Introduction
An increased rate of aerobic glycolysis followed by
lactate fermentation, named the Warburg Effect, is a hallmark of
cancer cells, and it assists cancer cells to survive despite an
insufficient oxygen supply in the tumor mass due to a disordered
blood vessel system (1). Glycolysis
does not produce extensive energy and building blocks alone in
order to support the survival and proliferation of cancer cells.
However, intermediates of glycolysis involved in certain
biochemical processes provide the nutrients for the rapid
proliferation of cancer cells. For example, glucose-6-phosphate
participates in the pentose phosphate pathway to generate NADPH, an
important antioxidant defense product, in addition to
ribose-5-phosphate and erythrose 4-phosphate to assist with the
synthesis of fatty acids, nucleotides and aromatic amino acids in
cells (2,3). Furthermore, glucose-6-phosphate supports
the generation of glycogen (2).
Dihydroxyacetone phosphate participates in triacylglyceride and
phospholipid synthesis (3). Pyruvate
participating in the citric acid cycle is responsible for the
production of ATP, isoprenoids, cholesterol and fatty acids
(2). As the end product of
glycolysis, lactate produces an acidic intercellular
microenvironment surrounding a tumor, which favors tumor invasion
(4).
Previous studies have demonstrated that suppressing
the expression and activity of glycolytic enzymes is efficient in
reducing the glycolytic rate and inhibiting the development of
cancer (1–3). Hypoxia-inducible factor (HIF), a
transcriptional complex overexpressed and activated in cancer
cells, induces the expression of glycolytic enzymes (5). Inhibiting HIF expression and activity
reduced aerobic glycolysis in cancer cells, which may contribute to
the inhibition of survival and growth of cancer cells (5). A number of HIF inhibitors have been
under clinical and preclinical development for use in cancer
treatment (6). In addition to the
indirect inhibition of glycolysis by targeting HIF, direct
inhibition of glycolysis was developed as novel anticancer
treatment (1,2). RNA interference and chemical inhibitors
of glucose and lactate transporters reduced the glycolysis rate by
inducing the decrease of glycolysis precursors and the increase of
glycolysis products (1,2,7,8). Reducing the expression of glycolytic
enzymes using small interfering RNA and targeting associated
microRNAs additionally downregulated the glycolytic pathway and
suppressed the growth of various cancer types (7,8). Chemical
inhibitors targeting glycolytic enzymes have been developed with
promising translational applications in treating cancer (2). A number of these inhibitors which
exhibit a potent efficiency in suppressing cancer have been used in
clinical and pre-clinical trials, including hexokinase inhibitors
2-deoxyglucose, 3-bromopyruvate and lonidamine, phosphofructose
kinase inhibitor 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one and
lactate dehydrogenase (LDH) inhibitor FX11 (2).
Previously, it has been revealed that
naphthaquinones including shikonin, vitamin K3 and
vitamin K5 are efficient inhibitors of glycolysis
(9,10). Inhibition of the rate of glycolysis is
toxic to cancer cells (11). Pyruvate
kinase M2 (PKM2) was demonstrated to be a target responsible for
the inhibited glycolytic rate in previous studies (9–12).
Replacing PKM2 with PKM1 partially reduced the death of cancer
cells induced by naphthaquinones with increased pyruvate kinase
activity (10). As the inhibition of
glycolysis by naphthaquinones was only partially due to the
suppressed activity of PKM2, it was hypothesized in the present
study that shikonin, vitamin K3 and vitamin
K5 may be able to interrupt the activity of other
glycolytic enzymes in addition to PKM2. In the present study, the
enzyme profile inhibited by shikonin, vitamin K3 and
vitamin K5 in the pathway from glucose to lactate was
screened in order to determine whether there were other targets
involved in the inhibition of glycolysis in cancer cells aside from
PKM2.
Materials and methods
Reagents
RPMI 1640, fetal calf serum,
penicillin-streptomycin, trypsin and HEPES were purchased from
Gibco, Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Mammalian
protein extraction reagent containing protease inhibitor cocktail
kit and the BCA protein assay kit were from Thermo Fisher
Scientific, Inc. Shikonin was purchased from the Tokyo Chemistry
Industry Co., Ltd. (Tokyo, Japan). Vitamin K3 was
purchased from Sigma-Aldrich, Merck KGaA (Darmstadt, Germany).
Vitamin K5 was purchased from Wako Pure Chemical
Industries, Ltd. (Okasa, Japan). Triphosphopyridine nucleotide
(NADP+), nicotinamide adenine dinucleotide
(NDA+), nicotinamide adenine dinucleotide, reduced form
(NADH), adenosine diphosphate (ADP) and phosphoenolpyruvic acid
were purchased from Roche Diagnostics (Basel, Switzerland).
Glucose, fructose-6-phosphate, glyceraldehyde-3-phosphate,
3-phosphoglycerate, 2-phosphoglycerate, adenosine monophosphate
(AMP), adenosine triphosphate (ATP), dimethylsulfoxide (DMSO),
fructose 1,6-bisphosphate, pyruvate, glucose-6-phosphate
dehydrogenase, triose phosphate isomerase (TPI), α-glycerophosphate
dehydrogenase, aldolase, glyceraldehyde phosphate dehydrogenase,
enolase and LDH were from Sigma-Aldrich Merck KGaA. All enzymes
used in the enzyme-coupled assays were quantified with their
activity (unit). One unit enzyme converts 1 mol substrates into
products in 1 min at the temperature and PH as indicated in the
following protocols.
Cell cultures
Breast cancer cell line MCF-7 was purchased from the
Type Culture Collection of the Chinese Academy of Sciences Cell
Bank (Shanghai, China), and were maintained in RPMI 1640 containing
10% fetal calf serum and 100 U/ml penicillin-streptomycin. Cells
were grown in a humidified CO2 incubator at 37°C, and
subcultured with 0.25% trypsin containing 0.02% EDTA, as previously
described (10).
Preparation of cell extract
MCF-7 cells were trypsinized and collected, and then
were lysed using a mammalian protein extraction reagent containing
protease inhibitor cocktail. Following centrifugation at 17,000 ×
g, 4°C for 15 min, and the protein content in the supernatant was
determined using a BCA protein assay kit according to the
manufacturer's protocol.
Measurement of intracellular
accumulation of shikonin
A total of 1×106 MCF-7 cells were plated
in each well of a 6-well plate. Subsequent to attachment, the cells
were incubated with 1, 5, 10 and 25 µM shikonin or 1/1,000 (v/v)
DMSO at 37°C for 0, 1, 3 or 4 h. Cells were washed rapidly using
ice-cold PBS three times, and intracellular shikonin was extracted
using 500 µl ethanol. Cellular debris was spun at 17,000 × g at 4°C
for 5 min. The supernatant was then collected for high performance
liquid chromatography (HPLC).
The HPLC experiment was conducted using a Series
1100 from Hewlett Packard (Palo Alto, CA, USA). An ODS Hypersil C18
column (5 µm, 125×4 mm; Hewlett Packard) was used at 30°C. Solvent
A [H2O and methanol (95:5, v/v) with 0.1%
trifluoroacetic acid], solvent B [H2O and methanol
(5:95, v/v) with 0.1% trifluoroacetic acid] were used, and 0.1 ml
sample was loaded. The quantitative determination of shikonin was
achieved by a gradient elution starting from a 50 to 100% solvent B
in 15 min with a flow rate of 1 ml/min, detected at wavelength 515
nm, at 30°C.
The intracellular concentration of shikonin was
calculated based on the value of the measured shikonin content in
one million cells, and converted to a micromolar value. The average
cell volume is 6.024×10−6 µl, which is calculated on the
basis of the diameter (measured microscopically) of >500
cells.
