Introduction
Transcatheter arterial chemoembolisation (TACE) is
recommended by the American Association for the Study of Liver
Disease as the first-line treatment for unresectable hepatocellular
carcinoma (HCC) (1), and is widely
performed in clinical practice due to its favourable efficacy
compared with conservative medical management or systematic
treatment (2–4) and minimal invasion. However, the
survival benefit following TACE remains limited (2). The present study reviewed the literature
and revealed that TACE, combined with hepatic arterial infusion
chemotherapy (HAIC), may achieve significantly longer
progression-free survival (PFS) times (8.0–9.3 months) compared
with patients treated with TACE alone (PFS, 4.5 months) (5,6).
Presumably due to technical refinements in HAIC protocol, no
bleeding, thrombus, infection or other associated complications
caused by the indwelling catheter in the HAIC procedure were
identified in recent studies (6,7),
suggesting that continuous transarterial infusion of 5-fluorouracil
(5-FU) is a generally safe treatment, and it is more effective
compared with TACE alone for patients with advanced HCC.
However, the underlying cause of the improving
efficacy of HAIC remains unclear. It appears that the primary
difference between TACE and TACE + HAIC is the approach of
administrating chemotherapeutic agents: TACE is administered via
bolus and HAIC is administered via a prolonged, continuous infusion
(8). Theoretically, the prolonged,
high regional concentration of the chemotherapeutic agent at the
tumour site would be expected to increase anti-tumour effects in
HAIC, particularly for time- and concentration-dependent agents
(9,10); 5-FU was one of these time-dependent
drugs (9).
5-FU was introduced as an anti-tumour drug in the
1950s (11) and it remains the
primary agent of various chemotherapy regimens. However, the
metabolism of 5-FU largely differs among individuals depending on
age (12), sex (12,13) and
hepatic insufficiency due to HCC and gene polymorphisms in the
dihydropyrimidine dehydrogenase gene (DPYD), which encodes DPD and
is involved in the catabolism of 5-FU (14–16).
Despite this, in previous studies, HAIC using regimens containing
5-FU was performed with favourable efficacy compared with
conventional TACE or best supportive care (17–19). Due
to the clinical efficacy of HAIC and individual variation of 5-FU
described above, the present study aimed to explore more detailed
associations, and to additionally explain the improved efficacy
achieved by HAIC. However, studies on the pharmaceutics of
transarterial 5-FU infusions are lacking, particularly in patients
with advanced HCC and hypohepatia, which limits additional
investigation. Therefore, the present study was initiated to
evaluate the peripheral concentration time curves of 5-FU
administrated through the hepatic artery, to provide an explanation
of its clinical efficacy and a basis for optimization of this
efficacy.
Materials and methods
Patient characteristics
The present study was conducted in the First
Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China).
The primary eligibility criteria included patients with
histologically-confirmed (20) HCC
with an Eastern Cooperative Oncology Group performance status
(21) of ≤2, those with a Child-Pugh
score (22) A, those with Barcelona
Clinic liver cancer stage C (23) and
those receiving 2 days of continuous HAIC following conventional
TACE. The primary exclusion criteria included patients with severe
coronary heart disease, those with severe active infection
[>grade 2, National Cancer Institute Common Terminology Criteria
for Adverse Events v4.0 criteria (24)], those with HIV infection, those with
renal insufficiency (creatinine level >2 mg/dl) or those with
allergies to platinum compounds, 5-FU or contrast media. All
patients provided written informed consent. The present study was
approved by the First Affiliated Hospital of Sun Yat-sen University
Ethics Committee. Baseline evaluation included the maximal diameter
measurement of viable tumours using dynamic contrast-enhanced
computed tomography according to the modified Response Evaluation
Criteria in Solid Tumours criteria (25) and biochemical examination such as
levels of α-fetoprotein, albumin, bilirubin, alanine
aminotransferase, aspartate transaminase, status of hepatitis B
virus and hepatitis C virus infection and prothrombin time.
Treatment protocol
A total of 10 patients totally underwent 18 cycles
of TACE + HAIC. The duration of each TACE + HAIC cycle was 3 days,
with a hospital stay ranging from 8 to 12 days. The interval
between each cycle was 3 weeks. All patients underwent a 2-day HAIC
treatment regimen using a folinic acid, fluorouracil and
oxaliplatin (FOLFOX4) regimen: 85 mg/m2 oxaliplatin
(Eloxatin®; Sanofi S.A., Paris, France) for 2 h on day
1; 200 mg/m2 leucovorin (Lingnan Pharmaceutical, Ltd.,
Guangdong, China) for 2 h on days 1 and 2; 400 mg/m2
5-FU (Sinochem Group, Beijing, China) bolus on days 1 and 2; and
600 mg/m2 5-FU on days 1 and 2, via an ambulatory
infusion pump (MR-508; Zhuhai MeiRuiHua Medical Technology Co.,
Ltd., Guangdong, China) following conventional TACE. TACE and HAIC
were performed as mentioned below.
