Introduction
Intestinal metaplasia (IM) induced by bile acid is
considered to be the most important precancerous lesion of
intestinal type gastric cancer (1).
IM is characterized by the expression of caudal-related homeobox 2
(CDX2), a transcription factor that is important for intestinal
tract development and differentiation during embryogenesis
(2,3).
In adult humans, CDX2 acts as a tumor suppressor, which is strictly
confined to the intestine and cannot be detected in normal gastric
mucosa. However, the aberrant expression of CDX2 in the upper
digestive tract is pro-oncogenic, and is observed in gastric IM and
gastric carcinoma (4). It has been
reported that transgenic mice overexpressing CDX2 caused extensive
IM progression in the stomach, which may be associated with the
downstream intestinal specific genes of CDX2 (5). Therefore, CDX2 is considered to be a
trigger in the progression of gastric IM.
Farnesoid X receptor (FXR) is a member of nuclear
metabolic receptors. FXR also functions as a transcription factor
and directly binds to DNA as a heterodimer with common partner
retinoid X receptor to regulate the expression of downstream genes
when activated by bile acid. It has been demonstrated that FXR is
involved in the bile acid-induced progression of IM and the
expression of various intestinal specific genes, including CDX2
(6,7).
However, the molecular mechanism remains to be fully
elucidated.
In the present study, it was found that FXR and CDX2
were upregulated and positively correlated in gastric IM tissues.
In addition, the results demonstrated that the molecular mechanism
by which the expression of CDX2 is mediated involved the direct
transcriptional regulation of small heterodimer partner (SHP) by
the nuclear receptor FXR.
Materials and methods
Tissue specimens and
immunohistochemistry
Biopsy specimens of gastric IM (61 patients) and
gastritis (55 patients) were obtained under endoscopy following the
provision of informed consent from patients at the Department of
Pathology of Xijing Hospital (Xi'an, China) between March 2011 and
February 2013. Corresponding clinical data were obtained from
medical records. The present study was approved by the
Institutional Review Board of Xijing Hospital.
The tissues were fixed in 10% formalin and
paraffin-embedded. IM and gastritis were diagnosed through standard
hematoxylin and eosin staining. For immunohistochemistry, tissue
sections (4 µm) were incubated with rabbit polyclonal anti-CDX2
antibody (cat. no. sc-134468; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA; dilution 1:100) and anti-FXR antibody (cat. no.
sc-13063, Santa Cruz Biotechnology, Inc.; dilution 1:100) at 4°C
overnight. Negative controls were incubated with rabbit IgG (Dako,
Glostrup, Denmark). The intensity of staining was divided into four
grades (intensity scores): Negative (0), weak (1), moderate (2) and strong (3). The histological score was determined
using the following formula: Overall score=percentage score ×
intensity score. An overall score of 0–12 was calculated and graded
as negative (score 0–2), or positive (score 3–12).
Cell culture and treatment
The normal GES-1 human gastric epithelial cell line,
AGS, BGC-823 and MKN-45 human gastric adenocarcinoma cell lines,
and SGC-7901 human gastric carcinoma cell line were obtained from
the Academy of Military Medical Science (Beijing, China) and
cultured in RPMI-1640 medium. All cells were maintained at 37°C in
a humidified incubator containing 5% CO2.
Chenodeoxycholic acid (CDCA; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany; cat. no. c9377), GW4064 (FXR agonist; Tocris
Bioscience, Bristol, UK; cat. no, 2473), and the FXR antagonist
guggulsterone (Gug; Sigma-Aldrich; Merck Millipore; cat. no.
1302214) were dissolved in dimethyl sulfoxide. The cells
(1–5×105) were seeded in six-well and 6 cm cell culture
dish respectively. When the cells reached 80–90% confluence, they
were serum-deprived overnight and then treated with CDCA (0, 50,
100 and 200 µM) in the presence or absence of Gug (5, 50 and 100
µM) or GW4064 (0.25, 0.5, 1, 5 and 10 µM) for 24 h under 37°C.
Plasmid construction and
transfection
The small interfering RNA (siRNA) oligonucleotides
for FXR, SHP and the negative control were designed and synthesized
by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The sequences
were as follows: FXR siRNA: Sense: 5′-GCCACUUCUUGAUGUGCUATT-3′,
anti-sense: 5′-UAGCACAUCAAGAAGUGGCTT-3′, SHP siRNA: Sense:
5′-GCAGUGGCUUCAAUGCUGUTT-3′ anti-sense:
5′-ACAGCAUUGAAGCCACUGCTT-3′. Transient transfection was performed
using DharmaFect Transfection reagent (Thermo Fisher Scientific,
Inc., Waltham, MA, USA; cat. no. T-2002-03) according to the
manufacturer's protocols. The cells were collected 48 h following
transfection.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA from the cultured cells was extracted
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol, and cDNA was synthesized using the
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China; cat. no. DRR037A). qPCR was performed using SYBR Premix Ex
Taq II (Takara Biotechnology Co., Ltd.; cat. no. DRR820A) and
measured in a LightCycler 480 system (Roche Diagnostics, Basel,
Switzerland). The thermocycling procedure was as follows: Initial
denaturation at 95°C for 5 min, followed by 39 cycles at 95°C for 5
sec, and at 60°C for 30 sec. GAPDH was used as the endogenous
control and relative quantification was evaluated using the
2−ΔΔCq method (8). The
primers are listed in Table I.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Forward primer
(5–3) | Reverse primer
(5–3) |
|---|
| CDX2 |
GCTGGAGCTGGAGAAGGAGTT |
CCTTTGCTCTGCGGTTCTG |
| MUC2 |
CAACCAGCACGTCATCCTGAA |
GATGCAAATGCTGGCATCAAAG |
| KLF4 |
AAGAGTTCCCATCTCAAGGCAA |
GGGCGAATTTCCATCCACAG |
| Villin1 |
GCTTGGCAACTCTAGGGACTGG |
TGAGGTTGCTGTTAGCATTGACAC |
| SHP |
CAAGGAATATGCCTGCCTGA |
TTCCAGGACTTCACACAGCAC |
| GAPDH |
ATGTCGTGGAGTCTACTGGC |
TGACCTTGCCCACAGCCTTG |
Western blot analysis
Proteins were prepared from the cultured cells with
complete cell lysis with protease and phosphatase inhibitor (Roche
Diagnostics). Protein concentrations were determined using
bicinchoninic acid Protein Assay kit (Thermo Fisher Scientific,
Inc.; cat. no. 23227). The denatured proteins (10–50 µg) were
separated on SDS-PAGE (10% gel) and transferred onto nitrocellulose
filter membranes. The membranes were blocked in 10% skimmed milk in
TBST (0.1% Tween-20) for 1 h at room temperature and then incubated
with the following primary antibodies: Rabbit polyclonal CDX2 (Cell
Signaling Technology, Inc.; cat. no. 12306; 1:1,000), rabbit
polyclonal kruppel like factor 4 (KLF4; Cell Signaling Technology,
Inc.; cat. no. 12173; 1:1,000), rabbit polyclonal villin-1 (Cell
Signaling Technology, Inc.; cat. no. 2369; 1:1,000), rabbit
polyclonal mucin 2 (MUC2; Abcam, Cambridge, MA, USA; cat. no.
