Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an
aggressive and lethal disease with an overall 5-year survival rate
of <5% (1). In China, more than
90,100 new PDAC cases and an estimated 79,400 deaths from PDAC
occurred in 2015 (2). While surgical
resection is the most effective treatment for PDAC, most patients
have little opportunity for surgery and a poor prognosis due to its
aggressive invasion and metastasis. Even if patients have the
opportunity for surgery, some are likely to undergo an R1
resection. The majority of patients will develop local or distant
tumor recurrence after undergoing a resection. Early detection and
dynamic monitoring of the disease progression are difficult.
Conventional imaging examinations and carbohydrate antigen 19-9
(CA19-9) assessment often fail to detect the early stage of a
primary tumor or metastasis (3).
Novel biomarkers are urgently needed for an early diagnosis and
detection of disease progression.
Circulating tumor cells (CTCs) are defined as
neoplastic cells that are disseminated from primary tumors and
secondary deposits, and the detection of CTCs may be a new method
to diagnose pancreatic cancer, a so called ‘fluid biopsy.’ Various
CTC isolation technologies have been developed, based on both
physical and biological properties. Technology has allowed the
identification CTCs using an epithelial marker, such as epithelial
cellular adhesion molecule (EpCAM) or cytokeratin (CK), but this
method exhibited a very low rate of detection in pancreatic cancer
patients (4,5), possibly due to the
epithelial-mesenchymal transition (EMT). The EMT has been described
as the process in which cancer cells lose some of their epithelial
characteristics and gain features of a more mesenchymal phenotype
during metastatic progression. This process makes cancer cells more
mobile and invasive, which are the main characteristics of CTCs.
Epithelial-marker-based technologies potentially miss CTCs with a
mesenchymal phenotype, which is important for metastasis. Some
studies have employed a size-based or mesenchymal marker to isolate
CTCs, which improves the detection rate (6–9).
In this study, we applied a detection platform
called EpCAM independent subtraction and
immunostaining-fluorescence in situ hybridization
(SET-iFISH), which facilitates the improvement of pancreatic CTC
detection (10). This platform
depletes white blood cells (WBCs) using an anti-CD45 antibody and
then identifies CTCs by karyotypic identification of centromere
probe 8 (CEP8), which can be performed regardless of EpCAM
expression and size variations (11).
The aim of this study is to investigate whether CTCs and
circulating tumor microemboli (CTMs) are related to the
clinicopathological factors and overall survival (OS) of PDAC
patients.
Materials and methods
Patients and sample collection
This prospective study was performed at the Peking
Union Medical College Hospital (Beijing, China) from August 2014 to
April 2015. All research subjects were hospitalized patients or
healthy individuals undergoing routine physical examinations.
Nineteen PDAC patients (including 3 stage IIa, 11 stage IIb, 4
stage III and 1 stage IV patients) were entered into a
prospectively collected database. PDAC was confirmed by
pathological assessment. Patients' demographic characteristics,
operative parameters, and postoperative outcomes were prospectively
collected in the database. Peripheral venous blood (7.5 ml) was
collected from each patient in customized acid citrate dextrose
(ACD)-anticoagulant tubes (Cytelligen, San Diego, CA, USA) before
the operation and at 10 days, 1, 3 and 7 months after the
operation. All patients were treated with chemotherapy. The blood
was processed immediately after collection. This study was approved
by the Institutional Review Board of Peking Union Medical College
Hospital and written informed consent was obtained from all
subjects.
Enrichment and identification of
CTCs
The process of the enrichment and identification of
pancreatic CTCs was performed according to the kit instructions
(Cytelligen). In brief, 7.5 ml of peripheral venous blood was
centrifuged to deplete the serum. The remaining components were
mixed with hCTCs Separation Matrix and centrifuged to remove red
blood cells. The remaining sample was incubated with immunomagnetic
particles conjugated to anti-CD45 monoclonal antibodies
(Cytelligen) for the separation of WBCs. The resulting cell pellet
was placed onto CTCs coated slides for iFISH. Hybridization
solution containing a CEP8 probe labeled with Spectrum Orange
(Cytelligen) was added to the slides, which were mounted and
denatured. Then, the cells were incubated with monoclonal anti-CK18
conjugated to Alexa Fluor 488 and monoclonal anti-CD45 conjugated
to Alexa Fluor 594 (Cytelligen). Finally, the nuclear dye
4′,6-diamidino-2-phenylindole (Cytelligen) was added, and the
slides were subsequently subjected to microscopic observation.
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation or the median (minimum, maximum), and
categorical variables are expressed as percentages. Continuous
variables were compared using the independent-samples T test and
Wilcoxon rank test, and categorical variables were compared using
the Pearson chi-square test. The primary end point was OS, which
was calculated using the Kaplan-Meier method. Survival differences
were assessed using the log-rank test. Two-sided P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were conducted by using IBM SPSS version 18.0
(BM Corp., Armonk, NY, USA).