A 5-F catheter was inserted into the femoral artery
using the Seldinger technique (26)
following routine preoperative preparation including fasting for 6
h and pubic hair removal. Arteriography of the celiac trunk and
hepatic and superior mesenteric arteries was performed to visualise
the arterial vascularisation of the tumour and to evaluate portal
vein patency, respectively. Guided by fluoroscopy, the tip of the
catheter, or microcatheter if necessary, was superselected into the
tumour-feeding branches using a guidewire. The embolisation of
target tumour-feeding vessels was performed by injecting a gelatine
sponge [Nanjing Jingling Pharmaceutical (Group) Co., Ltd., Nanjing,
China] or polyvinyl alcohol particles (Hangzhou Alicon
Pharmaceutical SCI&TEC Co., Ltd., Hangzhou, China). Following
embolisation, the catheter was inserted and the patient was
returned to the ward for FOLFOX4 administration. Regular analgesia
(Tramadol, 0.1 g intramuscularly, Hexal AG, Holzkirchen, Germany)
and nausea-controlling drugs (Palonosetron, 0.25 mg intravenously;
Qilu Pharmaceutical Co., Ltd., Jinan, China) were administered
during the HAIC. As soon as consecutive transarterial FOLFOX4
treatment was finished, the catheter was removed, the puncture site
was stanched by compression for ~15 min and pressure bandaging was
applied.
Pharmacokinetic blood sampling
A total of 11 peripheral venous blood samplings were
collected from all 10 patients, and each contained 3 ml blood from
prior the infusion at admission and at 0, 0.5, 1, 1.5, 2, 5, 10,
15, 22 and 23 h following the initiation of infusion on day 1.
Immediately after drawing, blood samples were centrifuged at room
temperature at 1,118.0 × g for 10 min, and the supernatant was
stored in clean 1.5 ml polypropylene tubes and stored at −80°C.
Optimising the extraction method and
liquid chromatography-mass spectrometry (LC-MS) conditions
The extraction method described by Remaud et
al (27) was simplified by
removing the evaporation procedure. Firstly, 100 mg ammonium
sulfate was added into 100 µl plasma samples to precipitate plasma
proteins. Following vortex mixing at 50.31 × g at room temperature
for 1 min, 300 µl internal standard (IS) solution (5-Br;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added. The
samples were gently mixed at room temperature for 1 min in a rotary
stirrer (45 rotations/min) and centrifuged for 10 min at 25,758.7 ×
g and 4°C. A total of 100 µl supernatant was then transferred to an
autosampler vial prior to injection onto the column. Volume
injection was set at 20 µl for 5-FU and 5-bromopyrimidine
(5-Br).
The standard curve of 5-FU was prepared by adding 20
µl standard solution (50 µg/ml) of industrial pure 5-FU (provided
by National Institute for Food and Drug Control, Beijing, China)
and 10 µl IS (5-Br) to 980 µl control human plasma. IS
concentration was set at 50 ng/ml. Final generated concentrations
were 10, 20, 50, 200, 600 and 1,000 ng/ml for 5-FU. Quality
controls of 10, 30 and 100 ng/ml of 5-FU were considered low,
moderate and high concentrations, respectively, and used to verify
the standard curve delineated by 10, 20, 50, 200, 600 and 1,000
ng/ml 5-FU. All samples were then treated according to the
extraction method and high-performance liquid chromatography (HPLC)
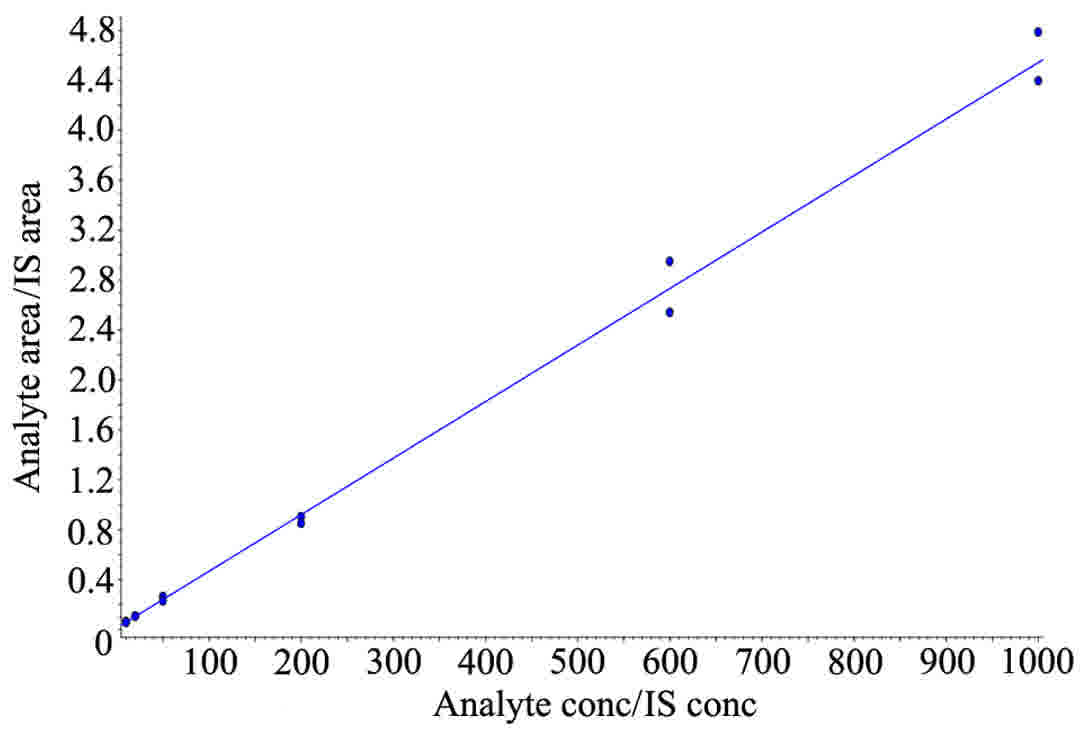
at room temperature as follows. Standard curves for 5-FU were
generated by plotting the peak area ratio to that of the IS vs. the
concentration of each compound.