ab76774; 1:1,000), rabbit polyclonal FXR (Abcam; cat. no. ab155124;
1:1,000), rabbit polyclonal SHP (Santa Cruz Biotechnology, Inc.;
cat. no. sc-2305; 1:1,000), mouse monoclonal β-actin
(Sigma-Aldrich; Merck Millipore; cat. no. A2228; 1:2,000) at 4°C
overnight. Subsequent to be washed with TBST for 3 time, membranes
were incubated with anti-Immunoglobulin G conjugated with
horseradish peroxidase antibody (Cell Signaling Technology, Inc.;
cat. no. 8457/3700; 1:2,000) at room temperature for 1 h. Then the
bands were scanned using the ChemiDocXRS+ imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and quantified using
Quantity One v4.6 software (Bio-Rad Laboratories, Inc.).
Chromatin immunoprecipitation (ChIP)
assay
The GES-1 cells were prepared for the ChIP assay
using a ChIP assay kit (Thermo-Fisher Scientific, Inc.; cat. no.
26156) according to the manufacturer's protocols.
Immunoprecipitation was performed with rabbit polyclonal anti-FXR
antibody (Abcam; cat. no. ab28676; 10 µg/ml) and normal rabbit IgG
(Abcam; cat. no. ab172730; 4 µg/ml) as a control at 4°C overnight.
The resulting precipitated DNA specimens were analyzed using PCR
(25 µl, total reaction system with 2 µl DNA) to amplify a 159-bp
region of the SHP promoter with the following primers: Forward,
5′-GGCTGGCTTCCTGGCTTAGC-3′ and reverse 5′-CTGCCCCTTATCAGATGACTC-3′.
The thermocycling procedure was as follows: Initial denaturation at
98°C for 5 min, followed by 33 cycles at 98°C for 30 sec, 58°C for
20 sec and at 68°C for 20 sec. The PCR products were resolved
electrophoretically on a 3% agarose gel and stained with ethidium
bromide. The gel was subjected to Peiqing JS-380A auto-focus gel
image analyzing system (P&Q Science & Technology Inc.
Shanghai, China, http://www.peiqing.com/.).
Statistical analysis
SPSS software (version 12.0; SPSS Inc., Chicago, IL,
USA) was used for statistical analyses. Continuous data are
presented as the mean ± standard error of the mean. Differences
between two groups were analyzed by an unpaired two-tailed
Student's t-test and differences between multiple groups were
analyzed by one-way analysis of variance with Tukey's post-hoc
test. Categorical variables are presented as rate and were compared
between two groups with a χ2 test. The linear
correlation coefficient (Pearson's R) was calculated to determine
the correlation between the expression of FXR and CDX2 in tissues.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CDCA-induced expression of CDX2 and
