Introduction
Chronic obstructive pulmonary disease (COPD) and
lung cancer remain major public concerns due to the increased
morbidity and mortality with which they are globally associated
(1,2).
COPD is a risk factor for lung cancer, and studies into
associations between COPD and lung cancer have suggested strategies
for decreasing the burden of the conditions (3). Smoking tobacco is a leading cause of
COPD (4–7). However, only a small proportion of
smokers develop COPD, and only a subsection of all patients with
COPD develop lung cancer (3).
Therefore, host differences serve important functions in the
pathogenesis of these diseases. The present study hypothesized that
environmental risk factors and a genetic predisposition may
facilitate the development of COPD and concurrent lung cancer.
Genome-wide association studies have provided statistically
significant associations between COPD and multiple gene variants
that may function in its pathogenesis (8–10).
Therefore, improved understanding of the genetic background of COPD
and of COPD with lung cancer is crucial to uncovering the
pathogenic mechanisms of, and developing novel therapeutic
strategies for, these diseases.
Macrophages serve a key function in defending
against respiratory infections. Macrophage scavenger receptors
(MSRs) are located on the surface of alveolar macrophages and are
associated with a range of functions, including phagocytosis,
antigen presentation, apoptotic cell clearance and bacterial and
particle uptake (11,12). A decreased ability of alveolar
macrophages to phagocytose apoptotic cells and bacteria results in
inflammation, which exacerbates COPD (13). Hirayama et al (14) demonstrated that a scavenger receptor
was also an independent prognostic factor in lung squamous cell
carcinoma, and that it may generate tumor-promoting
microenvironments. Therefore, MSR1 may serve a function in COPD and
in COPD with lung cancer.
MSR1 is located on chromosome 8p22 and is composed
of 11 exons (15). Bossé et al
(8) provided an overview of genes
associated with COPD, including MSR1. Seven missense mutations or
sequence variants (ex3P36A_C>G, ex3S41Y_C>A, ex4V113A_T>C,
ex4P174Y_G>T, ex6P275A_C>G, ex10G369S_G>A, and
ex11H441R_A>G) and one nonsense mutation at codon 293
(ex6R293×_C>T) have been reported to serve an important role in
the structure and function of MSR1 (16). A previous study revealed that a coding
single-nucleotide polymorphism (SNP; ex6P275A_C>G) of MSR1 was
associated with COPD susceptibility, COPD-associated measures of
lung function and abnormalities (10). To the best of our knowledge, there
have been no studies evaluating the association between MSR1
variants and COPD with lung cancer susceptibility. In the present
study, the association between the variants of MSR1 and COPD with
or without lung cancer in China was assessed.
Materials and methods
Patients
From 1 July 2015 to 20 February 2016, 100 patients
with COPD with lung cancer, 100 patients with COPD without lung
cancer and 100 healthy smokers were enrolled at the Department of
Pulmonary & Critical Care Medicine, Shanghai Institute of
Respiratory Disease, Shanghai Ruijin Hospital Affiliated to the
School of Medicine, Shanghai Jiao Tong University (Shanghai,
China). The age of patients with COPD, patients with COPD with lung
cancer subjects and healthy smokers was 68.33±6.47 years,
65.79±7.77 years and 66.47±9.67 years (data are presented as mean ±
standard deviation), respectively, and the male distribution of the
three groups was 76, 79 and 72%, respectively. COPD was diagnosed
according to the criteria proposed by the Global Initiative for
Chronic Obstructive Lung Disease (GOLD) (17). Lung carcinoma was diagnosed by
pathological examination by Dr. Yuan (Department of Pathology,
Shanghai Ruijin Hospital Affiliated to the School of Medicine,
Shanghai Jiao Tong University). Any patient with a history of
bronchial asthma, diabetes, hypertension or any other associated
pulmonary disease was excluded from the study. The healthy controls
exhibited normal lung function without any history of COPD, cancer,
asthma or any other pulmonary infectious diseases. The healthy
smokers were recruited when they attended the Ruijin Hospital for
medical tests from July 2015 to February 2016. The healthy controls
were age-, sex- and cigarette packs smoked/year-matched for
patients with COPD with and without lung cancer. In the present
study, the phenotypes of COPD based on the computed tomography (CT)
imaging of the chest were divided into 3 types: Emphysema, airway
obstruction (airway) and mixed type (hybrid). In patients with COPD
without lung cancer, 21% exhibited an emphysema phenotype, 12%
exhibited airway phenotype and the rest exhibited a hybrid
phenotype. In patients with COPD with lung cancer, 30% exhibited an
emphysema phenotype, 4% exhibited an airway phenotype and the rest
exhibited a hybrid phenotype. All patients who participated in the
present study provided written informed consent. The protocol was
reviewed and approved by the Ruijin Hospital Ethics Committee of
Shanghai Jiao Tong University, School of Medicine.
