primary NK cells
RFA has been reported to induce inflammatory cell
infiltration. Inflammation is associated with immune activity;
therefore, the innate immune responses in the tumor cells were
assessed. As one of the most abundant components of the innate
immune system, NK cells account for 10–15% of peripheral blood
lymphocytes, and are defined by the expression of CD56.
Furthermore, the CD69 differentiation antigen is one of the
earliest cell surface molecules expressed following NK cell
activation; therefore, FACS was used to determine the NK cell
proportion in the peripheral blood via calculation of the
CD56+CD69+ cell percentage. Compared with
cells in the normal control rabbit, the
CD56+CD69+ NK cell number in the VX2 rabbit
was significantly decreased (P<0.01), indicating the suppression
of immune activity following tumor growth prior to RFA treatment.
Following 1 week of RFA treatment, the NK cell number was increased
(P<0.05), indicating a quick stimulation of the innate immune
system. The percentage of CD56+CD69+ NK cells
was continuously upregulated in the fourth week following RFA
treatment (Fig. 3A).
As the major cytokines released by NK cells are
IFN-γ and TNF-α, an ELISA assay was used to measure the levels of
cytokines in the cell medium. Similar to the increase in the NK
cell population, the IFN-γ and TNF-α levels were also significantly
elevated following RFA treatment in the VX2 animals. Their levels
were the highest following the first week of RFA treatment and
maintained through the fourth week after RFA treatment, indicating
the innate immune system was induced and activated by RFA treatment
(Fig. 3B and C).
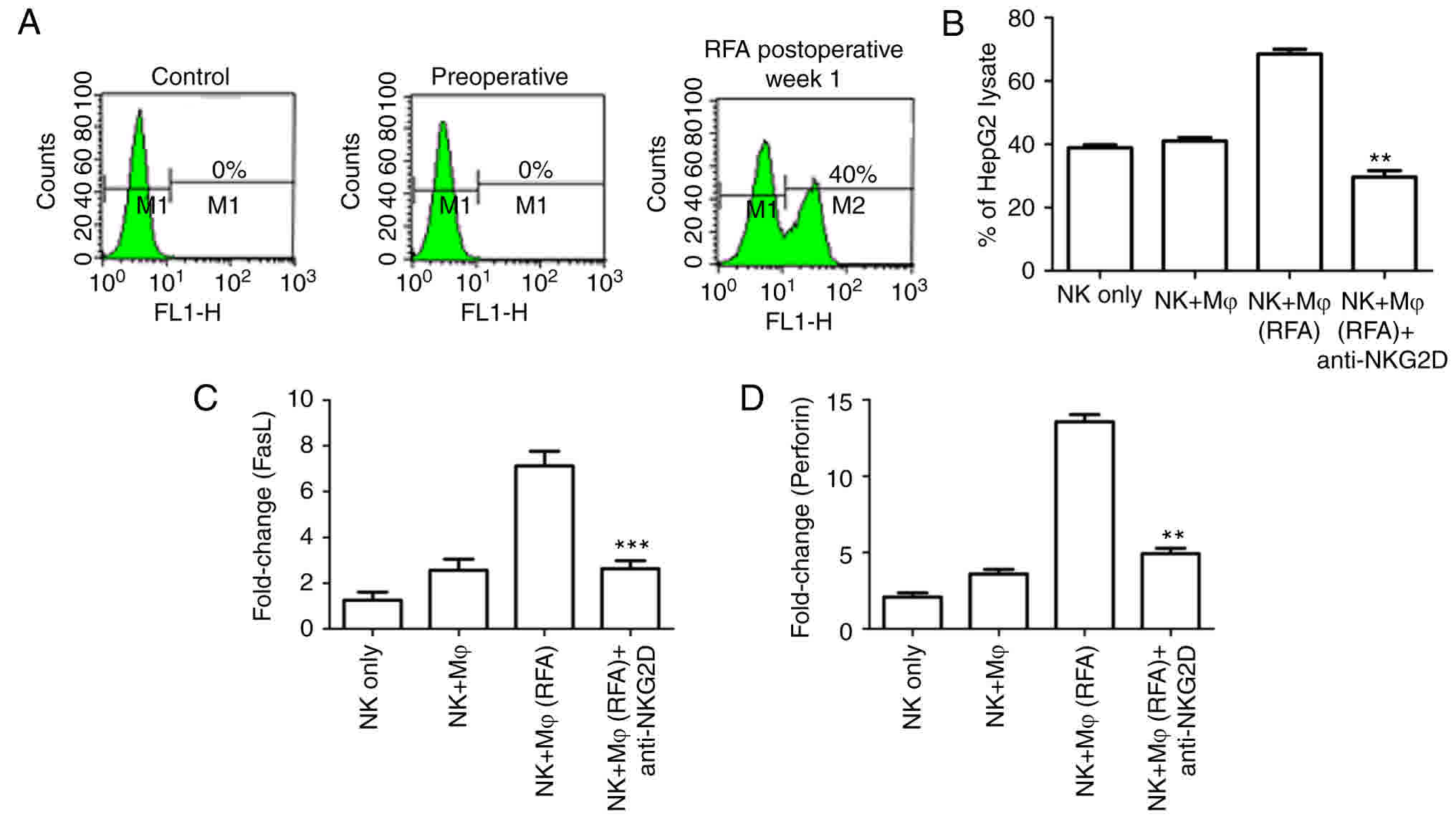
RFA enhances NKG2D expression and NK
cell function
NKG2D is the most important activating receptor
present on the surface of NK cells. A variety of immune cell
therapies in cancer treatments rely on the recognition of tumor
targets via the expression of the NKG2D ligand. To explore whether
NKG2D contributed to the enhanced immune activity following RFA
treatment, the expression of NKG2D was examined using FACS
analysis. It was identified that the NKG2D expression was notably
low prior to RFA treatment; however, as depicted in Fig. 4A, NKG2D was markedly upregulated at
one week following RFA treatment.
In order to further verify the role of NKG2D in
enhancing the killing activity of NK cells, NK cells sorted from