Hexokinase activity assay
Hexokinase activity of the cell extract was measured
using a glucose-6-phosphate dehydrogenase coupled assay. A total of
200 ng/µl cell extract were incubated with naphthaquinones
(shikonin, vitamin K3 and vitamin K5) at
concentrations of 5, 10, 20, 25, 40 or100 µM for 1 h at 25°C. The
reaction solution contained 50 mM HEPES (pH 7.5), 5 mM
MgCl2, 0.1 M glucose, 0.5 mM ATP, 0.2 mM
NADP+ and 1 unit glucose-6-phosphate dehydrogenase per
ml reaction solution. Relative hexokinase activity was calculated
at 25°C by comparing the changes of absorbance at 340 nm between
the vehicle controls (200 ng/µl cell extract treated with 0.1%
(v/v) DMSO) and naphthaquinone incubated groups once cell extract
added to the reaction solution, as previously described (13,14).
Phosphoglucose isomerase activity
assay
Phosphoglucose isomerase activity of the cell
extract was measured using a glucose-6-phosphate dehydrogenase
coupled assay. A total of 200 ng/µl cell extract was incubated with
1 mM naphthaquinone (shikonin, vitamin K3 and vitamin
K5) for 1 h at 25°C. The reaction solution contained 50
mM HEPES (pH 7.5), 5 mM MgCl2, 0.2 mM NADP+,
2 mM fructose-6-phosphate and 1 unit glucose-6-phosphate
dehydrogenase per ml reaction solution. Relative phosphoglucose
isomerase activity was calculated at 25°C by comparing the change
in absorbance at 340 nm between the vehicle controls [200 ng/µl
cell extract treated with 1% (v/v) DMSO] and naphthaquinone
incubated groups once the cell extract was added to the reaction
solution, as previously described (15).
Phosphofructokinase-1 (PFK-1) activity
assay
PFK-1 activity of the cell extract was measured
using a three-enzyme-coupled assay. A total of 200 ng/µl cell
extract was incubated with naphthaquinones (shikonin, vitamin
K3 and vitamin K5) with concentrations of 5,
20, 50, 100, 500 or 1,000 µM for 1 h at 25°C. The reaction solution
contained 50 mM HEPES (pH 7.5), 100 mM KCl, 5 mM MgCl2,
5 mM Na2HPO4, 1 mM NH4Cl, 0.1 mM
AMP, 0.2 mM NADH, 5 mM fructose-6-phosphate, 2 mM ATP, 5 units TPI
per ml reaction solution, 1 unit adolase per ml reaction solution
and 1 unit glycerol-3-phosphate dehydrogenate per ml reaction
solution. Relative PFK-1 activity was calculated at 25°C by
comparing the change in absorbance at 340 nm between vehicle
controls [200 ng/µl cell extract treated with 1% (v/v) DMSO] and
naphthaquinone incubated groups once the cell extract was added to
the reaction solution, as previously described (13,16,17).
Fructose bisphosphate aldolase
activity assay
Fructose bisphosphate aldolase activity of the cell
extract was measured using a two-enzyme-coupled assay. A total of
200 ng/µl cell extract was incubated with naphthaquinones
(shikonin, vitamin K3 and vitamin K5) with
concentrations of 10, 100, 200, 500 or 1,000 µM for 1 h at 25°C.
The reaction solution contained 50 mM HEPES (pH 7.5), 1 mM
fructose-1,6-biphosphate, 0.2 mM NADH, 1 unit glycerol-3-phosphate
dehydrogenate per ml reaction solution and 5 units TPI per ml
reaction solution. Relative fructose bisphosphate aldolase activity
was calculated at 25°C by comparing the change in absorbance at 340
nm between vehicle controls [200 ng/µl cell extract treated with 1%
(v/v) DMSO] and naphthaquinone incubated groups once the cell
extract was added to the reaction solution, as previously described
(18).
TPI activity assay
TPI activity of the cell extract was measured using
a glycerol-3-phosphate dehydrogenate coupled assay. A total of 200
ng/µl cell extract was incubated with naphthaquinone (shikonin,
vitamin K3 and vitamin K5) with
concentrations of 1 mM for 1 h at 25°C. The reaction solution
contained 50 mM HEPES (pH 7.5), 0.5 mM EDTA, 0.2 mM NADH, 1 mM
glyceraldehyde-3-phosphate and 1 unit glycerol-3-phosphate
dehydrogenate per ml reaction solution. Relative TPI activity was
calculated at 25°C by comparing the change in absorbance at 340 nm
between vehicle controls [200 ng/µl cell extract treated with 1%
(v/v) DMSO] and naphthaquinone incubated groups once the cell
extract was added to the reaction solution, as previously described
(19).
Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) activity assay
GAPDH activity of the cell extract was measured
using a one-step assay from D-glyceraldehyde-3-phosphate to
D-1,3-biphosphateglycerate. A total of 200 ng/µl cell extract was
incubated with naphthaquinones (shikonin, vitamin K3 and
vitamin K5) with concentrations of 10, 100, 200, 500 or
1,000 µM for 1 h at 25°C. The reaction solution contained 50 mM
HEPES (pH 7.5), 0.2 mM EDTA, 1 mM NAD+, 50 mM
Na2HPO4 and 1 mM glyceraldehyde-3-phosphate.
Relative glyceraldehydes-3-phosphate dehydrogenase activity was
calculated at 25°C by comparing the change in absorbance at 340 nm
between vehicle controls [200 ng/µl cell extract treated with 1%
(v/v) DMSO] and naphthaquinone incubated groups once the cell
extract was added to the reaction solution, as previously described
(20).
Phosphoglycerate kinase (PGK) activity
assay
PGK activity of the cell extract was measured using
a GAPDH coupled assay. Total of 200 ng/µl cell extract was
incubated using 1 mM naphthaquinone (shikonin, vitamin
K3 and vitamin K5) for 1 h at 25°C. The
reaction solution contained 50 mM HEPES (pH 7.5), 6 mM
MgSO4, 0.2 mM EDTA, 2 mM ATP, 0.2 mM NADH, 2 mM
3-phosphoglycerate and 2 units glyceraldehydes-3-phosphate
dehydrogenase per ml reaction solution. Relative PGK activity was
calculated at 25°C by comparing the change in absorbance at 340 nm
between vehicle controls [200 ng/µl cell extract treated with 1%
(v/v) DMSO] and naphthaquinone incubated groups once the cell
extract was added to the reaction solution, as previously described
(21).
Phosphoglycerate mutase (PGAM)
activity assay
PGAM activity of the cell extract was measured using
an enolase coupled assay. A total of 200 ng/µl cell extract was
incubated with 1 mM naphthaquinones (shikonin, vitamin
K3 and vitamin K5) for 1 h at 25°C. The
reaction solution contained 50 mM HEPES (pH 7.5), 5 mM
MgCl2, 2 mM 3-phosphoglycerate and 0.2 unit enolase per
ml reaction solution. Relative PGAM activity was calculated at 25°C
by comparing the change in absorbance at 240 nm between vehicle
controls [200 ng/µl cell extract treated with 1% (v/v) DMSO] and
naphthaquinone incubated groups once the cell extract was added to
the reaction solution, as previously described (22).
Enolase activity assay
Enolase activity of the cell extract was measured
using a one step assay from 2-phosphateglycerate to
phosphoenolpyruvate. A total of 200 ng/µl cell extract was
incubated with 1 mM naphthaquinones (shikonin, vitamin
K3 and vitamin K5) for 1 h at 25°C. The
reaction solution contained 50 mM HEPES (pH 7.5), 1 mM
MgCl2, 50 mM KCl, 1 mM EDTA and 0.5 mM
2-phosphoglycerate. Relative enolase activity was calculated at
25°C by comparing the change in absorbance at 240 nm between
vehicle controls [200 ng/µl cell extract treated with 1% (v/v)
DMSO] and naphthaquinone incubated groups once the cell extract was
added to the reaction solution, as previously described (23).
