Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common mature B-cell lymphoma and accounts for 38–50% of incident
lymphomas each year (1). The
prognosis of patients with DLBCL was predominately estimated
according to the International Prognostic Index (IPI) (2), which was proposed prior to the rituximab
era of treatment. The IPI was also confirmed as a prognostic tool
for predicting overall survival (OS), progression-free survival
(PFS) and event-free survival (EFS) (3) following the introduction of rituximab,
even though certain studies have claimed that it has lost its value
in the rituximab era (4) and requires
modifications (5). At present, the
IPI divides patients into four groups (low-risk group,
low-intermediate risk group, high-intermediate risk group and
high-risk group), with different survival rates (1).
Subsequent to investigating the cell-of-origin, Hans
et al (6) published an
algorithm based on immunohistochemistry, classifying DLBCL by the
cell-of-origin into germinal center B-cell (GCB) and non-GCB
activated B-cell (ABC and type III) subtypes (6). When comparing the
immunohistochemically-determined non-GCB and GCB subtypes with the
results of gene expression profiling (6,7), the
positive predictive value of this algorithm was 87% for the GCB
group and 73% for the non-GCB group, and the concordance with the
gene expression profile (GEP) was 86% (6). Following this, Choi et al
(8) developed an additional algorithm
with a higher accuracy, as 93% of algorithm predictions matched
their GEP. Meyer et al (9)
published a study in 2011 comparing several established algorithms,
and also constructed 2 novel ones; the modified Hans and the
modified Choi algorithms. The Choi algorithm exhibited an 87%
concordance with GEP, the Hans algorithm exhibited an 86%
concordance and the modified Hans and modified Choi algorithm each
exhibited an 87% concordance with GEP. The 2 modified algorithms
omitted the B-cell lymphoma 6 protein (Bcl-6) antigen
determination, yet retained their concordance with GEP (9). The 2016 revision of the WHO
classification of lymphoid neoplasms recommends the Hans algorithm
for the classification of GCB and ABC subtypes, but also allows the
application of other algorithms (10).
The ABC subtype is definitely associated with
inferior survival, as demonstrated by Hans, Choi, Meyer and
Alizadeh (6–9). Previously, numerous studies have
addressed this subject, aiming to identify a more aggressive
front-line treatment for the ABC subtype (11). At present, the majority of patients
are treated with the standard rituximab and cyclophosphamide,
doxorubicin, vincristine and prednisone (R-CHOP) therapies
(1). However, novel agents including
bortezomib (12), lenalidomide
(13) and ibrutinib (14) are being widely investigated in the
treatment of the ABC subtype of DLBCL.
The aim of the present study was to assess the
accuracy of each of the 4 most commonly-used and relatively
easy-to-perform algorithms in classifying patients into the ABC and
GCB subgroups, and to also evaluate which of the algorithms more
proficiently stratified patients into the GCB subgroup according to
the OS. The expression of cluster of differentiation (CD)19 and
CD20 antigens on the ABC and GCB subtypes was also evaluated.
Patients and methods
Patients
A total of 127 patients with de novo DLBCL
were included in the present study from April 2004 to December
2010. All were >18 years of age, HIV-negative and had a
histological tissue sample removed for accurate histological
diagnosis prior to any treatment either with surgical resection of
involved tissue (16 patients) or lymph node biopsy (111 patients).
They were treated with R-CHOP (intravenous cyclophosphamide 750
mg/m2, doxorubicin 50 mg/m2, vincristine 1.4
mg/m2; maximum dose 2 mg; rituximab 375 mg/m2
on day 1 and oral prednisolone 40 mg/m2 on days 1–5,
administered every 21 days) or CHOP-like regimens (etoposide 100
mg/m2 instead of doxorubicin) for 2–10 treatment cycles
between April 2004 and December 2010 at the Institute of Oncology
(Ljubljana, Slovenia). Detailed data of their age at diagnosis,
stage of the disease, number of treatment cycles and consolidation
with radiotherapy was obtained from the records of the patients.
The IPI was also determined. Ki-67 was determined on the available
samples (96 patients, as other samples were too damaged for
accurate analyses), and subgroups were created for each 10%
measurement. For each patient, the response to first-line treatment
was defined as complete remission (CR), partial remission (PR),
stable disease (SD) or progressive disease (PD) based on the
revised criteria of Cheson et al (15), and the PFS and OS were calculated. The
PFS was defined as the period from the end of first-line treatment
(either chemotherapy or consolidation radiotherapy) until verified
progression or mortality by any cause for patients achieving CR or
PR. The OS was defined as the period from the first day of
treatment until mortality by any cause for all patients. Data on
the cause and dates of mortality was obtained from the Cancer
Registry of the Republic of Slovenia on 15 July, 2016, so that each
patient had a minimum of 5.5 years observation time.
All patients signed an informed consent form to
participate in the study, and the study was conducted in accordance
with the Declaration of Helsinki. The present study was approved by
the Republic of Slovenia National Medical Ethics Committee.
Immunohistochemistry
For the tissue microarray (TMA), hematoxylin and
eosin-stained [H&E; stained in Leica automatic Multistainer
ST5020 (Leica Microsystems, Inc., Buffalo Grove, IL, USA) by use of
Mayer's hematoxylin (Merck & Co, Inc., Whitehouse Station, NJ,
USA) for 10 min, Scott's solution (Merck & Co, Inc.) for 1 min
and eosin-floxine (Merck & Co, Inc.) for 3 min at room
temperature] sections from each paraffin-embedded, formalin-fixed
block were used to define the diagnostic areas. A total of two
representative 2 mm cores were obtained from each sample and
inserted into a recipient block using a manual tissue arrayer
(Beecher Instruments Inc., Silver Springs MD, USA. A 3–4 µm thick
section was cut from each TMA and stained by H&E, as
aforementioned. They were also subject to antigen retrieval and
antibody staining. The immunoperoxidase stains were performed on
either a Benchmark XT (Ventana Medical Systems, Inc., Tucson, AZ,
USA) using a Cell conditioning solution for antigen unmasking (CC1;
Ventana Medical Systems, Inc.) and Ultraview universal
diaminobenzidine detection kits (Ventana Medical Systems, Inc.) or
an Labvision 720 autostainer (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) using the Envision Flex High pH visualisation
system (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA).
