Introduction
Hepatocellular carcinoma (HCC) is one of the most
common and aggressive human malignancies worldwide (1). The majority of the cancer burden (85%)
is present in developing countries, with a particularly high
prevalence in regions where hepatitis B virus (HBV) infection is
endemic, including South-East Asia and Sub-Saharan Africa (2). The majority of HCC remains incurable
once it has become metastatic and has a poor prognosis, although
there have previously been advances in cancer treatment with
respect to surgery, chemotherapy and biological therapy (3). The major causes of HCC are viral
infections, alcohol and tobacco use and HBV infection (4). Despite efforts made to identify
appropriate prognostic markers for HCC, including primary tumor
size, elevated α-fetoprotein (AFP) levels and gene expression
markers in the primary tumor, these methods have not proven
adequate to predict the prognosis of all patients with HCC
(5,6).
Therefore, a reliable clinical biomarker for HCC diagnosis and
prediction of clinical outcome is urgently required.
Long non-coding RNAs (lncRNAs) are >200
nucleotides in length and do not code for proteins but interact
with them (7). Although lncRNAs are
not as well characterized as small non-coding microRNAs, lncRNAs
are critical in the regulation of mechanisms underlying cellular
processes, including stem cell pluripotency, cell growth, cell
cycle, apoptosis, metabolism and cancer migration (8–13).
Functional lncRNAs can be used for cancer diagnosis and prognosis,
in addition to serving as potential therapeutic targets. Tang et
al (14) identified three
lncRNAs, RP11-160H22.5, XLOC_014172 and LOC149086, which were
upregulated in HCC tissues in comparison with in the cancer-free
controls of their study. Furthermore, XLOC_014172 and LOC149086
were confirmed to be highly expressed in patients with metastatic
HCC (14). The majority of patients
demonstrated a decreased expression level of the three lncRNAs
following surgery. In 2013, Xu et al (15) identified a lncRNA, lncRNA-LALR1, which
participated in liver regeneration. In addition, in 2014, Yuan
et al (16) observed a lncRNA,
lncRNA-ATB, that was upregulated in HCC tissues and modulated
tumorigenesis and tumor progression. In 2014, Wang et al
(17) revealed that the oncofetal
long noncoding RNA PVT1 promoted proliferation and stem cell-like
properties of HCC cells.
Functional lncRNAs are considered to be promising
candidates for future cancer diagnosis and therapeutic strategies
(18). BRAF-activated non-protein
coding RNA (BANCR) is a recurrently overexpressed, previously
unannotated 693-baspair transcript on chromosome 9 with a potential
functional role in melanoma cell migration (19,20). BANCR
is strongly associated with V600EBRAF, the most frequent mutation
type of the BRAF gene. Furthermore, high frequencies of
V600EBRAF mutations are detected in malignant melanoma (70%),
papillary thyroid cancer (36–53%) and colorectal cancer (CRC;
5–22%) (21). In 2014, Li et
al (22) demonstrated that BANCR
promoted proliferation in malignant melanoma by regulating the
mitogen-activated protein kinase signaling pathway activation. Wang
et al (23) revealed that
BANCR contributed to cell proliferation and activated autophagy in
papillary thyroid carcinoma cells. In a study investigating CRC,
Guo et al (24) demonstrated
that BANCR contributed to colorectal cancer migration by inducing
epithelial-mesenchyme transition and the results suggested the
potential application of BANCR in the therapeutic treatment of CRC;
however, the expression pattern and biological functions of BANCR
in HCC remain unknown. In the present study, we investigated the
expression level of BANCR in HCC tumor tissues by performing
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). The results revealed that BANCR expression level is
significantly lower in HCC tumors compared with in para-cancerous
tissues. In the tumors tissues samples, lower BANCR expression
level was closely associated with serum AFP levels and HCC tumor
number.
Materials and methods
Patients and specimens
Patient's data were accessed from the databank of
the Department of Hepatobiliary Surgery of Beijing 302 Hospital
(Beijing, China) of 46 consecutive patients (39 male and 7 female;
mean age, 52.2±10.1 years) with HBV-associated HCC. The patients
enrolled in the present study underwent surgery at Beijing 302
Hospital between October 2013 and March 2015. None of the patients
had received preoperative chemotherapy or radiation therapy prior
to surgery. All the diagnoses of HCC were histopathologically
confirmed. The present study was approved by the Ethics Committee
of Beijing 302 Hospital. Written informed consent was obtained from
all patients prior to collection of tumor tissues and
para-cancerous liver tissues samples. The resected tumor tissues
and para-cancerous tissue samples were immediately snap-frozen in
RNA ladder following resection and stored in the tissue bank until
analysis. All the clinical data were collected by doctors, and the
experimental operators were blinded to the clinical data.
