Introduction
Pancreatic adenocarcinoma is the fourth leading
cause of cancer-associated mortality in the USA, and pancreatic
ductal adenocarcinoma (PDAC) is a highly metastatic disease with a
high mortality rate (1). The majority
of patients are diagnosed at a late stage and, despite recent
advances in chemotherapeutic approaches, the prognosis of
pancreatic adenocarcinoma is extremely poor compared with other
types of cancer (2,3). Tumor growth and migration are largely
responsible for the high mortality rate of patients with pancreatic
adenocarcinoma; therefore, it is important for researchers to
investigate methods for preventing pancreatic adenocarcinoma cell
proliferation and migration in order to improve the treatment of
this disease (3,4).
Na+/H+ exchanger regulatory
factor 1 (NHERF1; also known as sodium-hydrogen antiporter 3
regulator 1 or ERM-binding protein 50) is a multi-functional
scaffolding protein that has different functions in a variety of
types of cancer through its interactions with oncogenic or
tumor-suppressive proteins (4,5). In breast
cancer, NHERF1 has been demonstrated to inhibit proliferation by
influencing the transduction of growth signals induced by epidermal
growth factor receptor (EGFR) and platelet-derived growth factor
receptor, and by modulating the expression of phosphatase and
tensin homolog (6,7). By contrast, in prostate cancer, the
expression of NHERF1 has been demonstrated to be increased,
suggesting that NHERF1 may be associated with the carcinogenic
potential of this cancer type (8).
However, the function of NHERF1 in the proliferation and migration
of pancreatic adenocarcinoma cells remains unresolved.
The objective of the present study was to determine
the effects of NHERF1 expression on proliferative and migratory
abilities of pancreatic adenocarcinoma cells by overexpressing
NHERF1 in MIAPaCa-2 cells. The results revealed that NHERF1 may be
able to inhibit the proliferative and migratory abilities of
pancreatic adenocarcinoma cells by downregulating the
phosphorylation of protein kinase B (Akt).
Materials and methods
Immunohistochemical (IHC) data,
plasmids and cell lines
The IHC-based protein expression data, including
high-resolution images, were downloaded from the Human Protein
Atlas web portal, using NHERF1 as the search term
(pancreatic cancer database; www.proteinatlas.org). All IHC images from pancreatic
cancer and normal tissues were collected, and the sum of the
integrated optical density (IOD) values of images were analyzed
using ImagePro Plus software (version 6.0; Media Cybernetics, Inc.,
Rockville, MD, USA). The mean IOD values of these images were
counted, which reflected the relative NHERF1 expression level in
pancreatic cancer and normal pancrease tissues, respectively. The
pBK-CMV-HA-NHERF1 wild-type (wt) plasmid and the empty vector
plasmid (pBK-CMV-HA) were designed and synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China), and G418 resistance was
encoded by the plasmid. MIAPaCa-2 human PDAC cells were obtained
from the American Type Culture Collection (ATCC; Manassas, VA,
USA).
Identification of stably transfected
cells
MIAPaCa-2 cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in an incubator with 5% CO2.
DMEM was supplemented with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). For stable overexpression of
NHERF1, MIAPaCa-2 cells were transfected with 2 µg
pBK-CMV-HA-NHERF1 wt plasmid or the pBK-CMV-HA plasmid (negative
control) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Stably transfected cells were selected using 300 µg/ml G418
(Amresco, LLC, Solon, OH, USA) for 4 weeks and then maintained in
maintenance culture medium containing G418 (150 µg/ml).
Western blotting
Cells were collected and total protein was extracted
from cells stably expressing NHERF1 and from empty
vector-transfected cells using radioimmunoprecipitation lysis
buffer (Beijing CoWin Biotech Co., Ltd., Beijing, China) containing
Halt™ Protease and Phosphatase Inhibitor Cocktail (100X; Thermo
Fisher Scientific, Inc.). Protein levels were quantified using
bicinchoninic acid assays (Beijing CoWin Biotech Co., Ltd.).
