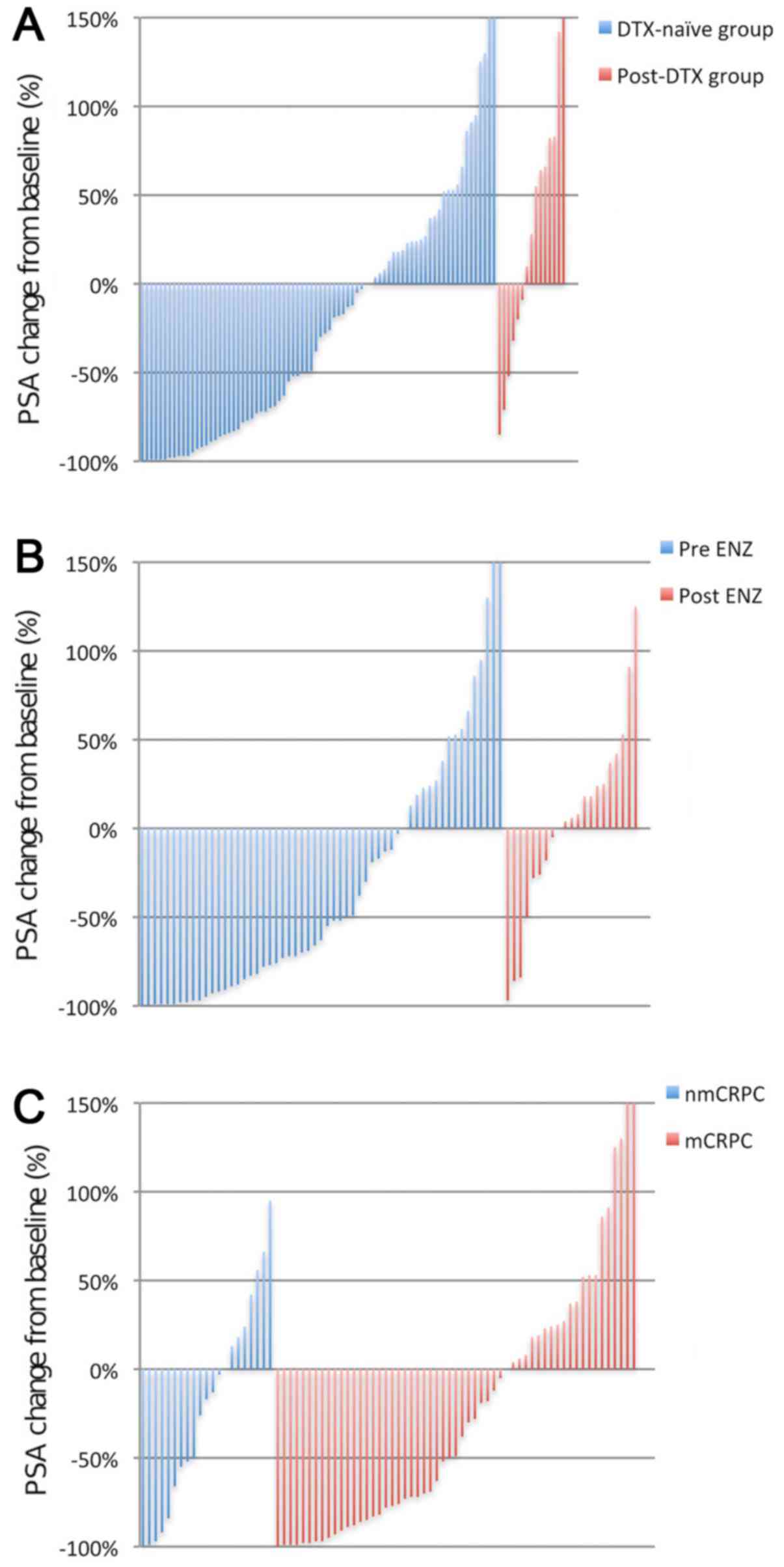
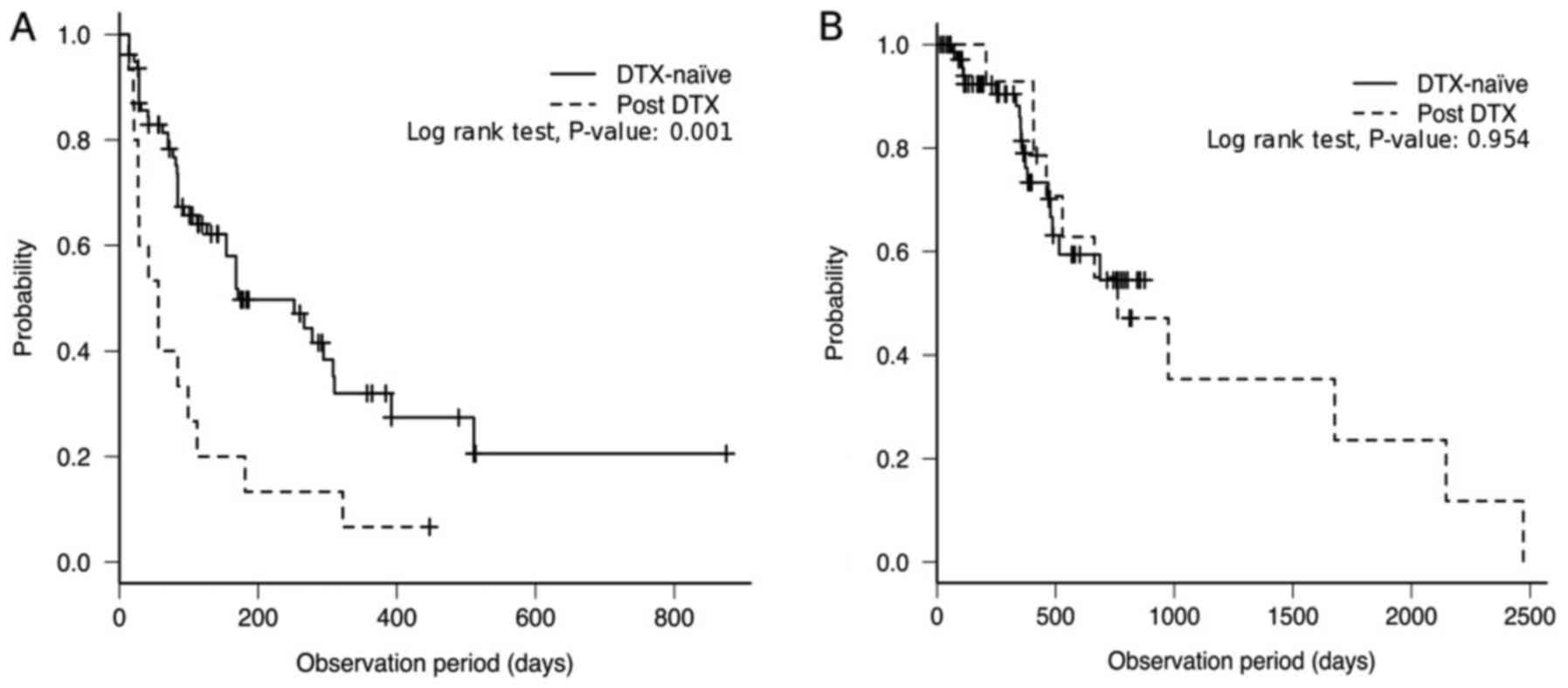
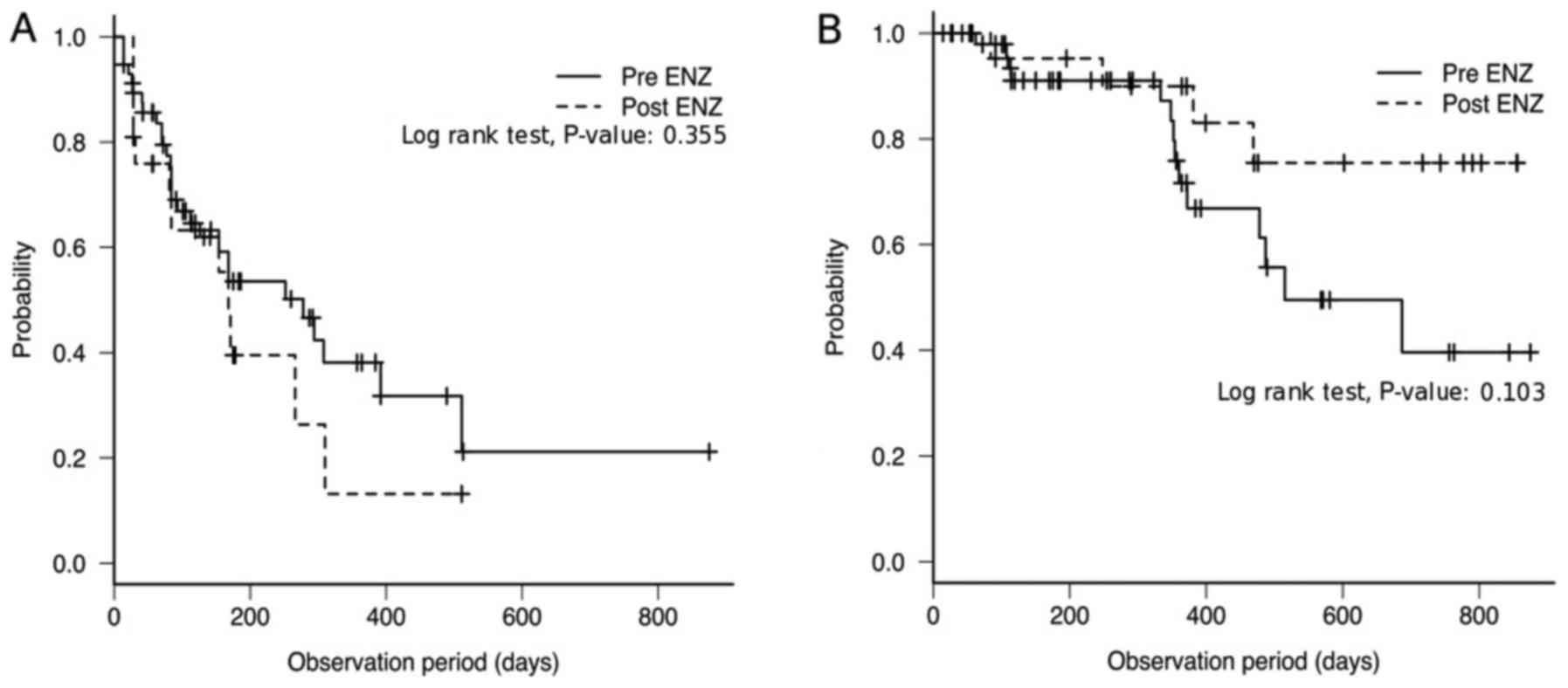
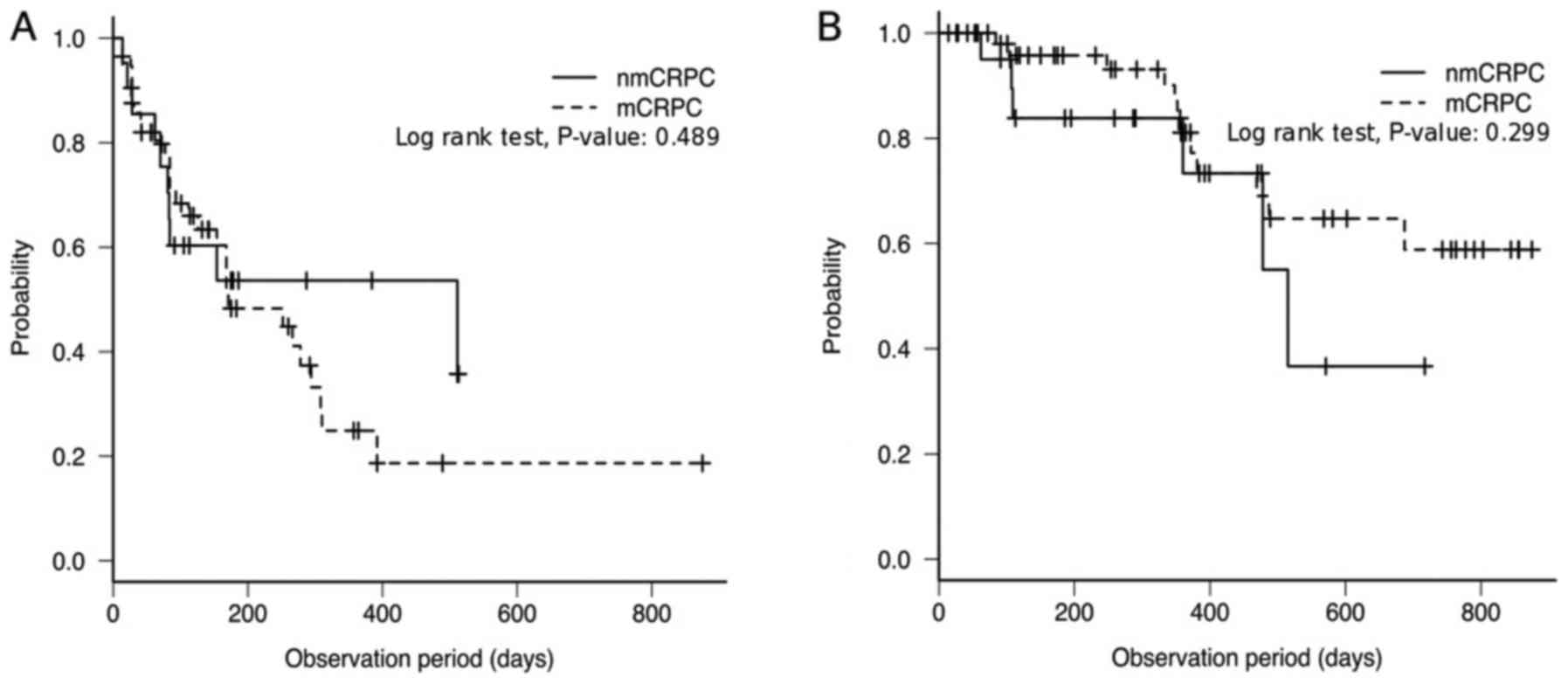
|
1
|
Huggins C and Hodges CV: Studies on
prostatic cancer. I. The effect of castration, of estrogen and
androgen injection on serum phosphatases in metastatic carcinoma of
the prostate. CA Cancer J Clin. 22:232–240. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walczak JR and Carducci MA: Prostate
cancer: A practical approach to current management of recurrent
disease. Mayo Clin Proc. 82:243–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chi KN, Bjartell A, Dearnaley D, Saad F,