RNA preparation and RT-qPCR
Total RNA from frozen HCC tissues and para-cancerous
tissues samples (n=46) was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacturer's protocol. The RNA integrity
was evaluated using a NanoDrop 1000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). cDNA from all
samples was synthesized from 50 ng total RNA from each sample.
BANCR expression levels were quantified by RT-qPCR, which was
performed using the ABI7500 system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and Thermo Fisher Scientific Maxima
SYBR® Green qPCR Master kit (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Briefly, reactions
were performed in a 20 µl mixture containing 100 ng cDNA template,
10 µl 2X PCR Mix and 0.5 µl each of forward and reverse primers.
The thermocycling conditions used were as follows: 95°C for 10 min,
followed by 25 cycles of 95°C for 10 sec, 60°C for 30 sec and 72°C
for 30 sec. RT-PCR was performed in duplicate for each sample. The
expression level of GAPDH was also detected as the endogenous
control, and all the samples were normalized to human GAPDH. The
primer sequences used in the present study were as follows: BANCR
forward, 5′-ACAGGACTCCATGGCAAACG-3′ and reverse,
5′-ATGAAGAAAGCCTGGTGCAGT-3′; and GAPDH forward,
5′-CAGCCTCAAGATCATCAGCA-3′ and reverse, 5′-TGTGGTCATGAGTCCTTCCA-3′.
The median from experiments performed in triplicate was used to
determine relative lncRNA concentrations (ΔCq=Cq median lncRNAs-Cq
median GAPDH). Expression fold changes were calculated using the
2−ΔΔCq method (25).
Statistical analysis
Statistical significances between groups were
determined by two-tailed Student's t-test. A receiver operating
characteristics (ROC) curve was plotted to determine how well the
expression level of BANCR discriminated between HBV HCC tumor
tissues and para-cancerous tissues. The association between BANCR
expression level and clinicopathological characteristics was
analyzed using one-way analysis of variance with Bonferroni
correction. All statistical analyses were performed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
lncRNA BANCR is downregulated in
HBV-associated HCC tissues compared with in para-cancerous