Subsequently, 30 µg protein from each sample was subjected to
SDS-PAGE (10% gel). Proteins were then transferred to
nitrocellulose membranes (Sigma-Aldrich; Merck KGaA). The membranes
were blocked with 5% skimmed milk (dissolved in TBST) for 1 h at
25°C, prior to incubation with rabbit anti-human primary antibodies
against NHERF1 (1:1,000 dilution, cat. no. ab88238), and GAPDH
(1:5,000 dilution, cat. no. ab70699) (both from Abcam, Cambridge,
UK), Akt (1:1,000 dilution, cat. no. 9272; Cell Signaling
Technology, Inc., Danvers, MA, USA) or phospho-Akt (p-AKT)
(phospho-Ser473, 1:2,000 dilution, cat. no. 4060; Cell Signaling
Technology, Inc.) overnight at 4°C, followed by incubation with
goat anti-rabbit horseradish peroxidase-conjugated secondary
antibodies (1:1,000 dilution, cat. no. ab6721; Abcam) for 1 h at
room temperature. Detection was facilitated using an enhanced
chemiluminescence western blot kit (Beijing CoWin Biotech Co.,
Ltd.) and images were analyzed using ImageJ software (version 1.62;
National Institutes of Health, Bethesda, MD, USA).
Cell proliferation assay
Cells stably expressing NHERF1 were plated at a
density of 5×103 cells/well in 96-well plates at 37°C in
an incubator with 5% CO2. Cell proliferation was then
assessed every 24 h for 96 h using a Cell Counting Kit-8 (CCK-8;
Sigma-Aldrich; Merck KGaA) according to the manufacturer's
instructions. For each sample at each time-point, 6 wells were
analyzed, and the experiment was repeated independently three
times.
Wound healing assay
Cells were plated at 3×105 cells/well in
6-well plates and grown to 100% confluence. Scratches were created
with 1-ml pipette tips in the cell monolayer, and an image was
immediately captured (0 h). Subsequent images were captured every
12 h, and the migration (scratch width) relative to 0 h was
calculated using Image-Pro Plus analysis software (version 6.0;
Media Cybernetics).