Pyruvate kinase activity assay
Pyruvate kinase activity of the cell extract was
measured using a LDH coupled assay. A total of 200 ng/µl cell
extract was incubated with naphthaquinones (shikonin, vitamin
K3 and vitamin K5) at concentrations of 5,
10, 20, 50, 100, 150, 200 or 1,000 µM for 1 h at 25°C. The reaction
solution contained 50 mM HEPES (pH 7.5), 100 mM KCl, 10 mM
MgCl2, 0.2 mM NADH, 2 mM ADP, 2 mM phosphoenolpyruvate
and 8 units LDH per ml reaction solution. Relative pyruvate kinase
activity was calculated at 25°C by comparing the change in
absorbance at 340 nm between vehicle controls [200 ng/µl cell
extract treated with 1% (v/v) DMSO] and naphthaquinones incubated
groups after the cell extract was added to the reaction solution
(9,10).
LDH activity assay
LDH activity of the cell extract was measured using
a one step assay from pyruvate to lactate. A total of 200 ng/µl
cell extract was incubated with 1 mM naphthaquinones (shikonin,
vitamin K3 and vitamin K5) for 1 h at 25°C.
The reaction solution contained 50 mM HEPES (pH 7.5), 0.2 mM NADH
and 2 mM pyruvate. Relative LDH activity was calculated at 25°C by
comparing the change in absorbance at 340 nm between vehicle
controls [200 ng/µl cell extract treated with 1% (v/v) DMSO] and
naphthaquinone incubated groups once the cell extract was added to
the reaction solution, as previously described (9,10).
Statistical analysis
All data were expressed as the mean ± the standard
deviation from at least three independent experiments and analyzed
using a paired Student's t-test by Excel 2007 (Microsoft, Redmond,
Washington, USA). P<0.05 was considered to indicate a
statistically significant difference. The half maximal inhibitory
concentration (IC50) was analyzed using SigmaPlot 10.0
(Alfasoft, London, UK).
Results
Enzyme inhibition assays using cell
lysate may mimic in vivo assays
In one previous study, it was revealed that although
the IC50 of shikonin for recombinant monomeric and
allosteric activated PKM2 were only 0.3 and 0.8 µM, respectively,
the IC50 of shikonin for PKM2 in vivo is ~10 µM
(10), ~30 fold higher (10). Thus, it is worth investigating whether
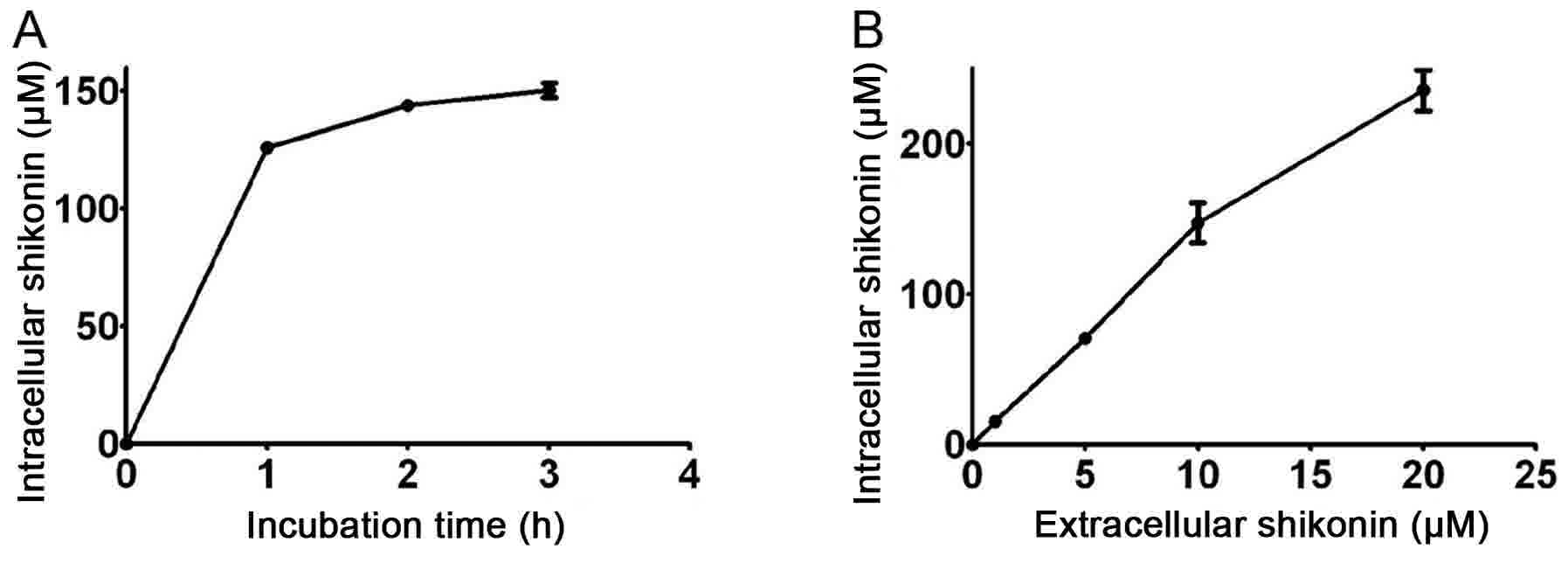
intracellular shikonin may accumulate to a similarly high level. By
incubating cells with 10 µM shikonin, it was revealed that
steady-state shikonin accumulation may be reached ~1 h after
incubation, and MCF-7 cells demonstrated a substantial capacity for
the uptake of shikonin (Fig. 1A).
While incubating the cells for 1 h, shikonin accumulated in the
cells in a dose-dependent manner, and the intracellular
concentration of shikonin was ~14 times higher compared with the
extracellular concentration (Fig.
1B). No further experiments were conducted into why cancer
cells are able to accumulate a high concentration of shikonin and
why the inhibitory efficiency of shikonin on purified recombinant
PKM2 and endogenous PKM2 differed. It may be that shikonin is
sequestered by proteins including PKM2 and that the inhibitory
effect of shikonin on PKM2 may be disrupted by shikonin binding
proteins, PKM2 binding complex and PKM2 allosteric structure in
cells (10,24,25). The
inhibitory efficiency of shikonin on the activity of pyruvate
kinase derived from cell lysate was investigated, and revealed that
12.2 µM shikonin was able to inhibit 50% of the activity of
pyruvate kinase (Table I), which is
similar to the IC50 of shikonin for PKM2 in vivo.
The inhibitory effect of naphthaquinones on enzymes in cell lysate
(Table I) is consistent with that
using intracellular enzymes (10),
and that cell lysate contains factors to retain allosteric and
contextual conditions of enzymes (10,24,25). Thus,
in order to study the influence of shikonin, vitamin K3
and K5 on the activity of endogenous glycolytic enzymes
in the breast cancer cell line MCF-7 and their physiological
effect, it is more appropriate to measure enzymatic activity in
cell extracts as opposed to purified recombinant enzymes.
 | Table I.Concentration of naphthaquiones to
inhibit 50% of the activity of glycolytic enzymes or the activity
of glycolytic enzymes in the presence of 1 mM naphthaqinones. |
Table I.
Concentration of naphthaquiones to
inhibit 50% of the activity of glycolytic enzymes or the activity
of glycolytic enzymes in the presence of 1 mM naphthaqinones.
|
|
IC50 (µM)a |
|---|
|
|
|
|---|
| Naphthaquiones | Hexokinase | Phosphoglucose
isomerase | Phosphofructo
kinase-1 | Fructose
bisphosphate aldolase | Triose phosphate
isomerase |
Glyceraldehyde-3-phosphate
dehydrogenase | Phospho glycerate
kinase | Phospho glycerate
mutase | Enolase | Pyruvate
kinase | Lactate
dehydrogenase |
|---|
| Shikonin |
9.7±0.1b | 96.8±5.4%, 1
mMc |
17.2±0.7b |
158.4±2.3b | n.d.d |
1052.0±10.7b | 89.5±3.7%, 1
mMc | n.d.d | n.d.d |
12.2±0.1b | n.d.d |
| Vitamin
K3 |
16.7±0.2b | 90.4±0.8%, 1
mMc |
1,192.4±4.9b | n.d.d | n.d.d | 82.1±0.6%, 1
mMc | 71.6±2.3%, 1
mMc | n.d.d | n.d.d | 70.5±5.4%, 1
mMc | n.d.d |
| Vitamin K5 |
20.8±0.1b | 86.6±2.6%, 1
mMc |
35.9±0.5b | n.d.d | n.d.d |
462.0±1.8b | 63.3±0.8%, 1
mMc | n.d.d | n.d.d | 73.6±1.4%, 1
mMc | n.d.d |
Shikonin, vitamin K3 and
vitamin K5 inhibited hexokinase activity potently
Glucose is phosphorylated to form
glucose-6-phosphate by hexokinase intracellularly, which is the
first reaction of glycolysis (1–3). In
addition to glycolysis, glucose-6-phosphate serves as the precursor
of the pentose phosphate pathway, glycogenesis and the hexosamine
biosynthetic pathway, which makes hexokinase an important regulator
of anabolism and catabolism in cells (3,26,27). A number of classical inhibitors of
hexokinase have been used in clinical trials to treat patients with
cancer (28,29). In the present study, a
glucose-6-phosphate dehydrogenase couple assay was performed in
order to test whether the inhibition of glycolysis in MCF-7 cells
induced by naphthaquinones was partially caused by the decrease of
hexokinase activity. As presented in Fig.