Notably, unlike LC conditions in previous studies
(27–33), the mobile phase solvent A in the
present study contained 0.1% (v/v) formic acid in water, and the
mobile phase solvent B contained 100% acetonitrile. The mobile
phase composition was 5% solvent A and 95% solvent B, which was
pumped at a flow rate of 380 µl/min for 10 min. Other parameters
are summarised in Tables I and
II.
 | Table I.Liquid chromatography mass
spectrometry system information. |
Table I.
Liquid chromatography mass
spectrometry system information.
| Instrument | Version | Supplier |
|---|
| Mass
spectrometer | AB Sciex API
2000 | AB Sciex Pte. Ltd.,
Warrington, UK |
| Liquid
chromatography system | Agilent
Technologies 1200 series | Agilent
Technologies, Inc., Santa Clara, CA, USA |
| Chromatographic
column | Agilent Eclipse
XDB-C18, 4.6 × 150 mm, 5 µm | Agilent
Technologies, Inc., Santa Clara, CA, USA |
| Data acquisition
and analysis system | Analyst 1.6, AB
Sciex | AB Sciex Pte. Ltd.,
Warrington, UK |
 | Table II.Mass spectrometer settings for the
analysis of 5-fluoruracil in human plasma. |
Table II.
Mass spectrometer settings for the
analysis of 5-fluoruracil in human plasma.
| Detector
parameters | Setting |
|---|
| Ion source | Electrospray
ionization |
| Ionization
modes | Negative |
| Scanning mode | Multiple reaction
monitoring scanning |
| Selected reaction
monitoring transition | 5-FU:129.0→42.1;
5-Br:188.9→52.0 |
| De-clustering
potential | −20
V |
| Focusing
potential | −400 V |
| Entrance
potential | −10
V |
| Collision
energy | −18
V |
| Collision cell exit
potential | −5
V |
| Curtain gas | 20 TSI |
| Collision gas | 8 |
| Ion spray gas | −4500 V |
| Temperature | 400°C |
| Ion source gas
1 | 55 PSI |
| Ion source gas
2 | 50 PSI |
| Flow rate | 380 µl/min |
DPD levels were also analysed in this research.
Direct approaches of determining DPD, such as genotyping,
quantification of DPD mRNA have been proved cumbersome to realise
(16,34). An alternative method by measuring the
ratio of plasma uracil (U) and dihydrouracil (UH2) through HPLC was
adopted in this research as DPD is responsible for catabolism of U
to UH2 (35,36). Elevated ratios (U:UH2 >2) have been
reported highly correlated to DPD deficiency (37–40).
Results
Prior to receiving the results, it was hypothesised
that the steady-state concentration of 5-FU would range between
200–300 ng/ml, due to previous data from studies examining
colorectal cancer (41–43).
Baseline characteristics of 10 patients are
summarised in Table III.
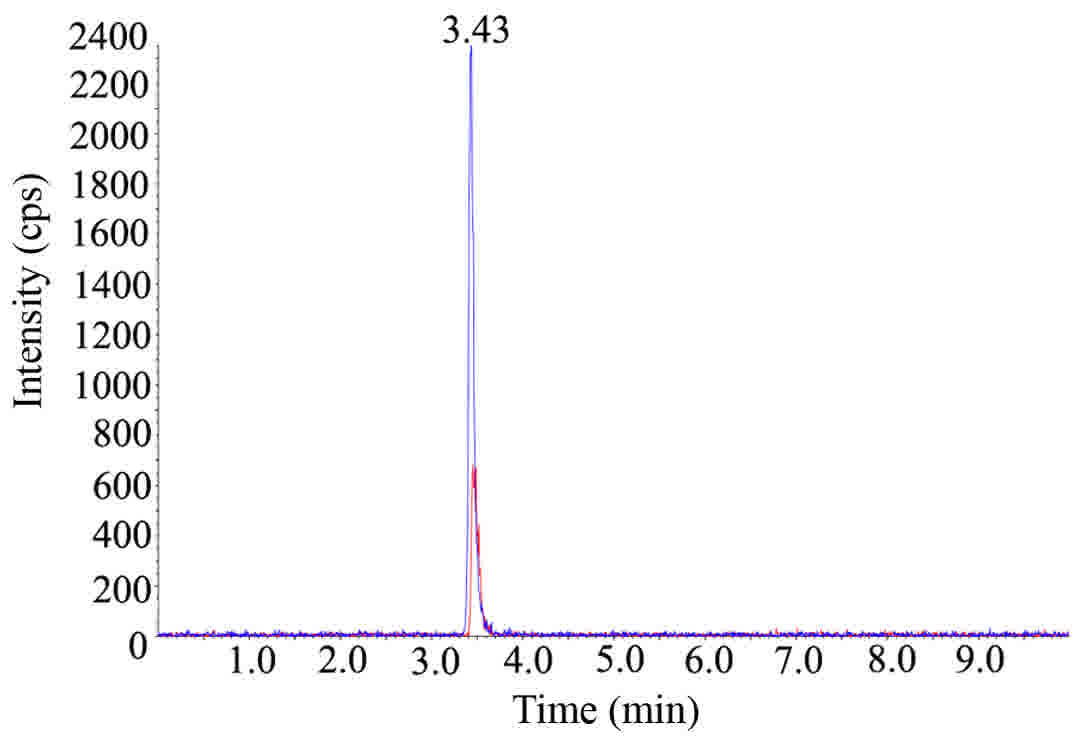
Representative chromatograms and linearity of 5-FU added into
control plasma are presented in Figs.