Antibodies used in the present study were as follows: B-cell
lymphoma 2 (Bcl2; clone 124; host, mouse; Dako, Agilent
Technologies, Inc.; cat no. M0887; antigen retrieval using Flex;
1:40; incubated for 30 min at room temperature), Bcl6 (clone, GI
191E/A8; host, mouse; Cell Marque, Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany; cat no. 227M; antigen retrieval using CC1;
1:200; incubation for 60 min at 37°C), CD5 (clone, 4C7; host,
mouse; Novocastra, Leica Microsystems, Inc.; cat no. CD5-4C7-L-CE;
antigen retrieval using CC1; 1:400; incubation for 60 min at 37°C),
CD10 (clone, 56C6; host, mouse; Novocastra, Leica Microsystems,
Inc.; cat no. CD10-270-L-CE; antigen retrieval using CC1; 1:20;
incubation for 60 min at 37°C), CD20 (clone, L26; host, mouse;
Dako, Agilent Technologies, Inc.; cat no. M0755; antigen retrieval
using Flex; 1:50; incubation for 30 min at room temperature),
proliferation marker protein Ki-67 (Ki67; clone, MIB1; host, mouse;
Dako, Agilent Technologies, Inc.; cat no. M7240; antigen retrieval
with CC1; 1:200; incubation for 60 min at 37°C), multiple myeloma
oncogene 1 (MUM1; clone, MUM1p; host, mouse; Dako, Agilent
Technologies, Inc.; cat no. M7259; antigen retrieval using Flex;
1:100; incubation for 30 min at room temperature), Forkhead box
protein P1 (FOXp1; clone, SP133; host, rabbit; Cell Marque,
Sigma-Aldrich, Merck KGaA; cat no. 350R; antigen retrieval with
CC1; 1:200; incubation for 60 min at 37°C) and serpin A9 (GCET1;
clone, RAM 341; host, mouse; Abcam, Cambridge, UK; cat no. ab68889;
antigen retrieval with CC1; incubation for 60 min at 37°C).
Antigens retrieved using CC1 (Bcl6, CD5, CD10, Ki-67
and GCET1) were treated for 88 min at 98°C and FOXp1 was treated
for 56 min at 98°C, then blocked using an OptiView Peroxidase
Inhibitor (Ventana Medical System, Inc.; 3%) at 37°C for 4 min.
Antigens retrieved using the Envision Flex high pH Retrieval
solution were treated for 10 min at 100°C in a microwave and
blocked using Peroxidase-Blocking Solution (Dako; Agilent
Technologies, Inc.) at room temperature for 8 min. All of the
assays were validated using proper negative and positive controls
by use of mutitumor tissue blocks consisting of tonsil, appendix,
liver, melanoma, mantle cell lymphoma and classical Hodgkin's
lymphoma tissue. Results were evaluated using a light microscope
(Olympus BX51) at a ×200 magnification on at least randomly
selected five fields. For each case, the core with a higher
percentage of stained tumor cells was used for the analysis.
Scoring of the antibodies was estimated visually in 10% increments.
The intensity of staining was also evaluated but was not used to
determine positivity as the variability in tissue fixation and
processing appeared to affect the intensity of staining. GCET1 and
FOXp1 were considered positive if ≥80% of the tumor cells were
stained. The MUM1 was considered positive if ≥30% of the tumor
cells were positive for the Hans and modified Hans algorithms and
≥80% for the Choi and modified Choi algorithms. The positive
cut-off for all other antibodies was considered to be 30% (8). The Ki-67 proliferative index was
evaluated as a percentage value calculated by scoring 500 tumor
cell nuclei. The TMA evaluations and the classification of patients
according to the algorithms were all performed by a
hematopathologist (Gorana Gašljević, MD, PhD, Institute of
Oncology, Ljubljana, Slovenia) who was blinded to all clinical
data.
Cytological samples and flow
cytometric quantification of CD19 and CD20 expression
For 39 patients, the cytological sample obtained via
fine needle aspiration prior to the first therapy was available. A
total of 1 skin lesion sample, 1 liver lesion sample, 1 nasal tumor
sample and 36 lymph node samples were obtained for the flow
cytometric immunophenotyping (FCI) analyses. The preparation of
samples for FCI was performed according to the in-house protocol,
previously described by Prevodnik et al (16). The FACSCalibur flow-cytometer and FACS
Canto II flow-cytometer were used (BD Biosciences) and the
CellQuest program version 5.1 (BD Biosciences) was applied for the
analyses. SPHERO Rainbow calibration beads (Spherotech, Inc.,
Illinois, USA) were used for the quantification of the expression
of CD19 and CD20. The beads have been routinely used in our
laboratory for monitoring the stability and linear performance of
the cytometer since 2001 (16). As
these beads and the cytological samples included in the present
study were tested with equal flow-cytometric settings, the beads
were available for use for the quantification of CD19 and CD20
expression. The level of CD19 and C20 expression was determined
with molecules of equivalent soluble fluorochrome using PMT
Linearity QC Record software version RCP-30-5a (Spherotech, Inc.)
(17).
Statistical analysis
The median age, number of treatment cycles, stage at
the time of diagnosis and proliferation marker protein Ki-67
(Ki-67) index were determined. The PFS and OS were estimated using
the Kaplan-Meier method. To compare the response to first-line
treatment, a χ2 test was performed comparing two groups;
CR and PR vs. SD and PD, with the exception of Ki-67, which was
analyzed using logistic regression. Regression models were used to
compare PFS and OS by testing one algorithm individually. The
present study focused on the subgroup of patients who were
differently classified according to the 4 algorithms; this subgroup
was referred to as ‘heterogeneous’ (those who were classified as
GCB by one and as ABC by other algorithms, and vice versa) and it
was observed which of the 4 algorithms was best in identifying the
patients in this subgroup that actually have the same OS as the
subgroup unanimously classified as the GCB by all the four
algorithms. For example, the algorithm that will have the HR of
this subgroup as close to 1 as possible. The Mann-Whitney test was
used for comparing the numeric measurements of CD19 and CD20
expression. P<0.05 was considered to indicate a statistically
significant difference. R Statistical Software (version 3.2.2., R
Foundation for Statistical computing, Vienna, Austria) and the
GraphPad Prism program (version 3.02, GraphPad Software, Inc., La
Jolla, CA, USA) were used for the analyses.