2A-a, the increase of absorbance at 340 nm demonstrated the
production of NADPH, and shikonin, vitamin K3 and
K5 decreased the production of NADPH, compared with the
vehicle control, revealing the inhibitory effect of naphthaquinones
on the hexokinase activity of the cell extract. Once the inhibition
of hexokinase activity using a series of concentrations of
naphthaquinones was measured, it was revealed that the inhibition
was dose-dependent (Fig. 2A-b), and
the IC50 of shikonin, vitamin K3 and
K5 on the hexokinase activity of MCF-7 cells were 9.7,
16.7 and 20.8 µM respectively (Table
I), whilst 40 µM shikonin, 100 µM vitamin K3 and 100
µM vitamin K5 inhibited >80% hexokinase activity (Fig. 2A-a and b). Shikonin was the most
potent of the three compounds in inhibiting hexokinase, and vitamin
K3 was a significantly more potent hexokinase inhibitor
compared with vitamin K5, as the inhibition induced by
25 µM vitamin K3 was significantly stronger compared
with 25 µM vitamin K5 (P=0.044; Fig. 2A-b). The results suggested that the
three naphthaquinones were potent inhibitors of hexokinase in MCF-7
cells.
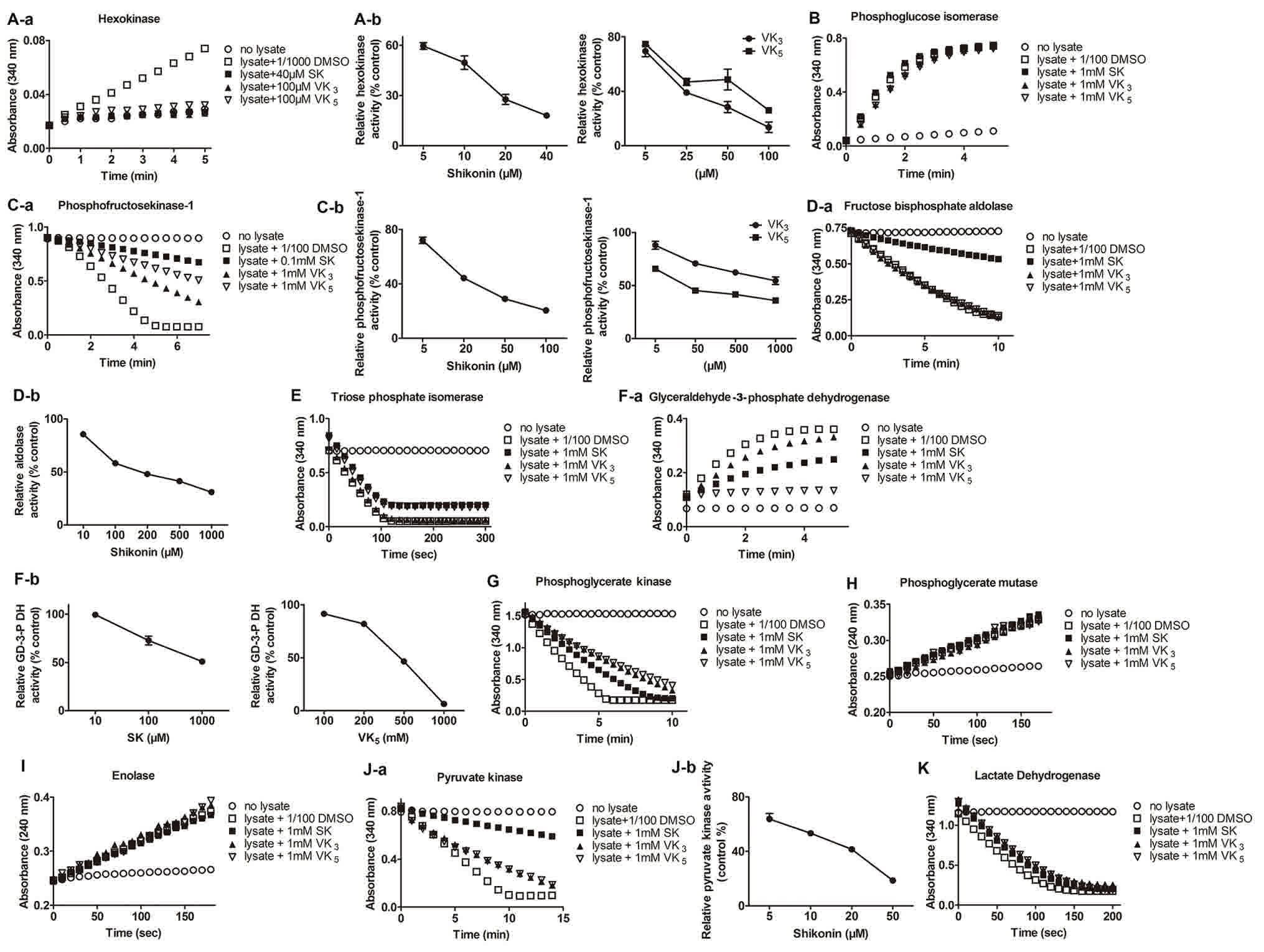 | Figure 2.Activity of glycolytic enzymes in the
absence or presence of naphthaquinones (SK, VK3 and
VK5) in cell lysate of MCF-7. Activity of (A-a and b)
hexokinase, (B) phosphoglucose isomerase, (C-a and b)
phosphofructokinase-1, (D-a and b) fructose bisphophate aldolase,
(E) triose phosphate isomerase, (F-a and b)
glyceraldehyde-3-phosphate dehydrogenase, (G) phosphoglycerate
kinase, (H) phosphoglycerate mutase, (I) enolase, (J-a and b)
pyruvate kinase and (K) lactate dehydrogenase following treatment
with SK, VK3 and VK5. SK, shikonin;
VK3, vitamin K3; VK5, vitamin
K5. *P<0.05 and **P<0.01. GD-3-P,
glyceraldehydes-3-phosphate dehydrogenase. |
Shikonin, vitamin K3 and
vitamin K5 inefficiently inhibit phosphoglucose
isomerase activity
Phosphoglucose isomerase is a housekeeping enzyme
that catalyzes the reversible reaction from glucose-6-phosphate to
fructose-6-phosphate and serves an important function in glycolysis
and glucogenesis (2). Phosphoglucose
isomerase is secreted by tumor cells as a motility factor promoting
the migration, invasion and metastasis of cancer, and its
overexpression in cancer is associated with a poor prognosis
(30,31). Inhibiting the activity of tumor
derived phosphoglucose isomerase has potential applications in the
treatment of cancer (2). In the
present study, the ability of glycolysis inhibitors (shikonin,
vitamin K3 and K5) to serve as inhibitors of
phosphoglucose isomerase was tested. The activity of phosphoglucose
isomerase was measured using a glucose-6-phosphate dehydrogenase
couple assay. The change of absorbance at 340 nm revealed the
reduction of NADP+ demonstrating the activity of
phosphoglucose isomerase (Fig. 2B).