Lung function evaluation
Lung function tests were performed using a
MasterScreen™ spirometry machine (BD Biosciences, Franklin Lakes,
NJ, USA) 20 min prior to and after inhaling 400 µg salbutamol
(GlaxoSmithKline, Plc., Brentford, UK) Forced expiratory volume in
1 sec (FEV1) and forced vital capacity (FVC) were
measured. The patients were instructed to take the deepest breath
they could and then exhale with maximum intensity into the
spirometry machine for at least 6 sec. The bronchial dilation test
was performed after the patients had inhaled 400 µg salbutamol.
Short-acting inhaled drugs were not administered during the 12 h
prior to testing. Long-acting bronchodilator therapy was ceased for
24 h prior to testing. Theophylline sustained-release tablets and
atropine were not administered for 24 and 8 h, respectively, prior
to spirometry. The present study was not a long term experiment for
one subject. Blood was drawn and the lung function test was
performed when patients were enrolled. The short-acting inhaled
drugs, long-acting bronchodilator therapy, theophylline
sustained-release tablets and atropine were not used during the
study to avoid any effects of the medicine on the lung function
tests. All pulmonary function tests were performed according to the
GOLD criteria (18).
DNA extraction and genotyping
Genomic DNA was extracted from patient whole blood
containing EDTA using a DNeasy Blood & Tissue kit (cat. no.
69506; Qiagen, Inc., Valencia, CA, USA) according to the
manufacturer's protocol, and stored at −20°C until use. The genomic
DNA underwent polymerase chain reaction (PCR) in a 50 µl reaction
mixture (2×Taq PCR Master mix, cat. no. KT201; Tiangen Biotech Co.,
Ltd., Beijing, China). The template was replaced with DEPC water
(Invitrogen; Thermo Fisher Scientific, Inc.) for use as the
negative control. The details of the primers are summarized in
Table I. The PCR reactions consisted
of 35 cycles of 30 sec at 95°C, 30 sec at 55°C and 1 min at 72°C. A
total of 200 patients and 100 healthy controls were tested for
eight SNPs (ex3P36A_C/G, ex3S41Y_C/A, ex4V113A_T/C, ex4P174Y_G/T,
ex6P275A_C/G, ex6R293×_C/T, ex10G369S_G/A and ex11H441R_A/G) in the
5 exonic regions of the MSR1 gene (Table
I). Due to the primer design, the size of the PCR products was
increased compared with that of the exon. Therefore, variants in
the adjacent intron, including rs13306550, were observed.
 | Table I.Characteristics of the SNPs of the
macrophage scavenger receptor 1 gene and associated primer
sequences. |
Table I.
Characteristics of the SNPs of the
macrophage scavenger receptor 1 gene and associated primer
sequences.