1 and 2. Plasma concentration vs.
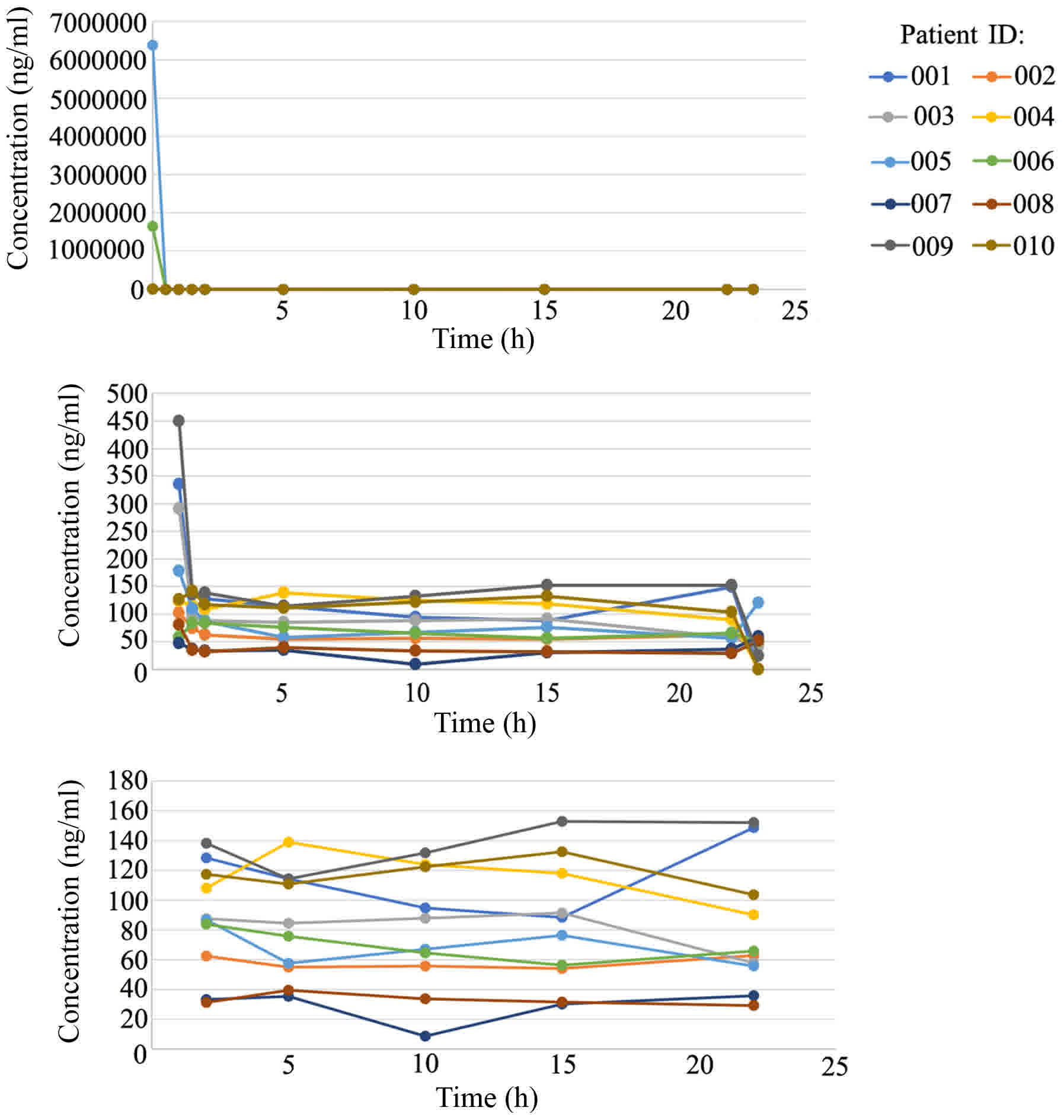
time curves of 5-FU are illustrated in Fig. 3 and detailed information is summarised
in Table IV. At 0 h of continuous
infusion of 5-FU, the plasma concentration of 5-FU was markedly
high compared with the normal range (200–300 ng/ml) due to previous
5-FU bolus; this concentration rapidly decreased to relatively
normal levels within 2 h and then fluctuated mildly to reach a
steady state. According to Fig. 3, a
steady-state plasma concentration of 5-FU, administered through the
hepatic artery, was achieved after 15 h; this concentration widely
varied in the 10 patients, ranging from 8.64–152 ng/ml. The ratio
of U and UH2 (U:UH2) fluctuated from 1.98–2.06, indicating mild DPD
deficiency in these 10 patients with HCC.
 | Table III.Baseline characteristics and tumour
responses of 10 patients. |
Table III.