While incubating MCF-7 cell lysate with shikonin, vitamin
K3 and vitamin K5 with a concentration of 1
mM, the highest concentration shikonin, vitamin K3 and
vitamin K5 could reach in the incubation solution,
phosphoglucose isomerase activity decreased very little compared
with DMSO treated cell lysate (Fig.
2B). Additionally, 4.2, 9.6 and 13.4% of phosphoglucose
isomerase activity was inhibited by shikonin, vitamin K3
and K5, respectively (Table
I). These results demonstrate that shikonin, vitamin
K3 and K5 are not efficient inhibitors of
phosphoglucose isomerase.
Shikonin, vitamin K3 and
vitamin K5 inhibited PFK-1 activity efficiently
Another rate-limiting enzyme, PFK-1 phosphorylates
fructose-6-phosphate into fructose-1,6-phosphate (1,3).
Downregulation of PFK-1 may reduce the rate of aerobic glycolysis,
biosynthesis, viability and anchorage-independent growth of cancer
cells (32,33), suggesting that PFK-1 serves an
important role in the metabolic reprogramming of cancer cells, and
targeting PFK-1 activity has potential for uses in the development
of novel cancer therapies. In order to study whether shikonin,
vitamin K3 and vitamin K5 exert a negative
effect on PFK-1 activity, a fructose bisphosphate aldolase, TPI and
glycerol-3-phosphate dehydrogenate coupled assay was performed.
PFK-1 activity was observed by the reduction of absorbance at 340
nm revealing the oxidation of NADH (Fig.
2C-a). The result revealed that shikonin, vitamin K3
and K5 reduced the rate of the reaction dose-dependently
(Fig. 2C-b), with an IC50
of 17.2, 1192.4 and 35.9 µM respectively (Table I), indicating that shikonin may
inhibit PFK-1 more potently compared with the other two
naphthaquinones. Additionally, vitamin K5 exhibited
significantly more potent inhibition compared with vitamin
K3 at concentrations of 50, 500 and 1,000 µM (P=0.0093,
P=0.0041 and P=0.021, respectively).
Shikonin is an efficient inhibitor of
fructose bisphosphate aldolase
Fructose bisphosphate aldolase catalyzes the
reversible conversion of fructose-1,6-bisphosphate to
glyceraldehyde-3-phosphate and dihydroxyacetone phosphate (2). In addition to its important role in
glycolysis, fructose bisphophate aldolase is involved in other
biological processes including signal transduction, vesicle
trafficking and cell motility (34,35). Thus,
altering the activity of fructose bisphophate aldolase in cancer
cells has potential uses in translational medical studies. In the
present study, TPI and glycerol-3-phosphate dehydrogenase coupled
assays were used to measure the effect of shikonin, vitamin
K3 and K5 on the activity of MCF-7 cell
derived fructose bisphophate aldolase. Fructose bisphophate
aldolase activity was revealed by the reduction of absorbance at
340 nm representing the oxidation of NADH (Fig. 2D-a). Results revealed that, compared
with the vehicle control, shikonin inhibited the fructose
bisphophate aldolase activity efficiently and dose-dependently with
an IC50 of 158.4 µM, however vitamin K3 and
K5 did not reduce the activity of fructose bisphophate
aldolase even at their highest concentration (1 mM) in the
incubation system (Fig. 2D-b;
Table I).
Shikonin, vitamin K3 and
vitamin K5 did not inhibit TPI activity
TPI is a homodimeric enzyme that catalyzes the
reversible conversion from dihydroxyacetone phosphate into
glyceraldehyde-3-phosphate (2).
Glyceraldehyde-3-phosphate may complete glycolysis, whilst
dihydroxyacetone phosphate participates in the pentose phosphate
pathway, a key source of reduced NADPH and a cofactor for anabolic
pathways in maintaining redox balance (3). Thus, as a regulator of distributed
metabolites between the glycolysis and pentose phosphate pathway
(36), TPI is a potential target to
disrupt the metabolism of cancer cells. In the present study, the
potential of shikonin, vitamin K3 and K5 to
be inhibitors of TPI was tested using a glycerol-3-phosphate
dehydrogenate coupled assay in which the TPI activity was
demonstrated by the absorbance at 340 nm representing the oxidation
of NADH (Fig. 2E). Results suggested
that shikonin, vitamin K3 and vitamin K5 did
not affect TPI activity even up to the soluble limit (1 mM) in the
incubation system (Fig. 2E; Table I).
Shikonin and vitamin K5
markedly inhibited the activity of GAPDH
GAPDH catalyzes the reversible conversion from
glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate coupled with
the production of NADH, an essential source of reducing power in
cancer cells (1,2). GAPDH is regarded to be a housekeeping
gene expressed in a majority of cells (2). However, its expression is upregulated in
cancer cells, and the expression level is associated with cancer
aggressiveness and drug resistance (36). Certain naphthalene derivatives have
exhibited inhibitory effects on GAPDH activity (37). In the present study, the influence of
shikonin, vitamin K3 and vitamin K5 on GAPDH
activity in the human breast cell line MCF-7 was examined, defined
by the velocity of the conversion from NAD+ to NADH catalyzed by
GAPDH exhibited by the increase in absorbance at 340 nm (Fig. 2F-a). The results indicated that
shikonin and vitamin K5 were able to inhibit the
activity of GAPDH with an IC50 of 1,052.0 and 462.0 µM,
respectively, whilst vitamin K3 at a high concentration
had little inhibitory effect on GAPDH (Fig. 2F-a and b; Table I).
Shikonin, vitamin K3 and
vitamin K5 inhibited the activity of PGK
PGK catalyzes the sixth step of glycolysis to
reversibly convert 1,3-bisphosphoglycerate to 3-phosphoglycerate
coupled with the generation of ATP from ADP (2). Human cells have two isoforms of PGK
(2). An increase in the expression of
PGK1 has been demonstrated in metastatic cancer cells (38–41). The
overexpression of PGK1 has been proven to promote invasion and
metastasis in the colon (38),
gastric (39) and prostate cancer
(40), and radioresistance in
astrocytoma (41). As PGK1 activity
is not limited to glycolysis intracellularly but additionally in
the microenviroment of tumor masses as a reductase, it is a
potential target for manipulating cancer growth despite the fact
that its role in the progression of different cancer types is
inconsistent (40). In the present
study, the effect of shikonin, vitamin K3 and vitamin
K5 on regulating PGK activity in MCF-7 cells was
measured using a glyceraldehyde-3-phosphate dehydrogenase coupled
assay. PGK activity was exhibited by the decrease of absorbance at
340 nm representing the conversion of NADH to NAD+ (Fig. 2G). The result demonstrated that
shikonin, vitamin K3 and K5 exerted a mild
inhibitory effect on PGK activity and inhibited 10.5, 28.6 and
36.7% PGK activity, respectively, even at the soluble limit of 1 mM
(Fig. 2G; Table I).
Shikonin, vitamin K3 and
vitamin K5 did not inhibit the activity of PGAM
PGAM catalyzes the interconversion from
3-phosphoglycerate to 2-phosphoglycerate in glycolysis (2). It is a regulator of glycolysis and
biosynthesis, as most of the glycolytic intermediates that are used
as precursors for anabolism are upstream of this step (42). Thus, PGM is a promising therapeutic
target for cancer. Aromatic compound MJE3 (43) and PGM1-004A (42) were potent inhibitors of PGAM, and were
toxic to cancer cells. In the present study, the influence of
aromatic compounds shikonin, vitamin K3 and
K5 on PGAM were measured using enzymatic assays. The
experiment was performed using an enolase coupled reaction to
measure the production of phosphoenolpyruvate indicated by the
absorbance at 240 nm (Fig. 2H). The
result demonstrated that shikonin, vitamin K3 and
K5 were unable to inhibit the activity of PGAM (Fig. 2H; Table
I).