| SNP | Primer sequence | Size (bp) | Region | Allele |
|---|
| ex3P36A | F:
5′-CCAAAAGATCAAACAAGC-3′ | 713 | Exon | C/G |
| ex3S41Y | R:
5′-GTCACCCACATTAGAAGG-3′ |
|
| C/A |
| ex4V113A | F:
5′-AATAGGAAAGGGAGAATG-3′ | 741 | Exon | T/C |
| ex4P174Y | R:
5′-TCAGGGTAAACAGGATGA-3′ |
|
| G/T |
| ex6P275A | F:
5′-ATAGAAAAGTGTTAGGCACA-3′ | 733 | Exon | C/G |
| ex6R293ⅹ | R:
5′-GATTTATTCAACGCAAAG-3′ |
|
| C/T |
| ex10G369S | F:
5′-ATTAGTCCTTGCTTGCCTTTT-3′ | 707 | Exon | G/A |
|
| R:
5′-ACGCTGGTCTTGAACTCATTTA-3′ |
|
|
|
| ex11H441R | F:
5′-CCAAGACCCTTTGACATA-3′ |
|
|
|
|
| R:
5′-GACATAAAATAGTAAGCATGAA-3′ | 708 | Exon | A/G |
Statistical analysis
The distribution of age, sex and smoking pack/year
of patients with COPD with or without lung cancer and healthy
smokers all conformed to the law of normal distribution. Data were
expressed as the mean ± standard deviation. Data regarding the lung
function and the COPD Assessment Test (CAT) score did not conform
to the normal distribution; these were expressed as the median
(Q3-Q1). The Mann-Whitney U test was used to
analyze the difference in lung function tests and CAT scores
between patients with COPD with and without rs13306550. An exact
test was used to assess the variation in SNP rs13306550 from the
Hardy-Weinberg equilibrium in all three groups (P>0.05). Data on
the age, sex and smoking pack/year between the three groups were
assessed using a one-way analysis of variance with
Student-Newman-Keuls test performed as a post-hoc test. The and the
variation of each SNP was assessed using Fisher's exact tests. For
all tests, two-sided P-values of <0.05 were considered to
indicate a statistically significant difference. All data were
analyzed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Each
assay was performed in triplicate and independently repeated at
least 3 times.
Results
Study patients
A total of 300 patients were enrolled in the present
study. The characteristics of all patients are listed in Table II. Among the 100 patients with COPD,
the mean age was 68.33±6.47 and 76% were male. All patients with
COPD were diagnosed according to the GOLD criteria (17). The patients with COPD smoked a mean
pack/year of 45.59±32.34. CT scanning serves a notable role in the
identification of the different phenotypes of COPD. Although a
majority of patients with COPD have some form of overlap between
airway morphologic changes and parenchymal destruction, it is
relatively easy to differentiate between those having an emphysema
predominant disease and those presenting with predominant airway
abnormalities. As a result, in this study, the types of COPD based
on the computed tomography imaging of the chest have been divided
into 3 types: Emphysema; airway obstruction (airway); and mixed
type (hybrid) as reported by Grenier et al (19). The phenotype of the patients with COPD
included emphysema (21%), airway (12%) and hybrid (67%). Among the
100 patients with COPD with lung cancer, the mean age was
65.79±7.77 and 79% were male. The phenotype of these patients
included emphysema (30%), airway (4%) and hybrid (66%). These
patients smoked a mean pack/year of 44.94±20.86; 12% of these
patients developed concurrent small cell lung cancer, 39% with
squamous carcinoma and 49% with adenocarcinoma. The mean age of the
healthy smokers was 66.47±9.67 and 72% were male. The healthy
smokers smoked a mean pack/year of 38.13±19.08.
 | Table II.Characteristics of the study
patients. |
Table II.
Characteristics of the study
patients.
| Characteristic | Patients with COPD
(N=100) | Patients with COPD
+ CA (N=100) | Healthy controls
(N=100) | P-value |
|---|
| Age (years) | 68.33±6.47 | 65.79±7.77 | 66.47±9.67 | 0.07a |
| Sex (male %) | 76 | 79 | 72 | 0.51 |
| Cigarette packs
smoked/year | 45.59±32.34 | 44.94±20.86 | 38.13±19.08 | 0.06a |
| Phenotype of
COPD |
|
| – | – |
|
Emphysema | 21 (21%) | 30 (30%) |
|
|
|
Airway | 12 (12%) | 4 (4%) |
|
|
|
Hybrid | 67 (67%) | 66 (66%) |
|
|
| FEV1/FVC
(%) | 49.81±11.50 | 62.18±15.90 | – | – |
| FEV1%
predicted | 39.82±18.00 | 60.70±20.91 | – | – |
| Type of cancer |
|
| – | – |
| Small
cell lung cancer |
| 12 (12%) |
|
|
|
Squamous carcinoma |
| 39 (39%) |
|
|
|
Adenocarcinoma |
| 49 (49%) |
|
|
| Large
cell carcinoma |
| 0 |
|
|
MSR1 exon variants is associated with
risk of COPD with/without lung cancer
In the present study, eight SNPs (ex3P36A_C>G,
ex3S41Y_C>A, ex4V113A_T>C, ex4P174Y_G>T, ex6P275A_C>G,
ex6R293×_C>T, ex10G369S_G>A and ex11H441R_A>G) were
genotyped in 300 patients, including 100 healthy smokers, 100
patients with COPD without lung cancer and 100 patients with COPD
with lung cancer (Table III).
 | Table III.Association between macrophage
scavenger receptor 1 exon variants and the risk of COPD with and
without lung cancer. |
Table III.