its downstream intestinal-specific genes MUC2, KLF4 and villin-1 in
GES-1 cells
To investigate the possible effects of bile acid on
the progression of gastric IM, the present study investigated the
expression of CDX2 and its downstream genes, MUC2, KLF4 and
villin-1, in the GES-1 normal human gastric epithelial cell line
following treatment with CDCA. As shown in Fig. 1A, when the GES-1 cells were treated
with CDCA (0, 50, 100 and 200 µmol/l) for 12 h, the expression of
endogenous CDX2 and downstream MUC2, KLF4 and villin-1 increased at
the mRNA levels in a dose-dependent manner. Compared with the
control group, the highest mRNA expression levels of CDX2, MUC2,
KLF4 and villin-1 were observed in the 200 µmol/l group
(P<0.01). Significantly increased protein expression levels of
CDX2, MUC2 and villin-1 were also observed, as measured using
western blot analysis, in the 200 µmol/l CDCA-treated GES-1
cells.
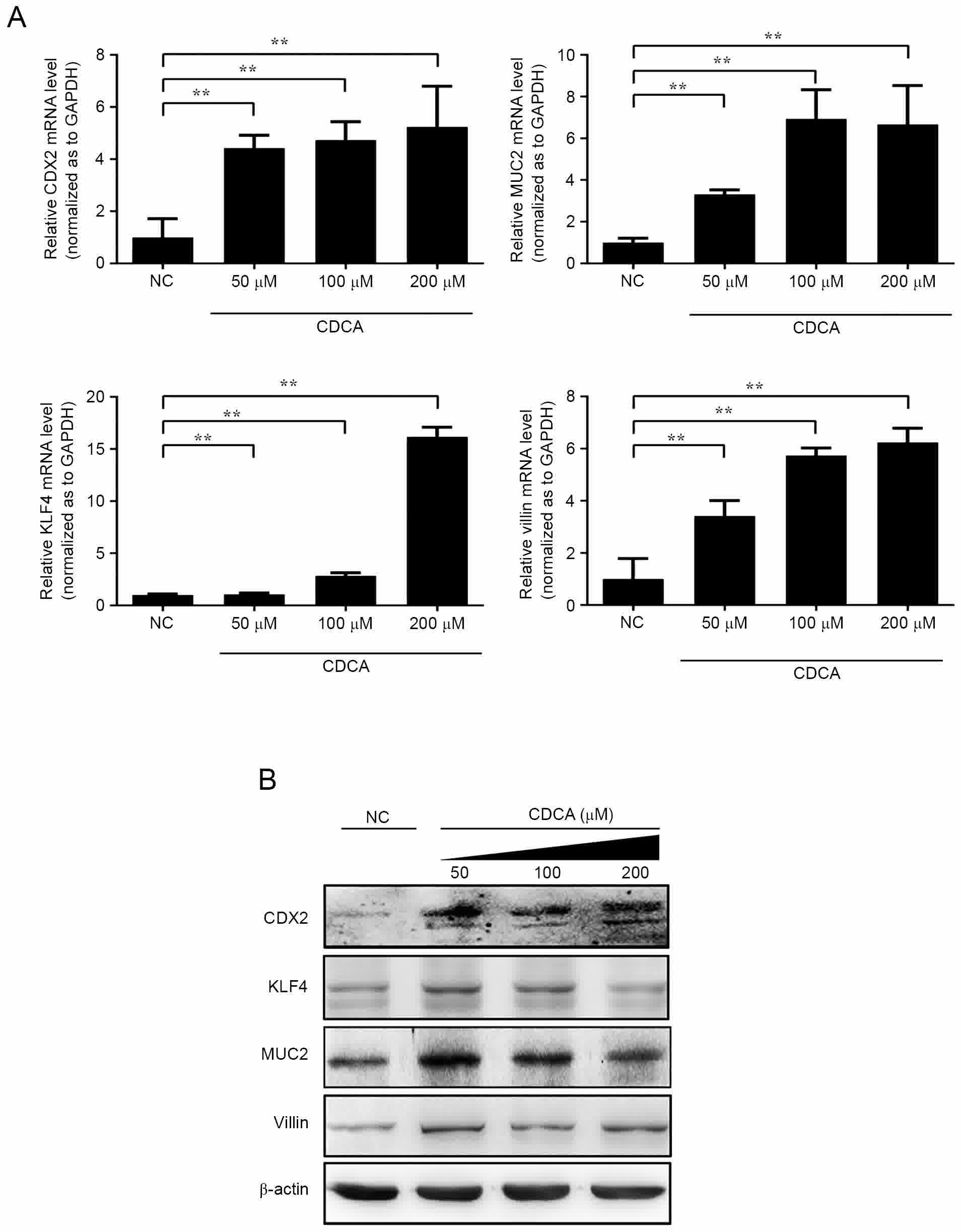 | Figure 1.Expression of CDX2, MUC2, KLF4 and
villin-1 following treatment with CDCA. CDCA induced the expression
of CDX2 and target genes in GES-1 cells. (A) mRNA expression levels
of CDX2, MUC2, KLF4 and villin-1 were detected via reverse
transcription-quantitative polymerase chain reaction analysis with
GAPDH as a control. (B) Protein expression levels of CDX2, MUC2,
KLF4 and villin-1 were analyzed using western blot analysis using
specific antibodies against CDX2, MUC2, KLF4, villin-1, and β-actin
as a control (**P<0.01). CDX2, caudal-related homeobox 2; MUC2,
mucin 2; KLF4, kruppel like factor 4; CDCA; chenodeoxycholic acid;
NC, negative control. |
CDCA increases the expression of CDX2
via the activation of FXR
FXR is a member of the nuclear hormone receptor
superfamily and is involved in the homeostasis of bile acid,
cholesterol and lipid. Previous studies have reported that the
expression of FXR is confined to the liver, intestine, kidney and
adrenal glands (9). To investigate
whether the activation of FXR is involved in the CDCA-induced
expression of CDX2, the present study aimed to identify the
baseline expression pattern of FXR in several human gastric
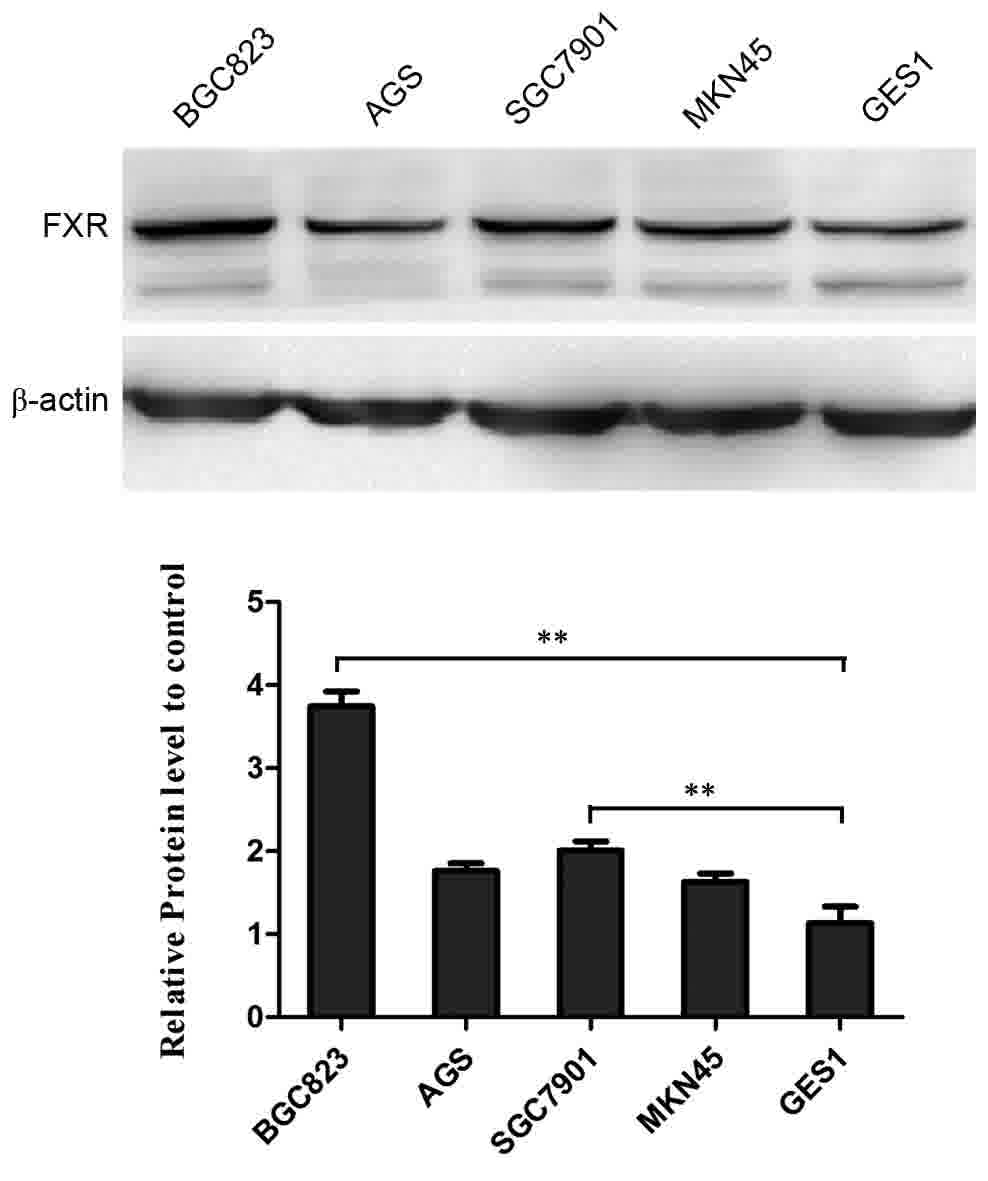
epithelial cells and gastric cancer cells. As shown in Fig. 2, FXR was expressed in the normal human
gastric epithelial cell and human gastric cancer cell lines. Of
note, the expression level was significantly lower in the GES-1
cells, compared with that in the other gastric cancer cells.