tissues
To assess the potential clinical significance of
BANCR, its expression levels in HCC tissues and para-cancerous
tissue samples were analyzed. Firstly, the expression level of
BANCR in HBV HCC tissues samples and para-cancerous tissues samples
were determined by RT-qPCR. The results demonstrated that the
expression levels of BANCR were markedly decreased in HCC tissues
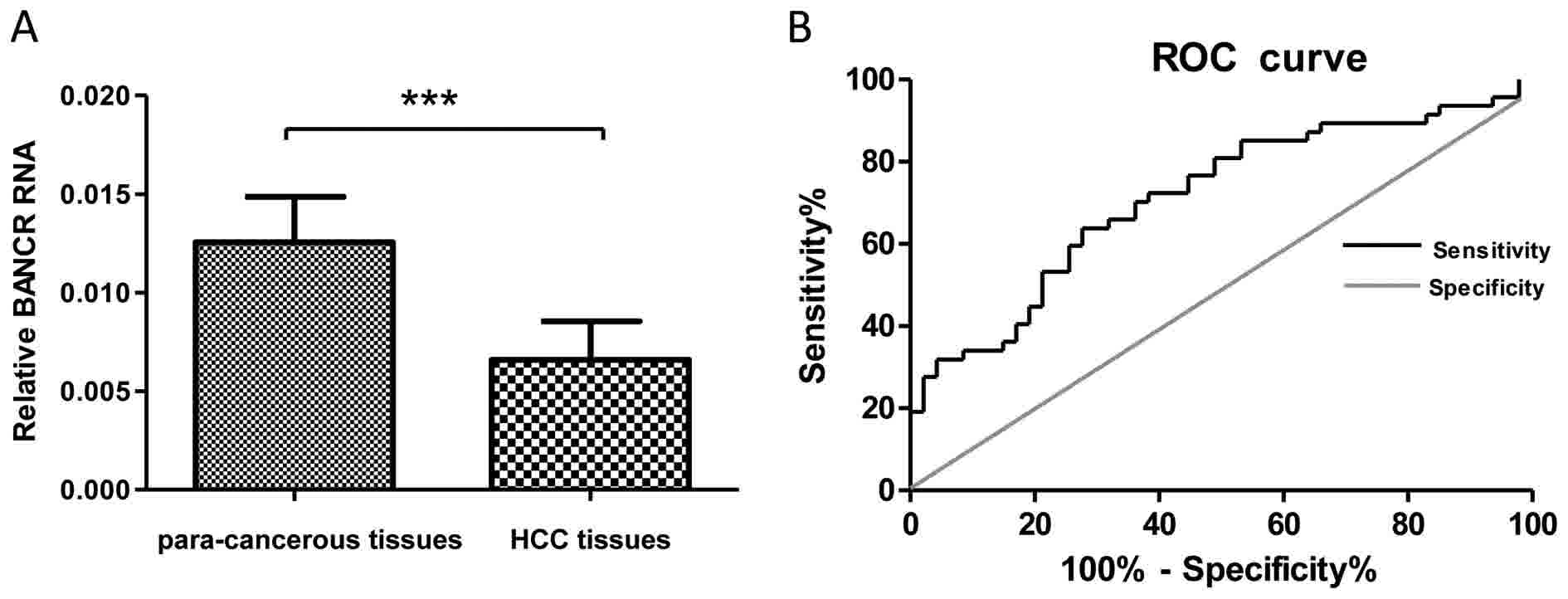
compared with in para-cancerous tissues (P<0.001; Fig. 1A). Subsequently, ROC analysis was used
to evaluate the suitability of BANCR expression level to
discriminate between the tumor and control tissue samples. Total
area under the curve (AUC) for BANCR was 0.76 (Fig. 1B), which suggested that BANCR has
adequate sensitivity and specificity to discriminate between HCC
and para-cancerous tissue samples. These results revealed that the
expression levels of BANCR significantly decreased in
HBV-associated HCC tissues compared with in para-cancerous tissue
samples.
lncRNA BANCR expression level is
associated with the AFP levels and tumor numbers in patients with
HBV-associated HCC
To investigate whether the expression levels of
lncRNA BANCR in the HBV-associated HCC tissues are associated with
the disease clinicopathological parameters, the present study
analyzed the expression profiles of BANCR in tumor tissue samples
according to AFP levels and tumors numbers. Since serum AFP level
is a critical tumor biomarker for patients with HCC, the evidence
resulted in the investigation of whether there is any association
between BANCR expression level and serum AFP concentration status
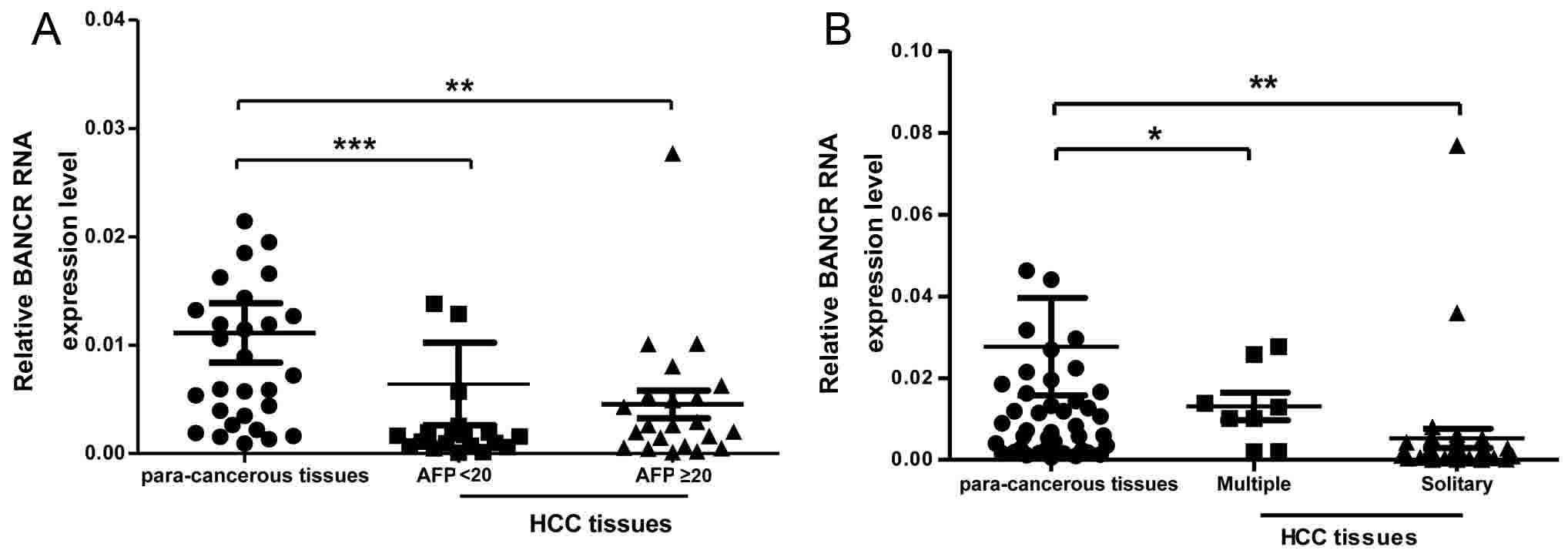
in patients with HBV-associated HCCs. As presented in Fig. 2A, the BANCR expression level was lower
in the tissue samples with serum AFP ≥20 ng/ml compared with in
tissue samples that had serum AFP <20 ng/ml from patients with
HBV HCC. In addition, the present study compared the BANCR
expression level and solitary or multiple tumor numbers in patients
with HBV-associated HCC (Fig. 2B);
these results revealed that the BANCR expression level and tumor
numbers were positively associated in these patients.