Association between macrophage
scavenger receptor 1 exon variants and the risk of COPD with and
without lung cancer.
| SNP | Genotype | Patients with COPD
(N=100) | Patients with COPD
+ CA (N=100) | Healthy controls
(N=100) |
|---|
| ex3P36A | CC | 100 | 100 | 100 |
| ex3S41Y | CC | 100 | 100 | 100 |
| ex4V113A | TT | 100 | 100 | 100 |
| ex4P174Y | GG | 100 | 100 | 100 |
| rs13306550 | AA | 90 | 87 | 99 |
|
| AG | 10 | 13 | 1 |
|
| GG | 0 | 0 | 0 |
|
ex6P275A | CC | 98 | 100 | 99 |
|
| CG | 2 | 0 | 1 |
|
| GG | 0 | 0 | 0 |
| ex6R293ⅹ | CC | 100 | 100 | 100 |
| ex10G369S | GG | 100 | 100 | 100 |
| ex11H441R | AA | 100 | 100 | 100 |
The genotype frequencies of these eight variants did
not significantly differ among the three groups. It was identified
that the SNP rs13306550 is significantly associated with an
increased risk of COPD. However, no significant difference in the
distribution of the SNP rs13306550 was demonstrated between those
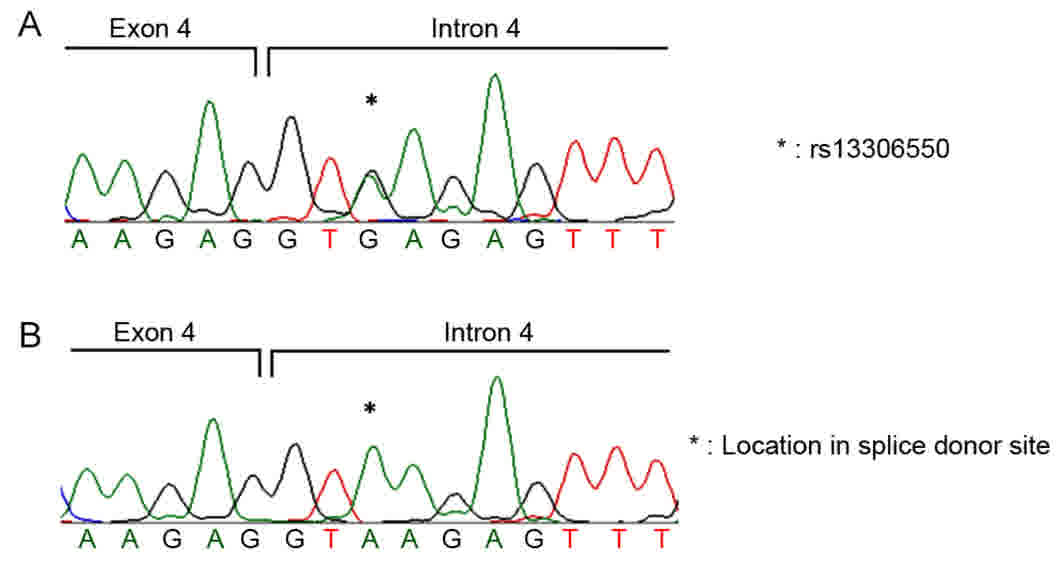
COPD patients with and without lung cancer (P=0.51; Table IV). Of the patients with COPD, 10%
had heterozygous mutations of rs13306550 (Fig. 1A); 13% of patients with COPD with lung
cancer were positive for this variant, all of which were the
heterozygous mutation (Fig. 1A). The
genotyping results of patients without this polymorphism are
presented in Fig. 1B.
 | Table IV.Association of the macrophage
scavenger receptor 1 gene polymorphism rs13306550 with the risk of
COPD and coexistence of COPD and lung cancer. |
Table IV.