Results
Whole cohort analysis
The patient characteristics are summarized in
Table I. For 5 patients, the response
to first-line treatment was not able to be unequivocally
determined. Relapse was documented in 41 patients (32%) during the
course of follow-up. A total of 52 patients (41%) succumbed: 11
from a non-lymphoma-associated condition (heart failure, myocardial
infarction, road accident, different infections and sepsis) and 3
of unknown causes.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Patient
characteristics | N |
|---|
| Age, median
(range) | 62 (24–84) |
| Sex, n (%) |
|
| Male | 65 (51) |
|
Female | 62 (49) |
| Stage, n (%) |
|
| I | 20 (16) |
| II | 29 (23) |
| III | 34 (27) |
| IV | 44 (35) |
| Disease presentation,
n (%) |
|
| B | 39 (31) |
| X | 29 (23) |
| S | 26 (20) |
| Treatment cycles,
median (range) | 8 (2–10)a |
| Consolidation
radiotherapy | 57 (45) |
| Treatment response, n
(%) |
|
| CR | 103 (81) |
| PR | 8 (6) |
| SD | 1 (1) |
| PD | 10 (8) |
| IPI Group, n
(%) |
|
|
Low | 37 (29) |
| Low
intermediate | 36 (28) |
| High
intermediate | 30 (24) |
|
Highb | 24 (19) |
| Ki-67 expression,
median % (range) | 80 (50–95) |
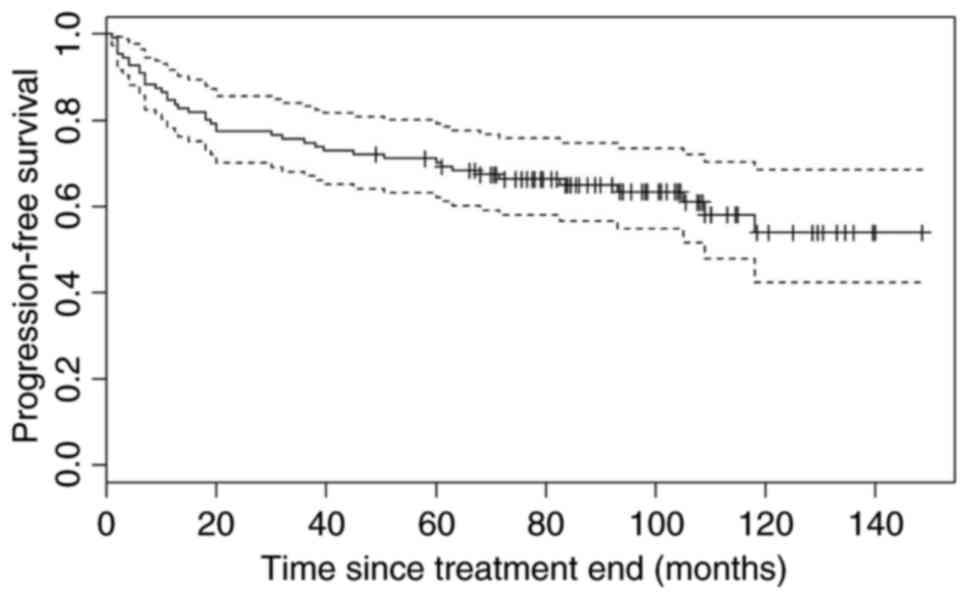
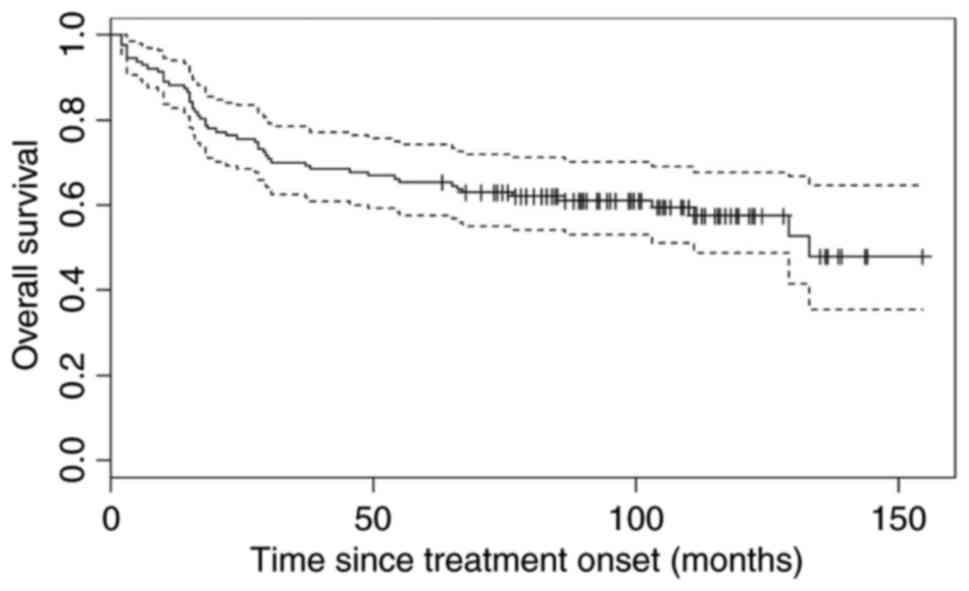
The median PFS was not reached during the course of
the present study [95% confidence interval (CI), 109-not yet
reached) and the median OS was 133 months, 95% CI, 111-not yet
reached (Figs. 1 and 2). The median observation period was 6 years
and 11 months.
Due to relatively small number of cases exhibiting a
bad treatment response (SD or PD), the power of any algorithm to
distinguish between cases, was very low. Original
immunohistochemistry data and flow cytometric data are presented in
Table II.
 | Table II.Original immunohistochemical and flow
cytometric data. |
Table II.
Original immunohistochemical and flow
cytometric data.