Association between BANCR expression
level and the clinicopathological parameters of patients with
HBV-associated HCC
To further analyze the association between lncRNA
expression level and various clinicopathological parameters of
patients with HBV HCC, 46 patients were divided into high (n=23)
and low (n=23) BANCR expression level groups, according to the mean
value of the expression levels of BANCR in tumor tissue samples. As
presented in Table I,
clinicopathological factors were investigated between the two
groups and the low BANCR expression level group demonstrated
significantly higher serum AFP levels (P=0.008) and more advanced
tumor numbers (P=0.047) compared with the high BANCR expression
level group. However, no significant association was revealed
between BANCR expression level and other clinicopathological
features, including age, gender, tumor size, clinical stage and
liver cirrhosis (P>0.05).
 | Table I.Association between BANCR expression
and clinicopathological characteristics of patients with
HBV-associated HCC. |
Table I.
Association between BANCR expression
and clinicopathological characteristics of patients with
HBV-associated HCC.
|
|
| BANCR expression
level, n |
|
|---|
|
|
|
|
|
|---|
| Parameters | Total, n | Low | High | P-value |
|---|
| Gender |
|
|
| 0.665 |
| Male | 40 | 19 | 21 |
|
|
Female | 6 | 4 | 2 |
|
| Age |
|
|
| 0.475 |
| <60
years | 36 | 17 | 19 |
|
| ≥60
years | 10 | 6 | 4 |
|
| Tumor size |
|
|
| 0.767 |
| <5
cm | 25 | 12 | 13 |
|
| ≥5
cm | 21 | 11 | 10 |
|
| AFP |
|
|
| 0.008b |
| <20
ng/ml | 21 | 15 | 6 |
|
| ≥20
ng/ml | 25 | 8 | 17 |
|
| Histological
grade |
|
|
| 0.349 |
|
Well | 41 | 20 | 21 |
|
|
Moderately-poorly | 5 | 3 | 2 |
|
| TNM stage |
|
|
| 0.491 |
|
I–II | 35 | 19 | 16 |
|
|
III | 11 | 4 | 7 |
|
| Liver
cirrhosis |
|
|
| 0.326 |
|
Absence | 33 | 15 | 18 |
|
|
Presence | 13 | 8 | 5 |
|
| Tumor number |
|
|
| 0.047a |
|
Solitary | 38 | 22 | 16 |
|
|
Multiple | 8 | 1 | 7 |
|
Discussion
With advancements in genome wide sequencing and
high-resolution microarray technology, more attention has been
received by lncRNAs (26–28). A previous study revealed that
dysexpression of lncRNAs is associated with numerous types of
disease (29). Furthermore, lncRNAs
have been identified to perform an important function in the
progression of various types of cancer. Numerous well-studied
lncRNAs, including Hox transcription antisense RNA (30–32),
maternally expressed 3 (33) and
LOC285194 (34) have been reported to
be strongly associated with survival of patients with cancer; thus,
they have been determined to be prognostic factors for specific
types of cancer.
