Introduction
Hepatocellular carcinoma (HCC) is a common cancer
with poor prognosis in Chinese patients. In 2011, there were a
reported ~355,595 new cases and ~322,416 incidences of mortality
owing to liver cancer in China; the incidence and mortality rates
of liver cancer were 26.39/100,000 and 23.93/100,000, respectively
(1). Among all cancer types, deaths
from liver cancer are increasing at the highest rate, with liver
cancer incidence rates increasing rapidly in the United States
(2). The burden of liver cancer is
growing worldwide (2). Despite the
substantial progress in surgical, interventional and targeted
treatments, the long-term survival rates of patients with HCC
remain bleak owing to postoperative recurrence and metastasis. The
recurrence and metastasis of HCC are mainly intrahepatic following
radical hepatectomy, which supports the theory that the peritumoral
microenvironment may provide a suitable environment for
colonization and proliferation of subclinical metastatic tumor
cells (3,4). Removal of the primary tumor does not
alter the peritumoral microenvironment, which remains suitable for
HCC initiation and progression (3).
Furthermore, the HCC tumor biomarkers currently under intensive
investigation are primarily derived from cancerous tissues to
predict early recurrence and prognosis (5). By contrast, previous studies have
demonstrated that patients with HCC exhibit a large degree of
spatial and temporal genomic heterogeneity and that the extent of
intratumor heterogeneity varies considerably among these patients
(6–8).
One tumor lesion may contain intratumor subregions with distinct
genomes (8), and therefore the
postoperative recurrence of disease may not share the same invasion
characteristics as the primary HCC lesions (8). Therefore, biomarkers extracted from only
one cancerous region may not represent the various HCC genomes
owing to the substantial heterogeneity and subclonal diversity.
This makes it critical to identify novel biomarkers, particularly
those from peritumoral liver tissues, which may contribute to the
prediction of HCC recurrence.
Zinc-binding protein-89 (ZBP-89), a ubiquitously
expressed Krüppel-type zinc-finger transcription factor, binds to
GC-rich DNA sequences and is involved in a number of cellular
functions, including cellular proliferation, differentiation, and
apoptosis (9). It has been reported
that ZBP-89 possesses the properties of a transactivator and a
tumor suppressor owing to its bifunctional regulatory domains
(8). ZBP-89 is capable of
transcriptionally activating the expression of a battery of genes,
including BCL2 antagonist/killer 1, p21waf1, matrix
metalloproteinases, human programmed cell death protein 4, and
proto-oncogene β-catenin (CTNNB1) (9–14).
However, ZBP-89 acts as a suppressor for other genes, including
gastrin, vimentin, p16, and ornithine decarboxylase promoter
(15–19). The ZBP-89 protein is involved in
several human cancer types, including HCC (20), gastric cancer (21), esophageal squamous cell cancer
(22), colorectal cancer (CRC)
(23), clear-cell renal cell
carcinoma (24), and pancreatic
cancer (25). However, the expression
of ZBP-89 in cancerous tissues has been inconsistently associated
with prognosis in patients with different tumor types. For
instance, HCC patients with high ZBP-89 expression in intratumoral
tissues exhibited superior survival rates to those with low ZBP-89
expression. On the contrary, high expression of intratumoral ZBP-89
is associated with decreased survival rates in patients with
esophageal squamous cell cancer and clear-cell renal cell carcinoma
(22,24). To the best of our knowledge, little
research has investigated whether the expression of ZBP-89 in
peritumoral liver tissues is associated with improved patient
prognosis.
The present study investigated the expression of
peritumoral ZBP-89 in 102 HCC patients who had received curative
hepatectomy by immunohistochemistry. The aim of the present study
was to reveal the possible association between peritumoral ZBP-89
expression and HCC patient survival, including disease-free
survival (DFS) and overall survival (OS) rates.
Materials and methods
Patients and their clinicopathological
data
In total, 93 men and 9 women, aged 31–81 years
(median, 58.0 years), were involved in the present study. Archived,
formalin-fixed, paraffin-embedded peritumoral liver tissue
specimens were obtained from 102 patients with pathologically
proven HCC and underwent curative resection between November 1995
and May 2017 at the Prince of Wales Hospital (Hong Kong, China).