| Sample no. | Hans algorithm | Modified Hans
algorithm | Choi algorithm | Modified Choi
algorithm | CD20 | CD19 |
|---|
| 1 | ABC | GCB | ABC | ABC |
|
|
| 2 | GCB | GCB | ABC | ABC |
|
|
| 3 | ABC | ABC | ABC | ABC |
|
|
| 4 | ABC | ABC | ABC | ABC |
|
|
| 5 | GCB | GCB | GCB | GCB |
|
|
| 6 | GCB | GCB | GCB | GCB | 183927 | 19560 |
| 7 | ABC | GCB | ABC | ABC |
|
|
| 8 | ABC | ABC | ABC | ABC | 49558 | 35198 |
| 9 | GCB | GCB | GCB | GCB |
|
|
| 10 | ABC | ABC | ABC | ABC |
|
|
| 11 | GCB | GCB | GCB | ABC | 68587 | 9570 |
| 12 | GCB | GCB | GCB | GCB | 129675 | 6525 |
| 13 | ABC | ABC | ABC | ABC | 75860 | 5571 |
| 14 | GCB | GCB | GCB | GCB |
|
|
| 15 | GCB | GCB | GCB | GCB |
|
|
| 16 | GCB | GCB | GCB | ABC | 169656 | 6498 |
| 17 | GCB | GCB | GCB | ABC | 30038 | 7991 |
| 18 | GCB | GCB | GCB | ABC | 35145 | 12937 |
| 19 | ABC | GCB | ABC | ABC | 74789 | 9174 |
| 20 | ABC | ABC | GCB | ABC |
|
|
| 21 | ABC | GCB | ABC | ABC |
|
|
| 22 | ABC | ABC | ABC | ABC |
|
|
| 23 | ABC | GCB | ABC | ABC |
|
|
| 24 | GCB | GCB | GCB | ABC |
|
|
| 25 | GCB | GCB | GCB | GCB |
|
|
| 26 | GCB | GCB | GCB | GCB |
|
|
| 27 | ABC | ABC | ABC | GCB |
|
|
| 28 | GCB | GCB | GCB | GCB | 150369 | 7713 |
| 29 | GCB | GCB | GCB | ABC | 11070 | 8838 |
| 30 | GCB | GCB | ABC | GCB | 173921 | 34158 |
| 31 | ABC | ABC | ABC | GCB |
|
|
| 32 | GCB | GCB | GCB | GCB | 63821 | 42365 |
| 33 | GCB | GCB | GCB | ABC | 149328 | 50691 |
| 34 | ABC | GCB | ABC | ABC |
|
|
| 35 | ABC | ABC | ABC | ABC | 4460 | 979 |
| 36 | GCB | GCB | GCB | ABC |
|
|
| 37 | GCB | GCB | GCB | GCB | 69712 | 6555 |
| 38 | ABC | ABC | ABC | ABC |
|
|
| 39 | ABC | GCB | ABC | ABC |
|
|
| 40 | ABC | ABC | ABC | GCB |
|
|
| 41 | GCB | GCB | GCB | GCB | 177781 | 14775 |
| 42 | ABC | ABC | ABC | ABC |
|
|
| 43 | ABC | GCB | ABC | ABC |
|
|
| 44 | ABC | ABC | ABC | ABC |
|
|
| 45 | GCB | GCB | GCB | GCB |
|
|
| 46 | ABC | ABC | ABC | ABC | 44264 | 3753 |
| 47 | ABC | ABC | ABC | ABC | 30091 | 2520 |
| 48 | GCB | GCB | GCB | GCB | 55441 | 6266 |
| 49 | ABC | ABC | ABC | ABC |
|
|
| 50 | GCB | GCB | GCB | GCB | 23355 | 2495 |
| 51 | GCB | GCB | GCB | GCB |
|
|
| 52 | ABC | ABC | ABC | ABC |
|
|
| 53 | GCB | GCB | GCB | GCB |
|
|
| 54 | ABC | ABC | ABC | GCB |
|
|
| 55 | GCB | GCB | GCB | GCB |
|
|
| 56 | ABC | ABC | ABC | ABC |
|
|
| 57 | GCB | GCB | GCB | GCB |
|
|
| 58 | GCB | GCB | GCB | GCB | 221029 | 20212 |
| 59 | GCB | GCB | GCB | ABC |
|
|
| 60 | GCB | GCB | ABC | ABC |
|
|
| 61 | GCB | GCB | ABC | ABC |
|
|
| 62 | GCB | GCB | ABC | ABC |
|
|
| 63 | ABC | ABC | GCB | GCB |
|
|
| 64 | ABC | ABC | ABC | GCB |
|
|
| 65 | GCB | GCB | ABC | ABC |
|
|
| 66 | ABC | ABC | ABC | ABC |
|
|
| 67 | GCB | GCB | GCB | GCB |
|
|
| 68 | ABC | GCB | ABC | ABC |
|
|
| 69 | GCB | GCB | GCB | GCB |
|
|
| 70 | GCB | GCB | GCB | GCB |
|
|
| 71 | GCB | GCB | GCB | GCB | 141135 | 22678 |
| 72 | GCB | GCB | ABC | ABC | 42681 | 13042 |
| 73 | GCB | GCB | GCB | GCB | 87892 | 12640 |
| 74 | GCB | GCB | GCB | ABC | 123789 | 5534 |
| 75 | ABC | ABC | GCB | ABC | 24403 | 2203 |
| 76 | GCB | GCB | GCB | ABC | 120392 | 3452 |
| 77 | GCB | GCB | GCB | GCB | 31825 | 10442 |
| 78 | ABC | ABC | ABC | ABC | 56768 | 12963 |
| 79 | GCB | GCB | GCB | GCB | 28606 | 41482 |
| 80 | GCB | GCB | GCB | ABC |
|
|
| 81 | ABC | ABC | ABC | ABC | 106388 | 34158 |
| 82 | GCB | GCB | GCB | GCB |
|
|
| 83 | GCB | GCB | ABC | ABC |
|
|
| 84 | GCB | GCB | ABC | ABC |
|
|
| 85 | ABC | ABC | GCB | GCB |
|
|
| 86 | GCB | GCB | GCB | GCB |
|
|
| 87 | ABC | ABC | ABC | ABC |
|
|
| 88 | ABC | ABC | ABC | ABC |
|
|
| 89 | GCB | GCB | GCB | GCB |
|
|
| 90 | ABC | ABC | ABC | ABC |
|
|
| 91 | ABC | ABC | ABC | ABC | 4474 | 3320 |
| 92 | ABC | ABC | ABC | ABC |
|
|
| 93 | ABC | ABC | ABC | ABC |
|
|
| 94 | GCB | GCB | ABC | ABC |
|
|
| 95 | GCB | GCB | GCB | GCB | 160240 | 37501 |
| 96 | ABC | ABC | ABC | ABC |
|
|
| 97 | GCB | GCB | GCB | GCB |
|
|
| 98 | ABC | ABC | ABC | ABC | 1444 | 6066 |
| 99 | ABC | ABC | ABC | GCB |
|
|
| 100 | ABC | ABC | ABC | ABC | 21125 | 18333 |
| 101 | GCB | GCB | GCB | ABC |
|
|
| 102 | GCB | GCB | GCB | GCB |
|
|
| 103 | ABC | ABC | GCB | ABC |
|
|
| 104 | GCB | GCB | GCB | GCB |
|
|
| 105 | ABC | ABC | ABC | ABC |
|
|
| 106 | GCB | GCB | GCB | GCB |
|
|
| 107 | ABC | ABC | GCB | ABC | 147365 | 21812 |
| 108 | GCB | GCB | GCB | GCB |
|
|
| 109 | GCB | GCB | ABC | ABC |
|
|
| 110 | GCB | GCB | GCB | ABC |
|
|
| 111 | ABC | ABC | ABC | ABC |
|
|
| 112 | ABC | ABC | ABC | ABC |
|
|
| 113 | GCB | GCB | GCB | GCB |
|
|
| 114 | GCB | GCB | GCB | GCB |
|
|
| 115 | GCB | GCB | GCB | ABC |
|
|
| 116 | GCB | GCB | GCB | ABC |
|
|
| 117 | GCB | GCB | GCB | ABC |
|
|
| 118 | GCB | GCB | GCB | ABC |
|
|
| 119 | GCB | GCB | GCB | GCB |
|
|
| 120 | ABC | ABC | ABC | GCB |
|
|
| 121 | ABC | GCB | GCB | / |
|
|
| 122 | / | GCB | GCB | GCB |
|
|
| 123 | GCB | GCB | ABC | ABC | 2960 | 664 |
| 124 | GCB | GCB | GCB | GCB |
|
|
| 125 | ABC | ABC | ABC | ABC |
|
|
| 126 | ABC | ABC | ABC | ABC |
|
|
| 127 | ABC | ABC | ABC | GCB | 299806 | 24765 |
Hans algorithm
Patients with the ABC subtype (56 patients) did not
exhibit an inferior response to first-line treatment (P=0.345) when
compared with the GCB type (70 patients). The difference in OS was
not significant [P=0.083; hazard ratio (HR)=1.61 (95% CI,
0.36–1.07)], and PFS was not significantly different among patients
with the ABC and GCB subtypes of DLBCL [P=0.339; HR=1.3 (95% CI,
0.42–1.41)].