Transwell assay
For the Transwell assay, DMEM containing 10% fetal
bovine serum was added to the lower chamber of Transwell culture
plates. Subsequently, three groups of cells (untransfected, empty
vector and NHERF1-overexpressing) were seeded into the upper
chambers of Transwell 24-well culture plates in serum-free DMEM.
Following incubation for 24 h at 37°C, the cells on the upper
membrane were removed with a cotton swab, and the cells that had
migrated through the membrane were fixed in 4% paraformaldehyde for
15 min at 25°C, and stained with 0.5% crystal violet for 15 min at
25°C. The mean number of cells that had traversed the membrane was
calculated in five random fields under a light microscope
(magnification, ×400).
Statistical analysis
All experiments were repeated at least three times.
SPSS software (version 21.0; IBM Corp., Armonk, NY, USA) and
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) were
used to analyze data. IHC data were analyzed using an independent
samples t-test. Growth curves, Transwell assay and wound healing
assay results were analyzed using a repeated-measures analysis of
variance with Fisher's least significant difference post hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of NHERF1 is downregulated
in pancreatic adenocarcinoma tissues
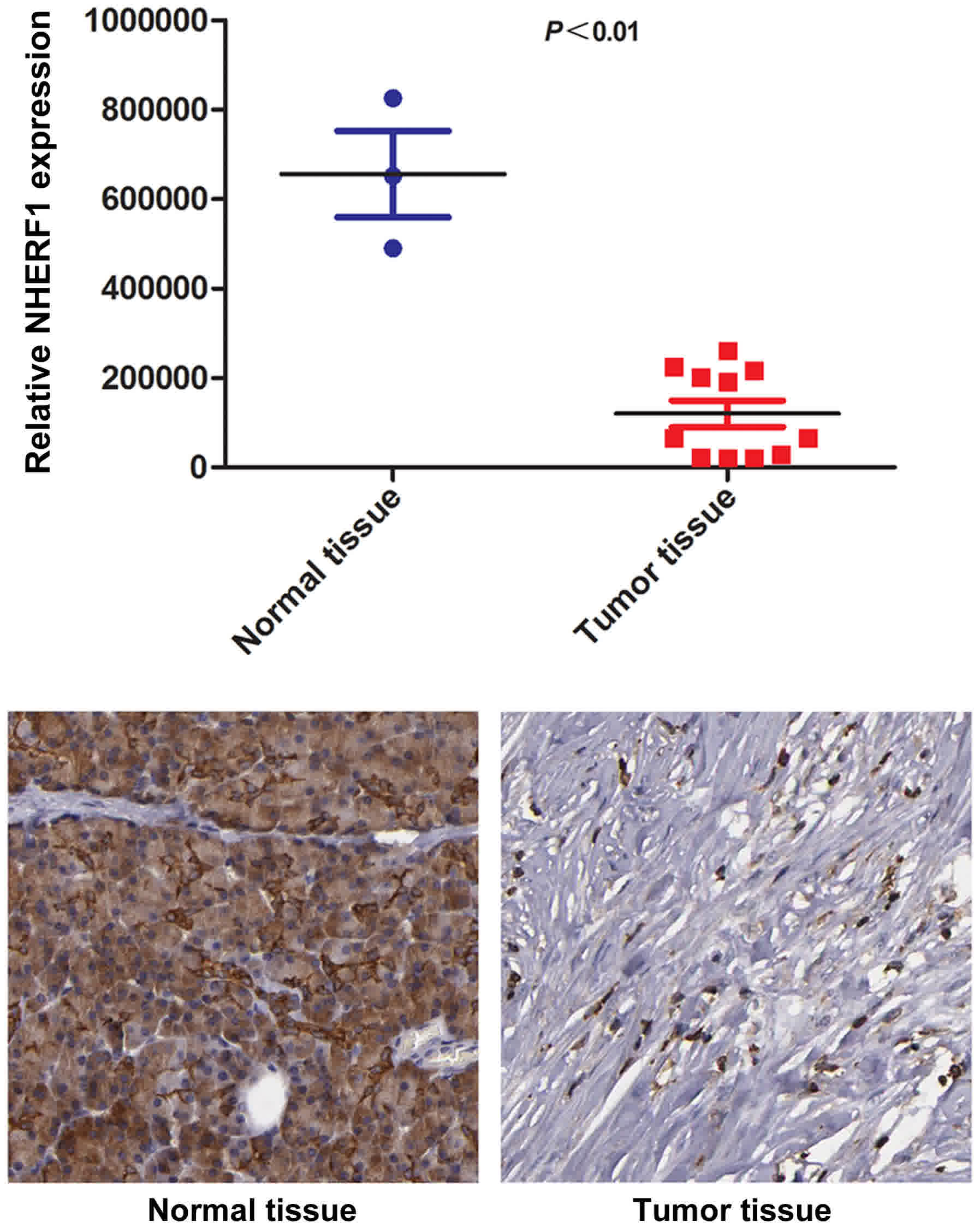
To identify the expression level of NHERF1 in
pancreatic adenocarcinoma tissues, sum of the IOD values of NHERF1
expression levels in pancreatic adenocarcinoma tissues (n=3) and
adjacent normal pancreatic tissues (n=11) were compared using a
Human Protein Atlas Database dataset. The results confirmed that
the expression of NHERF1 was significantly downregulated in
pancreatic adenocarcinoma tissues compared with adjacent tissues
(P<0.01; Fig. 1), indicating that
decreased NHERF1 expression may be associated with pancreatic
cancer progression.