All patients involved in the present study were hepatitis B virus
(HBV)-associated HCC patients. The study was performed in strict
accordance with the Reporting Recommendations for Tumor Marker
Prognostic Studies (REMARK) and the Transparent Reporting of a
Multivariable Prediction Model for Individual Prognosis or
Diagnosis (TRIPOD) Statement (26,27).
Informed written consent was obtained from all patients involved in
the present study. Curative resection was defined as the complete
removal of cancer tissue, with tumor-negative resection margins.
The resection edge was at least 2 cm from the tumor margin. Tumor
differentiation was graded by the Edmondson grading system
(28). Patients had no signs of
distant metastasis and did not receive any anticancer therapy prior
to surgery. Following curative resection, all liver specimens were
histologically evaluated by two independent pathologists blinded to
all patient-associated information. Biochemical markers, including
α-fetoprotein (AFP), albumin, alanine aminotransferase (ALT), and
bilirubin, were acquired from the patients' medical records. All
detailed clinicopathological features are listed in Table I. The present study was approved by
the Joint Chinese University of Hong Kong-New Territories East
Cluster Clinical Research Ethics Committee (Hong Kong, China).
 | Table I.Demographic, biochemical and clinical
characteristics of the 102 HCC patients. |
Table I.
Demographic, biochemical and clinical
characteristics of the 102 HCC patients.
| Variable | Value |
|---|
| Age, years | 58 (31–81) |
| Sex,
male/female | 93/9 |
| Albumin, g/l | 41 (26–49) |
| ALT, U/l | 48 (11–283) |
| Total bilirubin,
g/l | 9 (3–83) |
| HCC diameter,
cm | 4 (0.7–15) |
| AFP, ng/ml | 91 (1–625,000) |
Patient follow-up
All patients were followed up until May 2017, with a
median observation time of 179 months. Patients were followed up by
clinic visits every 3 months in the first year after surgery, every
4 months during the second year after surgery, and every 6 months
thereafter. A contrast-enhanced abdomen computed tomography or
magnetic resonance imaging scan was performed at least every three
months during the postoperative follow-up. Mortality information of
patients was obtained from the social security death index, medical
records, or notifications from the family of the deceased.
Immunohistochemistry and western blot
analysis
The expression of ZBP-89 was assessed in peritumoral
hepatocytes, which are defined as liver tissues at least 2 cm from
the tumor margin. The adjacent non-cancerous tissues were
continuously sectioned into paraffin slices, and one slide/patient
was processed for IHC staining and counted. Immunohistochemistry,
western blot analysis and scoring was performed according to
previously described protocols (20).
On each slide, 1,000 cells were randomly selected, counted, and
scored. Negative controls were prepared using PBS instead of the
primary antibody. All the primary antibodies for ZBP-89 (sc-48811
X) were diluted at 1:200, were purchased from Santa Cruz
Biotechnology (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
The extent of IHC staining was defined as: (+), <10% of
peritumor cells were positive; (++), 10–50% of peritumor cells were
positive; (+++), >50% of peritumoral cells were positive; (−),
negative staining. Negative and (+) positive staining were defined
as low expression, whereas (++) and (+++) positive staining were
defined as high expression. All sections were observed by light
microscopy (magnification, ×200), and scoring was performed
separately by two independent pathologists. The METAVIR scoring
system was used for liver fibrosis scoring (29). F0=no fibrosis, F1=portal fibrosis
without septa, F2=portal fibrosis with few septa, F3=numerous septa
without cirrhosis, F4=cirrhosis. All patients involved in our
present study were divided into three groups, F0-1, F1-2, and
F3-4.
Hematoxylin and eosin (H&E)
staining
Routine H&E staining was conducted on paraffin
slices of peritumoral liver tissue according to the previous
H&E staining protocol (30).
Paraffin slices would underwent the subsequent procedures at room
temperature: Deparaffinisation using: Xylene I for 5 min and Xylene
II for 5 min; followed by rehydration in a descending alcohol
series of 90% alcohol for 5 min and 70% alcohol for 5 min. Samples
were then washed with distilled water for 10 min. Nuclear Staining
was conducted with Harris' haematoxyl solution for 8 min and
following washing with distilled water for 2 min; cytoplasmic
Staining with 1% eosin 1 min was conducted.
Statistical analysis
All statistical analyses were performed using the
software SPSS version 19.0 (IBM Corp., Armonk, NY, USA). The
association between ZBP-89 expression and clinicopathological
variables was assessed by applying Pearson's χ2 test.