Modified Hans algorithm
Patients with the ABC subtype (46 patients) did not
exhibit an inferior response to first-line treatment (P=0.543) when
compared with the GCB type (81 patients). There was no difference
observed in the OS [P=0.282; HR=1.35 (95% CI, 0.43–1.28)] or PFS
[P=0.632; HR=1.17 (95% CI, 0.46–1.60)] between the two
subtypes.
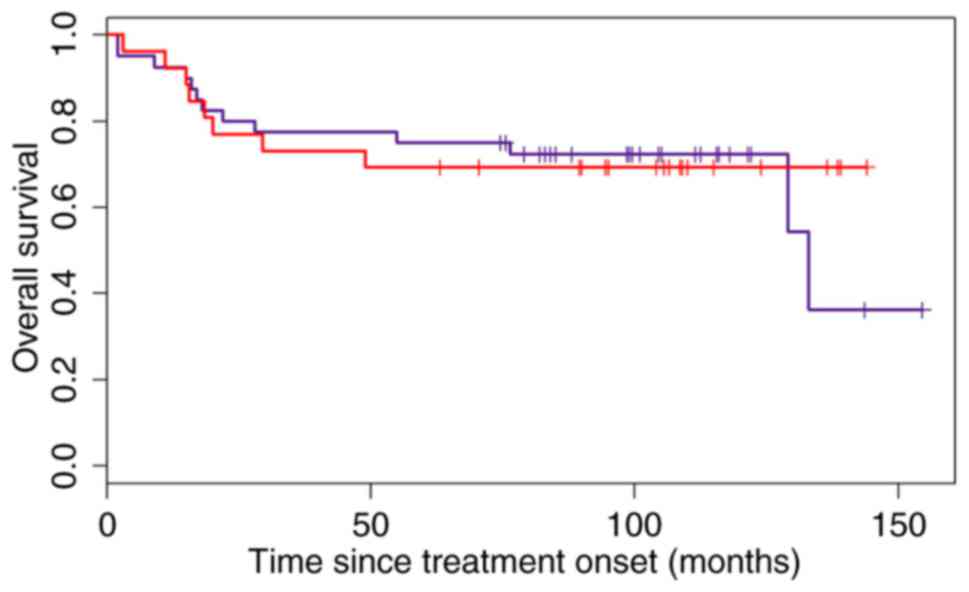
Choi algorithm
There was no difference in response to front-line
treatment between the ABC (61 patients) and the GCB subtype (66
patients) (P=0.116). In the ABC subtype, the OS was significantly
shorter [5-year OS, 73% in the GCB subtype and 5-year OS, 57% in
the ABC subtype; P=0.019, HR=1.91 (95% CI, 0.30–0.91)]; the PFS was
also shorter (5-year PFS in the GCB subtype, 77% and the 5-year PFS
in the ABC subtype, 63%; HR=1.56, 95% CI, 0.35–1.18), but the
difference was not statistically significant (P=0.151).
Modified Choi algorithm
In the ABC subtype (74 patients), the response to
front-line treatment did not differ when compared to the GCB
subtype (52 patients; P=0.364). The difference in the OS was not
significant [P=0.047; HR=1.8 (95% CI, 0.31–1.00)], but no
statistical difference was identified between the PFS in the two
groups [P=0.903; HR=1.75 (95% CI, 0.29–1.10)].
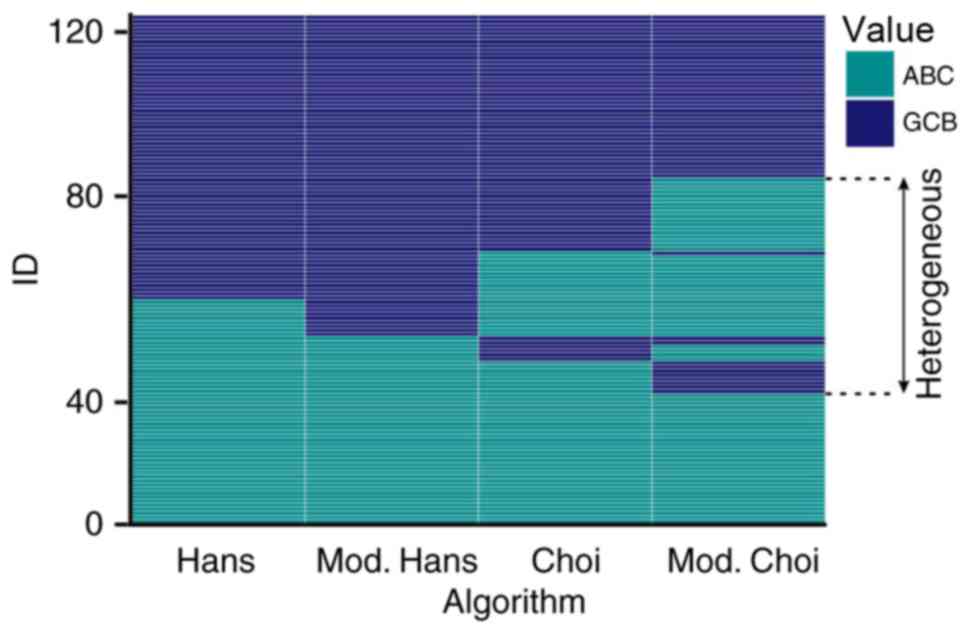
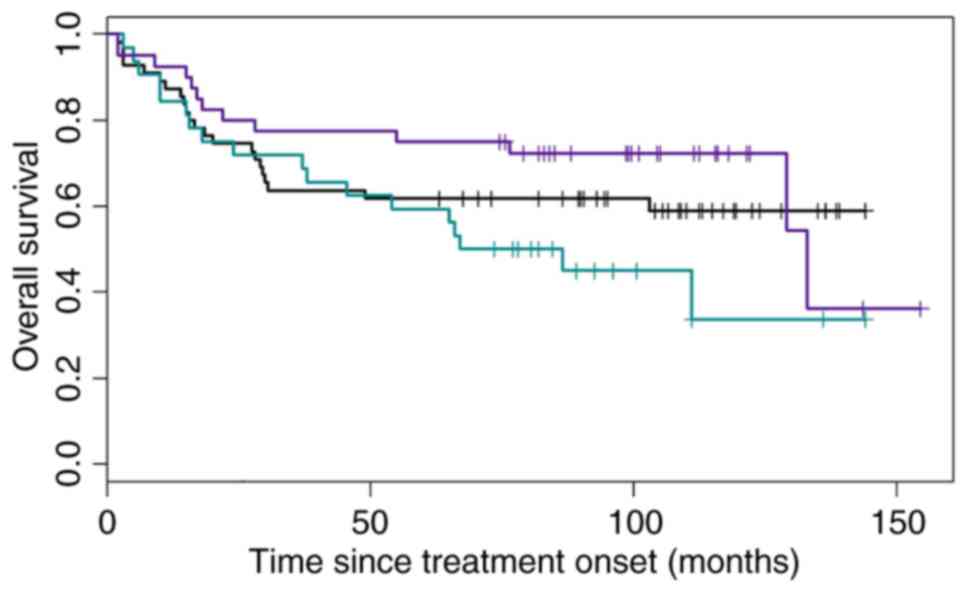
According to the results of all 4 algorithms,
patients were subdivided into three categories, as previously
described above. These are indicated in Fig. 3, and have been divided as such: When
all of the algorithms classified patients as having the GCB type;
when all of the algorithms classified the patients as having the
ABC type; and ‘heterogeneous’ types. The OS of the three groups is
presented in Fig. 4.