Schröder FH, Sternberg C, Tombal B and Visakorpi T:
Castration-resistant prostate cancer: From new pathophysiology to
new treatment targets. Eur Urol. 56:594–605. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berthold DR, Pond GR, Soban F, de Wit R,
Eisenberger M and Tannock IF: Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer: Updated
survival in the TAX 327 study. J Clin Oncol. 26:242–245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seruga B, Ocana A and Tannock IF: Drug
resistance in metastatic castration-resistant prostate cancer. Nat
Rev Clin Oncol. 8:12–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in metastatic prostate
cancer. N Engl J Med. 364:1995–2005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ryan CJ, Smith MR, de Bono JS, Molina A,
Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng
S, et al: Abiraterone in metastatic prostate cancer without
previous chemotherapy. N Engl J Med. 368:138–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beer TM, Armstrong AJ, Rathkopf DE, Loriot
Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J,
Chowdhury S, et al: Enzalutamide in metastatic prostate cancer
before chemotherapy. N Engl J Med. 371:424–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Attard G, Belldegrun AS and de Bono JS:
Selective blockade of androgenic steroid synthesis by novel lyase
inhibitors as a therapeutic strategy for treating metastatic
prostate cancer. BJU Int. 96:1241–1246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamasaki M, Yuasa T, Yamamoto S, Hayashi
T, Ogawa M, Sakura M, Masuda H, Fukui I and Yonese J: Efficacy and
safety profile of enzalutamide for Japanese patients with
castration-resistant prostate cancer. Anticancer Res. 36:361–365.