DFS was defined as the interval between the date of surgery and
recurrence, whereas OS was defined as the dates of surgery and
mortality. OS and DFS were assessed using the Kaplan-Meier method
and compared using the log-rank test in the 102 HCC patients.
Univariate Cox-regression model was performed against all the
clinicopathological features as covariates. Multivariate Cox
proportional hazards analysis was performed on the significant
factors determined by univariate analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of ZBP-89 in HCC
peritumoral tissues by immunohistochemistry and western blot
analysis
The clinicopathological characteristics of the 102
patients are presented in Table I.
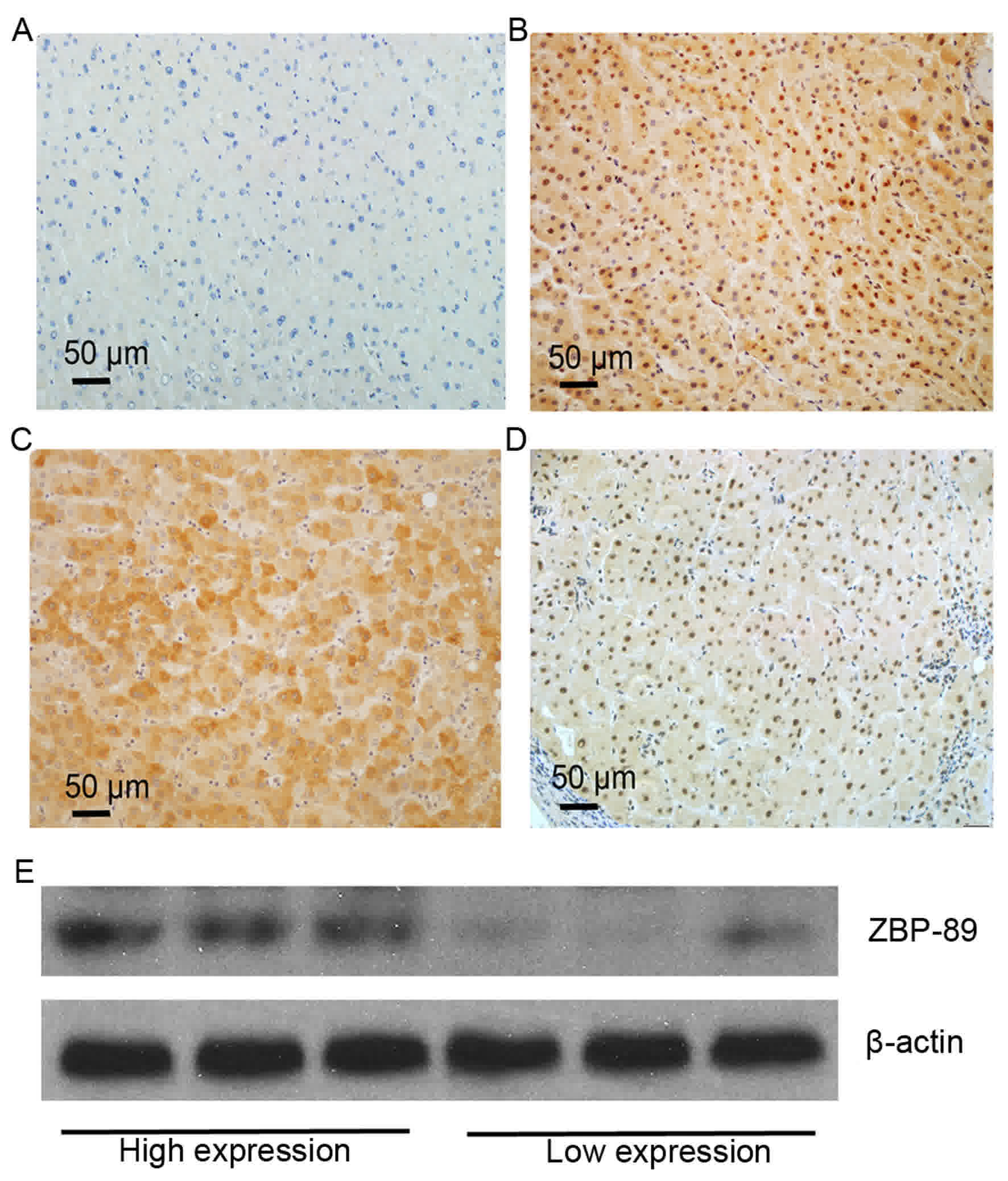
IHC staining revealed that positive staining of ZBP-89 protein was
mainly observed in the cytoplasm of peritumoral hepatocytes
(Fig. 1). Negative peitumoral
cytoplasmic/nuclei staining for ZBP-89 (Fig. 1A). High peritumoral cytoplasmic and
nuclear staining for ZBP-89 (Fig.