Furthermore, the accuracy of the Choi and modified
Choi algorithms (as they presented a difference in OS between ABC
and GCB subgroups) in classifying the ‘heterogeneous’ subgroup into
the GCB subtype according to the OS, presuming that these patients
have the same risk as the patients in the GCB subgroup and an HR
that was as close to 1 as possible, was examined. The HR for the
Choi algorithm in the whole cohort was 0.91, but the confidence
interval was large (0.38–2.21; Fig.
5).
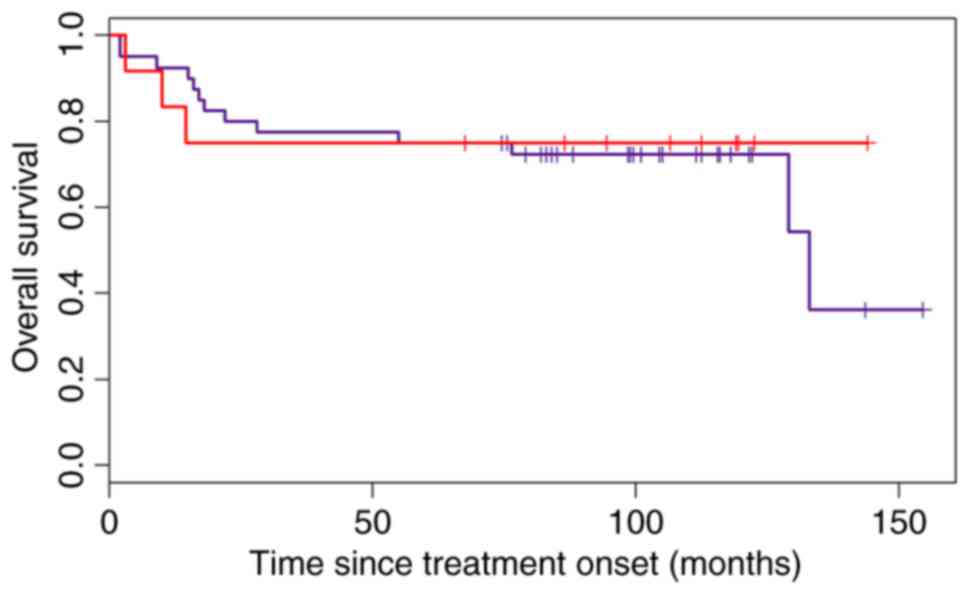
The modified Choi algorithm was not as successful in
classifying the ‘heterogeneous’ subgroup into the GCB subtype as
the Choi algorithm with an HR=0.82 (95% CI 0.23–2.88; Fig. 6).
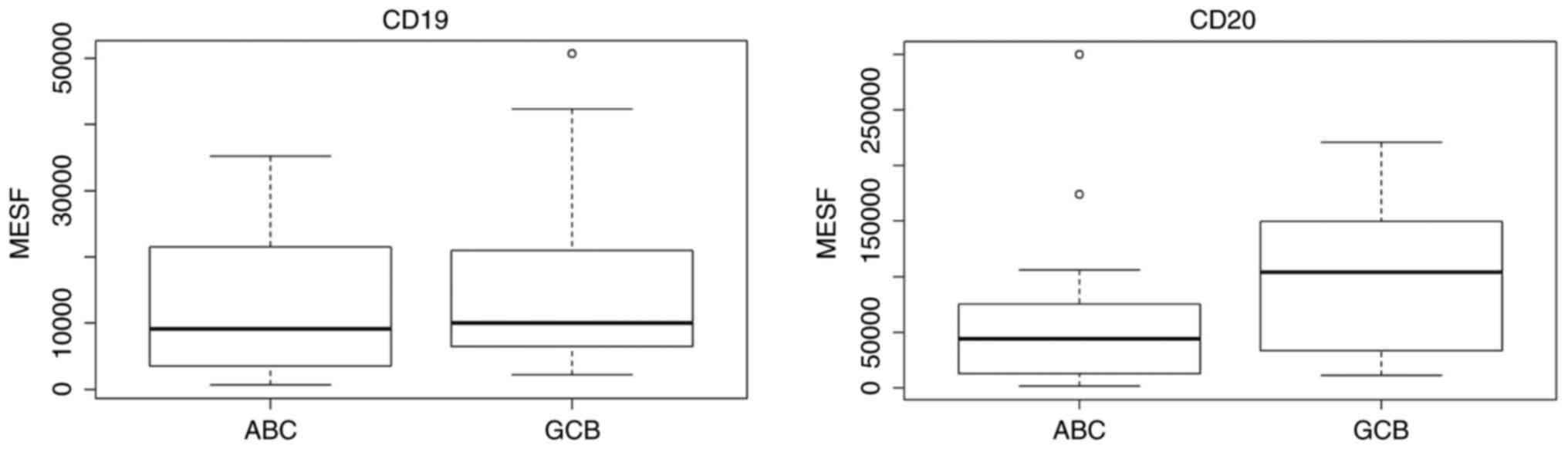
For the CD19 and CD20 expression studies, patients
were divided into the GCB and ABC groups according to the Choi
algorithm, as it was identified to be the most accurate in our
analyses. The results are demonstrated in Fig. 7. There was no difference in the CD19
expression (P=0.427) observed, but the CD20 expression was lower in
the ABC subtype (P=0.058) compared with the GCB subtype.