2016.PubMed/NCBI
|
|
13
|
Ramalingam S, Humeniuk MS, Hu R, Rasmussen
J, Healy P, Wu Y, Harrison MR, Armstrong AJ, George DJ and Zhang T:
Prostate-specific antigen response in black and white patients
treated with abiraterone acetate for metastatic castrate-resistant
prostate cancer. Urol Oncol. 35:418–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mezynski J, Pezaro C, Bianchini D, Zivi A,
Sandhu S, Thompson E, Hunt J, Sheridan E, Baikady B, Sarvadikar A,
et al: Antitumour activity of docetaxel following treatment with
the CYP17A1 inhibitor abiraterone: Clinical evidence for
cross-resistance? Ann Oncol. 23:2943–2947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Soest RJ, Nieuweboer AJ, de Morrée ES,
Chitu D, Bergman AM, Goey SH, Bos MM, van der Meer N, Hamberg P, de
Wit R and Mathijssen RH: The influence of prior novel androgen
receptor targeted therapy on the efficacy of cabazitaxel in men
with metastatic castration-resistant prostate cancer. Eur J Cancer.
51:2562–2569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Design and end points of clinical trials for
patients with progressive prostate cancer and castrate levels of
testosterone: Recommendations of the prostate cancer clinical
trials working group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, National cancer
institute of the United States, National cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: ISUP Grading Committee: The 2005 international society
of urological pathology (ISUP) consensus conference on gleason
grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsubara N, Uemura H, Satoh T, Suzuki H,
Nishiyama T, Uemura H, Hashine K, Imanaka K, Ozono S and Akaza H: A
phase 2 trial of abiraterone acetate in Japanese men with
metastatic castration-resistant prostate cancer and without prior
chemotherapy (JPN-201 study). Jpn J Clin Oncol. 44:1216–1226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Satoh T, Uemura H, Tanabe K, Nishiyama T,
Terai A, Yokomizo A, Nakatani T, Imanaka K, Ozono S and Akaza H: A
phase 2 study of abiraterone acetate in Japanese men with
metastatic castration-resistant prostate cancer who had received
docetaxel-based chemotherapy. Jpn J Clin Oncol. 44:1206–1215. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyake H, Hara T, Terakawa T, Ozono S and
Fujisawa M: Comparative assessment of clinical outcomes between
abiraterone acetate and enzalutamide in patients with
docetaxel-naive metastatic castration-resistant prostate cancer:
Experience in real-world clinical practice in Japan. Clin
Genitourin Cancer. 15:313–319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antonarakis ES, Lu C, Wang H, Luber B,
Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et
al: AR-V7 and resistance to enzalutamide and abiraterone in
prostate cancer. N Engl J Med. 371:1028–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamada Y, Matsubara N, Tabata KI, Satoh T,
Kamiya N, Suzuki H, Kawahara T, Uemura H, Yano A and Kawakami S:
Abiraterone acetate after progression with enzalutamide in
chemotherapy-naïve patients with metastatic castration-resistant
prostate cancer: A multi-center retrospective analysis. BMC Res
Notes. 9:4712016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noonan KL, North S, Bitting RL, Armstrong
AJ, Ellard SL and Chi KN: Clinical activity of abiraterone acetate
in patients with metastatic castration-resistant prostate cancer
progressing after enzalutamide. Ann Oncol. 24:1802–1807. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loriot Y, Bianchini D, Ileana E, Sandhu S,
Patrikidou A, Pezaro C, Albiges L, Attard G, Fizazi K, De Bono JS
and Massard C: Antitumour activity of abiraterone acetate against
metastatic castration-resistant prostate cancer progressing after
docetaxel and enzalutamide (MDV3100). Ann Oncol. 24:1807–1812.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Virgo KS, Basch E, Loblaw DA, Oliver TK,
Rumble RB, Carducci MA, Nordquist L, Taplin ME, Winquist E and
Singer EA: Second line hormonal therapy for men with