the VX2 model cells were co-cultured with human hepatoblastoma
HepG2 cells, and the neutralizing antibody against NKG2D was added
to the co-culture. The in vitro killing assay demonstrated
that a notable increase in killing activity was observed in NK
cells with macrophages from RFA-treated animals, compared with NK
cells with macrophages from non-treated animals. Blocking NKG2D
using a specific antibody notably impaired the RFA-induced immune
activity, as the NK cell-mediated killing activity was
significantly downregulated (Fig.
4B). This confirmed that NKG2D is an important activating
receptor in RFA-induced NK cell activation.
Perforin-mediated cytotoxicity and Fas ligand (FasL)
dependent antitumor activity are important targets for NK cell
therapy (39). The anti-metastatic
activity following RFA treatment was assessed. As depicted in
Fig. 4C and D, RFA treatment induced
the notable upregulation of perforin and FasL expression, compared
with the control NK cells from untreated animals, or the NK cells
incubated with a control isotype antibody. In cells cultured with
the NKG2D neutralizing antibody, the expression levels of the NK
cell surface marker CD69 and the NK cell effectors perforin and
FasL were significantly decreased. Taken in combination, these
experiments demonstrated that RFA treatment could suppress tumor
metastases, depending on the sensitivity of the tumor to perforin
or FasL, and reduce the expression of NKG2D ligands.
Discussion
In the present study, it was demonstrated that RFA
treatment could effectively eliminate liver tumors through
enhancement of NK-mediated antitumor activity and NKG2D
expression.
Curative resection has been considered to be the
first choice therapeutic strategy for a number of malignant tumor
types (40); however, only ~20% of
the patients with liver cancer are eligible for surgical resection
due to cancer multifocality, including severe impairment of hepatic
functional reserve, extrahepatic metastases, involvement of the
portal vein and severe extrahepatic disease (7–9). To date,
various treatment strategies for liver cancer, including local
ablative therapies, transarterial embolization and liver
transplantation, have been developed. RFA has gained widespread
acceptance as a local ablative treatment option for patients, due
to it being highly effective, minimally invasive and a generally
safe therapy for primary and secondary hepatic malignancies
(10,11,14,41).