Shikonin, vitamin K3 and
vitamin K5 did not inhibit the activity of enolase
Enolase catalyzes the interconversion of
2-phosphoglycerate to phosphoenolpyruvate (2). The expression of enolase is upregulated
and associated with the invasion ability of cancer (44,45).
Enolase is regarded to be a potential target for cancer treatment.
An aromatic compound, ENOblock, was identified to exhibit potent
inhibition of enolase activity and anticancer effects (46). In the present study, whether the
glycolysis inhibitors shikonin, vitamin K3 and
K5 affect the activity of enolase was examined. The
assay was performed by detecting the production of
phosphoenolpyruvate indicated by the increase of the absorbance at
240 nm (Fig. 2I). The result
demonstrated that shikonin, vitamin K3 and vitamin
K5 did not exert any inhibition on the activity of
enolase, even at their soluble limits (Fig. 2I; Table
I).
Shikonin notably inhibited the
activity of pyruvate kinase derived from MCF-7 lysate
Pyruvate kinase catalyzes the last rate limiting
step of glycolysis, converting phosphoenolpyruvate to pyruvate
irreversibly (2). PKM2, an isoform of
pyruvate kinase, is expressed specifically and ubiquitously in
cancer cells (47,48). Interrupting its activity inhibits the
glycolytic flux and growth of cancer cells (47). PKM2 has become highly focused on in
the development of novel cancer therapy methods (1,3,11). A number of chemical inhibitors of PKM2
have been identified (9,10,12). In
previous studies, shikonin, vitamin K3 and K5
were revealed to inhibit the activity of purified recombinant PKM2
potently, with an IC50 of 0.3, 150 and 28 µM
respectively (9,10). However, it was additionally revealed
that shikonin may inhibit 50% of PKM2 activity in MCF-7 cells with
a concentration of up to 10 µM (10).
The inconsistent effect on the inhibitory effect on PKM2 activity
in vitro and in vivo may be caused by the different
allosteric structures of PKM2 and the conjugation of
naphothaquinones with the various components of cells (24,25). In
the present study, a LDH coupled assay was applied in order to
measure the inhibitory effect of shikonin, vitamin K3
and K5 on PKM2 in MCF-7 cell lysate. The result was
indicated by the consumption of NADH with the absorbance at 340 nm
(Fig. 2J-a). It was revealed that,
compared with the vehicle control, shikonin exhibited a notable
inhibitory efficiency with an IC50 of 12.2 µM, while
vitamin K3 and K5 inhibited PKM2 in cell
extract very little, reducing 29.5 and 26.4% of PKM2 activity,
respectively, at their soluble limits (Fig. 2J-a and b; Table I).
Shikonin, vitamin K3 and
vitamin K5 did not inhibit the activity of LDH
LDH converts the pyruvate to lactate reversibly
(2). LDHA and LDHB are the two major
isoforms of LDH in mammalian cells (2). LDHA preferably transforms pyruvate to
lactate and LDHB favors the reverse conversion (49). Chemical inhibitors of LDHA have been
proven to possess antitumor activity; and of these inhibitors,
gossypol was used in clinical trials (50). In the present study, the activity of
LDH in catalyzing the conversion from pyruvate to lactate in the
presence of shikonin, vitamin K3 or K5 was
measured, which was indicated by the consumption of NADH at an
absorbance of 340 nm (Fig. 2K). The
results demonstrated that shikonin, vitamin K3 and
vitamin K5 were unable to inhibit the LDH activity of
MCF-7 cell lysate, even at their soluble limits (Fig. 2K; Table
I).
Discussion
Shikonin, vitamin K3 and K5
are glycolytic suppressors, and have been previously revealed to
inhibit PKM2 in cancer cells (9,10). The
present study firstly screened the inhibition profile of glycolytic
enzymes of identified PKM2 inhibitors, shikonin, vitamin
K3 and K5. The inhibitory effect of
naphthaquinones on enzymes in cell lysate (Table I) is consistent with that using
intracellular enzymes (10), and that
cell lysate contains factors to retain allosteric and contextual
conditions of enzymes (10,24,25). Thus,
in order to study the influence of shikonin, vitamin K3
and K5 on the activity of endogenous glycolytic enzymes
in the breast cancer cell line MCF-7 and their physiological
effect, it is more appropriate to measure enzymatic activity in
cell extracts as opposed to purified recombinant enzymes, thus
using a systematic method of exploring inhibitors of biological
enzymes.
Hexokinase II (HK II), an isoform of hexokinase, is
specifically upregulated in cancer cells (27) and is mainly expressed in MCF-7 cells
(51). The expression of HK II is
associated with a poor prognosis in patients with cancer, and the
survival and growth of cancer cells (27). Therefore, HK II is regarded to be an
important target in cancer treatment (1–3). Chemicals
including 2-deoxyglucose, lonidamine, 3-bromopyruvate and imatinib
that compete with the substrate of hexokinase (glucose) or inhibit
the activity of HK II, exert a notable effect on treating cancer
alone or in combination with traditional chemotherapy and
radiotherapy (1,2). Imatinib has been approved for clinical
usage (2). Lonidamine and
2-deoxyglucose have been used in clinical trial stages II/III and
I/II respectively (1,2), while 3-bromopyruvate has been used in
pre-clinical trials (1). A potent HK
II inhibitor, 3-bromopyruvate, with a concentration of 25 µM, may
inhibit ~40% of the hexokinase activity of MCF-7 cells (29), suggesting that an IC50
>25 µM higher compared with the IC50 of shikonin,
vitamin K3 and K5. The results of the present
study indicated that shikonin, vitamin K3 and
K5 inhibited the hexokinase activity of MCF-7 cells more
potently compared with 3-bromopyruvate.
PFK1 is an allosteric enzyme activated by
fructose-2,6-phosphate. Decreased fructose-2,6-phosphate may reduce
the rate of aerobic glycolysis, biosynthesis, viability and
anchorage-independent growth of cancer cells indirectly due to
allosterically downregulated PFK1 (32,33),
suggesting that PFK1 serves an important role in the metabolic
reprogramming of cancer cells, and targeting PFK1 activity has
potential uses in the development of novel cancer therapies. A
number of small chemical inhibitors were discovered to dissociate
PFK1's tetrameric form and reduce its activity directly (52,53).
Acetylsalicylic acid and salicylic acid, medicines proven to be
efficient in preventing breast cancer in clinical trials (54), inhibited the PFK1 activity of MCF-7
cells, with an IC50 of ~10 mM (52). Resveratrol, naturally present in
grapes, inhibited the glycolytic rate and growth of MCF-7 cells
through decreasing PFK1 activity, with an IC50 of around
100 µM (53). Compared with the
inhibitory effect of acetylsalicylic acid, salicylic acid and
resveratrol on the PFK1 activity of MCF-7 cells, shikonin and
vitamin K5 were notably more efficient inhibitors.
Aldolase A, a muscle specific isoform, is highly
expressed in cancer cells and the expression level is associated
with the metastasis and poor prognosis of cancer (34,35),
suggesting that it may be a potential target in treating cancer. A
number of aromatic compounds were discovered to be efficient
inhibitors of muscle specific aldolase, with a minimum
IC50 of ~30 µM required to inhibit purified recombinant
aldolase A (55). In the present
study, it was revealed that 158.4 µM shikonin may suppress 50% of
the aldolase activity of MCF-7 cell lysate (Table I). As cell lysate was used as opposed
to purified enzyme in the inhibition assays, it is unreasonable to
compare the inhibitory activity between shikonin and classical
aldolase inhibitors. Taking into consideration the fact that the
inhibitory activity was interrupted by complicated components in
the cell lysate, shikonin exhibited potent efficiency in
suppressing aldolase activity.