Generation of PDAC cells
overexpressing NHERF1
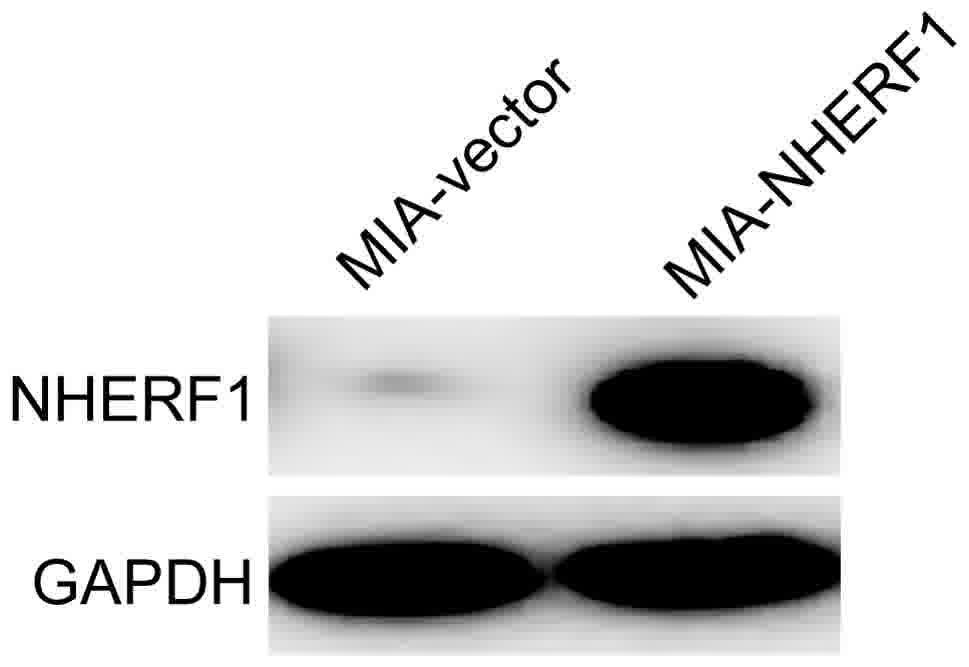
As presented in Fig.
2, NHERF1 was overexpressed in MIAPaCa-2 cells transfected with
the NHERF1 plasmid compared with the cells transfected with the
empty vector. These cells were used to verify whether NHERF1 is
able to attenuate the malignant phenotype in subsequent
experiments. Cells transfected with NHERF1 were designated
MIA-NHERF1 and cells transfected with the empty vector were
designated MIA-vector.
NHERF1 overexpression inhibits the
proliferative ability of MIAPaCa-2 cells
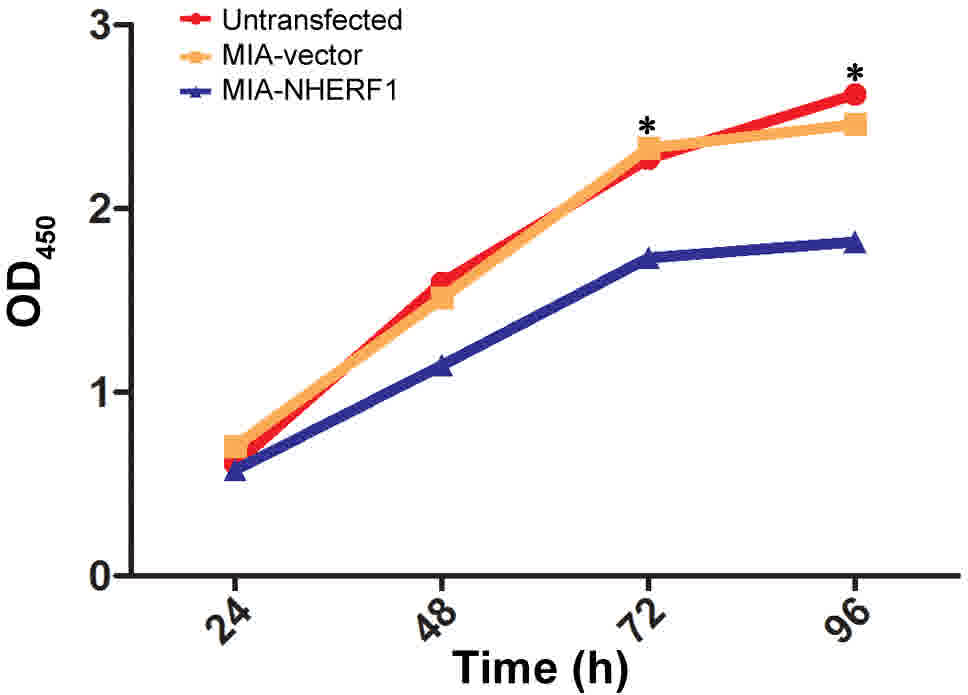
The ability of NHERF1 to modulate the proliferation
of PDAC cells was analyzed using a CCK-8 assay. The results
suggested that NHERF1 overexpression significantly inhibited the
proliferative capacity of MIAPaCa-2 cells following 48 h of
incubation, compared with that of control cells (P<0.05;
Fig. 3). There were no significant
differences observed between MIA-vector and untransfected MIAPaCa-2
cells.