Subsequently, the expression of CDX2 was detected
following treatment of the GES-1 and BGC-823 cells with the FXR
agonist (GW4064). The results of the western blot analysis showed
that the expression of CDX2 was significantly upregulated by
different concentrations of GW4064 (1, 5 and 10 µmol/l; Fig. 3A). Gug is reported to compete for FXR
ligand-binding domain with CDCA and to be an antagonist ligand for
FXR (10). Accordingly, GES-1 and
BGC-823 cells were treated with CDCA in the absence or presence of
different concentrations of Gug. As shown in Fig. 3B, the effect of the promotion of CDCA
on the expression of CDX2 was eliminated by Gug in a dose-dependent
manner.
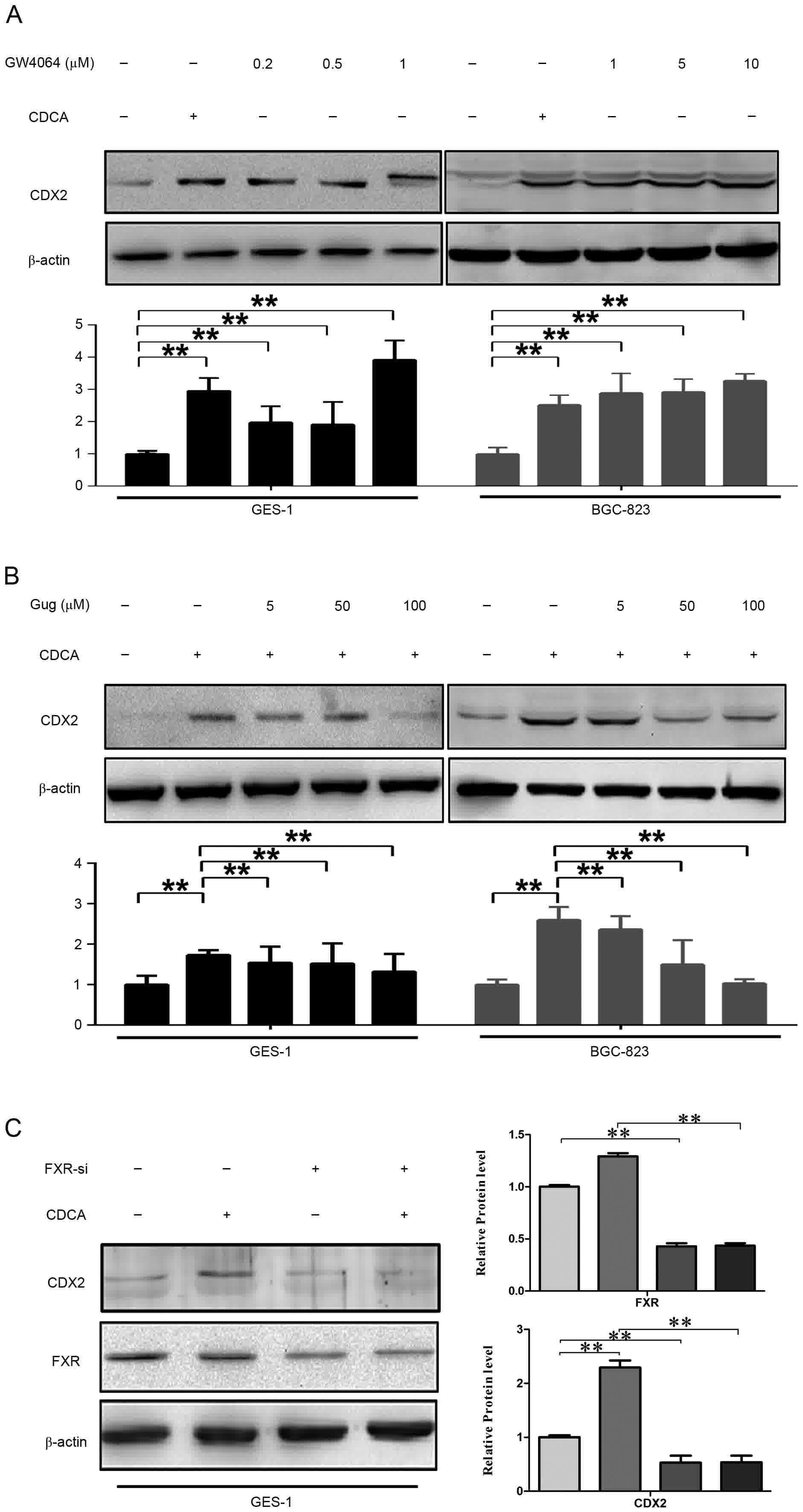 | Figure 3.Effects of FXR on CDCA-induced
expression of CDX2. Cell extracts from each sample were separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
analyzed using western blot analysis with specific antibodies
against FXR, CDX2 and β-actin as a control. (A) CDCA (200 µmol/l)
and GW4064 (1, 5 and 10 µmol/l) treatment increase the protein
expression of CDX2. The cells were exposed for 24 h with the
indicated concentrations of CDCA and GW4064. (B) Protein expression
of CDX2 was eliminated by Gug in a dose-dependent manner. Following
treatment with CDCA at 200 µmol/l, cells were exposed for 24 h to
different concentration of Gug (5, 50 and 100 µmol/l). (C) Protein
expression of CDX2 was eliminated by FXR-siRNA. Following 48 h
transfection, the cells were treated for 24 h with CDCA at 200
µmol/l. *P<0.05, **P<0.01. FXR, farnesoid X receptor; CDCA,
chenodeoxycholic acid; CDX2, caudal-related homeobox 2; siRNA,
small interfering RNA; Gug, guggulsterone. |
To further investigate the role of FXR in the
upregulation of CDX2, the siRNA of FXR was transfected into GES-1
cells. As shown in the Fig. 3C, the
FXR-siRNA significantly reduced the protein level of FXR, and the
knockdown of FXR eliminated the protein expression of CDX2 induced
by CDCA.