Baseline characteristics and tumour
responses of 10 patients.
| Patient ID | Age, years | Sex | Hepatitis B
virus | Hepatitis C
virus | Child-Pugh
score | AFP | BCLC stage | Blood supply | Previous
treatments | Response |
|---|
| 001 | 40 | M | Positive | Negative | A | Negative | B | Hypervascular | TACE, intravenous
FOLFOX4 | PR |
| 002 | 30 | M | Positive | Negative | A | Negative | B | Hypervascular | TAE + HAIC
(FOLFOX4) | Lost |
| 003 | 38 | M | Positive | Negative | A | Positive | B | Hypervascular | None | Lost |
| 004 | 42 | M | Positive | Negative | A | Positive | C | Hypervascular | Resection,
ablation, TACE | PR |
| 005 | 51 | M | Positive | Negative | A | Negative | C | Hypovascular | TACE + HAIC
(FOLFOX4) | SD |
| 006 | 51 | M | Positive | Negative | A | Positive | C | Hypovascular | TACE | SD |
| 007 | 33 | M | Positive | Negative | A | Positive | B | Hypovascular | Resection | SD |
| 008 | 37 | M | Positive | Negative | A | Negative | C | Hypervascular | None | SD |
| 009 | 42 | M | Positive | Negative | A | Negative | C | Hypovascular | TACE + HAIC
(FOLFOX4) | Lost |
| 010 | 46 | M | Positive | Negative | A | Positive | B | Hypervascular | Resection, TACE +
HAIC (FOLFOX4) | CR |
 | Table IV.Detail information of 5-FU and DPD
levels of 10 patients. |
Table IV.
Detail information of 5-FU and DPD
levels of 10 patients.
|
|
5-FU levels
(ng/ml) |
|
|---|
|
|
|
|
|---|
| Patient ID | 0 h | 0.5 h | 1 h | 1.5 h | 2 h | 5 h | 10 h | 15 h | 22 h | 23 h | U:UH2 (prior the
infusion at admission) |
|---|
| 001 | 12,477.0 | 1,771.0 | 336.1 | 106.6 | 128.3 | 114.3 | 94.7 | 88.4 | 148.7 | 0.0 | 2.02 |
| 002 | 10,118.9 | 237.1 | 102.4 | 74.8 | 62.5 | 54.9 | 55.8 | 54.1 | 62.8 | 44.7 | 2.05 |
| 003 | 11,452.4 | 1,150.8 | 291.4 | 93.9 | 87.5 | 84.5 | 87.9 | 91.4 | 57.8 | 41.2 | 2.02 |
| 004 | 2,200.0 | 219.0 | 123.0 | 115.0 | 108.0 | 139.0 | 124.0 | 118.0 | 90.1 | 0.0 | 2.08 |
| 005 | 6,390,000.0 | 963.0 | 178.0 | 110.0 | 86.8 | 57.5 | 67.1 | 76.3 | 55.7 | 121.0 | 1.98 |
| 006 | 1,650,000.0 | 169.0 | 57.4 | 83.1 | 83.8 | 75.8 | 64.6 | 56.3 | 65.8 | 49.4 | 2.00 |
| 007 | 10,500.0 | 508.0 | 46.7 | 36.1 | 33.3 | 35.4 | 8.6 | 30.2 | 35.8 | 58.5 | 2.04 |
| 008 | 12,000.0 | 336.0 | 80.7 | 35.5 | 31.2 | 39.5 | 33.7 | 31.6 | 29.2 | 52.1 | 2.01 |
| 009 | 14,953.7 | 1,965.2 | 449.5 | 135.1 | 138.1 | 114.3 | 131.7 | 152.9 | 152.1 | 24.6 | 2.04 |
| 010 | 11,204.7 | 280.8 | 125.7 | 142.3 | 117.3 | 110.8 | 122.4 | 132.5 | 103.6 | 0.0 | 2.06 |
Discussion
To the best of our knowledge, this is the first
study to quantitatively evaluate plasma concentration time curves
of 5-FU continuously administered through the hepatic artery for
>44 h in patients with advanced HCC. As the therapeutic agent
first passes through the liver in HAIC, which is the organ involved
in its eventual metabolism, lower peripheral blood concentration
and fewer systemic side effects are anticipated (8). A conventional HPLC was not used, as the
lower limit of quantification (LLOQ) was 100 ng/ml (44,45), and
thus it may not detect lower concentrations of 5-FU in peripheral
blood during HAIC. A more sensitive method with an LLOQ of 5–10
ng/ml was identified from previous studies (27,31–33,46),
and the procedure was simplified to meet clinical requirements. It
should be noted that specific parameters, primarily the
aforementioned LC conditions, of the LC-MS performed in the present
study were not exactly the same in different centres with different
instruments when the methods published previously were repeated
(27–33). The results of the present study
suggest that 5-FU was better extracted by precipitate plasma
proteins with ammonium sulfate compared with simple liquid-liquid
extraction with acetonitrile or formic acid in acetonitrile.
Additionally, with the evaporation procedure, the ultimate peak
intensity may be more marked compared with any other procedure, but
took a longer time. For clinical application, the evaporation
process was removed.
The half-life of 5-FU in the human body is 10–20 min
(47). The majority of drugs will
reach steady state following 5 half-lives during continuous
intravenous administration (48). In
the present study, a steady-state plasma concentration of 5-FU was
achieved after 15 h, which is much longer compared with the 5
half-lives of 5-FU. This conclusion was consistent with results
obtained by Kaldate et al (49), which revealed that more factors than
half-life alone affect the steady-state plasma concentration of
5-FU, particularly in patients with levels of liver dysfunction,
including HCC. For additional studies on the steady-state
concentration of 5-FU in peripheral blood and the efficacy of TACE
+ HAIC, the average time-points of 15 and 22 h are recommend for
the measurement of the steady-state plasma concentration.