Via the heating of tumor tissue to temperatures
exceeding 50°C, RFA produces localized tumor coagulative necrosis,
which results in the final destruction of the tumor tissues
(21). Although the success rate for
completely eliminating small liver tumors is >85% with RFA,
incomplete ablation frequently occurs and leads to tumor
reoccurrence (10,11,41).
Depending on the patient's medical situation, the overall complete
ablation rate ranges from 50–93%. The tumor size is another risk
factor, for tumors of diameter ≤3, 3–5 and ≥5 cm, the complete
ablation rate is 77–100, 84–93.5 and 41–71%, respectively,
following a single treatment session (41). Additionally, local recurrence
following RFA limits its application, with a rate varying between
2–60% (42). The tumor recurrence
rate may be notably higher in a number of specific anatomical
locations (43). In the present
study, four weeks following thermal ablation the coagulation of the
tumor had become white, and the boundary between the tumor and
surrounding tissues remained undistinguishable, indicating complete
ablation in the animal model. However, tumor recurrence should
still be determined over a longer time period.
Innate and adaptive immune cells actively prevent
cancer development (44). A notable
inflammatory cell infiltration was observed following RFA, which
led to speculation that the immune system was activated following
RFA treatment. As one of the most important components of the
innate immune system, NK cells serve critical roles in host
immunity to cancer (45). NK cells
have the ability to lyse certain tumor cells in the absence of
prior stimulation (24). In response
to tumor invasion, NK cells exert their function via two principal
underlying mechanisms: i) Releasing cytoplasmic granules containing
perforin and granzymes that lead to tumor-cell apoptosis; ii)
secreting cytokines to modulate the functions of other immunocytes
(22). The cytotoxic activity of NK
cells is modulated by NK cell receptors and cytokines (24,25). The
results indicated that RPA activates primary NK cells and induces
the stimulation of cytokines, including IFN-γ and TNF-α, in VX2
rabbit cells. Antibody neutralization demonstrated that the
RFA-induced immune activation is dependent on the NK receptor
NKG2D. RFA treatment also induced the elevation of the stimulatory
receptor CD69 and effector molecules, including perforin and FasL;
this indicates that RFA treatment induces antitumor mechanisms. It
is important for the mechanisms underlying liver tumor control to
be further characterized using a greater number and variety of
in vivo models.
Collectively, the results indicated that the RFA
treatment for liver cancer is a promising therapeutic strategy, and
that RFA-induced immune activation serves an important role in
suppressing cancer in animal models. While the present study mainly
focused on liver cancer, further investigations should be conducted
using other cancer types and in vivo models.
Acknowledgements
The authors would like to thank FL Zhao for
assistance of the writing.
Funding
The present study was supported by the Natural
Science Foundation of Hainan Province (grant no. 20158292).
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Author's contributions
ZM and HL conceived this study and performed all
these experiments. SM and XF helped with the VX2 model. SC and JY
helped with the cell culture and data analysis. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments in the present study were
carried out according to the guidelines on animal welfare and
regulations of Hainan Province, China and were evaluated and
approved by the Institutional Animal Care and Use Committee of
Research Center for Drug Safety Evaluation of Hainan
(HNYWAPZX201607011).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davis GL, Dempster J, Meler JD, Orr DW,
Walberg MW, Brown B, Berger BD, O'Connor JK and Goldstein RM:
Hepatocellular carcinoma: Management of an increasingly common
problem. Proc (Bayl Univ Med Cent). 21:266–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127 5 Suppl 1:S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simonetti RG, Camma C, Fiorello F, Politi
F, D'Amico G and Pagliaro L: Hepatocellular carcinoma. A worldwide
problem and the major risk factors. Dig Dis Sci. 36:962–972. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global Cancer Statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Y, Sui C, Li B, Yin Z, Tan Y, Yang J
and Liu Z: Repeat hepatectomy for recurrent hepatocellular
carcinoma: A local experience and a systematic review. World J Surg
Oncol. 8:552010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee PH, Lin WJ, Tsang YM, Hu RH, Sheu JC,
Lai MY, Hsu HC, May W and Lee CS: Clinical management of recurrent
hepatocellular carcinoma. Ann Surg. 222:670–676. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanematsu T, Matsumata T, Takenaka K,
Yoshida Y, Higashi H and Sugimachi K: Clinical management of
recurrent hepatocellular carcinoma after primary resection. Br J
Surg. 75:203–206. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsujita E, Yamashita Y, Takeishi K,
Matsuyama A, Tsutsui S, Matsuda H, Toshima T, Taketomi A, Shirabe
K, Ishida T and Maehara Y: Poor prognostic factors after repeat
hepatectomy for recurrent hepatocellular carcinoma in the modern
era. Am Surg. 78:419–425. 2012.PubMed/NCBI
|
|
10
|
Ikeda K, Osaki Y, Nakanishi H, Nasu A,
Kawamura Y, Jyoko K, Sano T, Sunagozaka H, Uchino K, Minami Y, et
al: Recent progress in radiofrequency ablation therapy for
hepatocellular carcinoma. Oncology. 87 Suppl 1:S73–S77. 2014.