Association of the macrophage
scavenger receptor 1 gene polymorphism rs13306550 with the risk of
COPD and coexistence of COPD and lung cancer.
| A, |
|
|
| Patients with COPD
(N=100) | Healthy controls
(N=100) |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Genotype | N | % | N | % | Crude OR (95%
CI) | Adjusted OR (95%
CI) | P-value |
|---|
| AA | 90 | 90 | 99 | 99 | 1.00 | 1.00 |
|
| AG | 10 | 10 | 1 | 1 | 11.00
(1.38–87.64) | 12.07
(1.51–95.52) | 0.0053 |
| GG | 0 | 0 | 0 | 0 |
|
|
|
|
| B, |
|
|
| Patients with COPD+
(N=100) | Healthy controls
(N=100) |
|
|
|
|
|
|
|
|
|
|
| Genotype | N | % | N | % | Crude OR (95%
CI) | Adjusted OR (95%
CI) | P-value |
|
| AA | 87 | 87 | 99 | 99 | 1.00 | 1.00 |
|
| AG | 13 | 13 | 1 | 1 | 14.79
(1.90–115.4) | 15.30
(1.92–121.76) | 0.00088 |
| GG | 0 | 0 | 0 | 0 |
|
|
|
|
| C, |
|
|
| Patients with COPD+
CA (N=100) | Patients with COPD
(N=100) |
|
|
|
|
|
|
|
|
|
|
| Genotype | N | % | N | % | Crude OR (95%
CI) | Adjusted OR (95%
CI) | P-value |
|
| AA | 87 | 87 | 90 | 90 | 1.00 | 1.00 |
|
| AG | 13 | 13 | 10 | 10 | 1.35
(0.56–3.23) | 1.29
(0.53–3.13) | 0.51 |
| GG | 0 | 0 | 0 | 0 |
|
|
|
A significant difference in the distribution of SNP
rs11306550 was identified between patients with COPD with lung
cancer and the healthy controls, but not the patients with COPD
(Table IV). For the patients with
COPD without lung cancer and the controls, and the patients with
COPD with lung cancer and the controls, the odds ratio was >1,
which suggested that the rs13306550 SNP is a harmful factor for
COPD morbidity. This SNP is associated with an increased
susceptibility of COPD, and smokers with rs13306550 tend to develop
COPD more easily compared with those without the SNP. However,
there is no evidence about this polymorphism suggesting that it may
contribute to the pathogenesis of lung cancer in patients with
COPD.
Rs13306550 SNP is not significantly
associated with COPD severity
To assess whether SNP rs13306550 serves a crucial
function in COPD severity, the results of lung function tests and
the CAT score were analyzed between the patients with COPD with and
without this SNP. Data regarding lung function and the CAT score
did not conform to the normal distribution and thus were expressed
as the median (Q3-Q1). The Mann-Whitney U
test was used to analyze the data. The results indicated no
significant difference in lung function between patients with COPD
with and without the SNP rs13306550 (Table V). The results of FEV1/FVC,
FEV1% predicted and the CAT scores between the patients
and the controls did not significantly differ.
 | Table V.Association of the single-nucleotide
polymorphism rs13306550 with COPD severity. |
Table V.
Association of the single-nucleotide
polymorphism rs13306550 with COPD severity.
|
| Genotype |
|
|---|
|
|
|
|
|---|
| Severity | AA | AG + GG | P-value |
|---|
|
FEV1/FVC% | 48.55
(57.90–39.93) | 43.30
(64.00–41.30) | 0.9904 |
| FEV1%
predicted | 35.97
(48.86–28.08) | 32.81
(40.58–28.93) | 0.6502 |
| CAT | 25.50
(28.75–22.00) | 28.00
(30.00–25.00) | 0.4036 |
Discussion
To evaluate the association between MSR1 variants
and the risk of COPD with and without lung cancer, seven missense
mutations (Pro36Ala, Ser41Tyr, Val113Ala, Asp174Tyr, Pro275Ala,
Gly369Ser and His441Arg) and one nonsense mutation at codon 293
(Arg293X), which serve an important role in the function and
structure of MSR1, were identified (16). The SNP P275A is associated with COPD
susceptibility in smokers, as indicated through the results of the
spirometry tests. For the quantitative lung function measures
analyzed using spirometry in the total population of smokers,
subjects with the CG genotype at P275A exhibited a significantly
lower FEF25–75 compared with the CC subjects (P=0.03)
(10,20). However, these results are inconsistent
with those of the present study. In the present study, 100 patients
with COPD with lung cancer, 100 patients with COPD without lung
cancer and 100 healthy controls were genotyped. The results
revealed no significant association between the eight
aforementioned variants and COPD risk, which were inconsistent with
the studies performed by Ohar et al (10) and Ben et al (20). The heterogeneity observed in these
previous studies may be due to the genetic background and sample
size.