SHP is required for the FXR-induced
expression of CDX2
Extensive evidence suggests that the nuclear
receptor SHP is a classic downstream gene of FXR in bile acid
biosynthesis. SHP is reported to be required for the DCA-induced
expression of CDX1 (11). Therefore,
it was hypothesized that CDCA-FXR may induce the expression of CDX2
through SHP. First, to examine whether CDCA-FXR induces the
expression of SHP in gastric cells, the expression of SHP was
measured in GES-1 cells following CDCA treatment with or without
GW4064 and Gug. The mRNA and protein levels of SHP were induced by
CDCA and were considerably enhanced by GW4064 treatment. The
upregulation of the CDCA-induced protein expression of SHP was
eliminated by Gug (Fig. 4A). The
knockdown of SHP by siRNA resulted in a significant reduction in
the CDCA-induced expression of CDX2 (Fig.
4B).
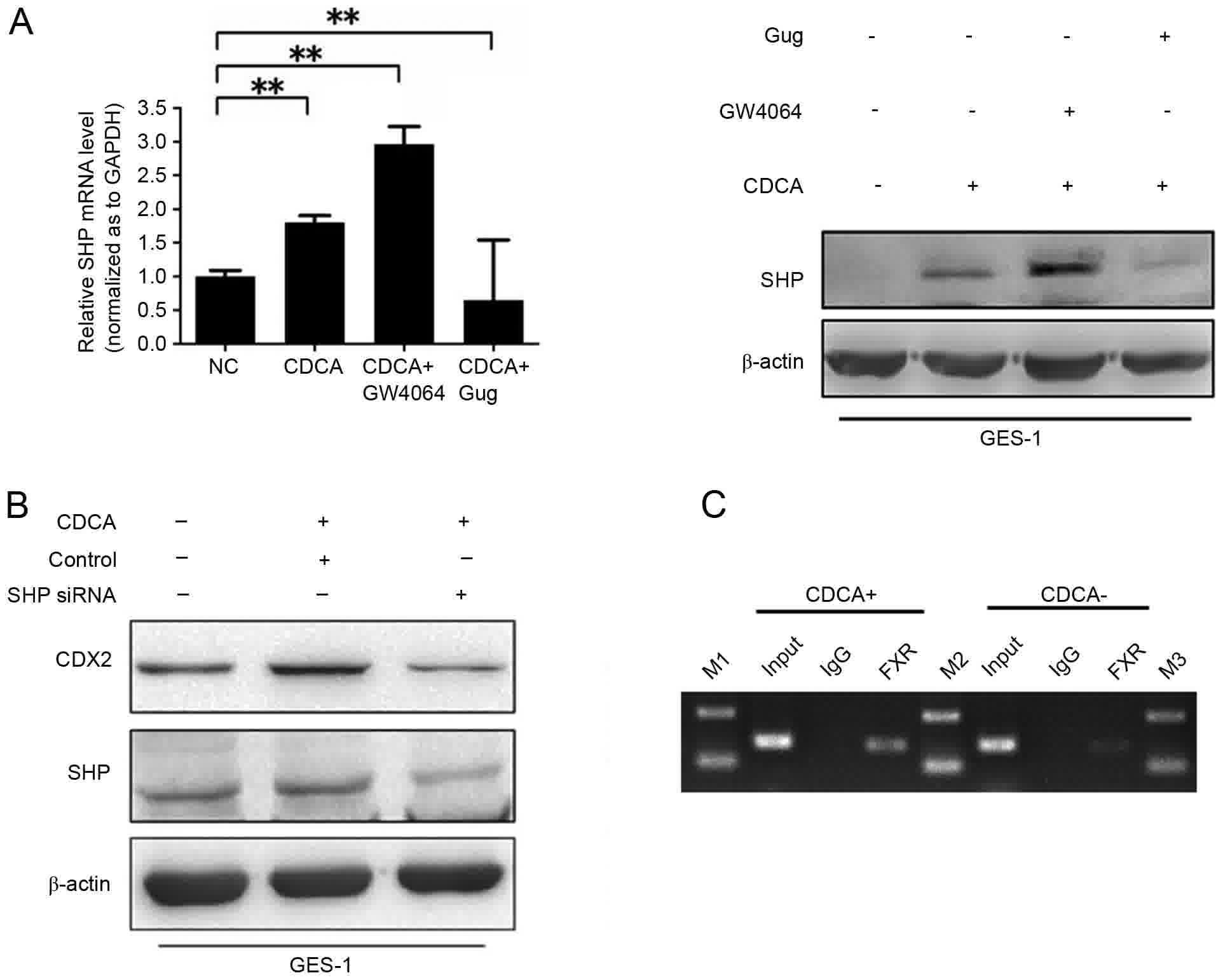 | Figure 4.Effect of SHP on the CDCA-induced
expression of CDX2. (A) Activation of FXR promoted the expression
of SHP at mRNA and protein levels. **P<0.01. Following treatment
with CDCA at 200 µmol/l for 12 h, GES-1 cells were exposed to
GW4064 at 1 µmol/l or Gug at 50 µmol/l. RT-qPCR analysis was
performed following incubation for 12 h (left) and western blot
analysis was performed following incubation for 24 h (right). (B)
Protein expression of CDX2 was eliminated by SHP-siRNA. Following
48 h transfection, the cells were treated for 24 h with CDCA at 200
µmol/l. (C) ChIP assay. Chromatins were isolated from GES-1 cells
following treatment with or without CDCA at 200 µmol/l. Binding of
FXR to the SHP promoter was examined using the ChIP assay.
Following immunoprecipitation with FXR antibody, precipitated DNAs
were used for PCR analysis. Rabbit IgG served as control and normal
cell lysates were subjected to PCR as input. FXR, farnesoid X
receptor; SHP, small heterodimer partner; CDCA, chenodeoxycholic
acid; CDX2, caudal-related homeobox 2; Gug, guggulsterone; siRNA,
small interfering RNA; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; M1-3, markers 1–3; NC, negative
control. |
In order to further confirm whether the increase in
the upregulation of SHP as a result of the expression of FXR is the
consequence of direct DNA-binding activity, a ChIP assay was
designed using the GES-1 cell line. As expected, the DNAs from the
chromatin sample binding with FXR antibody contained SHP mRNA, and
increased binding to the SHP promoter site was observed following
CDCA treatment (Fig. 4C). Taken
together, CDCA-FXR regulated the expression of SHP by binding to
its promoter site in gastric cells.