Results
Identification of CTCs patterns in
PDAC patients
Cells from the peripheral blood samples of PDAC
patients were identified using the SET-iFISH platform. Based on the
immunostaining of an epithelial marker (CK18), a WBC marker (CD45),
the cell nucleus (DAPI) and different numbers of chromosome ploidy
(CEP 8), CTCs were defined with the following three patterns: A,
CK18+/CD45-/DAPI+/CEP8≠2; B, CK18-/CD45-/DAPI+/CEP8≠2; and C,
CK18+/CD45-/DAPI+/CEP8=2. All CD45+ cells were defined as WBCs, and
CK-/CD45-/DAPI+/CEP8=2 cells were defined as indeterminate cells
(11,12). We detected these markers in 86
peripheral blood samples from 19 PDAC patients at different time
points. Several peripheral blood samples were not detected because
some patients were not able to meet our request on time due to
their personal reasons or deaths. Of these 86 samples, only one
sample was detected as having CK18+ CTCs preoperatively, while the
others were all negative for these cells. Furthermore, we found
some CTCs that were similar in size to WBCs, which we called small
CTCs (SCTCs), and we isolated some clusters of CTCs called CTMs
(Fig. 1). We did not detect CK18+ and
CD45+ in all the CTMs.
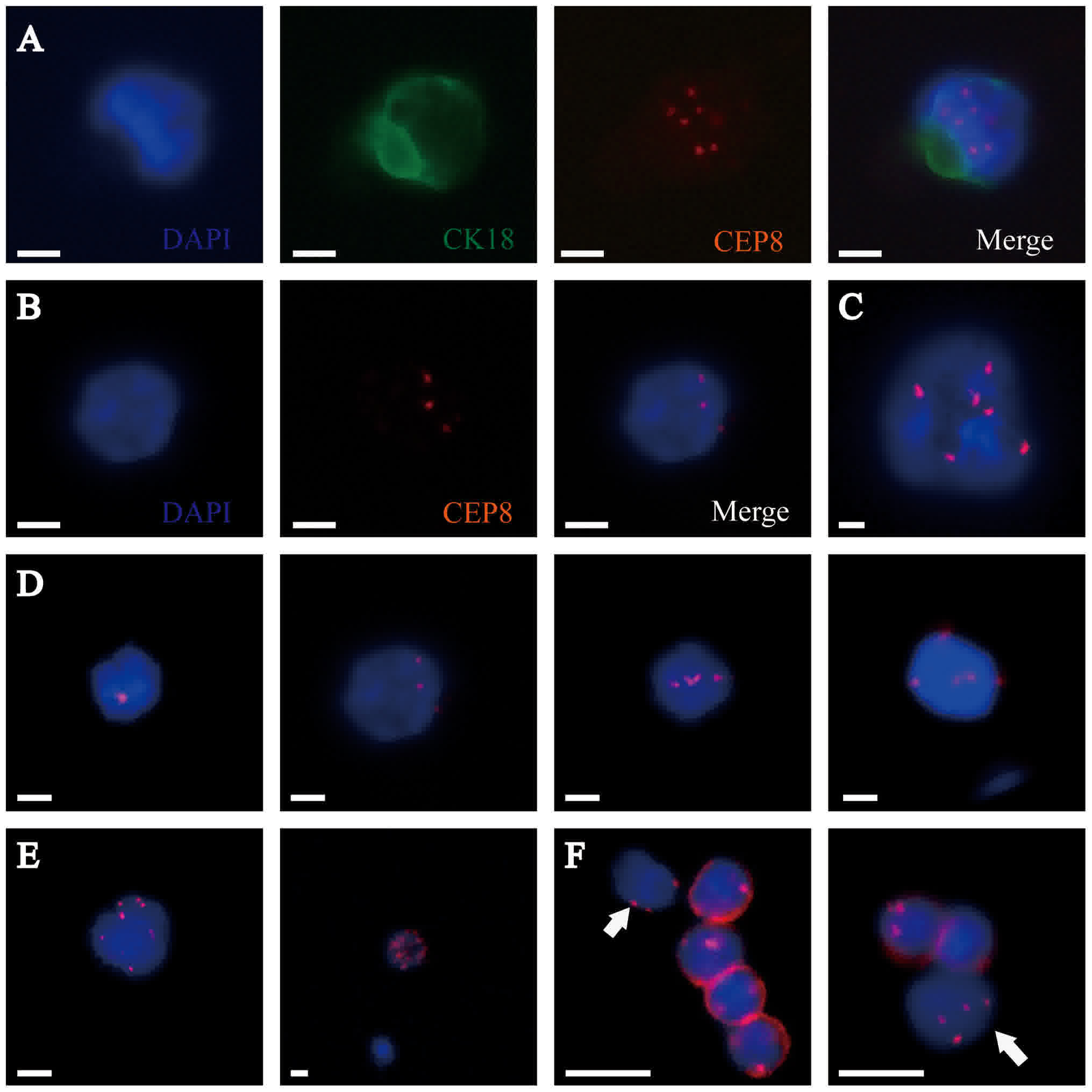 | Figure 1.Identification of CTCs in PDAC samples
by the SET-iFISH platform. CK18, green; CEP8, orange; DAPI, blue;
and CD45, red. (A) CTC: CK18+/CD45-/DAPI+/CEP8=6. (B) CTC:
CK18-/CD45-/DAPI+/CEP8=3. (C) CTM: Clusters of CTCs. (D) CTCs with
different numbers of CEP8 (CEP8=1, 3, 4 and 5, respectively). (E)
CTCs with CEP8 >5. (F) Small CTC: The diameter of the CTC is
<5 µm, which is similar to a white blood cell (indicated by
white arrows). Scale bars=5 µm. CTCs, circulating tumor cells;
PDAC, pancreatic ductal adenocarcinoma; CK, cytokeratin; CEP8,
centromere probe 8; CD, cluster of differentiation. |
CTCs in PDAC patients
All 19 PDAC patients included in this study were
histologically confirmed as having PDAC. The patient cohort
consisted of 47.4% males, with an average age of 59.1 years (range,
40–78 years). Preoperative CA19-9 levels were measured in all
patients. The mean CA19-9 level was 321.6 units/ml (range: 0.6–2074
units/ml), and 14 patients (73.7%) had a level greater than 37
units/ml, which is considered abnormally elevated. Sixteen (84.2%)
patients underwent resection, and 3 (15.8%) patients had
unresectable disease. Of the patients who underwent operation,
62.5% underwent resection by pancreaticoduodenectomy (n=10) and 6
(37.5%) had a distal pancreatectomy with splenectomy. The average
tumor size was 3.39 cm (range, 1.2–7 cm). The majority of the
adenocarcinomas were either moderately or poorly differentiated
(n=11, 68.7%). Thirteen (81.3%) patients had positive regional
lymph nodes. After analyzing the numbers of CTCs with the above
clinicopathologic features, no statistically significant
differences were found in regard to the age and sex of the patients
and the stage, grade and status of the lymph nodes. The number of