Although no significant association with regard to
these eight variants was observed, the SNP rs13306550 was
demonstrated to be associated with COPD morbidity. Of the patients
with COPD, 10% tested positive for the variant, while only one of
the healthy controls was positive for it. Furthermore, 13% of the
patients with COPD and lung cancer tested positive for the variant
but no significant difference was identified between patients with
COPD with and without lung cancer. That this polymorphism is
associated with COPD susceptibility, but does not influence the
pathogenesis of lung cancer in patients with COPD, could explain
the results obtained.
Scavenger receptors on alveolar macrophages are
crucial for identifying and eliminating airborne microorganisms and
inhaled particles (21). Irritants,
including cigarette smoke, may activate macrophages, resulting in
inflammation through the release of neutrophil, monocyte, and
T-lymphocyte chemotactic factors (22). Multiple mechanisms, including
infection, apoptosis and oxidative stress characterize COPD
pathogenesis (23). In innate
immunity, alveolar macrophages represent the initial line of host
immune defenses responsible for the reactions that cigarette smoke
may activate. Alveolar macrophages are also associated with
cytokine secretion (24,25). These functional responses result in
chronic inflammatory conditions and tissue destruction, which may
enhance the emphysema commonly observed in patients with COPD
(26–28). Kaku et al (29) revealed that MSR1 was overexpressed in
patients with COPD and may be associated with COPD
pathogenesis.
It was reported that MSR1 expression is decreased in
tumor tissues compared with in normal tissues, and that this
decreased MSR1 expression enhanced the growth and angiogenesis of
lung carcinoma in mice (20).
Therefore, the overexpression of MSR1 in patients with COPD may not
induce lung cancer pathogenesis and the SNP rs13306550 may not
influence lung cancer susceptibility in patients with COPD.
The present study confirmed the location of the SNP
rs13306550 in MSR1. Rs13306550 (IVS4+3A) is located in the splice
donor site of exon 4 of MSR1 (30).
During evolution, splice donor sites and splice acceptor sites are
commonly conserved. Mutations at the aforementioned splice site
have been revealed to be associated with multiple genetic disorders
(31). The creation of pseudo-exons
within introns, intron retention, exon skipping and the activation
of cryptic splice sites result from abnormal pre-mRNA splicing,
which usually results from splice site mutations (32). In the present study, the distribution
frequencies of the SNP rs13306550 differed significantly between
the patients and the controls. Considering the location of this
variant, it may be associated with COPD susceptibility.
As aforementioned, MSR1 is a macrophage scavenger
receptor that identifies and clears potential pathogens during COPD
exacerbation, including inhaled particles and microorganisms,
modified lipids and apoptotic cells (33,34).
Therefore, MSR1 is associated with the pathogenesis and
exacerbation of COPD. However, no significant association was
identified between the SNP rs13306550 and the lung function test
outcomes or the CAT scores. Therefore, this variant may influence
only COPD pathogenesis and not its exacerbation. However, the small
sample size renders conclusions regarding the function of the
rs13306550 SNP challenging.
To conclude, the present study demonstrated that the
SNP rs13306550 represented a risk factor for COPD susceptibility,
but does not influence lung cancer pathogenesis in patients with
COPD. However, the mechanisms underlying how this variant
influences the development and progression of COPD remain to be
fully understood and require further study.
Acknowledgements
The authors would like to thank Dr. Yuan from the
Department of Pathology, Shanghai Ruijin Hospital Affiliated to the
School of Medicine, Shanghai Jiao Tong University, Shanghai,
China.
Funding
The present study was supported by the Chinese
National Natural Science Foundation (grant no. 81570029) and
Shanghai Key Discipline for Respiratory Diseases (grant. no.,
2017ZZ02014).
Availability of data and material
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX performed the experiments and wrote the whole
manuscript. WC analyzed and interpreted the patient data with LX.
RD and BH produced the blood samples of the subjects. KZ and LZ
helped perform the experiments. MZ and PH contributed to the ideas
of the research and provided financial support.
Ethics approval and consent to
participate
The protocol was reviewed and approved by the Ruijin
Hospital Ethics Committee of Shanghai Jiao Tong University, School
of Medicine (Shanghai, China).