LncRNA BANCR was first revealed to have a potential
functional role in melanoma cell migration (22). Other previous studies also
investigated the expression levels and functions of BANCR in
various malignancies. BANCR has been demonstrated to be upregulated
in numerous types of solid tumors, including papillary thyroid
cancer (23) and CRC (21). Therefore, the present study
hypothesized that BANCR also had similar effects on the
tumorigenesis, development and progression of HCC. To confirm these
hypotheses, the present study firstly determined the BANCR
expression levels in 46 pairs of HBV-associated HCC tissues and
their para-cancerous tissues by RT-qPCR. HCC para-cancerous tissues
always demonstrate liver cirrhosis or chronic inflammations and
fibrosis (35); thus, para-cancerous
tissues were used as the control in the present study. It was
revealed that BANCR was significantly downregulated in
HBV-associated HCC and this differs from the overexpression of
BANCR observed in melanoma tissue samples. The results of the
present study may provide evidence for the tissue specificity of
lncRNA. However, the underlying molecular mechanisms of lncRNA
BANCR require further investigation. Only HBV-associated HCC
patients were included in the present study and it was demonstrated
that the expression level of BANCR in HBV-associated HCC tissues
were lower compared with in para-cancerous tissues. Furthermore,
the ROC curve analysis was performed in order to identify the
diagnostic significance of BANCR expression level in HBV-associated
HCC and the AUC area was 0.76, revealing a possible diagnostic
value of BANCR. However, a considerably larger cohort of patients
with HBV-associated HCC is required to better support these
findings.
Aberrant expression level of BANCR was previously
reported to be involved in the progression of numerous tumors and
can be used as a prognostic indicator (36). Subsequently, the present study
described the association between BANCR expression level and
various clinicopathological parameters. The results also
demonstrated positive associations between the BANCR expression
level and tumor number and serum AFP value. As a diagnostic
indicator, AFP serves an essential role in the diagnosis of HCC and
there was a significant association between BANCR expression level
and serum AFP in the present study. Thus, BANCR may be a biomarker
for the occurrence of HBV-associated HCC. However, no significant
association was identified between the BANCR expression level and
other clinic pathological features, including age, gender, tumor
size, clinical stage and liver cirrhosis. Further in vitro
and in vivo experiments are presently being performed to
investigate the biological function and molecular basis of lncRNA
BANCR expression in patients with HCC.
In conclusion, the results of the present study
demonstrated, to the best of our knowledge, for the first time that
lncRNA BANCR expression level was significantly lower in
HBV-associated HCC tissues, and downregulation of lncRNA BANCR was
positively associated with serum AFP level and tumor number in
patients with HBV-associated HCC. These results suggested that
lncRNA BANCR may be a potential novel diagnosis biomarker for
HBV-associated HCC.
Acknowledgements
This work was supported by the funding from Natural
Science Foundation of Beijing Municipality (7162185).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: A global and regional perspective. Oncologist. 15 Suppl
4:5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blum HE: Hepatocellular carcinoma: Therapy
and prevention. World J Gastroenterol. 11:7391–7400.
2005.PubMed/NCBI
|
|
4
|
Liu YR, Tang RX, Huang WT, Ren FH, He RQ,
Yang LH, Luo DZ, Dang YW and Chen G: Long noncoding RNAs in
hepatocellular carcinoma: Novel insights into their mechanism.
World J Hepatol. 7:2781–2791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan K, Liang XT, Zhang HK, Zhao JJ, Wang
DD, Li JJ, Lian Q, Chang AE, Li Q and Xia JC: Characterization of
bridging integrator 1 (BIN1) as a potential tumor suppressor and
prognostic marker in hepatocellular carcinoma. Mol Med. 18:507–518.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tu ZQ, Li RJ, Mei JZ and Li XH:
Down-regulation of long non-coding RNA GAS5 is associated with the
prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol.