1B). High peritumoral cytoplasmic staining for ZBP-89 (Fig. 1C). High peritumoral nuclear staining
for ZBP-89 (Fig. 1D). The expression
of ZBP-89 protein was categorized into low- and high-ZBP-89
expression samples. To confirm the results of IHC, western blotting
was used to detect the protein levels of ZBP-89 in 6 HCC
peritumoral tissues, 3 of which were assessed as exhibiting high
expression of ZBP-89 by IHC and 3 were low expression (Fig. 1E). High ZBP-89 expression (++ and +++)
was detected in 65.6% (67/102) of the patients, whereas low ZBP-89
expression (− and +) was detected in 33.4% (34/102) of the
patients.
Association between ZBP-89 expression
in HCC peritumoral tissues and clinicopathological variables
The association between clinicopathological features
and peritumoral ZBP-89 expression in 102 HCC patients is summarized
in Table II. Peritumoral ZBP-89
expression was positively associated with the presence of liver
cirrhosis, ALT and albumin (P<0.05), whereas no statistically
significant association was observed with the remaining
clinicopathological parameters, which included histological grade,
tumor size, multinodular tumor, capsular infiltration, AFP,
bilirubin, age and sex (P=0.167, 0.532, 0.804, 0.349, 0.676, 1.000,
0.488, and 0.087, respectively). In the peritumoral tissues, ZBP-89
levels in patients from the F2-4 groups were significantly higher
(76.4%, 42/55) than those of in patients from the F0-1 group
(53.2%, 25/47).
 | Table II.Association of ZBP-89 protein
expression in peritumoral tissues with clinicopathological
characteristics. |
Table II.
Association of ZBP-89 protein
expression in peritumoral tissues with clinicopathological
characteristics.
|
| ZBP-89
expression |
|
|---|
|
|
|
|
|---|
| Parameter | Positive | Negative | P-value |
|---|