The sub-cellular location of GAPDH is associated
with the apoptosis of cells (2). One
previous study on the manipulation of the location of GAPDH to the
nucleus or mitochondria in order to induce cell death shed light on
the development of cancer therapies (56). Nevertheless, preventing GAPDH activity
in glycolysis provides a different perspective in order to invent
novel treatments of cancer (2). Given
that GAPDH has a high affinity and activity toward NAD+,
biochemists have synthesized naphthalene analogs with a similar
chemical structure to NAD+ in order to compete with NAD+ in docking
with the binging site (37). These
naphthalene derivatives demonstrated a potent inhibition of GAPDH
activity derived from trypanosome but not in human cells (37). In the present study, shikonin and
vitamin K5, two naphthalene derivatives, exhibited
efficient inhibitory activity on GAPDH in human derived cells,
which provides an indication of how to distinguish regulatory
characteristics of GAPDH derived from human and trypanosome.
PKM2, the major pyruvate kinase isoform in MCF-7
cells, had been previously proven to be substantially inhibited by
shikonin, vitamin K3 and vitamin K5 (9,10).
However, the inhibitory efficiency was measured using assays
applying purified recombinant PKM2. The results revealed that
vitamin K3 and vitamin K5 exhibit mild but
notable suppression of PKM2 activity in MCF-7 cell lysate with
various components. Shikonin exhibited a similar inhibitory effect
on PKM2 derived from cell lysate and living cells (10). Given that factors regulating the
allosteric structure of PKM2 (12,47,48) and
proteins binding PKM2 (24,25) to form complexes are reserved in cell
lysate as intracellularly, using cell lysate in inhibition assays
may mimic the inhibitory efficiency of shikonin in physiological
condition as opposed to using purified recombinant PKM2.
In conclusion, shikonin, vitamin K3 and
vitamin K5 may inhibit a number of glycolytic enzymes
simultaneously, and the suppression of the glycolytic rate by
shikonin, vitamin K3 and vitamin K5 was due
to the reduced activity of certain enzymes. Among the inhibited
enzymes, three rate-limiting enzymes (hexokinase,
phosphofructokinase-1 and pyruvate kinase) were main targets of the
naphthaquinones, which primarily contributed to the decreased
consumption of glucose and the production of lactate in cancer
cells.
Previously, the amount of glucose and lactate in
culture media was measured in order to examine the inhibitory
effect of shikonin, vitamin K3 and vitamin K5
on the glycolysis process. The IC50 of shikonin, vitamin
K3 and K5 in inhibiting glucose consumption
were 10, 50 and 105 µM, respectively, and the IC50 in
inhibiting lactate production were 4, 30 and 45 µM, respectively
(9,10). The IC50 of shikonin,
vitamin K3 and vitamin K5 toward the
reduction of glucose in culture media were inconsistent with those
toward the increase of lactate, which indicates that the
transporters that assist the intercellular flux of glucose and
lactate (31) may be partially
blocked by naphthaquinones. Suppression of glycolysis in
vivo may be attributed partially to the inhibition of the
activity of enzymes and partly to the reduced import and export of
glucose and lactate (1). In the
present study, vitamin K5 inhibited glycolytic enzymes
notably more potently compared with vitamin K3 (Table I), but the previous study demonstrated
a lower efficiency in inhibiting glycolysis (9), indicating that vitamin K3 may
be stronger in inhibiting transporters of glucose and lactate
compared with vitamin K5.
Overall, the present study revealed that
naphthaquinones, shikonin, vitamin K3 and K5
inhibit aerobic glycolysis efficiently by reducing the activity of
glycolytic enzymes in cancer cells, which provides evidence for the
development of novel chemical drugs to treat cancer.
Acknowledgements
The authors would like to thank Dr Gongchu Li
(College of Life Sciences, Zhejiang Sci-Tech University, Zhejiang,
China) for useful instructions during the writing of this
manuscript.
Funding
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant no. LQ14C090003 to
Dr Jing Chen) and the National Natural Science Foundation of China
(grant no. 31700886 to Dr Jing Chen, grant no. 51102152 to Dr
Jingjie Cui).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
JCh performed the experiments, analyzed the data
and wrote the manuscript. XH conceived and designed the study, and
supervised the whole project. JCu provided technical support.
Ethics and consent to participate
The present study was conducted on cell line MCF-7.
There was no ethical issue in the present study.
Consent for publication
No human participant was involved in this
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Porporato PE, Dhup S, Dadhich RK, Copetti
T and Sonveaux P: Anticancer targets in the glycolytic metabolism
of tumors: A comprehensive review. Front Pharmacol. 2:492011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pelicano H, Martin DS, Xu RH and Huang P:
Glycolysis inhibition for anticancer treatment. Oncogene.
25:4633–4646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamanaka RB and Chandel NS: Targeting
glucose metabolism for cancer therapy. J Exp Med. 209:211–215.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gatenby RA, Gawlinski ET, Gmitro AF,
Kaylor B and Gillies RJ: Acid-mediated tumor invasion: A
multidisciplinary study. Cancer Res. 66:5216–5223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Courtnay R, Ngo DC, Malik N, Ververis K,
Tortorella SM and Karagiannis TC: Cancer metabolism and the Warburg
effect: The role of HIF-1 and PI3K. Mol Biol Rep. 42:841–851. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poon E, Harris AL and Ashcroft M:
Targeting the hypoxia-inducible factor (HIF) pathway in cancer.
Expert Rev Mol Med. 11:e262009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lew CR and Tolan DR: Targeting of several
glycolytic enzymes using rna interference reveals aldolase affects
cancer cell proliferation through a non-glycolytic mechanism. J
Biol Chem. 287:42554–42563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan B, Manley J, Lee J and Singh SR: The
emerging roles of microRNAs in cancer metabolism. Cancer Lett.
356:301–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Jiang Z, Wang B, Wang Y and Hu X:
Vitamin K(3) and K(5) are inhibitors of tumor pyruvate kinase M2.
Cancer Lett. 316:204–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Xie J, Jiang Z, Wang B, Wang Y and
Hu X: Shikonin and its analogs inhibit cancer cell glycolysis by
targeting tumor pyruvate kinase-M2. Oncogene. 30:4297–4306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christofk HR, Vander Heiden MG, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vander Heiden MG, Christofk HR, Schuman E,
Subtelny AO, Sharfi H, Harlow EE, Xian J and Cantley LC:
Identification of small molecule inhibitors of pyruvate kinase M2.
Biochem Pharmacol. 79:1118–1124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rady P, Arany I, Bojan F and Kertai P:
Activities of four glycolytic enzymes (HK, PFK, PK and LDH) and
isozymic pattern of LDH in mouse lung tumor induced by urethan. J
Cancer Res Clin Oncol. 95:287–289. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boyland E, Goss GC and Williamsashman HG:
The hexokinase activity of animal tumours. Biochem J. 49:321–325.
1951. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gracy RW and Tilley BE: Phosphoglucose
isomerase of human erythrocytes and cardiac tissue. Methods
Enzymol. 41:392–400. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vora S, Halper JP and Knowles DM:
Alterations in the activity and isozymic profile of human
phosphofructokinase during malignant transformation in vivo and in
vitro: Transformation- and progression-linked discriminants of
malignancy. Cancer Res. 45:2993–3001. 1985.PubMed/NCBI
|
|
17
|
El-Bacha T, de Freitas MS and Sola-Penna
M: Cellular distribution of phosphofructokinase activity and
implications to metabolic regulation in human breast cancer. Mol
Genet Metab. 79:294–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pontremoli S, Melloni E, Salamino F,
Sparatore B, Michetti M and Horecker BL: Changes in activity of
fructose-1,6-bisphosphate aldolase in livers of fasted rabbits and
accumulation of crossreacting immune material. Proc Natl Acad Sci
USA. 76:6323–6325. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rieder SV and Rose IA: The mechanism of
the triosephosphate isomerase reaction. J Biol Chem. 234:1007–1010.
1959.PubMed/NCBI
|
|
20
|
Brenneman FN and Volk WA: Glyceraldehyde
phosphate dehydrogenase activity with triphosphopyridine nucleotide
and with diphosphopyridine nucleotide. J Biol Chem. 234:2443–2447.