IPI
A group of patients with an IPI <2 (low risk
patients) were compared with those with an IPI ≥2 (all other risk
groups). The response (CR+PR) to first-line treatment was observed
in 97.3% of patients with an IPI<2, and in 86.2% of those with
an IPI ≥2, which was not significant (P=0.065). Patients with an
IPI ≥2 had a higher risk of progression, but it was not
statistically significant [P=0.161; HR=1.83 (95% CI, 0.7–4.25)].
Patients with an IPI ≥2 exhibited a higher risk of
lymphoma-associated mortality [P=0.032; HR=2.59 (95% CI,
1.09–6.19)]. The prognosis for OS according to the IPI was also
significant when assessing the risk of mortality by any cause
[P=0.018; HR=2.36 (95% CI, 1.15–4.85)].
Ki-67
A higher expression of Ki-67 had no effect on the
response to first-line treatment (P=0.130). There was also no
difference in the PFS or OS regarding the Ki-67 expression
[P=0.815; HR (for a 10% difference)=1.04 (95% CI, 0.73–1.5)] and
[P=0.164; HR (for a 10% difference)=1.28 (95% CI 0.91–1.8)],
respectively.
Discussion
When comparing the 4 selected immunohistochemical
algorithms (Hans, Choi, modified Hans and modified Choi) in the
present study, only the Choi algorithm was identified to be
significantly prognostic of OS. The modified Choi algorithm was
close to significance in terms of OS, while the modified Hans and
Hans algorithm were the least reliable in discriminating between
the two groups in the cohort of the present study. The Choi
algorithm was also quite accurate in predicting the GCB subtype,
but with a large confidence interval, therefore, it is not accurate
to state that it is precise enough to classify the GCB subtype
definitely. The modified Choi algorithm was less accurate in our
series in terms of predicting the GCB subtype compared with the
Choi algorithm. However, the overall treatment response was very
high, so statistical power of this subdivision is low.
The present study focused on the determination of
the GCB subtype due to the existence of novel studies with novel
drugs included in the front-line treatment of the ABC subtype
(11–14). Therefore, a patient classified as
having the ABC subtype of DLBCL is supposed to be treated more
aggressively and with novel drugs, while one classified as
exhibiting the GCB type will receive the standard R-CHOP regimen.
Therefore, the primary concern of the present study was the
potential insufficient treatment of future patients who may be
classified as GCB, but were actually the ABC subtype.
As the revised WHO lymphoma classification (10) advises the use of the Hans algorithm,
the results of the present study may provide an interesting
contrast. At present, there have been a number of studies
suggesting that the Hans algorithm does not have prognostic value
in terms of OS (18–21). A study by Meyer et al (9) also concluded that the Choi algorithm had
the highest predictive power when compared with gene-expression
profiling and in terms of OS, but that it is the least
user-friendly as it includes 2 antibodies (GCET1 and FOXP1) not
routinely applied in a number of laboratories. This study also
suggested the use of Tally's algorithm for prognostic purposes, as
it was identified to have the ability to predict OS slightly less
accurately than Choi (9). The present
study did not assess the Tally algorithm, as it includes LMO2
antibody staining, which was not performed as it is not routinely
performed at the Institute of Oncology Ljubljana, Slovenia for
diffuse large B-cell lymphoma.
To the best of our knowledge, there has been no FCI
quantification of the CD19 and CD20 expression using the
quantitative measurements of fluorescence intensity on the ABC or
GCB subtype of DLBCL. At present, the majority of studies applied
the immunohistochemical CD20 staining, while Johnson et al
(22) used the semi-quantitative
evaluation of CD19 and CD20 expression in mean fluorescence
intensity units. They subdivided their study population to the
‘bright’ and ‘dim’ subgroups of CD19 and CD20 expression. They
categorized patients with dim CD20 expression and bright CD19
expression into a ‘discordant CD20 group’. The authors also noticed
that a high proportion of these ‘discordant’ patients exhibited the
ABC subtype, and stated that the cell-of-origin may be a
confounding factor in the prognostic effect of the discordant CD20
expression. However, they did not evaluate the CD19 and CD20
expression quantitatively. The ABC subtype exhibited a lower
expression of CD20 when compared with the GCB subtype in the sample
cohort of the present study, and additional studies are required to
confirm this result.
The present study confirmed the prognostic value of
IPI in the group of patients regarding the OS, but not the PFS.
Ziepert et al (3) clearly
demonstrated that IPI is a prognostic factor of PFS, EFS and OS.
Certain other studies also confirmed these data (20,23).
The Ki-67 index was identified not to be prognostic
in any aspect, which is inconsistent with the results of several
larger studies (20,24) and meta-analyses (25). Yoon et al (24) set a cut-off level of 85%, and Salles
et al (20) at 75%, to
differentiate between the two subgroups (lower vs. higher
expression) with a different OS. In the largest meta-analysis in
this field of study by He et al (25), the cut-off level was not set, but
simply confirmed that a higher Ki-67 expression was associated with
inferior survival. The present study observed a trend of shorter
survival associated with higher Ki-67 values, but the association
was not significant.
Based on the results of the present study, it may be
concluded that only the Choi algorithm significantly predicts OS in
the ABC and GCB subgroups. The Choi algorithm also appeared to be
quite accurate in defining the GCB subtype according to the OS, but
additional studies with larger cohorts of patients are required. A
lower expression of CD20 was observed in the ABC subtype. The IPI
was confirmed to be prognostic for OS, but the Ki-67 index was not
identified to be prognostic for the OS, PFS or response rate.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ministry of
Science and Technology, Slovenia (grant no. P3-0321).
Availability of data and materials
The datasets generated and analyzed in the present
study are stored at the authors' institution (Institute of Oncology
Ljubljana, Slovenia) and may be obtained upon reasonable request
from the corresponding author.
Authors' contributions
LB wrote the manuscript and gathered the clinical
data, VKP gathered the cytological data and revised the manuscript,
MPP performed the statistical analyses and revised the manuscript,
GG performed the pathology work and revised the manuscript, BJN
provided the design of the study, clinical data and revised the
manuscript.
Ethics and consent to participate
All participants provided written informed consent
for participation in the present study. The present study was
approved by the Republic of Slovenia National Medical Ethics
Committee.