Relative expression levels of CDX2 and
FXR in gastritis and IM
In order to further investigate whether there is a
correlation between FXR and CDX2, the expression levels of FXR and
CDX2 were investigated in clinical samples of IM and gastritis
tissues. The clinical characteristics of all patients are shown in
Table II. The results of the
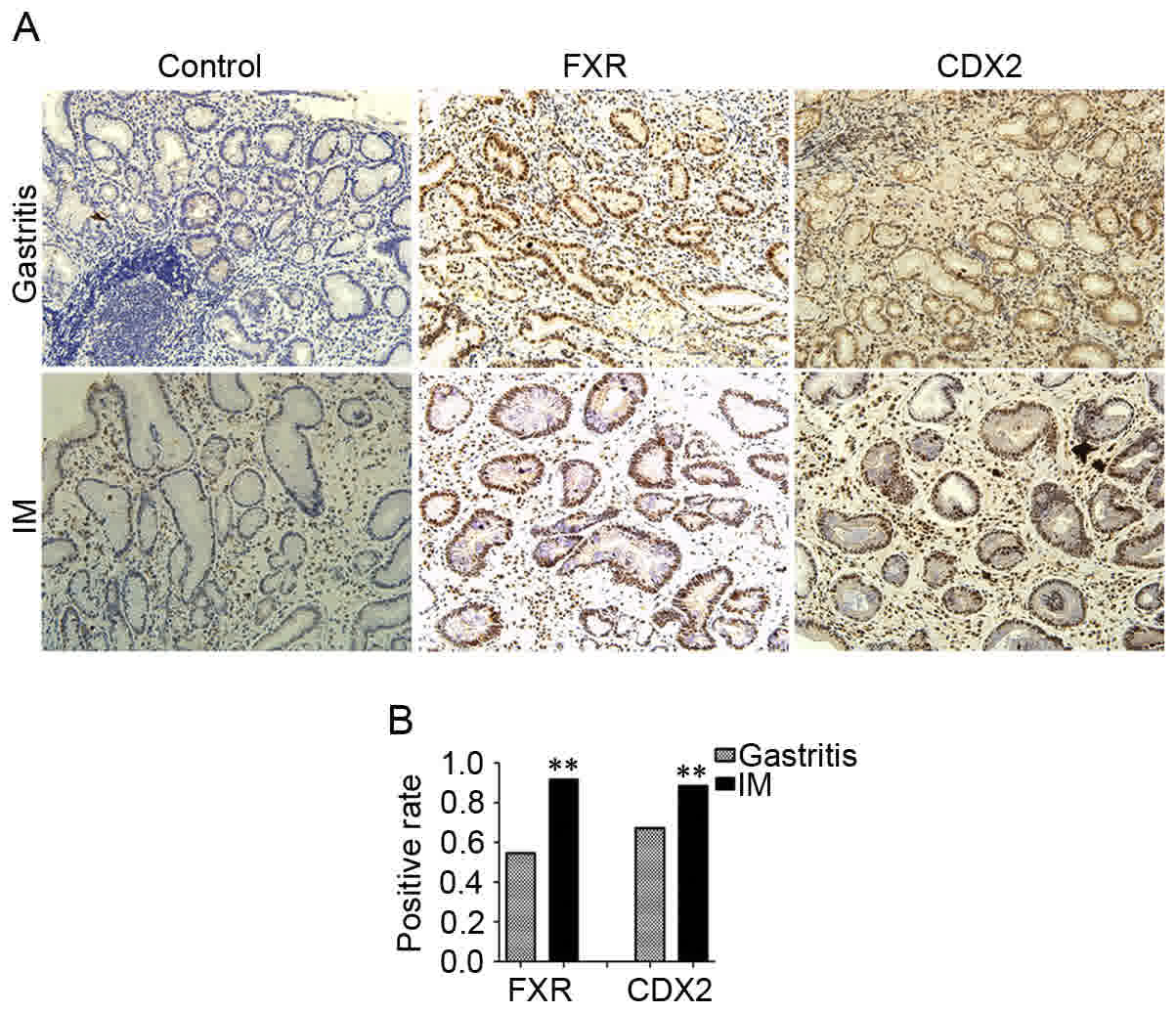
immunohistochemistry showed that the positive rate of FXR nuclear
staining was 91.8% (56/61) in IM tissues, which was significantly
higher than the 54.5% (30/55) in the gastritis tissues (P<0.01).
In addition, elevated expression of CDX2 nuclear staining was
detected in 88.5% (54/61) of the IM tissues, compared with 67.2%
(37/55) of gastritis tissues (P<0.01; Fig. 5A and B). The expression levels of FXR
and CDX2 were positively correlated in the gastritis tissues
(R=0.764, P<0.001) and IM tissues (R=0.642, P<0.001; Table III).
 | Table II.Clinical characteristics of
patients. |
Table II.
Clinical characteristics of
patients.
| Sample | n | Male/female | Mean age
(years) |
|---|
| Gastritis | 55 | 33/22 | 48.5 |
| Intestinal
metaplasia | 61 | 42/19 | 55.1 |
 | Table III.Positive rate of FXR and CDX2 nuclear
staining in IM and gastritis tissues. |
Table III.
Positive rate of FXR and CDX2 nuclear
staining in IM and gastritis tissues.
| FXR | CDX2+ | CDX2- | P-/R-value |
|---|
| Gastritis |
|
|
<0.001/0.764 |
| + | 30 | 0 |
|
| − | 7 | 18 |
|
| IM |
|
|
<0.001/0.642 |
| + | 53 | 3 |
|
| − | 1 | 4 |
|
Discussion
Previous studies using cell lines have showed that
the activation of FXR may be involved in the induction of the
expression of CDX2 and IM (6,7). However, the association between the
expression of FXR and CDX2 in vivo and the exact molecule
mechanisms remain to be fully elucidated. In the present study, the
in vivo results showed that FXR and CDX2 were concomitantly
overexpressed and were positively correlated in IM tissues. In
addition, the extensive in vitro results indicated that the
upregulation of CDX2 by FXR was dependent on the direct
transcriptional induction of SHP.