1959.PubMed/NCBI
|
|
21
|
Yoshida A and Watanabe S: Human
phosphoglycerate kinase. I. Crystallization and characterization of
normal enzyme. J Biol Chem. 247:440–445. 1972.PubMed/NCBI
|
|
22
|
Rose ZB and Dube S: Phosphoglycerate
mutase. Kinetics and effects of salts on the mutase and
bisphosphoglycerate phosphatase activities of the enzyme from
chicken breast muscle. J Biol Chem. 253:8583–8592. 1978.PubMed/NCBI
|
|
23
|
Wold F and Ballou CE: Studies on the
enzyme enolase. II. Kinetic studies. J Biol Chem. 227:313–328.
1957.PubMed/NCBI
|
|
24
|
Christofk HR, Vander Heiden MG, Wu N,
Asara JM and Cantley LC: Pyruvate kinase M2 is a
phosphotyrosine-binding protein. Nature. 452:181–186. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mazurek S, Zwerschke W, Jansen-Durr P and
Eigenbrodt E: Metabolic cooperation between different oncogenes
during cell transformation: Interaction between activated ras and
HPV-16 E7. Oncogene. 20:6891–6898. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roberts DJ and Miyamoto S: Hexokinase II
integrates energy metabolism and cellular protection: Akting on
mitochondria and TORCing to autophagy. Cell Death Differ.
22:248–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang D, Li J, Wang F, Hu J, Wang S and
Sun Y: 2-Deoxy-D-glucose targeting of glucose metabolism in cancer
cells as a potential therapy. Cancer Lett. 355:176–183. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu L, Xu J, Yuan W, Wu B, Wang H, Liu G,
Wang X, Du J and Cai S: The reversal effects of 3-bromopyruvate on
multidrug resistance in vitro and in vivo derived from human breast
MCF-7/ADR cells. PLoS One. 9:e1121322014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yanagawa T, Funasaka T, Tsutsumi S,
Watanabe H and Raz A: Novel roles of the autocrine motility
factor/phosphoglucose isomerase in tumor malignancy. Endocr Relat
Cancer. 11:749–759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiu CG, St-Pierre P, Nabi IR and Wiseman
SM: Autocrine motility factor receptor: A clinical review. Expert
Rev Anticancer Ther. 8:207–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ros S and Schulze A: Balancing glycolytic
flux: The role of 6-phosphofructo-2-kinase/fructose
2,6-bisphosphatases in cancer metabolism. Cancer Metab. 1:82013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Phan LM, Yeung SC and Lee MH: Cancer
metabolic reprogramming: Importance, main features and potentials
for precise targeted anti-cancer therapies. Cancer Biol Med.
11:1–19. 2014.PubMed/NCBI
|
|
34
|
Long F, Cai X, Luo W, Chen L and Li K:
Role of aldolase A in osteosarcoma progression and metastasis:
In vitro and in vivo evidence. Oncol Rep.
32:2031–2037. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun Y, Long JH and Zhou Y:
Angiopoietin-like 4 promotes melanoma cell invasion and survival
through aldolase A. Oncol Lett. 8:211–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lincet H and Icard P: How do glycolytic
enzymes favour cancer cell proliferation by nonmetabolic functions?
Oncogene. 34:3751–3759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kennedy KJ, Bressi JC and Gelb MH: A
disubstituted NAD+ analogue is a nanomolar inhibitor of
trypanosomal glyceraldehyde-3-phosphate dehydrogenase. Bioorg Med
Chem Lett. 11:95–98. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahmad SS, Glatzle J, Bajaeifer K, Bühler
S, Lehmann T, Königsrainer I, Vollmer JP, Sipos B, Ahmad SS,
Northoff H, et al: Phosphoglycerate kinase 1 as a promoter of
metastasis in colon cancer. Int J Oncol. 43:586–590. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zieker D, Konigsrainer I, Tritschler I,
Löffler M, Beckert S, Traub F, Nieselt K, Bühler S, Weller M,
Gaedcke J, et al: Phosphoglycerate kinase 1 a promoting enzyme for
peritoneal dissemination in gastric cancer. Int J Cancer.
126:1513–1520. 2010.PubMed/NCBI
|
|
40
|
Wang J, Ying G, Wang J, Jung Y, Lu J, Zhu
J, Pienta KJ and Taichman RS: Characterization of phosphoglycerate
kinase-1 expression of stromal cells derived from tumor
microenvironment in prostate cancer progression. Cancer Res.
70:471–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ding H, Cheng YJ, Yan H, Zhang R, Zhao JB,
Qian CF, Zhang WB, Xiao H and Liu HY: Phosphoglycerate kinase 1
promotes radioresistance in U251 human glioma cells. Oncol Rep.
31:894–900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hitosugi T, Zhou L, Elf S, Fan J, Kang HB,
Seo JH, Shan C, Dai Q, Zhang L, Xie J, et al: Phosphoglycerate
mutase 1 coordinates glycolysis and biosynthesis to promote tumor
growth. Cancer Cell. 22:585–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Evans MJ, Saghatelian A, Sorensen EJ and
Cravatt BF: Target discovery in small-molecule cell-based screens
by in situ proteome reactivity profiling. Nat Biotechnol.
23:1303–1307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song Y, Luo Q, Long H, Hu Z, Que T, Zhang
X, Li Z, Wang G, Yi L, Liu Z, et al: Alpha-enolase as a potential
cancer prognostic marker promotes cell growth, migration and
invasion in glioma. Mol Cancer. 13:652014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Principe M, Ceruti P, Shih NY,
Chattaragada MS, Rolla S, Conti L, Bestagno M, Zentilin L, Yang SH,
Migliorini P, et al: Targeting of surface alpha-enolase inhibits
the invasiveness of pancreatic cancer cells. Oncotarget.
6:11098–11113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jung DW, Kim WH, Park SH, Lee J, Kim J, Su
D, Ha HH, Chang YT and Williams DR: A unique small molecule
inhibitor of enolase clarifies its role in fundamental biological
processes. ACS Chem Biol. 8:1271–1282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iqbal MA, Gupta V, Gopinath P, Mazurek S
and Bamezai RN: Pyruvate kinase M2 and cancer: An updated
assessment. FEBS Lett. 588:2685–2692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mazurek S, Boschek CB, Hugo F and
Eigenbrodt E: Pyruvate kinase type M2 and its role in tumor growth
and spreading. Semin Cancer Biol. 15:300–308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miao P, Sheng S, Sun X, Liu J and Huang G:
Lactate dehydrogenase A in cancer: A promising target for diagnosis
and therapy. IUBMB Life. 65:904–910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Doherty JR and Cleveland JL: Targeting
lactate metabolism for cancer therapeutics. J Clin Invest.
123:3685–3692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu CL, Qin L, Liu HC, Candas D, Fan M and
Li JJ: Tumor cells switch to mitochondrial oxidative
phosphorylation under radiation via mTOR-mediated hexokinase II
inhibition-a Warburg-reversing effect. PLoS One. 10:e01210462015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Spitz GA, Furtado CM, Sola-Penna M and
Zancan P: Acetylsalicylic acid and salicylic acid decrease tumor
cell viability and glucose metabolism modulating
6-phosphofructo-1-kinase structure and activity. Biochem Pharmacol.
77:46–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gomez LS, Zancan P, Marcondes MC,
Ramos-Santos L, Meyer-Fernandes JR, Sola-Penna M and Da Silva D:
Resveratrol decreases breast cancer cell viability and glucose
metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie.
95:1336–1343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao YS, Zhu S, Li XW, Wang F, Hu FL, Li
DD, Zhang WC and Li X: Association between NSAIDs use and breast
cancer risk: A systematic review and meta-analysis. Breast Cancer
Res Treat. 117:141–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Blonski C, De Moissac D, Périé J and
Sygusch J: Inhibition of rabbit muscle aldolase by phosphorylated
aromatic compounds. Biochem J. 323:71–77. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Colell A, Green DR and Ricci JE: Novel
roles for GAPDH in cell death and carcinogenesis. Cell Death
Differ. 16:1573–1581. 2009. View Article : Google Scholar : PubMed/NCBI
|