View Article : Google Scholar
|
|
11
|
Feng K and Ma KS: Value of radiofrequency
ablation in the treatment of hepatocellular carcinoma. World J
Gastroenterol. 20:5987–5998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Q, Kobayashi S, Ye X and Meng X:
Comparison of hepatic resection and radiofrequency ablation for
small hepatocellular carcinoma: A meta-analysis of 16,103 patients.
Sci Rep. 4:72522014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khajanchee YS, Hammill CW, Cassera MA,
Wolf RF and Hansen PD: Hepatic resection vs. minimally invasive
radiofrequency ablation for the treatment of colorectal liver
metastases a markov analysis. Arch Surg. 146:1416–1423. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu C, Liu N, Deng Q, Li X, Ma K and Bie P:
Radiofrequency ablation vs. surgical resection on the treatment of
patients with small hepatocellular carcinoma: A system review and
meta-analysis of five randomized controlled trials.
Hepatogastroenterology. 61:1722–1729. 2014.PubMed/NCBI
|
|
15
|
Nguyen T, Hattery E and Khatri VP:
Radiofrequency ablation and breast cancer: A review. Gland Surg.
3:128–135. 2014.PubMed/NCBI
|
|
16
|
van der Ploeg IM, van Esser S, van den
Bosch MA, Mali WP, van Diest PJ, Rinkes Borel IH and van
Hillegersberg R: Radiofrequency ablation for breast cancer: A
review of the literature. Eur J Surg Oncol. 33:673–677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiraki T, Gobara H, Iguchi T, Fujiwara H,
Matsui Y and Kanazawa S: Radiofrequency ablation as treatment for
pulmonary metastasis of colorectal cancer. World J Gastroenterol.
20:988–996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fegrachi S, Besselink MG, van Santvoort
HC, van Hillegersberg R and Molenaar IQ: Radiofrequency ablation
for unresectable locally advanced pancreatic cancer: A systematic
review. HPB (Oxford). 16:119–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McGhana JP and Dodd GD: Radiofrequency
ablation of the liver: Current status. AJR Am J Roentgenol.
176:3–16. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Navarra G, Bartolotta M, Scisca C and
Barbera A: Ultrasound-guided radiofrequency-assisted segmental
arterioportal vascular occlusion in laparoscopic segmental liver
resection. Surg Endosc. 22:1724–1728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu CH, Yu CY, Chang WC, Dai MS, Hsiao CW
and Chou YC: Radiofrequency ablation of hepatic metastases: Factors
influencing local tumor progression. Ann Surg Oncol. 21:3090–3095.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waldhauer I and Steinle A: NK cells and
cancer immunosurveillance. Oncogene. 27:5932–5943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morvan MG and Lanier LL: NK cells and
cancer: You can teach innate cells new tricks. Nat Rev Cancer.