NHERF1 overexpression inhibits the
migratory ability of MIAPaCa-2 cells
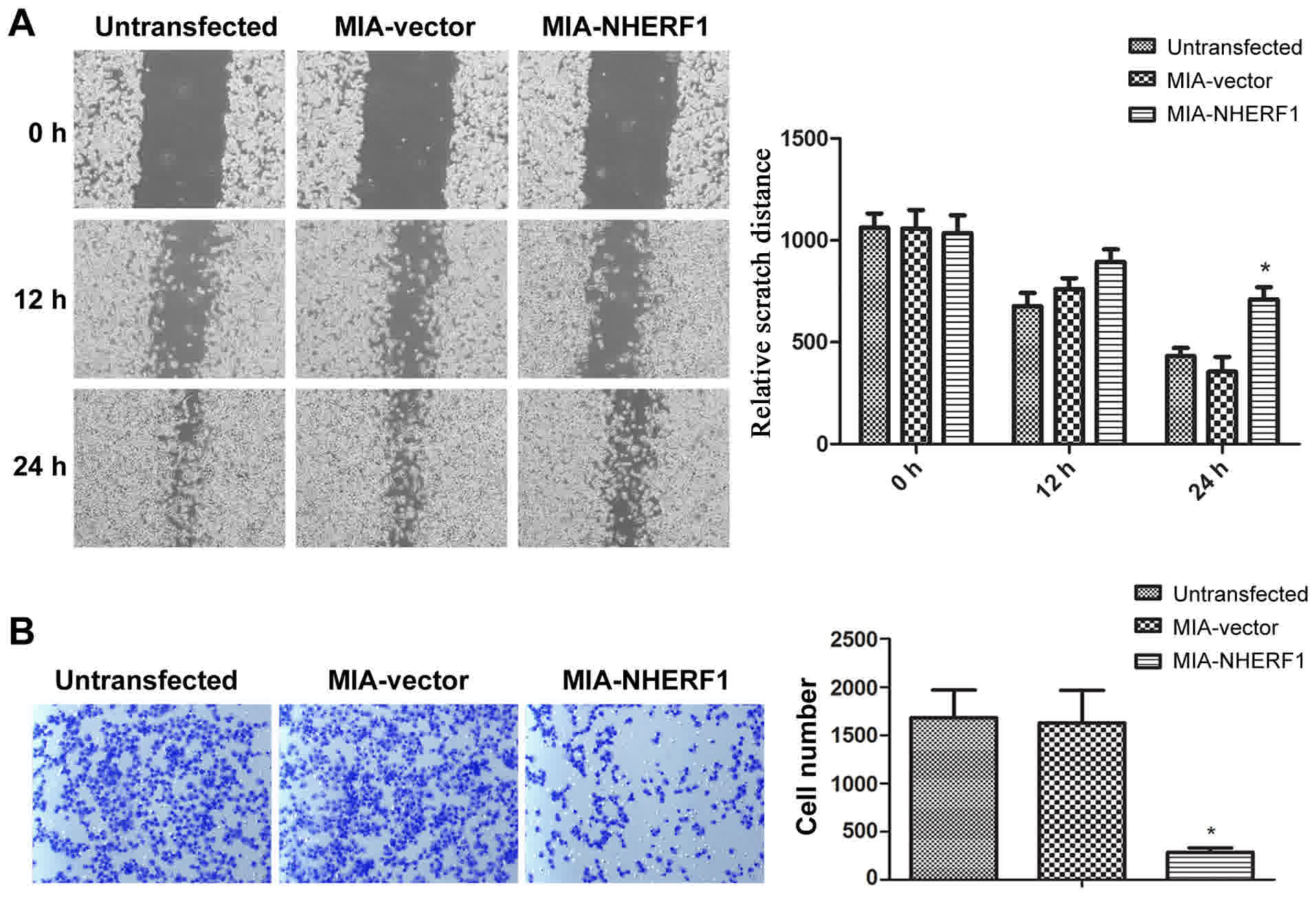
A proliferation assay indicated that NHERF1
exhibited the ability to inhibit tumor cell proliferation. Since
proliferation and migration are closely associated with tumor
progression (9), the ability of
NHERF1 to suppress cell migratory ability was investigated. The
wound-healing assay revealed that, after 24 h, the wound width was
significantly decreased in the MIA-vector and untransfected
MIAPaCa-2 group, while the width reduction was significantly
inhibited in the MIA-NHERF1 group (P<0.05), as presented in
Fig. 4A. Consistently, in the
Transwell assay, NHERF1 overexpression significantly decreased the
number of migrated cells compared with the vector control group
(P<0.05) (Fig. 4B). These results
suggested that NHERF1 overexpression was able to inhibit the
migratory abilities of MIAPaCa-2 cells.