It has been shown that the reflux of bile acid in
the stomach is the most important risk factor for the development
of IM in addition to Helicobacter pylori (H. pylori)
infection (12). Clinical studies
have showed that high concentrations of bile acid appear to be
associated with an elevated risk of IM regardless of H.
pylori infection (13,14). However, the exact underlying molecular
mechanisms remain to to be fully elucidated.
IM is characterized by the occurrence of goblet
cells, absorptive cells and Paneth cells within the gastric
epithelial mucosa. CDX2 is reported to be a direct transcriptional
activator of several intestine-specific genes involved in this
phenotype, including MUC2, villin-1, intestinal fatty acid-binding
protein, glucagon and guanylyl cyclase. Mutoh et al directly
showed that CDX2 contributed to the generation of IM in transgenic
mice (15). In addition, several
studies using primary cultured esophageal epithelial cells and
gastric epithelial cell lines have shown that CDX2 is associated
with bile acid-induced cell transformation (16,17). In
the present study, it was found that CDX2 and target genes were
induced by CDCA in normal and gastric cancer cell lines. The
results indicated that the induced expression of CDX2 was
functional. In support of the in vitro results, the in
vivo results indicated that CDX2 was expressed in IM at a
significantly higher level, compared with that in gastritis
tissues. Taken together, these results revealed that the increased
expression of CDX2 was an early event, which occurred prior to the
development of IM.
The present study also investigated the upstream
signals involved in the induction of CDX2 by CDCA. FXR, acting as a
nuclear bile acid sensor, predominantly regulates the metabolism of
intracellular bile acid. Previously, the expression of FXR has been
reported to be associated with gastric cancer (18,19). In
the present study, baseline expression of FXR was observed in
normal epithelial and gastric cancer cell lines, and the expression
level of FXR was higher in the gastric cancer cell lines. These
results indicated that the upregulation of FXR was involved in
gastric carcinogenesis. The present study also examined the
expression level of FXR in IM and gastritis tissues. The results
showed that the level of FXR was significantly higher in IM,
compared with that in gastritis, and was positively correlated with
the level of CDX2. In addition, GW4064 further promoted the
expression of FXR and CDX2. By contrast, following inhibition of
the expression of FXR, the expression of CDX2 was almost
eliminated. These observations showed that the activation of FXR by
CDCA was involved in the induction of the expression of CDX2.
However, the role of FXR in the gastrointestinal
system remains controversial. It has been reported that activation
of the FXR pathway protects the gastric mucosa against
inflammation-mediated damage (20,21). These
findings suggest that the appropriate stimulation of FXR can serve
as a protective mechanism. By contrast, other studies have
suggested that FXR has a pro-inflammatory role (21,22). The
reasons for these contradicting results are unclear, however, they
may be associated with the differences in ligands and the genetic
disparity of cell lines. Further tissue and cell-specific
manipulation of FXR in vivo may elucidate the underlying
mechanisms.
The activation of epidermal growth factor receptors
(EGFRs) and TGR5 is also involved in gastric intestinal
carcinogenesis. Previous reports have suggested that bile acid
activates EGFR and TGR5 in the AGS cell line (23,24). In
addition, bile acid activates EGFR in other cells lines, including
colon cancer cells, cholangiocyte cells and esophageal
adenocarcinoma cells (25,26). Whether the activation of EGFR and TGR5
is involved in the regulation of CDX2 requires further
investigation.
SHP is a unique member of the nuclear receptor
downstream of FXR, which is important in metabolism and cancer.
Previous studies have shown that FXR regulates the expression of
SHP through directly binding to an LRH-1 binding site (27). In addition, Park et al
demonstrated that the increased expression of SHP is involved in
the protein expression of CDX1 in gastric cells induced by bile
acid (28). Therefore, it was
hypothesized that SHP may also regulate the bile acid-induced
expression of CDX2 downstream of the activation of FXR. The results
of the present study showed that the activation of FXR enhanced the
expression of SHP induced by CDCA, whereas Gug had the opposite
effect. In addition, SHP-knockdown eliminated the effects of CDCA
on the induced protein expression of CDX2. The results of the ChIP
assay showed that CDCA stimulated the DNA binding activity of FXR
on the SHP promoter, thus promoting the expression of SHP. However,
the molecular mechanisms underlying the effect of SHP on the
enhancement of CDX2 remain to be fully elucidated. Previous studies
have demonstrated that SHP functionally interacts with NF-κB as a
co-regulator for target gene expression (28). As the CDX2 promoter has two binding
sites for NF-κB, the expression of CDX2 may be augmented by the
direct interaction of SHP and NF-κB. CDX2 can also bind to its own
promoter and induce its expression by an effective auto-regulatory
loop (29).
In conclusion, the present study demonstrated in
vivo and in vitro that the activation of FXR is involved
in the bile acid-induced expression of CDX2 to generate IM.
Specifically, the results indicated that bile acid activated the
expression of FXR, which then directly unregulated the expression
of SHP at the transcriptional level. SHP-knockdown eliminated the
effects of CDCA on the induced protein expression of CDX2. Taken
together, the observations of the present study suggested that
augmentation of the FXR/SHP signal was associated with the
phenotypic change of intestinal metaplasia induced by bile acid and
gastric carcinogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81270445, 81370484
and 81470805).
Availability of data and materials
All datasets generated in the present study are
provided in full in the results section of this manuscript.
Author's contributions
HZ, LZ and YS conceived and designed the study. HZ,
TL, and LS performed the experiments. ZN and NL analyzed the
results. ZN and HZ wrote the paper. ZN and YS reviewed and edited
the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The study was approved by the Insititutional Review
Board of Xijing Hospital. All patients provided written informed
consent.
Consent for publication
All patients provided written informed consent for
the publication of their data and any potentially identifying
information was removed.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IM
|
intestinal metaplasia
|
|
CDX2
|
caudal-related homeobox 2
|
|
FXR
|
farnesoid X receptor
|
|
SHP
|
small heterodimer partner
|
|
CDCA
|
chenodeoxycholic acid
|
|
EGFR
|
epidermal growth factor receptor
|
|
NF-kB
|
nuclear factor-kB
|
References
|
1
|
Correa P, Piazuelo MB and Wilson KT:
Pathology of gastric intestinal metaplasia: Clinical implications.
Am J Gastroenterol. 105:493–498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hryniuk A, Grainger S, Savory JG and
Lohnes D: Cdx function is required for maintenance of intestinal
identity in the adult. Dev Biol. 363:426–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silberg DG, Swain GP, Suh ER and Traber
PG: Cdx1 and cdx2 expression during intestinal development.