chemotherapy-Naïve, castration-resistant prostate cancer: American
Society of Clinical Oncology provisional clinical opinion. J Clin
Oncol. 35:1952–1964. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fizazi K, Tran N, Fein L, Matsubara N,
Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S,
Protheroe A, et al: Abiraterone plus prednisone in metastatic,
castration-sensitive prostate cancer. N Engl J Med. 377:352–360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
James ND, de Bono JS, Spears MR, Clarke
NW, Mason MD, Dearnaley DP, Ritchie AWS, Amos CL, Gilson C, Jones
RJ, et al: Abiraterone for prostate cancer not previously treated
with hormone therapy. N Engl J Med. 377:338–351. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lolli C, Caffo O, Scarpi E, Aieta M,
Conteduca V, Maines F, Bianchi E, Massari F, Veccia A, Chiuri VE,
et al: Systemic immune-inflammation index predicts the clinical
outcome in patients with mCRPC treated with abiraterone. Front
Pharmacol. 7:3762016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Facchini G, Caffo O, Ortega C, D'Aniello
C, Di Napoli M, Cecere SC, Della Pepa C, Crispo A, Maines F, Ruatta
F, et al: Very early PSA response to abiraterone in mCRPC patients:
A novel prognostic factor predicting overall survival. Front
Pharmacol. 7:1232016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chi KN, Kheoh T, Ryan CJ, Molina A,
Bellmunt J, Vogelzang NJ, Rathkopf DE, Fizazi K, Kantoff PW, Li J,
et al: A prognostic index model for predicting overall survival in
patients with metastatic castration-resistant prostate cancer
treated with abiraterone acetate after docetaxel. Ann Oncol.
27:454–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Praet C, Rottey S, Van Hende F,
Pelgrims G, Demey W, Van Aelst F, Wynendaele W, Gil T, Schatteman
P, Filleul B, et al: Which factors predict overall survival in
patients with metastatic castration-resistant prostate cancer
treated with abiraterone acetate post-docetaxel? Clin Genitourin
Cancer. 15:502–508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Poon DM, Chan K, Lee SH, Chan TW, Sze H,
Lee EK, Lam D and Chan MF: Abiraterone acetate in metastatic
castration-resistant prostate cancer-the unanticipated real-world
clinical experience. BMC Urol. 16:122016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyake H, Hara T, Tamura K, Sugiyama T,
Furuse H, Ozono S and Fujisawa M: Independent association between
time to prostate-specific antigen (PSA) nadir and PSA
progression-free survival in patients with docetaxel-naïve,
metastatic castration-resistant prostate cancer receiving
abiraterone acetate, but not enzalutamide. Urol Oncol. 35:432–437.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McKay RR, Werner L, Fiorillo M,
Nakabayashi M, Kantoff PW and Taplin ME: Predictors of duration of
abiraterone acetate in men with castration-resistant prostate
cancer. Prostate Cancer Prostatic Dis. 19:398–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ryan CJ, Londhe A, Molina A, Smith MR, De
bono JS, Mulders P, Rathkopf DE, Saad F, Logothetis C, Fizazi k, et
al: Relationship of baseline PSA and degree of PSA decline to
radiographic progression-free survival (rPFS) in patients with
chemotherapy-naive metastatic castration-resistant prostate cancer
(mCRPC): Results from COU-AA-302. J Clin Oncol. 31:2013.
|
|
38
|
Rescigno P, Lorente D, Bianchini D,
Ferraldeschi R, Kolinsky MP, Sideris S, Zafeiriou Z, Sumanasuriya
S, Smith AD, Mehra N, et al: Prostate-specific antigen decline
after 4 weeks of treatment with abiraterone acetate and overall
survival in patients with metastatic castration-resistant prostate
cancer. Eur Urol. 70:724–731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ueda Y, Matsubara N, Tabata KI, Satoh T,
Kamiya N, Suzuki H, Kawahara T and Uemura H: Prostate-specific
antigen flare phenomenon induced by abiraterone acetate in
chemotherapy-naive patients with metastatic castration-resistant
prostate cancer. Clin Genitourin Cancer. 15:320–325. 2017.
View Article : Google Scholar : PubMed/NCBI
|