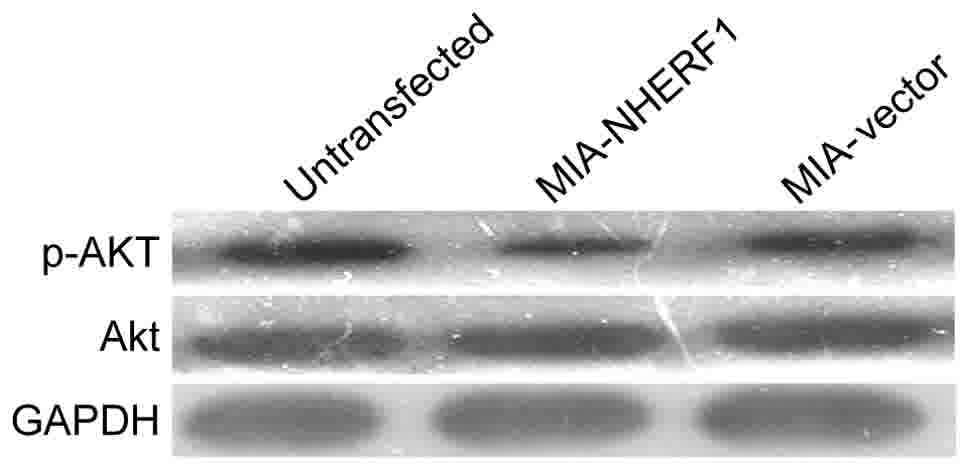
NHERF1 overexpression reduces the Akt
phosphorylation in MIAPaCa-2 cells
The activation of the phosphatidylinositol 3-kinase
(PI3K)/Akt signaling pathway contributes to cell growth and
survival of numerous types of cancer (10,11),
including pancreatic adenocarcinoma (12), and NHERF1 has been demonstrated to
regulate the PI3K/Akt signaling pathway in breast cancer (13). Thus, the activation of Akt was
examined in the present study. The results identified markedly
decreased levels of p-Akt in the MIA-NHERF1 group compared with the
Mia-vector group, whereas there was no notable difference between
the MIA-vector and untransfected MIAPaCa-2 cells. These results
suggest that NHERF1 may inhibit Akt phosphorylation in pancreatic
cancer cells (Fig. 5).
Discussion
In the present study, it was demonstrated that the
expression level of NHERF1 was downregulated in pancreatic
adenocarcinoma tissues, and that NHERF1 overexpression may inhibit
the proliferative and migratory abilities of MIAPaCa-2 PDAC cells
in vitro, while downregulating Akt phosphorylation.
Therefore, NHERF1 may represent a metastasis-suppressing protein in
pancreatic adenocarcinoma. The data suggest that NHERF1 expression
may be able to inhibit the malignant phenotype of pancreatic
adenocarcinoma cells via downregulating the expression of
p-Akt.
Abnormalities in NHERF1 expression have been
demonstrated to be associated with the occurrence, development and
metastasis of cancer (6,14). NHERF1 is hypothesized to directly or
indirectly affect adenocarcinoma behaviors via interaction with
other proteins and signal transduction (15). NHERF1 is known to form a protein
complex with EGFR (16), thereby
mediating the internalization and signal transduction of EGFR to
regulate oncogenic processes. NHERF1 also serves as a binding
partner for G protein-coupled estrogen receptor (GPER), and its
overexpression promotes the stability and activation of GPER in
estrogen receptor-positive invasive breast cancer (17). Furthermore, a previous report outlined
a complex function of NHERF1 in intestinal morphology and presented
evidence for its in vivo tumor-suppressive function upstream
of the Wnt-β-catenin and Hippo-YAP signaling pathways (18). However, the physiological function of
NHERF1 in pancreatic cancer has largely remained unresolved.