16:7–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marcus A, Gowen BG, Thompson TW, Iannello
A, Ardolino M, Deng W, Wang L, Shifrin N and Raulet DH: Recognition
of tumors by the innate immune system and natural killer cells. Adv
Immunol. 122:91–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hagerling C, Casbon AJ and Werb Z:
Balancing the innate immune system in tumor development. Trends
Cell Biol. 25:214–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moretta A, Marcenaro E, Parolini S,
Ferlazzo G and Moretta L: NK cells at the interface between innate
and adaptive immunity. Cell Death Differ. 15:226–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vivier E, Raulet DH, Moretta A, Caligiuri
MA, Zitvogel L, Lanier LL, Yokoyama WM and Ugolini S: Innate or
adaptive immunity? The example of natural killer cells. Science.
331:44–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vivier E, Nunès JA and Vély F: Natural
killer cell signaling pathways. Science. 306:1517–1519. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xuan XY, Zhang JF, Hu GM, Li QR, Liu PP
and Du Y: Upregulated expression of NKG2D and its ligands give
potential therapeutic targets for patients with thymoma. Cancer
Gene Ther. 22:368–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raneros Baragaño A, Suarez-Álvarez B and
López-Larrea C: Secretory pathways generating immunosuppressive
NKG2D ligands: New targets for therapeutic intervention.
Oncoimmunology. 3:e284972014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spear P, Wu MR, Sentman ML and Sentman CL:
NKG2D ligands as therapeutic targets. Cancer Immun.
13:82013.PubMed/NCBI
|
|
32
|
Dromi SA, Walsh MP, Herby S, Traughber B,
Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR, et al:
Radiofrequency ablation induces antigen-presenting cell
infiltration and amplification of weak tumor-induced immunity.
Radiology. 251:58–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zerbini A, Pilli M, Laccabue D, Pelosi G,
Molinari A, Negri E, Cerioni S, Fagnoni F, Soliani P, Ferrari C and
Missale G: Radiofrequency thermal ablation for hepatocellular
carcinoma stimulates autologous NK-cell response. Gastroenterology.
138:1931–1942. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Virmani S, Harris KR, Szolc-Kowalska B,
Paunesku T, Woloschak GE, Lee FT, Lewandowski RJ, Sato KT, Ryu RK,
Salem R, et al: Comparison of two different methods for inoculating
VX2 tumors in rabbit livers and hind limbs. J Vasc Interv Radiol.
19:931–936. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008:pdb.prot49862008.PubMed/NCBI
|
|
36
|
Hoppner M, Luhm J, Schlenke P, Koritke P
and Frohn C: A flow-cytometry based cytotoxicity assay using
stained effector cells in combination with native target cells. J
Immunol Methods. 267:157–163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seong NJ, Yoon CJ, Kang SG, Chung JW, Kim
HC and Park JH: Effects of arsenic trioxide on radiofrequency
ablation of VX2 liver tumor: Intraarterial versus intravenous
administration. Korean J Radiol. 13:195–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ohshima K, Suzumiya J, Shimazaki K, Kato
A, Tanaka T, Kanda M and Kikuchi M: Nasal T/NK cell lymphomas
commonly express perforin and Fas ligand: Important mediators of
tissue damage. Histopathology. 31:444–450. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scheele J, Stangl R and Altendorf-Hofmann
A: Hepatic metastases from colorectal carcinoma: Impact of surgical
resection on the natural history. Br J Surg. 77:1241–1246. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cucchetti A, Piscaglia F, Cescon M,
Ercolani G and Pinna AD: Systematic review of surgical resection
vs. radiofrequency ablation for hepatocellular carcinoma. World J
Gastroenterol. 19:4106–4118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mulier S, Ni Y, Jamart J, Ruers T, Marchal
G and Michel L: Local recurrence after hepatic radiofrequency
coagulation: Multivariate meta-analysis and review of contributing
factors. Ann Surg. 242:158–171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pawlik TM, Izzo F, Cohen DS, Morris JS and
Curley SA: Combined resection and radiofrequency ablation for
advanced hepatic malignancies: Results in 172 patients. Ann Surg
Oncol. 10:1059–1069. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gajewski TF, Schreiber H and Fu YX: Innate
and adaptive immune cells in the tumor microenvironment. Nat
Immunol. 14:1014–1022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheng M, Chen Y, Xiao W, Sun R and Tian Z:
NK cell-based immunotherapy for malignant diseases. Cell Mol
Immunol. 10:230–252. 2013. View Article : Google Scholar : PubMed/NCBI
|