Consent for publication
All patients who participated in the present study
provided written informed consent for publication of the associated
data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MSR
|
macrophage scavenger receptor
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
SNP
|
single-nucleotide polymorphism
|
|
GOLD
|
Global Initiative for Chronic
Obstructive Lung Disease
|
|
FEV1
|
forced expiratory volume in 1 sec
|
|
FVC
|
forced vital capacity
|
|
PCR
|
polymerase chain reaction
|
|
CAT
|
COPD Assessment Test
|
References
|
1
|
Ko FW, Chan KP, Hui DS, Goddard JR, Shaw
JG, Reid DW and Yang IA: Acute exacerbation of COPD. Respirology.
21:1152–1165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gridelli C, Rossi A, Carbone DP, Guarize
J, Karachaliou N, Mok T, Petrella F, Spaggiari L and Rosell R:
Non-small-cell lung cancer. Nat Rev Dis Primers. 1:150092015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Houghton AM: Mechanistic links between
COPD and lung cancer. Nat Rev Cancer. 13:233–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Putcha N, Barr RG, Han MK, Woodruff PG,
Bleecker ER, Kanner RE, Martinez FJ, Smith BM, Tashkin DP, Bowler
RP, et al: Understanding the impact of second-hand smoke exposure
on clinical outcomes in participants with COPD in the SPIROMICS
cohort. Thorax: thoraxjnl-2015-207487. 2016. View Article : Google Scholar
|
|
5
|
Dong R, Xie L, Zhao K, Zhang Q, Zhou M and
He P: Cigarette smoke-induced lung inflammation in COPD mediated
via LTB4/BLT1/SOCS1 pathway. Int J Chron Obstruct Pulmon Dis.
11:31–41. 2015.PubMed/NCBI
|
|
6
|
Cheng L, Liu J, Li B, Liu S, Li X and Tu
H: Cigarette smoke-induced hypermethylation of the GCLC gene is
associated with COPD. Chest. 149:474–482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim CH, Lee YC, Hung RJ, McNallan SR, Cote
ML, Lim WY, Chang SC, Kim JH, Ugolini D, Chen Y, et al: Exposure to
secondhand tobacco smoke and lung cancer by histological type: A
pooled analysis of the international lung cancer consortium
(ILCCO). Int J Cancer. 135:1918–1930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bossé Y: Updates on the COPD gene list.
Int J Chron Obstruct Pulmon Dis. 7:607–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hersh CP, DeMeo DL, Raby BA, Litonjua AA,
Sylvia JS, Sparrow D, Reilly JJ and Silverman EK: Genetic linkage
and association analysis of copd-related traits on chromosome 8p.
COPD. 3:189–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohar JA, Hamilton RF Jr, Zheng S,
Sadeghnejad A, Sterling DA, Xu J, Meyers DA, Bleecker ER and Holian
A: COPD is associated with a macrophage scavenger receptor-1 gene
sequence variation. Chest. 137:1098–1107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Canton J, Neculai D and Grinstein S:
Scavenger receptors in homeostasis and immunity. Nat Rev Immunol.
13:621–634. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peiser L, Mukhopadhyay S and Gordon S:
Scavenger receptors in innate immunity. Curr Opin Immunol.
14:123–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taylor AE, Finney-Hayward TK, Quint JK,
Thomas CM, Tudhope SJ, Wedzicha JA, Barnes PJ and Donnelly LE:
Defective macrophage phagocytosis of bacteria in COPD. Eur Respir
J. 35:1039–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirayama S, Ishii G, Nagai K, Ono S,
Kojima M, Yamauchi C, Aokage K, Hishida T, Yoshida J, Suzuki K and
Ochiai A: Prognostic impact of CD204-positive macrophages in lung
squamous cell carcinoma: Possible contribution of Cd204-positive
macrophages to the tumor-promoting microenvironment. J Thorac
Oncol. 7:1790–1797. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Emi M, Asaoka H, Matsumoto A, Itakura H,
Kurihara Y, Wada Y, Kanamori H, Yazaki Y, Takahashi E, Lepert M, et
al: Structure, organization, and chromosomal mapping of the human
macrophage scavenger receptor gene. J Biol Chem. 268:2120–2125.