| Age, years |
|
| 0.488 |
|
<50 | 21 | 8 |
|
|
≥50 | 46 | 27 |
|
| Sex |
|
| 0.087 |
|
Male | 63 | 29 |
|
|
Female | 4 | 6 |
|
| AFP, µg/l |
|
| 0.676 |
|
<400 | 39 | 22 |
|
|
≥400 | 28 | 13 |
|
| ALT, IU/l |
|
| 0.020 |
|
>80 | 16 | 2 |
|
|
≥80 | 51 | 33 |
|
| Bilirubin, g/l |
|
|
|
|
>20 | 3 | 1 | 1.000 |
|
≤20 | 64 | 34 |
|
| Albumin, g/l |
|
|
|
|
>35 | 52 | 33 | 0.048 |
|
≤35 | 15 | 2 |
|
| Tumor lesions |
|
| 0.804 |
|
Multiple | 16 | 7 |
|
|
Single | 51 | 28 |
|
| Tumor size, cm |
|
| 0.532 |
| ≥5 | 32 | 14 |
|
|
<5 | 35 | 21 |
|
| Vascular
invasion |
|
| 0.349 |
|
Absence | 47 | 28 |
|
|
Presence | 20 | 7 |
|
| Cirrhosis |
|
| 0.020a |
|
Presence | 42 | 13 |
|
|
Absence | 25 | 22 |
|
| Histological
grade |
|
| 0.167 |
| Well
and moderate | 53 | 32 |
|
|
Poor | 13 | 3 |
|
Association between ZBP-89 expression
in HCC peritumoral tissues and patient survival
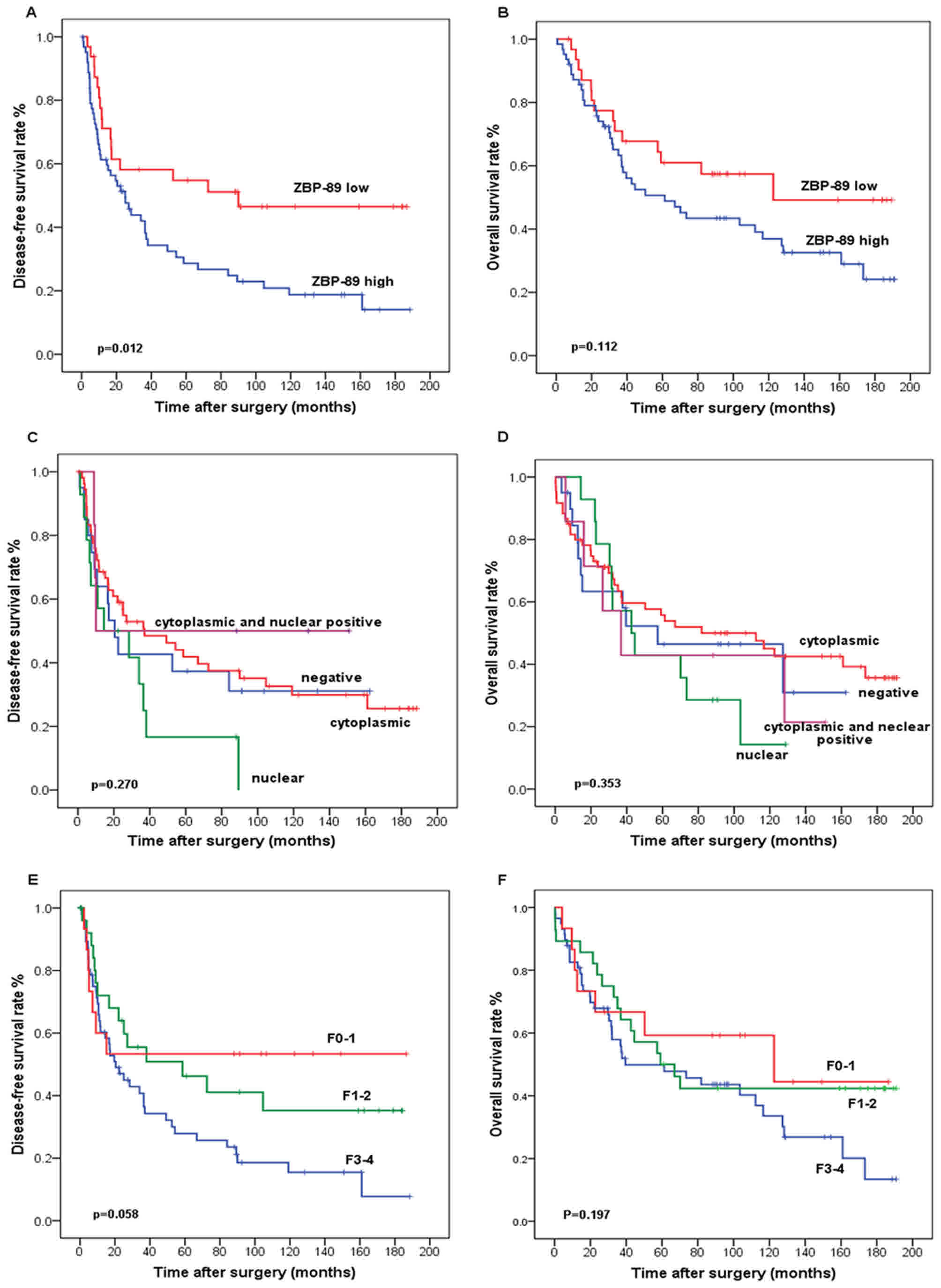
The association between peritumoral ZBP-89
expression in HCC and survival was analyzed by the Kaplan-Meier
method (Fig. 2). Prognostic values of
the peritumoral hepatocellular expression of ZBP-89 illustrated by
a Kaplan-Meier analysis of DFS and OS rate analysis of ZBP-89 in
102 patients with HCC who had received curative hepatectomy
(Fig. 2A and B). The prognostic
values of the subcellular localization of ZBP-89 expression in
peritumoral hepatocytes as shown by Kaplan-Meier analysis of DFS
and OS in 4 subgroups in the peritumoral liver tissue (Fig. 2C and D). Fig 2E and F illustrates the
Kaplan-Meier analysis of DFS and OS of the 3 subgroups in the
peritumoral liver tissue. Among the 102 HCC patients, 67 patients
had succumbed to disease and 35 were alive at the end of the
follow-up studies. The median observation period was 179 months
(range, 128–270 months).