Notably, despite the mild fluctuation of U:UH2 from
1.98–2.06 in the 10 patients, the steady-state concentration of
5-FU varied widely between patients, ranging from 8.64–152 ng/ml,
which indicates that factors separate from DPD levels require
consideration. Concurrently, the steady-state concentration of 5-FU
(30.2–152.9 ng/ml) in the present study was under the established
therapeutic range, when administered venously, was between 200–300
ng/ml in colorectal cancer (41–43).
However, it is noteworthy that the comparisons between arterial and
venous modes of administration, and between different types of
cancer is insufficient. Therefore, the association between the
steady-state concentration of 5-FU in peripheral blood and the
efficacy of TACE + HAIC requires additional study.
An additional notable result of the present study
was that the concentration of 5-FU at 23 h, which is the hour when
5-FU treatment was ceased, during the administration of folinic
acid, exhibited various changes; 3 cases decreased to 0 ng/ml, 4
decreased to a detectable degree (24.6–49.4 ng/ml) and 3 increased
to more than the respective steady-state concentration (52.1–121.0
ng/ml). Folinic acid serves as a coactivator of thymidylate
synthetase, which is the primary target of the continuous infusion
action of 5-FU, to increase the efficacy of 5-FU (50). Rebound increases in the concentration
of 5-FU during the administration of folinic acid were not expected
at the initiation of the present study.
The present study contained several limitations.
Firstly, the chemotherapy regimen used in the HAIC was FOLFOX4,
which contains a chemotherapeutic agent-oxaliplatin. The
anti-tumour role of oxaliplatin should not be neglected.
Theoretically, the combination of 5-FU and oxaliplatin yielded
additive or synergistic cytotoxic effects (51), but which one served the primary role
remains unknown, and requires additional study. Also, the present
study did not focus on the pharmacokinetics of oxaliplatin; the
group are developing an assay that is simpler and more sensitive
and cost-effective in clinical applications for quantitative
assessment, although a small number of previous studies have
described the use of laser ablation-inductively coupled plasma-mass
spectrometry and flameless atomic absorption spectrometry methods
(52,53).
An additional limitation was that the prognosis of
each patient was not completely assessed. A total of 3 of the 10
patients were lost to follow-up. However, the aim of the present
study was not to assess the efficacy. There may be several
prognostic factors of TACE + HAIC, including the materials used and
extent of embolisation. The studies of Gao et al (5,6) considered
embolisation as the basis and core of combination therapy. Perfect
embolisation may thoroughly block the blood supply of tumour and
lower the risk of catheter malposition during chemotherapy
(5). The present study only proposes
an additional potential prognostic factor-the area under the curve
of chemotherapeutic drugs. This is the basis for additional
response evaluation and prognosis analysis.
To conclude, continuous transarterial infusion of
5-fluorouracil is a generally safe treatment. Optimised LC-MS may
detect low concentrations of 5-FU. The steady-state concentration
of 5-FU administered through the hepatic artery was achieved after
15 h, which may provide a basis for additional therapeutic drug
monitoring practice, response prediction and efficacy
optimization.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: A
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rammohan A, Sathyanesan J, Ramaswami S,
Lakshmanan A, Senthil-Kumar P, Srinivasan UP, Ramasamy R and
Ravichandran P: Embolization of liver tumors: Past, present and
future. World J Radiol. 4:405–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao S, Zhu X, Yang R and Guo J: TACE
combined with hepatic arterial infusion chemotherapy using
oxaliplatin,5-fluorouracil and folinic acid for intermediate and
advanced hepatocellular carcinomas. J Int Radiol. 21:377–383.
2012.
|
|
6
|
Gao S, Zhang PJ, Guo JH, Chen H, Xu HF,
Liu P, Yang RJ and Zhu X: Chemoembolization alone vs combined
chemoembolization and hepatic arterial infusion chemotherapy in
inoperable hepatocellular carcinoma patients. World J
Gastroenterol. 21:10443–1052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishikawa M, Kakizawa H, Hieda M, Toyota N,
Katamura Y, Aikata H, Chayama K and Awai K: Long-term outcomes of
hepatic arterial port implantation using a coaxial microcatheter
system in 176 patients with hepatocellular carcinoma. Hiroshima J
Med Sci. 61:7–13. 2012.PubMed/NCBI
|
|
8
|
Paul SB and Sharma H: Role of
Transcatheter intra-arterial therapies for hepatocellular
carcinoma. J Clin Exp Hepatol. 4 Suppl 3:S112–S121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Obi S, Sato S and Kawai T: current status
of hepatic arterial infusion chemotherapy. Liver Cancer. 4:188–199.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song MJ: Hepatic artery infusion
chemotherapy for advanced hepatocellular carcinoma. World J
Gastroenterol. 21:3843–3849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heidelberger C, Chaudhuri NK, Danneberg P,
Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E and
Scheiner J: Fluorinated pyrimidines, a new class of
tumour-inhibitory compounds. Nature. 179:663–666. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milano G, Etienne MC, Cassuto-Viguier E,
Thyss A, Santini J, Frenay M, Renee N, Schneider M and Demard F:
Influence of sex and age on fluorouracil clearance. J Clin Oncol.