CTCs was significantly greater in patients with a tumor size >3
cm, perineural invasion or vascular invasion. No significant
differences were found in the numbers of SCTCs regarding any of the
clinicopathologic features (Table
I).
 | Table I.Patient characteristics and numbers of
CTCs, CTMs and SCTCs. |
Table I.
Patient characteristics and numbers of
CTCs, CTMs and SCTCs.
| Variable | All patients
(n=19) | CTCs, n (min,
max) | P-value | CTMs, n (min,
max) | P-value | SCTCs, n (min,
max) | P-value |
|---|
| Age (years) |
|
| 0.383 |
| 0.908 |
| 0.818 |
| ≤60 | 11 (57.9%) | 6 (1, 30) |
| 0 (0, 6) |
| 1 (1, 3) |
|
|
>60 | 8 (42.1%) | 4 (1, 22) |
| 0 (0, 1) |
| 1 (0, 10) |
|
| Gender |
|
| 0.511 |
| 0.909 |
| 0.316 |
| Male | 9 (47.4%) | 5 (1, 22) |
| 0 (0, 1) |
| 1 (0, 10) |
|
|
Female | 10 (52.6%) | 7 (1, 30) |
| 0 (0, 6) |
| 1 (0, 3) |
|
| Tumor size (cm),
(n=16)a |
|
| 0.019b |
| 0.036b |
| 0.855 |
| ≤3 | 9 (56.3%) | 3 (1, 9) |
| 0 (0, 0) |
| 1 (0, 3) |
|
|
>3 | 7 (43.7%) | 17 (1, 30) |
| 0 (0, 6) |
| 1 (1, 10) |
|
| CA19-9 level
(units/ml) |
|
| 0.963 |
| 1.000 |
| 0.570 |
|
<37 | 5 (26.3%) | 6 (2, 17) |
| 0 (0, 3) |
| 1 (1, 3) |
|
|
>37 | 14 (73.7%) | 5 (1, 30) |
| 0 (0, 6) |
| 1 (0, 10) |
|
| TNM Stage |
|
| 0.852 |
| 0.363 |
| 0.717 |
|
I–II | 14 (73.7%) | 5.5 (1, 30) |
| 0 (0, 6) |
| 1 (0, 3) |
|
|
III–IV | 5 (26.3%) | 5 (1, 22) |
| 0 (0, 1) |
| 1 (0, 10) |
|
| Differentiation
(n=16) |
|
| 0.078 |
| 0.213 |
| 0.192 |
|
Well/moderate | 11 (68.7%) | 6 (1, 30) |
| 0 (0, 6) |
| 1 (0, 10) |
|
|
Poor | 5 (31.3%) | 2 (1, 5) |
| 0 (0, 0) |
| 1 (1, 1) |
|
| Perineural invasion
(n=16) |
|
| 0.017b |
| 0.934 |
| 0.078 |
| No | 11 (68.7%) | 3 (1, 21) |
| 0 (0, 6) |
| 1 (0, 3) |
|
|
Yes | 5 (31.3%) | 15 (6, 30) |
| 0 (0, 1) |
| 2 (1, 10) |
|
| Vascular invasion
(n=16) |
|
| 0.043b |
| 0.036b |
| 0.583 |
| No | 9 (56.3%) | 3 (1, 9) |
| 0 (0, 0) |
| 1 (0, 3) |
|
|
Yes | 7 (43.7%) | 17 (1, 30) |
| 0 (0, 6) |
| 1 (1, 10) |
|
| Positive lymph
nodes (n=16) |
|
| 0.199 |
| 0.374 |
| 0.215 |
| No | 3 (18.7%) | 6 (6, 30) |
| 0 (0, 0) |
| 2 (1, 3) |
|
|
Yes | 13 (81.3%) | 3 (1, 22) |
| 0 (0, 6) |
| 1 (0, 10) |
|
CTMs in PDAC patients
CTMs were found in five patients (three stage IIb
patients, one stage III patient and one stage IV patient). Four of
these five patients were found CTMs before treatment. Before
treatment, a concentration of one CTM per 7.5 ml of blood was found
in two patients (one stage III patient and one stage IV patient),
and concentrations of three CTMs and six CTMs per 7.5 ml of blood
were found in two stage IIb patients, which showed that the number
of CTMS was not significantly different with TNM stage. No
statistically significant differences were observed in the numbers
of CTMs in regard to the age and sex of the patients and the stage,
grade and status of the lymph nodes. The number of CTMs was
significantly greater in patients with a tumor size >3 cm and
vascular invasion (Table I).