The median survival time of patients with high
peritumoral ZBP-89 expression levels was 44.5 months, whereas the
median survival time of patients with low peritumoral ZBP-89 levels
was 122.6 months. In the high-peritumoral-ZBP-89-expression group,
the cumulative 5-year survival rate was 45.6% (n=90), whereas in
the low-peritumoral-ZBP-89-expression group, the survival rate was
58.0%. The high peritumoral ZBP-89 expression group had a
significantly shorter duration of DFS (P=0.012) compared with the
ZBP-89-low group, whereas no significant association was observed
between peritumoral ZBP-89 expression and OS (Fig. 1A and D). Univariate analysis revealed
that a tumor size >5 cm, multiple tumors, and a poor
histological grade were all statistically significant predictors of
poor survival in patients with HCC (Table III). Meanwhile, multiple tumors and
macroscopic vascular invasion were also statistically associated
with poor DFS. The univariate Cox proportional hazard ratio (HR) of
high vs. low peritumoral ZBP-89 expression was 1.797 (95% CI:
0.972–3.322; P=0.061) for DFS rate (Table IV).
 | Table III.Cox proportional hazard regression
analysis of patients' overall survival rates. |
Table III.
Cox proportional hazard regression
analysis of patients' overall survival rates.
|
| Univariable | Multivariable |
|---|
|
|
|
|
|---|
| Parameter | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age (<50 vs. ≥50
years) | 1.275 | 0.677–2.398 | 0.452 | – | – | – |
| Gender (female vs.
male) | 0.957 | 0.309–2.965 | 0.940 | – | – | – |
| Cirrhosis (absence
vs. presence) | 1.614 | 0.932–2.794 | 0.087 | – | – | – |
| Fibrosis vs.
normal | 0.701 | 0.373–1.316 | 0.269 | – | – | – |
| Cirrhosis vs.
normal | 0.574 | 0.224–1.470 | 0.247 | – | – | – |
| Tumor size (<5
vs. > 5 cm) | 2.577 | 1.402–4.739 | 0.002a | 2.454 | 1.447–4.160 |
0.001a |
| AFP (<400 vs.
≥400 µg/l) | 0.999 | 0.561–1.777 | 0.996 | – | – | – |
| ALT (≤80 vs. >80
IU/l) | 0.697 | 0.335–1.452 | 0.335 |
|
|
|
| Albumin (>35 vs.
≤ 35 g/l) | 0.383 | 0.190–0.774 | 0.007 | 0.375 | 0.205–0.683 | 0.001 |
| Bilirubin (>20
vs. ≤20 µmol/l) | 2.239 | 0.592–8.471 | 0.235 | – | – | – |
| Histological grade
(moderate vs. well) | 0.665 | 0.313–1.416 | 0.290 | – | – | – |
| Histological grade
(poor vs. well) | 0.242 | 0.063–0.929 | 0.039 | 0.241 | 0.067–0.872 | 0.03 |
| Vascular invasion
(absent vs. present) | 0.672 | 0.363–1.246 | 0.207 | – | – | – |
| Number of tumor
lesions (single vs. multiple) | 2.577 | 1.402–4.739 | 0.002a | 2.98 |
1.647–5.390 | 0.001a |
| ZBP-89 (low vs.
high) | 0.989 | 0.523–1.870 | 0.972 | – | – | – |
 | Table IV.Cox proportional hazard regression
analysis of patients' disease-free survival rates. |
Table IV.
Cox proportional hazard regression
analysis of patients' disease-free survival rates.
|
| Univariable | Multivariable |
|---|
|
|
|
|
|---|
| Parameter | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age (<50 vs. ≥50
years) | 1.143 | 0.670–2.076 | 0.652 | – | – | – |
| Gender (female vs.
male) | 0.401 | 0.155–1.273 | 0.150 | – | – | – |
| Cirrhosis (absence
vs. presence) | 1.201 | 0.682–2.117 | 0.525 | – | – | – |
| Fibrosis vs.
normal | 0.831 | 0.439–1.572 | 0.570 | – | – | – |
| Cirrhosis vs.
normal | 0.608 | 0.246–1.505 | 0.282 | – | – | – |
| Tumor size (<5
vs. >5 cm) | 1.420 | 0.797–2.530 | 0.235 | – | – | – |
| AFP (<400 vs.