7:4303–4309. 2014.PubMed/NCBI
|
|
7
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: Past, present and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Xie G, Singh M, Ghanbarian AT,
Raskó T, Szvetnik A, Cai H, Besser D, Prigione A, Fuchs NV, et al:
Primate-specific endogenous retrovirus-driven transcription defines
naive-like stem cells. Nature. 516:405–409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Wang Y, Li J, Zhang Y, Yin H and
Han B: CRNDE, a long-noncoding RNA, promotes glioma cell growth and
invasion through mTOR signaling. Cancer Lett. 367:122–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leveille N, Melo CA, Rooijers K,
Díaz-Lagares A, Melo SA, Korkmaz G, Lopes R, Moqadam Akbari F, Maia
AR, Wijchers PJ, et al: Genome-wide profiling of p53-regulated
enhancer RNAs uncovers a subset of enhancers controlled by a
lncRNA. Nat Commun. 6:65202015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu JJ, Wang Y, Ding JX, Jin HY, Yang G
and Hua KQ: The long non-coding RNA HOTAIR promotes the
proliferation of serous ovarian cancer cells through the regulation
of cell cycle arrest and apoptosis. Exp Cell Res. 333:238–248.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui M, Xiao Z, Wang Y, Zheng M, Song T,
Cai X, Sun B, Ye L and Zhang X: Long noncoding RNA HULC modulates
abnormal lipid metabolism in hepatoma cells through an
miR-9-mediated RXRA signaling pathway. Cancer Res. 75:846–857.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang J, Jiang R, Deng L, Zhang X, Wang K
and Sun B: Circulation long non-coding RNAs act as biomarkers for
predicting tumorigenesis and metastasis in hepatocellular
carcinoma. Oncotarget. 6:4505–4515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu D, Yang F, Yuan JH, Zhang L, Bi HS,
Zhou CC, Liu F, Wang F and Sun SH: Long noncoding RNAs associated
with liver regeneration 1 accelerates hepatocyte proliferation
during liver regeneration by activating Wnt/b-catenin signaling.
Hepatology. 58:739–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-b promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Yuan JH, Wang SB, Yang F, Yuan SX,
Ye C, Yang N, Zhou WP, Li WL, Li W and Sun SH: Oncofetal long
noncoding RNA PVT1 promotes proliferation and stem cell-like
property of hepatocellular carcinoma cells by stabilizing NOP2.
Hepatology. 60:1278–1290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi K, Yan I, Haga H and Patel T:
Long noncoding RNA in liver diseases. Hepatology. 60:744–753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Flockhart RJ, Webster DE, Qu K,
Mascarenhas N, Kovalski J, Kretz M and Khavari PA: BRAFV600E
remodels the melanocyte transcriptome and induces BANCR to regulate
melanoma cell migration. Genome Res. 22:1006–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCarthy N: Epigenetics. Going places with
BANCR. Nat Rev Cancer. 12:4512012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li R, Zhang L, Jia L, Duan Y, Li Y, Bao L
and Sha N: Long non-coding RNA BANCR promotes proliferation in
malignant melanoma by regulating MAPK pathway activation. PLoS One.
9:e1008932014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Guo Q, Zhao Y, Chen J, Wang S, Hu
J and Sun Y: BRAF-activated long non-coding RNA contributes to cell
proliferation and activates autophagy in papillary thyroid
carcinoma. Oncol Lett. 8:1947–1952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo Q, Zhao Y, Chen J, Hu J, Wang S, Zhang
D and Sun Y: BRAF-activated long non-coding RNA contributes to
colorectal cancer migration by inducing epithelial-mesenchymal
transition. Oncol Lett. 8:869–875. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma H, Hao Y, Dong X, Gong Q, Chen J, Zhang
J and Tian W: Molecular mechanisms and function prediction of long
noncoding RNA. ScientificWorldJournal. 2012:5417862012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang JY, Lee JC, Chang YT, Hou MF, Huang
HW, Liaw CC and Chang HW: Long noncoding RNAs-related diseases,
cancers, and drugs. ScientificWorldJournal. 2013:9435392013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu MX, Chen X, Chen G, Cui QH and Yan GY:
A computational framework to infer human disease-associated long
noncoding RNAs. PLoS One. 9:e844082014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakagawa T, Endo H, Yokoyama M, Abe J,
Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T and Satoh K: Large
noncoding RNA HOTAIR enhances aggressive biological behavior and is
associated with short disease-free survival in human non-small cell
lung cancer. Biochem Biophys Res Commun. 436:319–324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu
WQ, Xie WP and Hou YY: Long non-coding RNA MEG3 inhibits NSCLC
cells proliferation and induces apoptosis by affecting p53
expression. BMC Cancer. 13:4612013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qi P, Xu MD, Ni SJ, Huang D, Wei P, Tan C,
Zhou XY and Du X: Low expression of LOC285194 is associated with
poor prognosis in colorectal cancer. J Transl Med. 11:1222013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zou H, Shao CX, Zhou QY, Zhu GQ, Shi KQ,
Braddock M, Huang DS and Zheng MH: The role of lncRNAs in
hepatocellular carcinoma: Opportunities as novel targets for
pharmacological intervention. Expert Rev Gastroenterol Hepatol.
10:331–340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan YH, Ye MH, Wu L, Wu MJ, Lu SG and Zhu
XG: BRAF-activated lncRNA predicts gastrointestinal cancer patient
prognosis: A meta-analysis. Oncotarget. 24:6295–6303. 2017.
|