Dynamic changes in CTCs and CTMs in
PDAC patients
This study detected CTCs in 86 peripheral blood
samples collected at different times from 19 PDAC patients. Of all
peripheral blood samples, 19 samples were obtained preoperatively,
19 samples were obtained at 10 days postoperation, 18 samples were
obtained at 1 month postoperation, 15 samples were obtained at 3
months postoperation (2 cycles of adjuvant gemcitabine therapy) and
15 samples were obtained at 7 months postoperation (6 cycles of
adjuvant gemcitabine therapy). We found that the number of CTCs
after surgery or chemotherapy was not significantly different than
the number of preoperative CTCs. Only samples obtained at 10 days
postoperation showed significantly different numbers of SCTCs than
samples obtained preoperatively (Table
II).
 | Table II.Number of CTCs, CTM and SCTCs at
different times. |
Table II.
Number of CTCs, CTM and SCTCs at
different times.
| Time | Number of
samples | CTCs, n (min,
max) | Difference | CTM, n (min,
max) | Difference | SCTCs, n (min,
max) | Difference |
|---|
| Pre-operative | 19 | 5 (1, 30) |
| 0 (0, 6) |
| 1 (0, 10) |
|
| 10 days
postoperation | 19 | 13 (6, 19) | 0.098 | 0 (0, 1) | 0.484 | 4 (0, 11) | 0.013a |
| 1 month
postoperation | 18 | 2.5 (0, 30) | 0.267 | 0 (0, 6) | 0.974 | 1 (0, 6) | 0.365 |
| 3 months
postoperation | 15 | 0 (0, 24) | 0.353 | 0 (0, 0) | 0.273 | 0 (0, 21) | 0.573 |
| 7 months
postoperation | 15 | 5.5 (2, 10) | 0.935 | 0 (0, 2) | 0.866 | 2.5 (0, 7) | 0.321 |
In four of five patients, CTMs were detected before
treatment. One month after surgery, the number of CTMs in a stage
III patient increased to 6 per 7.5 ml of blood, and one CTM was
detected in a new patient (stage IIb) who was negative before
surgery. After 2 cycles of adjuvant gemcitabine therapy, CTMs were
detected in two patients who were dead at approximately 7 months
postoperation, while after 6 cycles of adjuvant gemcitabine
therapy, two CTMs were found in one patient who had 3 CTMs detected
per 7.5 ml of blood before surgery (Table III).
 | Table III.CTM in 5 patients with pancreatic
ductal adenocarcinoma. |