10:1171–1175. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mueller F, Büchel B, Köberle D, Schürch S,
Pfister B, Krähenbühl S, Froehlich TK, Largiader CR and Joerger M:
Gender-specific elimination of continuous-infusional 5-fluorouracil
in patients with gastrointestinal malignancies: Results from a
prospective population pharmacokinetic study. Cancer Chemother
Pharmacol. 71:361–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Diasio RB and Harris BE: Clinical
pharmacology of 5-fluorouracil. Clin Pharmacokinet. 16:215–237.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Etienne MC, Lagrange JL, Dassonville O,
Fleming R, Thyss A, Renée N, Schneider M, Demard F and Milano G:
Population study of dihydropyrimidine dehydrogenase in cancer
patients. J Clin Oncol. 12:2248–2253. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Z, Zhang R and Diasio RB:
Dihydropyrimidine dehydrogenase activity in human peripheral blood
mononuclear cells and liver: Population characteristics, newly
identified deficient patients, and clinical implication in
5-fluorouracil chemotherapy. Cancer Res. 53:5433–5438.
1993.PubMed/NCBI
|
|
17
|
Sumie S, Yamashita F, Ando E, Tanaka M,
Yano Y, Fukumori K and Sata M: Interventional radiology for
advanced hepatocellular carcinoma: Comparison of hepatic artery
infusion chemotherapy and transcatheter arterial lipiodol
chemoembolization. AJR Am J Roentgenol. 181:1327–1334. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Monden M, Sakon M, Sakata Y, Ueda Y and
Hashimura E: FAIT Research Group: 5-fluorouracil arterial infusion
+ interferon therapy for highly advanced hepatocellular carcinoma:
A multicenter, randomized, phase II study. Hepatol Res. 42:150–165.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W,
Guo RP and Shi M: Hepatic artery infusion chemotherapy using
mFOLFOX versus transarterial chemoembolization for massive
unresectable hepatocellular carcinoma: A prospective non-randomized
study. Chin J Cancer. 36:832017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chinese Society of Liver Cancer, Chinese
Anti-Cancer Association; Chinese Society of Clinical Oncology,
Chinese Anti-Cancer Association; Liver Cancer: Expert consensus on
the scheme of pathological diagnosis of primary liver cancer.
Zhonghua Gan Zang Bing Za Zhi. 19:254–256. 2011.(In Chinese).
PubMed/NCBI
|
|
21
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Forner A, Reig ME, de Lope CR and Bruix J:
Current strategy for staging and treatment: the BCLC update and
future prospects. Semin Liver Dis. 30:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Cancer Institute: Common
Terminology Criteria for Adverse Events (CTCAE). NIH Publication;
pp. 0–71. 2010
|
|
25
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) Assessment for Hepatocellular Carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seldinger SI: Catheter replacement of the
needle in percutaneous arteriography; a new technique. Acta radiol.
39:368–376. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Remaud G, Boisdron-Celle M, Morel A and
Gamelin A: Sensitive MS/MS-liquid chromatography assay for
simultaneous determination of tegafur, 5-fluorouracil and
5-fluorodihydrouracil in plasma. J Chromatogr B Analyt Technol
Biomed Life Sci. 824:153–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishii H, Shimada M, Yamaguchi H and Mano
N: A simultaneous determination method for 5-fluorouracil and its
metabolites in human plasma with linear range adjusted by in-source
collision-induced dissociation using hydrophilic interaction liquid
chromatography-electrospray ionization-tandem mass spectrometry.
Biomed Chromatogr. 30:1882–1886. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peer CJ, McManus TJ, Hurwitz HI and Petros
WP: Development and utilization of a combined LC-UV and LC-MS/MS
method for the simultaneous analysis of tegafur and 5-fluorouracil
in human plasma to support a phase I clinical study of oral
UFT(R)/leucovorin. J Chromatogr B Analyt Technol Biomed Life Sci.
898:32–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Licea-Perez H, Wang S and Bowen C:
Development of a sensitive and selective LC-MS/MS method for the
determination of alpha-fluoro-beta-alanine, 5-fluorouracil and
capecitabine in human plasma. J Chromatogr B Analyt Technol Biomed
Life Sci. 877:1040–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Büchel B, Rhyn P, Schürch S, Bühr C,
Amstutz U and Largiadèr CR: LC-MS/MS method for simultaneous
analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and
5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug
monitoring and toxicity prediction in cancer patients. Biomed
Chromatogr. 27:7–16. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kosovec JE, Egorin MJ, Gjurich S and
Beumer JH: Quantitation of 5-fluorouracil (5-FU) in human plasma by
liquid chromatography/electrospray ionization tandem mass
spectrometry. Rapid Commun Mass Spectrom. 22:224–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carli D, Honorat M, Cohen S, Megherbi M,
Vignal B, Dumontet C, Payen L and Guitton J: Simultaneous
quantification of 5-FU, 5-FUrd, 5-FdUrd, 5-FdUMP, dUMP and TMP in
cultured cell models by LC-MS/MS. J Chromatogr B Analyt Technol
Biomed Life Sci. 877:2937–2944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boisdron-Celle M, Remaud G, Traore S,
Poirier AL, Gamelin L, Morel A and Gamelin E:
5-Fluorouracil-related severe toxicity: A comparison of different
methods for the pretherapeutic detection of dihydropyrimidine
dehydrogenase deficiency. Cancer Lett. 249:271–282. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garg MB, Sevester JC, Sakoff JA and
Ackland SP: Simple liquid chromatographic method for the
determination of uracil and dihydrouracil plasma levels: a
potential pretreatment predictor of 5-fluorouracil toxicity. J
Chromatogr B Analyt Technol Biomed Life Sci. 774:223–230. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang H, Jiang J, Hu P and Hu Y:
Measurement of endogenous uracil and dihydrouracil in plasma and
urine of normal subjects by liquid chromatography-tandem mass
spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci.