≥400 µg/l) | 0.966 | 0.556–1.680 | 0.904 | – | – | – |
| ALT (≤80 vs. >80
IU/l) | 1.271 | 0.637–2.535 | 0.497 | – | – | – |
| Albumin (>35 vs.
≤35 g/l) | 0.821 | 0.404–1.665 | 0.583 | – | – | – |
| Bilirubin (>20
vs. ≤20 µmol/l) | 1.608 | 0.447–5.786 | 0.468 | – | – | – |
| Histological grade
(moderate vs. well) | 0.996 | 0.439–2.258 | 0.991 | – | – | – |
| Histological grade
(poor vs. well) | 0.674 | 0.203–2.239 | 0.519 | – | – | – |
| Vascular invasion
(absent vs. present) | 0.553 | 0.307–0.996 |
0.048a | 0.543 | 0.321–0.918 | 0.023a |
| Number of tumor
lesions (single vs. multiple) | 2.796 | 1.504–5.196 |
0.001a | 3.145 | 1.783–5.547 | 0.001a |
| ZBP-89 (low vs.
high) | 1.797 | 0.972–3.322 | 0.061 | 2.031 | 1.152–3.580 | 0.014a |
Multivariate Cox proportional hazard analysis was
performed based on factors that had been demonstrated to be
significant in the univariate analysis. This analysis revealed that
multiple tumors and macroscopic vascular invasion independently and
significantly increased the recurrence of HCC. In the multivariate
model, the adjusted Cox proportional HR for peritumoral ZBP-89-high
patients was 2.031 (95% CI: 1.152–3.580; P=0.014) for DFS (Table IV). The univariate and the
multivariate model demonstrated that the association between
positive peritumoral ZBP-89 expression and OS rate in patients with
HCC were not statistically significant (Table III). Taken together, the results of
the present study indicated that peritumoral ZBP-89 expression may
be a good prognostic marker for DFS in HCC patients.
Discussion
ZBP-89, commonly expressed at a low level in a
number of adult tissues (31), has
been found to be increased in multiple types of cancer, including
HCC (20), breast cancer (21), esophageal squamous cell carcinomas
(ESCC) (22), melanoma, gastric
cancer, and CRC (21,32), but was reduced in clear-cell renal
cell carcinoma (24), 30% of
pancreatic adenocarcinomas, and Duke's B colon cancer (25). ZBP-89 expression differs in various
tumor types owing to the different cell origins. Furthermore,
positive ZBP-89 expression in different tumors may be indicative of
completely different outcomes. For instance, high ZBP-89 expression
in clear cell renal cell carcinoma and ESCC is associated with poor
survival (22,24), whereas ZBP-89 overexpression is
associated with prolonged OS and DFS times in patients with HCC and
CRC (stages I–IV) (20,30). The contrasting effects of high
intra-HCC and peri-HCC ZBP-89 expression levels on survival rates
are probably due to the different subcellular localization of
ZBP-89 in the HCC cell and nomal hepatocyte. However the specific
role of cytoplasmic ZBP-89 has not been confirmed and further
investigation is required. ZBP-89 expression in adjacent non-tumor
tissues and the associated prognostic impact on HCC has not, to the
best of our knowledge, been systematically studied at present. The
present study revealed an association between peritumoral ZBP-89
expression and patient survival, including DFS and OS rates.
The results of IHC staining identified high
peritumoral ZBP-89 expression in 66.7% of the peritumoral samples
from 102 HCC patients. ZBP-89 expression was localized
predominantly in the cytoplasm of the peritumoral hepatocytes. In
certain tissues with high cytoplasmic staining of ZBP-89 protein, a
few nuclei also exhibited non-uniform immunostaining. This
phenomenon may be associated with the heterogeneity present in
peritumoral liver tissues or due to technical issues with IHC. The
recurrence of HCC is a complex and multifactorial consequence,
including the infiltration of neoplastic into the peritumoral
tissues or the malignant transformation of previously untransformed
or precancerous hepatocytes. However, in the current study, the
later hypothesis is more plausible as there is no histopathological
evidence to support neoplastic hepatocyte infiltration to the
peritumoral tissues by the review of the corresponding
hematoxylin/eosin-stained slide.