Table III.
CTM in 5 patients with pancreatic
ductal adenocarcinoma.
| Patient | Recurrence
site | DFS (months) | OS (months) | Prior to
treatment | 10 days po | 1 months po | 3 months po | 7 months po |
|---|
| Patient 1 (stage
IIb) | Liver | 1.8 | 7.3 | 6 CTMs | 1 CTMs | 0 CTMs | 1 CTMs | / |
| Patient 2 (stage
IIb) | Liver | 3.2 | 20.8 | 3 CTMs | 0 CTMs | 0 CTMs | 0 CTMs | 2 CTMs |
| Patient 3 (stage
III) | Liver and lung | 1.8 | 3.9 | 1 CTMs | 0 CTMs | 6 CTMs | / | / |
| Patient 4 (stage
IV) | – | – | 7.2 | 1 CTMs | 0 CTMs | 0 CTMs | 3 CTMs | / |
| Patient 5 (stage
IIb) | Liver | 4.2 | 10.6 | 0 CTMs | 0 CTMs | 1 CTMs | 0 CTMs | 0 CTMs |
Survival analysis
The mean and median follow-up times of all patients
were 19.94 and 24.90 months, respectively. During the follow-up
period, 13 patients died due to disease progression, including 3
patients who did not undergo surgery. two patients remained alive
but exhibited disease progression. Four patients remained alive
without evidence of disease progression. No patients were lost to
follow-up. To evaluate the influence of the cutoff value of the
CTCs count on the hazard ratios of OS, different values for the
number of CTCs per 7.5 ml were tested for correlation with OS using
the Kaplan-Meier method. However, the number of CTCs was not
associated with survival. We also examined the correlation between
OS and the number of SCTCs, but no prognostic significance was
found. The relationship between the number of CTMs and survival was
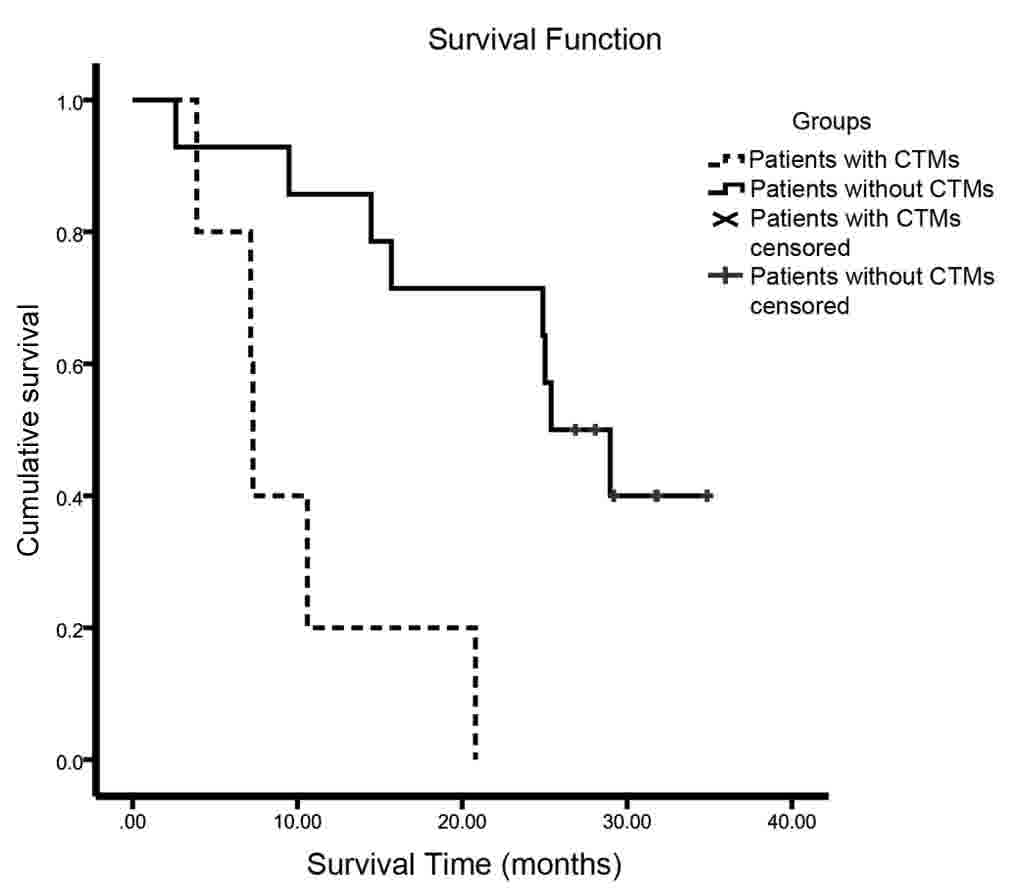
further explored. Patients with no CTMs survived significantly
longer than those with CTMs (25.4 vs. 7.3 months, P=0.001)
(Table IV) (Fig. 2). The median disease-free survival
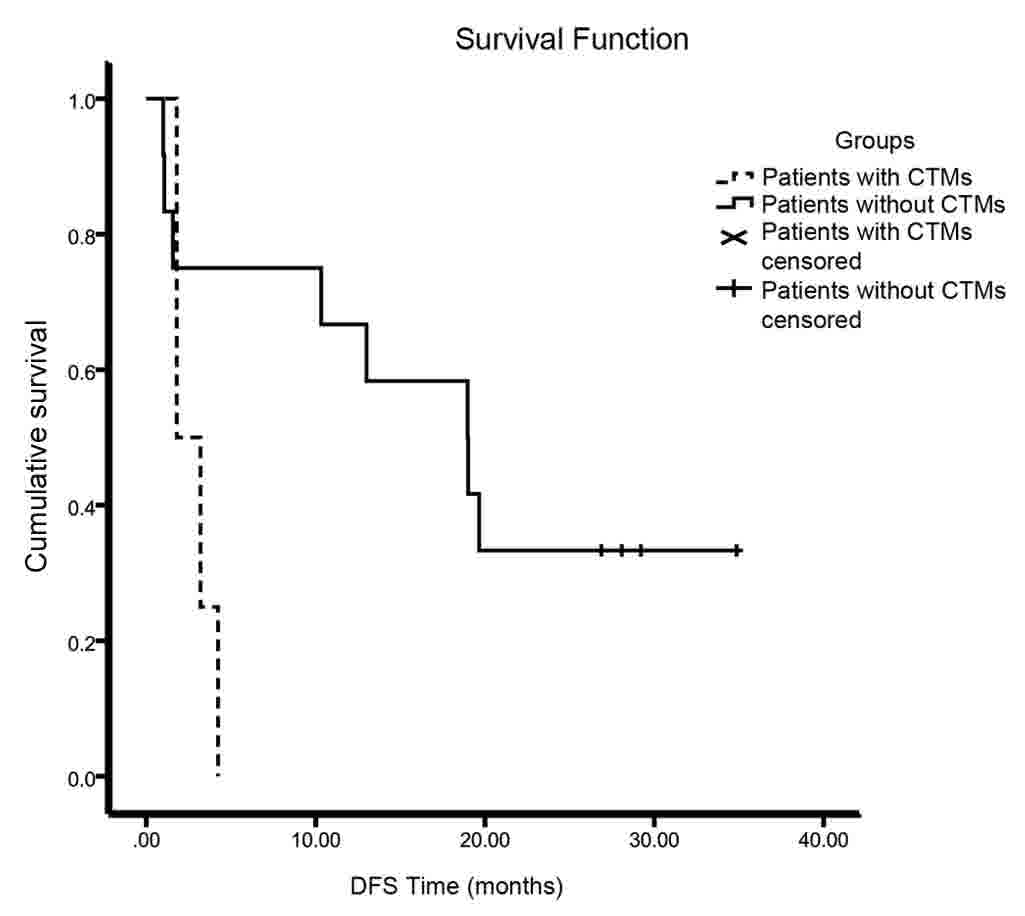
(DFS) of the patients with CTMs was significantly shorter than that
of patients without CTMs (18.97 vs. 1.8 months, P=0.037) (Fig. 3). The effects of tumor size, grade of
differentiation, TNM stage, perineural and vascular invasion,
resection margin status, and adjuvant chemoradiation therapy on
median OS were all evaluated using the Kaplan-Meier method.