Components of the PI3K/Akt/mTOR signaling pathway
are commonly upregulated in malignant tumors, and increased Akt
phosphorylation is commonly observed (19). The activation of Akt signaling is
associated with cell proliferation, migration and invasion
(20). Notably, it has been reported
that NHERF1 inhibits the migration and invasion of human breast
cancer cells via the PI3K/Akt signaling pathway (13). However, it remains unknown known
whether or not NHERF1 may be able to attenuate the malignant
phenotype of pancreatic cancer cells via inhibition of Akt
phosphorylation.
In pancreatic adenocarcinoma tissues, NHERF1
exhibited relatively low endogenous expression. NHERF1 expression
was increased using stable transfection with a pBK-CMV-HA-NHERF1 wt
plasmid. Analysis indicated that NHERF1 overexpression inhibited
the proliferation of MIAPaCa-2 cells, and also suppressed cell
migration. Cell proliferation and migration are associated with
tumor development and substantially contribute to the mortality of
patients with tumors (2–4). Thus, the results of the present study
are relevant to the understanding of tumor development and
therapeutics. With regard to the molecular mechanism underlying the
inhibition of cell proliferation and migration by NHERF1, two
possible mechanisms are hypothesized. The first is that NHERF1
interacts with molecular partners, which serve important functions
in proliferation and migration; for example, NHERF1 is reported to
interact with EGFR and regulate EGFR signaling (21). The other hypothesis is that NHERF1 may
be associated with the downregulation of Akt phosphorylation.
Further experimental studies are required to confirm these
hypotheses, and this may be a focus of future study.
NHERF1 participates in cell signaling and has
multiple physiological functions. The results of the present study
demonstrate that NHERF1 can regulate the malignant behaviors
(proliferative and migratory abilities) of MIAPaCa-2 PDAC cells by
downregulating Akt phosphorylation, and support that NHERF1 may
function as a metastasis-suppressing protein in pancreatic
adenocarcinoma. Thus, NHERF1 may be a potential therapeutic target
for the treatment of pancreatic adenocarcinoma.
Glossary
Abbreviations
Abbreviations:
|
NHERF1
|
Na+/H+ exchanger
regulatory factor 1
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
EGFR
|
epidermal growth factor receptor
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
Akt
|
protein kinase B
|
References
|
1
|
Mann KM, Ying H, Juan J, Jenkins NA and
Copeland NG: KRAS-related proteins in pancreatic cancer. Pharmacol
Ther. 168:29–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vijayvergia N and Cohen SJ: Personalized
medicine in sporadic pancreatic cancer without homologous
recombination-deficiency: Are we any closer? J Gastrointest Oncol.
7:727–737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miyoshi E and Kamada Y: Application of
glycoscience to the early detection of pancreatic cancer. Cancer
Sci. 107:1357–1362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng S, Li Y, Yang Y, Feng D, Yang L, Ma
Q, Zheng S, Meng R, Wang S, Wang S, et al: Breast cancer-derived
K172N, D301V mutations abolish Na+/H+
exchanger regulatory factor 1 inhibition of platelet-derived growth
factor receptor signaling. FEBS Lett. 587:3289–3295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shibata T, Chuma M, Kokubu A, Sakamoto M
and Hirohashi S: EBP50, a beta-catenin-associating protein,
enhances Wnt signaling and is over-expressed in hepatocellular
carcinoma. Hepatology. 38:178–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fraenzer JT, Pan H, Minimo L Jr, Smith GM,
Knauer D and Hung G: Overexpression of the NF2 gene inhibits
schwannoma cell proliferation through promoting PDGFR degradation.