1993.PubMed/NCBI
|
|
16
|
Walsh PC: Germline mutations and sequence
variants of macrophage scavenger receptor 1 gene are associated
with prostate cancer risk. J Urol. 169:1589–1590. 2003.PubMed/NCBI
|
|
17
|
Pauwels RA, Buist AS, Calverley PM,
Jenkins CR and Hurd SS: GOLD Scientific Committee: Global strategy
for the diagnosis, management, and prevention of chronic
obstructive pulmonary disease. NHLBI/WHO global initiative for
chronic obstructive lung disease (GOLD) workshop summary. Am J
Respir Crit Care Med. 163:1256–1276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rabe KF, Hurd S, Anzueto A, Barnes PJ,
Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R,
van Weel C, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 176:532–555. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grenier PA, Beigelman-Aubry C, Fetita CI
and Brillet PY: CT imaging of chronic obstructive pulmonary
disease: Role in phenotyping and interventions. Expert Opin Med
Diagn. 3:689–703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ben J, Jin G, Zhang Y, Ma B, Bai H, Chen
J, Zhang H, Gong Q, Zhou X, Zhang H, et al: Class A scavenger
receptor deficiency exacerbates lung tumorigenesis by cultivating a
procarcinogenic microenvironment in humans and mice. Am J Respir
Crit Care Med. 186:763–772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palecanda A and Kobzik L: Receptors for
unopsonized particles: The role of alveolar macrophage scavenger
receptors. Curr Mol Med. 1:589–595. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barnes PJ: Alveolar macrophages as
orchestrators of COPD. COPD. 1:59–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valacchi G, Maioli E, Sticozzi C,
Cervellati F, Pecorelli A, Cervellati C and Hayek J: Exploring the
link between scavenger receptor B1 expression and chronic
obstructive pulmonary disease pathogenesis. Ann N Y Acad Sci.
1340:47–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barnes PJ: Inflammatory mechanisms in
patients with chronic obstructive pulmonary disease. J Allergy Clin
Immunol. 138:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nurwidya F, Damayanti T and Yunus F: The
role of innate and adaptive immune cells in the immunopathogenesis
of chronic obstructive pulmonary disease. Tuberc Respir Dis
(Seoul). 79:5–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu G and Yang H: Modulation of macrophage
activation and programming in immunity. J Cell Physiol.
228:502–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng C, Huang C, Ma TT, Bian EB, He Y,
Zhang L and Li J: SOCS1 hypermethylation mediated by DNMT1 is
associated with lipopolysaccharide-induced inflammatory cytokines
in macrophages. Toxicol Lett. 225:488–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cosio MG, Saetta M and Agusti A:
Immunologic aspects of chronic obstructive pulmonary disease. N
Engl J Med. 360:2445–2454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaku Y, Imaoka H, Morimatsu Y, Komohara Y,
Ohnishi K, Oda H, Takenaka S, Matsuoka M, Kawayama T, Takeya M and
Hoshino T: Overexpression of CD163, CD204 and CD206 on alveolar
macrophages in the lungs of patients with severe chronic
obstructive pulmonary disease. PLoS One. 9:e874002014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lindmark F, Jonsson BA, Bergh A, Stattin
P, Zheng SL, Meyers DA, Xu J and Gronberg H: Analysis of the
macrophage scavenger receptor 1 gene in Swedish hereditary and
sporadic prostate cancer. Prostate. 59:132–140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng L, Liu W, Feng W, Wang X, Dang H, Gao
L, Yao J and Zhang X: A novel donor splice-site mutation of major
intrinsic protein gene associated with congenital cataract in a
Chinese family. Mol Vis. 19:2244–2249. 2013.PubMed/NCBI
|
|
32
|
Krawczak M, Reiss J and Cooper DN: The
mutational spectrum of single base-pair substitutions in mRNA
splice junctions of human genes: Causes and consequences. Hum
Genet. 90:41–54. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuronuma K, Sano H, Kato K, Kudo K,
Hyakushima N, Yokota S, Takahashi H, Fujii N, Suzuki H, Kodama T,
et al: Pulmonary surfactant protein A augments the phagocytosis of
Streptococcus pneumoniae by alveolar macrophages through a casein
kinase 2-dependent increase of cell surface localization of
scavenger receptor A. J Biol Chem. 279:21421–21430. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taylor PR, Martinez-Pomares L, Stacey M,
Lin HH, Brown GD and Gordon S: Macrophage receptors and immune
recognition. Annu Rev Immunol. 23:901–944. 2005. View Article : Google Scholar : PubMed/NCBI
|