Survival differences were assessed using the log-rank test
(Table III). The results of these
analyses indicated that TNM stage and vascular invasion were
significantly associated with OS.
 | Table IV.Kaplan-Meier analysis of OS. |
Table IV.
Kaplan-Meier analysis of OS.
|
| Univariate
analysis |
|---|
|
|
|
|---|
| Factor | Median OS
(months) | P-value |
|---|
| CA19-9 level
(<37/>37) (U/ml) | −/14.47 | 0.100 |
| Tumor size (≤3
cm/>3 cm)a | 10.60/28.97 | 0.187 |
| Differentiation
(well-moderate/poor) | 24.90/25.40 | 0.812 |
| TNM
(I–II/III–IV) | 25.40/9.50 | 0.008c |
| Perineural invasion
(no/yes) | 28.97/25.03 | 0.800 |
| Vascular invasion
(no/yes) | 28.97/10.60 | 0.017b |
| Resection margin
status (R0/not R0) | 25.40/7.30 | 0.194 |
| CTMs (no/yes) | 25.40/7.30 | 0.001c |
Discussion
PDAC has a dismal prognosis, which is commonly due
to the occurrence of invasion and metastasis before this disease is
diagnosed. In recent years, CTCs have attracted increasing
attention as a novel biomarker in clinical practice due to their
use in the early detection of tumors. Some studies have shown that
CTCs are present in pancreatic cancer (12–14). A
study on pancreatic cancer revealed that CTCs can enter the
bloodstream before tumor formation occurs (15). However, it is noteworthy that in the
past, most studies or clinical applications have employed the
CellSearch system, which depends upon the principle that CTCs can
be captured via an anti-EpCAM antibody. This method of CTCs capture
is based on epithelial expression and may result in decreased
sensitivity and loss of key information due to the EMT process,
which has is considered a critical factor in tumor cell invasion
and metastasis (16). The detection
of epithelial-marker-negative CTCs is very important for patients.
A study by Poruk et al (14)
assessed CTCs based on epithelial and mesenchymal markers in PDAC
patients and found that heterogeneous CTCs provide prognostic
utility for PDAC patients. Therefore, in our study, we detected
CTCs using the SET-iFISH platform instead of epithelial markers.
Surprisingly, we found that only one of 46 samples from the 19
patients exhibited epithelial CTCs along with CTMs in the
peripheral blood. If the traditional CellSearch system had been
used, this important information would have been missed.
In this study, the relationships of CTCs and CTMs
with clinicopathologic data were investigated. The numbers of CTCs
and CTMs were significantly associated with tumor size and vascular
invasion. CTCs, but not CTMs, were associated with perineural
invasion. Although the reasons for these relationships are unknown,
these findings could reflect the severity of the illness and the
effect that tumors have on vascular or perineural invasion. Zhang
et al (11) also found no
correlations between CTCs and the TNM stage, CA19-9 level or lymph
node status using the same platform. The results of our study were
consistent with this finding by Zhang et al (11). The lack of correlation between CTCs
and TNM stage was also demonstrated by the presence of CTCs in
patients with different stages of PDAC (17,18).
CA19-9 is the most commonly used tumor marker of pancreatic cancer,
but it was not elevated in a Lewisa-b− patient. CTCs and
CTMs were detected in patients with a normal CA19-9 level in our
study, which suggested that CTC and CTMs detection combined with
CA19-9 levels could improve the diagnosis rate (12,13). The
number of CTCs, especially the number of SCTCs, was increased 10
days after the operation compared with the level before surgery.
Another study also found the same phenomenon using SET-iFISH
(11). Some studies employing
epithelial markers to detect CTCs also showed a similar finding
(19,20). They proposed that the change in the
number of CTCs might be associated with dormant disseminated tumor
cells being reactivated and released into the circulation after the
primary tumors were removed. The reason for this phenomenon, which
could involve the stress of surgery, is still unknown.