Int J Oncol. 23:1493–1500. 2003.PubMed/NCBI
|
|
7
|
Yao W, Feng D, Bian W, Yang L, Li Y, Yang
Z, Xiong Y, Zheng J, Zhai R and He J: EBP50 inhibits EGF-induced
breast cancer cell proliferation by blocking EGFR phosphorylation.
Amino Acids. 43:2027–2035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Q, Jiao Y, Hao Y, Yan S, Lyu N, Gao H,
Li D, Liu Q, Zheng J and Song N: Targeting of NHERF1 through RNA
interference inhibits the proliferation and migration of metastatic
prostate cancer cells. Oncol Lett. 11:1149–1154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Yang H, Zhang S, Wang Z, Ye F,
Liang C, Wang H and Fang Z: A novel splice variant of supervillin,
SV5, promotes carcinoma cell proliferation and cell migration.
Biochem Biophys Res Commun. 482:43–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Talbert EE, Yang J, Mace TA, Farren MR,
Farris AB, Young GS, Elnaggar O, Che Z, Timmers CD, Rajasekera P,
et al: Dual inhibition of MEK and PI3K/Akt rescues cancer cachexia
through both tumor extrinsic and intrinsic activities. Mol Cancer
Ther. 16:344–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCubrey JA, Rakus D, Gizak A, Steelman
LS, Abrams SL, Lertpiriyapong K, Fitzgerald TL, Yang LV, Montalto
G, Cervello M, et al: Effects of mutations in Wnt/beta-catenin,
hedgehog, Notch and PI3K pathways on GSK-3 activity-diverse effects
on cell growth, metabolism and cancer. Biochim Biophys Acta.
1863:2942–2976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rumman M, Jung KH, Fang Z, Yan HH, Son MK,
Kim SJ, Kim J, Park JH, Lim JH and Hong S: HS-173, a novel PI3K
inhibitor suppresses EMT and metastasis in pancreatic cancer.
Oncotarget. 7:78029–78047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Zhang B, Liu Y and Yin C: EBP50
inhibits the migration and invasion of human breast cancer cells
via LIMK/cofilin and the PI3K/Akt/mTOR/MMP signaling pathway. Med
Oncol. 31:1622014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bellizzi A, Greco MR, Rubino R, Paradiso
A, Forciniti S, Zeeberg K, Cardone RA and Reshkin SJ: The
scaffolding protein NHERF1 sensitizes EGFR-dependent tumor growth,
motility and invadopodia function to gefitinib treatment in breast
cancer cells. Int J Oncol. 46:1214–1224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
James MF, Beauchamp RL, Manchanda N,
Kazlauskas A and Ramesh V: A NHERF binding site links the betaPDGFR
to the cytoskeleton and regulates cell spreading and migration. J
Cell Sci. 117:2951–2961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Curto M, Cole BK, Lallemand D, Liu CH and
McClatchey AI: Contact-dependent inhibition of EGFR signaling by
Nf2/Merlin. J Cell Biol. 177:893–903. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng R, Qin Q, Xiong Y, Wang Y, Zheng J,
Zhao Y, Tao T, Wang Q, Liu H, Wang S, et al: NHERF1, a novel GPER
associated protein, increases stability and activation of GPER in
ER-positive breast cancer. Oncotarget. 7:54983–54997. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Georgescu MM, Gagea M and Cote G:
NHERF1/EBP50 suppresses Wnt-beta-catenin pathway-driven intestinal
neoplasia. Neoplasia. 18:512–523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim HJ, Wang X, Crowe P, Goldstein D and
Yang JL: Targeting the PI3K/PTEN/AKT/mTOR pathway in treatment of
sarcoma cell lines. Anticancer Res. 36:5765–5771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao J, Lin HY, Zhu YY, Zhu YP and Chen
LW: miR-126 regulates proliferation and invasion in the bladder
cancer BLS cell line by targeting the PIK3R2-mediated PI3K/Akt
signaling pathway. Onco Targets Ther. 9:5181–5193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng Z, Wang Q, Zhang Y, He J and Zheng J:
EBP50 interacts with EGFR and regulates EGFR signaling to affect
the prognosis of cervical cancer patients. Int J Oncol.
49:1737–1745. 2016. View Article : Google Scholar : PubMed/NCBI
|