769:169–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang H, Lu J and Ji J: Circadian rhythm
of dihydrouracil/uracil ratios in biological fluids: A potential
biomarker for dihydropyrimidine dehydrogenase levels. Br J
Pharmacol. 141:616–623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ben Fredj R, Gross E, Ben Ahmed S, Hassine
H and Saguem S: The dihydrouracil/uracil ratio in plasma, clinical
and genetic analysis for screening of dihydropyrimidine
dehydrogenase deficiency in colorectal cancer patients treated with
5-fluorouracil. Pathol Biol (Paris). 57:470–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gamelin E, Boisdron-Celle M, Guérin-Meyer
V, Delva R, Lortholary A, Genevieve F, Larra F, Ifrah N and Robert
J: Correlation between uracil and dihydrouracil plasma ratio,
fluorouracil (5-FU) pharmacokinetic parameters, and tolerance in
patients with advanced colorectal cancer: A potential interest for
predicting 5-FU toxicity and determining optimal 5-FU dosage. J
Clin Oncol. 17:11051999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ciccolini J, Mercier C, Evrard A, Dahan L,
Boyer JC, Duffaud F, Richard K, Blanquicett C, Milano G, Blesius A,
et al: A rapid and inexpensive method for anticipating severe
toxicity to fluorouracil and fluorouracil-based chemotherapy. Ther
Drug Monit. 28:678–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seitz JF, Cano JP, Rigault JP, Aubert C
and Carcassonne Y: Chemotherapy of extensive digestive cancers with
5-fluorouracil: Relation between the clinical response and plasma
clearance of the drug. Gastroenterol Clin Biol. 7:374–380. 1983.(In
French). PubMed/NCBI
|
|
42
|
Gamelin EC, Danquechin-Dorval EM, Dumesnil
YF, Maillart PJ, Goudier MJ, Burtin PC, Delva RG, Lortholary AH,
Gesta PH and Larra FG: Relationship between 5-fluorouracil (5-FU)
dose intensity and therapeutic response in patients with advanced
colorectal cancer receiving infusional therapy containing 5-FU.
Cancer. 77:441–451. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gamelin E, Delva R, Jacob J, Merrouche Y,
Raoul JL, Pezet D, Dorval E, Piot G, Morel A and Boisdron-Celle M:
Individual fluorouracil dose adjustment based on pharmacokinetic
follow-up compared with conventional dosage: Results of a
multicenter randomized trial of patients with metastatic colorectal
cancer. J Clin Oncol. 26:2099–2105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alanazi FK, Yassin AE, El-Badry M, Mowafy
HA and Alsarra IA: Validated high-performance liquid
chromatographic technique for determination of 5-fluorouracil:
Applications to stability studies and simulated colonic media. J
Chromatogr Sci. 47:558–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Serve KM, Yáñez JA, Remsberg CM, Davies NM
and Black ME: Development and validation of a rapid and sensitive
HPLC method for the quantification of 5-fluorocytosine and its
metabolites. Biomed Chromatogr. 24:556–561. 2010.PubMed/NCBI
|
|
46
|
Licea-Perez H, Wang S and Bowen C:
Development of a sensitive and selective LC-MS/MS method for the
determination of alpha-fluoro-beta-alanine, 5-fluorouracil and
capecitabine in human plasma. J Chromatogr B Analyt Technol Biomed
Life Sci. 877:1040–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Blaschke M, Blumberg J, Wegner U,
Nischwitz M, Ramadori G and Cameron S: Measurements of 5-FU plasma
concentrations in patients with gastrointestinal cancer: 5-FU
levels reflect the 5-FU dose applied. J Cancer Ther. 3:28–36. 2012.
View Article : Google Scholar
|
|
48
|
Jianshi L: Pharmacology [M]: Tsinghua
University Press. 3:20–21. 2015.
|
|
49
|
Kaldate RR, Haregewoin A, Grier CE,
Hamilton SA and McLeod HL: Modeling the 5-fluorouracil area under
the curve versus dose relationship to develop a pharmacokinetic
dosing algorithm for colorectal cancer patients receiving FOLFOX6.
Oncologist. 17:296–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Machover D, Goldschmidt E, Chollet P,
Metzger G, Zittoun J, Marquet J, Vandenbulcke JM, Misset JL,
Schwarzenberg L, Fourtillan JB, et al: Treatment of advanced
colorectal and gastric adenocarcinomas with 5-fluorouracil and
high-dose folinic acid. J Clin Oncol. 4:685–696. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Raymond E, Faivre S, Chaney S, Woynarowski
J and Cvitkovic E: Cellular and molecular pharmacology of
oxaliplatin. Mol Cancer Ther. 1:227–235. 2002.PubMed/NCBI
|
|
52
|
Moraleja I, Esteban-Fernández D, Lázaro A,
Humanes B, Neumann B, Tejedor A, Mena Luz M, Jakubowski N and
Gómez-Gómez MM: Printing metal-spiked inks for LA-ICP-MS bioimaging
internal standardization: Comparison of the different nephrotoxic
behavior of cisplatin, carboplatin, and oxaliplatin. Anal Bioanal
Chem. 408:2309–2318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
LeRoy AF, Wehling ML, Sponseller HL,
Friauf WS, Solomon RE, Dedrick RL, Litterst CL, Gram TE, Guarino AM
and Becker DA: Analysis of platinum in biological materials by
flameless atomic absorption spectrophotometry. Biochem Med.
18:184–191. 1977. View Article : Google Scholar : PubMed/NCBI
|