Although several follow-up studies have confirmed
the general prognostic value of CTCs, the prognostic significance
of CTCs in pancreatic cancer is still controversial. A diversified
detection platform obtained different conclusions, and even results
obtained using the same detection platform were inconsistent. CTCs
positive patients were shown to have a shorter progression-free
survival in one study. However, another study arrived at the
opposite conclusion using the same CellSearch system (4,5). The
size-based CTCs detection platform also yielded different
conclusions. Recently, a study using the Isolation by Size of
Epithelial Tumor cells method found that CTCs could provide
prognostic utility for PDAC patients (14). Another study adopting a similar
size-based device failed to reveal the prognostic significance of
CTCs (21). Some studies using the
SET-iFISH platform showed that CTCs positive pancreatic cancer
patients exhibit a worse survival rate (11,12);
however, in this study, we did not find this correlation. Further
studies are needed to analyze this relationship.
Despite that CTCs were not associated with the
prognosis in our study, the presence of CTMs in patients was
significantly associated with a worse prognosis, which was
consistent with the results of a previous study (22). The authors found that the number of
CTMs, instead of CTCs, before treatment was an independent
predictor of OS in PDAC patients. In another study, the presence of
CTMs was used as an independent prognostic marker in small cell
lung cancer patients and was correlated with a worse clinical
outcome (23). Why do the patients
with CTMs have a dismal prognosis? Using mouse models with tagged
mammary tumors, Aceto et al (24) demonstrated that CTCs clusters are
oligoclonal precursors. Although they are rare in the circulation
compared with single CTCs, CTC clusters have a 23- to 50-fold
increased metastatic potential (24).
Some studies also speculate that tumor cells form CTMs to resist
anoikis, which is a survival advantage (25,26). The
increased metastatic potential and resistance of anoikis might
explain why patients with CTMs had a worse prognosis in the present
study.
In this study, the five patients with CTMs had
different TNM stages, and four of these patients underwent surgery.
The median OS of these patients was only 7.3 months. As the
previous studies have described, the median OS of metastatic
pancreatic cancer patients who undergo chemotherapy (gemcitabine,
nab-Paclitaxel plus Gemcitabine or FOLFIRINOX) is 6.7–11.1 months
(27,28), which is equivalent to or even better
than that of patients with CTMs in this study. Therefore, we
presumed that CTMs may be micrometastasis, which is irrelevant to
the clinical TNM stage. The TNM stage is mainly determined
according to imaging examinations before surgery at the present
time, which provide a reference for surgeons. However, this
micrometastasis in the blood cannot be detected by a traditional
clinical examination. Patients with CTMs exhibited very short
survival after surgery, which suggests that the surgeons should
cautiously consider surgery for patients with CTMs.
Compared with CTCs, CTMs effectively reflected the
severity of illness and dismal prognosis of patients in our study.
Although CTCs have been found in many studies, only a few
successful detections of CTC clusters have been reported,
especially in PDAC patients (29). We
believe that the role of CTMs in current clinical applications and
research is largely underestimated. However, this study has a small
sample size of only 19 patients, most of whom had either stage II
or III disease and the follow-up time is limited. Thus, a further
large-scale study is needed. CTMs should be given more attention
because their detection could be a powerful tool that can benefit
patients.
We used the SET-iFISH platform to detect CTCs and
CTMs in various pathological stages of pancreatic cancer. Unlike in
other studies, most of the CTCs in our study were CK18 negative.
CTMs effectively reflected the severity of the illness and the
dismal prognosis of patients and might be a new biomarker of PDAC,
independent of the current staging system.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GW performed the experiments, analyzed the data and
wrote the manuscript. RZ contributed towards the acquisition of the
data and manuscript preparation. YL contributed to the
interpretation of survival data. YZ assisted with the conception of
the study and revised the manuscript. MD contributed to the
conception of the study, designed the experiments and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Peking Union Medical College Hospital (Beijing,
China) and written informed consent was obtained from all
subjects.
Consent for publication
Written informed consent was obtained from all
patients enrolled.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CTM
|
circulating tumor microemboli
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
CTCs
|
circulating tumor cells
|
|
SCTCs
|
small CTCs
|
|
EpCAM
|
epithelial cellular adhesion
molecule
|
|
CK
|
cytokeratin
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SET-iFISH
|
EpCAM independent subtraction and
immunostaining-fluorescence in situ hybridization
|
|
WBCs
|
white blood cells
|
|
CEP8
|
centromere probe 8
|
|
OS
|
overall survival
|
|
ACD
|
acid citrate dextrose
|
|
DFS
|
disease-free survival
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chari ST, Kelly K, Hollingsworth MA,
Thayer SP, Ahlquist DA, Andersen DK, Batra SK, Brentnall TA, Canto
M, Cleeter DF, et al: Early detection of sporadic pancreatic
cancer: Summative review. Pancreas. 44:693–712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bissolati M, Sandri MT, Burtulo G, Zorzino
L, Balzano G and Braga M: Portal vein-circulating tumor cells
predict liver metastases in patients with resectable pancreatic
cancer. Tumour Biol. 36:991–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bidard FC, Huguet F, Louvet C, Mineur L,
Bouché O, Chibaudel B, Artru P, Desseigne F, Bachet JB, Mathiot C,
et al: Circulating tumor cells in locally advanced pancreatic
adenocarcinoma: The ancillary CirCe 07 study to the LAP 07 trial.