Gastroenterology. 119:961–971. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xin S, Huixin C, Benchang S, Aiping B,
Jinhui W, Xiaoyan L, Yu WB and Minhu C: Expression of Cdx2 and
claudin-2 in the multistage tissue of gastric carcinogenesis.
Oncology. 73:357–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Silberg DG, Sullivan J, Kang E, Swain GP,
Moffett J, Sund NJ, Sackett SD and Kaestner KH: Cdx2 ectopic
expression induces gastric intestinal metaplasia in transgenic
mice. Gastroenterology. 122:689–696. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li S, Chen X, Zhou L and Wang BM:
Farnesoid X receptor signal is involved in deoxycholic acid-induced
intestinal metaplasia of normal human gastric epithelial cells.
Oncol Rep. 34:2674–2682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y, Watanabe T, Tanigawa T, Machida H,
Okazaki H, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Oshitani
N and Arakawa T: Bile acids induce cdx2 expression through the
farnesoid x receptor in gastric epithelial cells. J Clin Biochem
Nutr. 46:81–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–428. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsubara T, Li F and Gonzalez FJ: FXR
signaling in the enterohepatic system. Mol Cell Endocrinol.
368:17–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Urizar NL, Liverman AB, Dodds DT, Silva
FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ and
Moore DD: A natural product that lowers cholesterol as an
antagonist ligand for FXR. Science. 296:1703–1706. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park MJ, Kim KH, Kim HY, Kim K and Cheong
J: Bile acid induces expression of COX-2 through the homeodomain
transcription factor CDX1 and orphan nuclear receptor SHP in human
gastric cancer cells. Carcinogenesis. 29:2385–2393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tatsugami M, Ito M, Tanaka S, Yoshihara M,
Matsui H, Haruma K and Chayama K: Bile acid promotes intestinal
metaplasia and gastric carcinogenesis. Cancer Epidemiol Biomarkers
Prev. 21:2101–2107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuhisa T and Tsukui T: Relation between
reflux of bile acids into the stomach and gastric mucosal atrophy,
intestinal metaplasia in biopsy specimens. J Clin Biochem Nutr.
50:217–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuhisa T, Arakawa T, Watanabe T,
Tokutomi T, Sakurai K, Okamura S, Chono S, Kamada T, Sugiyama A,
Fujimura Y, et al: Relation between bile acid reflux into the
stomach and the risk of atrophic gastritis and intestinal
metaplasia: A multicenter study of 2283 cases. Dig Endosc.
25:519–525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mutoh H, Hayakawa H, Sakamoto H, Sashikawa
M and Sugano K: Transgenic Cdx2 induces endogenous Cdx1 in
intestinal metaplasia of Cdx2-transgenic mouse stomach. FEBS J.
276:5821–5831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cooke G, Blanco-Fernandez A and Seery JP:
The effect of retinoic acid and deoxycholic acid on the
differentiation of primary human esophageal keratinocytes. Dig Dis
Sci. 53:2851–2857. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camilo V, Barros R, Sousa S, Magalhães AM,
Lopes T, Santos Mário A, Pereira T, Figueiredo C, David L and
Almeida R: Helicobacter pylori and the BMP pathway regulate CDX2
and SOX2 expression in gastric cells. Carcinogenesis. 33:1985–1992.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duan JH and Fang L: MicroRNA-92 promotes
gastric cancer cell proliferation and invasion through targeting
FXR. Tumour Biol. 35:11013–11019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gadaleta RM, Cariello M, Sabba C and
Moschetta A: Tissue-specific actions of FXR in metabolism and
cancer. Biochim Biophys Acta. 1851:30–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lian F, Xing X, Yuan G, Schäfer C, Rauser
S, Walch A, Röcken C, Ebeling M, Wright MB, Schmid RM, et al:
Farnesoid X receptor protects human and murine gastric epithelial
cells against inflammation-induced damage. Biochem J. 438:315–323.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fiorucci S, Mencarelli A, Cipriani S,
Renga B, Palladino G, Santucci L and Distrutti E: Activation of the
farnesoid-X receptor protects against gastrointestinal injury
caused by non-steroidal anti-inflammatory drugs in mice. Br J
Pharmacol. 164:1929–1938. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Capello A, Moons LM, Van de Winkel A,
Siersema PD, van Dekken H, Kuipers EJ and Kusters JG: Bile
acid-stimulated expression of the farnesoid X receptor enhances the
immune response in Barrett esophagus. Am J Gastroenterol.
103:1510–1516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yasuda H, Hirata S, Inoue K, Mashima H,
Ohnishi H and Yoshiba M: Involvement of membrane-type bile acid
receptor M-BAR/TGR5 in bile acid-induced activation of epidermal
growth factor receptor and mitogen-activated protein kinases in
gastric carcinoma cells. Biochem Biophys Res Commun. 354:154–159.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao W, Tian W, Hong J, Li D, Tavares R,
Noble L, Moss SF and Resnick MB: Expression of bile acid receptor
TGR5 in gastric adenocarcinoma. Am J Physiol Gastrointest Liver
Physiol. 304:G322–G327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Centuori SM, Gomes CJ, Trujillo J, Borg J,
Brownlee J, Putnam CW and Martinez JD: Deoxycholic acid mediates
non-canonical EGFR-MAPK activation through the induction of calcium
signaling in colon cancer cells. Biochim Biophys Acta.
1861:663–670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Werneburg NW, Yoon JH, Higuchi H and Gores
GJ: Bile acids activate EGF receptor via a TGF-alpha-dependent
mechanism in human cholangiocyte cell lines. Am J Physiol
Gastrointest Liver Physiol. 285:G31–G36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoeke MO, Heegsma J, Hoekstra M, Moshage H
and Faber KN: Human FXR regulates SHP expression through direct
binding to an LRH-1 binding site, independent of an IR-1 and LRH-1.
PLoS One. 9:e880112014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park WI, Park MJ, An JK, Choi YH, Kim HY,
Cheong J and Yang US: Bile acid regulates c-Jun expression through
the orphan nuclear receptor SHP induction in gastric cells. Biochem
Biophys Res Commun. 369:437–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barros R, da Costa LT, Pinto-de-Sousa J,
Duluc I, Freund JN, David L and Almeida R: CDX2 autoregulation in
human intestinal metaplasia of the stomach: Impact on the stability
of the phenotype. Gut. 60:290–298. 2011. View Article : Google Scholar : PubMed/NCBI
|