Consent for publication
All participants provided informed consent for the
publication of this data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tilly H, da Silva Gomes M, Vitolo U, Jack
A, Meignan M, Lopez-Guillermo A, Walewski J, André M, Johnson PW,
Pfreundschuh M, et al: Diffuse large B-cell lymphoma: ESMO clinical
practice guidelines, treatment and follow-up. Ann Oncol. 26 Suppl
5:v116–v125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
International non-Hodgkin's lymphoma
prognostic factors project: A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ziepert M, Hasenclever D, Kuhnt E, Glass
B, Schmitz N, Pfreundschuh M and Loeffler M: Standard International
prognostic index remains a valid predictor of outcome for patients
with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin
Oncol. 28:2373–2380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ngo L, Hee SW, Lim LC, Tao M, Quek R, Yap
SP, Loong EL, Sng I, Hwan-Cheong TL, Ang MK, et al: Prognostic
factors in patients with diffuse large B cell lymphoma: Before and
after the introduction of rituximab. Leuk Lymphoma. 49:462–469.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sehn LH, Berry B, Chhanabhai M, Fitzgerald
C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J,
et al: The revised international prognostic index (R-IPI) is a
better predictor of outcome than the standard IPI for patients with
diffuse large B-cell lymphoma treated with R-CHOP. Blood.
109:1857–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi WW, Weisenburger DD, Greiner TC,
Piris MA, Banham AH, Delabie J, Braziel RM, Geng H, Iqbal J, Lenz
G, et al: A new immunostain algorithm classifies diffuse large
B-cell lymphoma into molecular subtypes with high accuracy. Clin
Cancer Res. 15:5494–5502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meyer PN, Fu K, Greiner TC, Smith LM,
Delabie J, Gascoyne RD, Ott G, Rosenwald A, Braziel RM, Campo E, et
al: Immunohistochemical methods for predicting cell of origin and
survival in patients with diffuse large B-Cell lymphoma treated
with rituximab. J Clin Oncol. 29:200–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Molina TJ, Canioni D, Copie-Bergman C,
Recher C, Brière J, Haioun C, Berger F, Fermé C, Copin MC,
Casasnovas O, et al: Young patients with non-germinal center
B-cell-like diffuse large B-cell lymphoma benefit from intensified
chemotherapy with ACVBP plus rituximab compared with CHOP plus
rituximab: Analysis of data from the groupe d'Etudes des lymphomes
de l'Adulte/lymphoma study association phase III trial LNH 03-2B. J
Clin Oncol. 32:3996–4003. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ruan J, Martin P, Furman RR, Lee SM,
Cheung K, Vose JM, Lacasce A, Morrison J, Elstrom R, Ely S, et al:
Bortezomib plus CHOP-rituximab for previously untreated diffuse
large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol.
29:690–697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nowakowski GS, LaPlant B, Macon WR, Reeder
CB, Foran JM, Nelson GD, Thompson CA, Rivera CE, Inwards DJ,
Micallef IN, et al: Lenalidomide combined with R-CHOP overcomes
negative prognostic impact of non-germinal center B-cell phenotype
in newly diagnosed diffuse large B-Cell lymphoma: A phase II study.
J Clin Oncol. 33:251–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Younes A, Thieblemont C, Morschhauser F,
Flinn I, Friedberg JW, Amorim S, Hivert B, Westin J, Vermeulen J,
Bandyopadhyay N, et al: Combination of ibrutinib with rituximab,
cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)
for treatment-naive patients with CD20-positive B-cell non-Hodgkin
lymphoma: A non-randomised, phase 1b study. Lancet Oncol.
15:1019–1026. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E and Lister TA: Alliance,
Australasian Leukaemia and Lymphoma Group; Eastern Cooperative
Oncology Group: Recommendations for initial evaluation, staging,
and response assessment of Hodgkin and non-Hodgkin lymphoma: The
Lugano classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prevodnik VK, Lavrenčak J, Horvat M and
Novakovič BJ: The predictive significance of CD20 expression in
B-cell lymphomas. Diagn Pathol. 6:332011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwartz A, Gaigalas AK, Wang L, Marti GE,
Vogt RF and Fernandez-Repollet E: Formalization of the MESF unit of
fluorescence intensity. Cytometry B Clin Cytom. 57:1–6. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benesova K, Forsterova K, Votavova H,
Campr V, Stritesky J, Velenska Z, Prochazka B, Pytlik R and Trneny
M: The Hans algorithm failed to predict outcome in patients with
diffuse large B-cell lymphoma treated with rituximab. Neoplasma.
60:68–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar A, Lunning MA, Zhang Z, Migliacci
JC, Moskowitz CH and Zelenetz AD: Excellent outcomes and lack of
prognostic impact of cell of origin for localized diffuse large
B-cell lymphoma in the rituximab era. Br J Haematol. 171:776–783.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salles G, de Jong D, Xie W, Rosenwald A,
Chhanabhai M, Gaulard P, Klapper W, Calaminici M, Sander B, Thorns
C, et al: Prognostic significance of immunohistochemical biomarkers
in diffuse large B-cell lymphoma: A study from the lunenburg
lymphoma biomarker consortium. Blood. 117:7070–7078. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castillo JJ, Beltran BE, Song MK, Ilic I,
Leppa S, Nurmi H, Seki R, Uccella S, Li JM, Treaba DO, et al: The
Hans algorithm is not prognostic in patients with diffuse large
B-cell lymphoma treated with R-CHOP. Leuk Res. 36:413–417. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnson NA, Boyle M, Bashashati A, Leach
S, Brooks-Wilson A, Sehn LH, Chhanabhai M, Brinkman RR, Connors JM,
Weng AP and Gascoyne RD: Diffuse large B-cell lymphoma: Reduced
CD20 expression is associated with inferior survival. Blood.
113:3773–3780. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu K, Weisenburger DD, Choi WW, Perry KD,
Smith LM, Shi X, Hans CP, Greiner TC, Bierman PJ, Bociek RG, et al:
Addition of rituximab to standard chemotherapy improves the
survival of both the germinal center B-cell-like and non-germinal
center B-cell-like subtypes of diffuse large B-cell lymphoma. J
Clin Oncol. 26:4587–4594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoon DH, Choi DR, Ahn HJ, Kim S, Lee DH,
Kim SW, Park BH, Yoon SO, Huh J, Lee SW and Suh C: Ki-67 expression
as a prognostic factor in diffuse large B-cell lymphoma patients
treated with rituximab plus CHOP. Eur J Haematol. 85:149–157.
2010.PubMed/NCBI
|
|
25
|
He X, Chen Z, Fu T, Jin X, Yu T, Liang Y,
Zhao X and Huang L: Ki-67 is a valuable prognostic predictor of
lymphoma but its utility varies in lymphoma subtypes: Evidence from
a systematic meta-analysis. BMC Cancer. 14:1532014. View Article : Google Scholar : PubMed/NCBI
|