Ann Oncol. 24:2057–2061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin HK, Zheng S, Williams AJ, Balic M,
Groshen S, Scher HI, Fleisher M, Stadler W, Datar RH, Tai YC and
Cote RJ: Portable filter-based microdevice for detection and
characterization of circulating tumor cells. Clin Cancer Res.
16:5011–5018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harouaka RA, Zhou MD, Yeh YT, Khan WJ, Das
A, Liu X, Christ CC, Dicker DT, Baney TS, Kaifi JT, et al: Flexible
micro spring array device for high-throughput enrichment of viable
circulating tumor cells. Clin Chem. 60:323–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lecharpentier A, Vielh P, Perez-Moreno P,
Planchard D, Soria JC and Farace F: Detection of circulating tumour
cells with a hybrid (epithelial/mesenchymal) phenotype in patients
with metastatic non-small cell lung cancer. Br J Cancer.
105:1338–1341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Armstrong AJ, Marengo MS, Oltean S, Kemeny
G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ and
Garcia-Blanco MA: Circulating tumor cells from patients with
advanced prostate and breast cancer display both epithelial and
mesenchymal markers. Mol Cancer Res. 9:997–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin PP: Integrated EpCAM-independent
subtraction enrichment and iFISH strategies to detect and classify
disseminated and circulating tumors cells. Clin Transl Med.
4:382015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Wang F, Ning N, Chen Q, Yang Z,
Guo Y, Xu D, Zhang D, Zhan T and Cui W: Patterns of circulating
tumor cells identified by CEP8, CK and CD45 in pancreatic cancer.
Int J Cancer. 136:1228–1233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Zhu Y, Zhang Z, Zhang C, Huang X
and Yuan Z: Clinical significance of pancreatic circulating tumor
cells using combined negative enrichment and
immunostaining-fluorescence in situ hybridization. J Exp Clin
Cancer Res. 35:662016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kulemann B, Pitman MB, Liss AS, Valsangkar
N, Fernández-Del Castillo C, Lillemoe KD, Hoeppner J, Mino-Kenudson
M, Warshaw AL and Thayer SP: Circulating tumor cells found in
patients with localized and advanced pancreatic cancer. Pancreas.
44:547–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poruk KE, Valero V III, Saunders T,
Blackford AL, Griffin JF, Poling J, Hruban RH, Anders RA, Herman J,
Zheng L, et al: Circulating tumor cell phenotype predicts
recurrence and survival in pancreatic adenocarcinoma. Ann Surg.
264:1073–1081. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tjensvoll K, Nordgård O and Smaaland R:
Circulating tumor cells in pancreatic cancer patients: Methods of
detection and clinical implications. Int J Cancer. 134:1–8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sergeant G, Roskams T, van Pelt J,
Houtmeyers F, Aerts R and Topal B: Perioperative cancer cell
dissemination detected with a real-time RT-PCR assay for EpCAM is
not associated with worse prognosis in pancreatic ductal
adenocarcinoma. BMC Cancer. 11:472011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sandri MT, Zorzino L, Cassatella MC, Bassi
F, Luini A, Casadio C, Botteri E, Rotmensz N, Adamoli L and Nolè F:
Changes in circulating tumor cell detection in patients with
localized breast cancer before and after surgery. Ann Surg Oncol.
17:1539–1545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kulemann B, Liss AS, Warshaw AL, Seifert
S, Bronsert P, Glatz T, Pitman MB and Hoeppner J: KRAS mutations in
pancreatic circulating tumor cells: A pilot study. Tumour Biol.
37:7547–7554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang MC, Chang YT, Chen JY, Jeng YM, Yang
CY, Tien YW, Yang SH, Chen HL, Liang TY, Wang CF, et al: Clinical
significance of circulating tumor microemboli as a prognostic
marker in patients with pancreatic ductal adenocarcinoma. Clin
Chem. 62:505–513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou JM, Krebs MG, Lancashire L, Sloane R,
Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, et
al: Clinical significance and molecular characteristics of
circulating tumor cells and circulating tumor microemboli in
patients with small-cell lung cancer. J Clin Oncol. 30:525–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aceto N, Bardia A, Miyamoto DT, Donaldson
MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al:
Circulating tumor cell clusters are oligoclonal precursors of
breast cancer metastasis. Cell. 158:1110–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liotta LA, Saidel MG and Kleinerman J: The
significance of hematogenous tumor cell clumps in the metastatic
process. Cancer Res. 36:889–894. 1976.PubMed/NCBI
|
|
26
|
Friedl P and Gilmour D: Collective cell
migration in morphogenesis, regeneration and cancer. Nat Rev Mol
Cell Biol. 10:445–457. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong Y, Li Z and Zhang Q: A circulating
tumor cell cluster-based model for tumor metastasis (Hypothesis).
Oncology Lett. 12:4891–4895. 2016. View Article : Google Scholar
|