Among all the clinicopathological parameters, the
high expression of ZBP-89 in peritumoral hepatocytes is associated
with the presence of liver cirrhosis. In the present study, HCC
recurrence (that is, DFS time) as one indicator of poor patient
prognosis. The direct cause of this poor prognosis, including HCC
recurrence, in HCC patients with high ZBP-89 expression may be
cirrhosis. Significantly, HCC patients expressing high levels of
ZBP-89 in the corresponding non-tumor tissues exhibited a
substantially shorter DFS time than those expressing low
peritumoral ZBP-89 levels, which is consistent with the results of
a previous study (20). Cox
proportional hazard regression analysis of DFS and OS rates
revealed that peritumoral ZBP-89 was a more sensitive factor for
predicting HCC recurrence than cirrhosis. The results of the
present study thus demonstrated that ZBP-89 expression in
peritumoral liver tissues is a highly promising prognostic
biomarker for recurrence of HCC.
The present study revealed that peritumoral ZBP-89
expression is predominantly localized in the cytoplasm, and its
positive expression is associated with a higher risk of HCC
recurrence following hepatic resection. By contrast, ZBP-89 is
mainly expressed in the nuclei of HCC cells, and its overexpression
is associated with prolonged OS and DFS times. A similar phenomenon
also exists in gastric adenocarcinoma and CRC (21,32).
ZBP-89 is mainly expressed in the nuclei of CRC and gastric
adenocarcinoma cells; however, intensive cytoplasmic ZBP-89
staining is present in the surface epithelial cells in the areas of
atrophic gastritis with intestinal metaplasia, which is
pre-malignant (21). Similarly,
ZBP-89 staining is localized in the cytoplasm of adenoma cells,
which are precursors of adenocarcinoma. In familial adenomatous
polyposis, the expression of ZBP-89 increases steadily during the
transition from normal mucosa to adenoma and adenocarcinoma
(32). ZBP-89 expression then
decreases during the progression from stage I to stage IV CRC.
These results indicate that ZBP-89 expression is upregulated at
tumor initiation (23,32). Furthermore, the cytoplasmic
accumulation of ZBP-89 protein occurs in certain pre-malignant
states, particularly during the progression from normal mucosa to
adenocarcinoma (32). We hypothesized
that cytoplasmic ZBP-89 has a role in promoting cancer initiation,
but that nuclear ZBP-89 has a role in tumor suppression (20), and prognosis analysis was performed
concerning whether the sub-localization of the ZBP-89 protein
affects patient prognosis. Although there was a trend of different
survival rates for the four subtypes, the results were not
significant (Fig. 2C and D). Inspired
by this phenomenon, we hypothesized that the subcellular
localization of ZBP-89 in the cytoplasm and nuclei may have a
distinct role in HCC development. However, the molecular pathways
and regulatory mechanisms involved require further
investigation.
Previous studies have focused on the intratumoral
expression of ZBP-89, revealing that its antitumor properties
result from the binding of ZBP-89 to tumor protein p53 (hereafter
p53), which prevents nuclear export and results in an elevated
level of nuclear p53 (33,34). However, evidence indicates that ZBP-89
promotes tumor initiation (23,32,35).
Recent reports indicate that ZBP-89 suppresses the activity of p53;
therefore, reducing ZBP-89 expression could restore p53 activity
and protect against cancer development (23). In addition, a recent study revealed
that the ZBP-89 protein binds directly to the promoter of CTNNB1 to
induce transcription and drives a feed-forward loop of β-catenin
expression (9). The hyper-activation
of Wnt/β-catenin signaling is closely associated with tumor
aggressiveness and resistance to chemotherapeutic agents in HCC
(36–38). Furthermore, ZBP-89 and β-catenin
induce gene expression reciprocally and synergistically (9), which may be the mechanism by which
ZBP-89 promotes the neoplastic transformation in adjuvant
non-cancerous tissue.
Evidence indicates that patients with HBV infection
are more likely to exhibit high ZBP-89 expression, which could
drive a feed-forward loop of β-catenin expression. The reactivation
of the sustained Wnt/β-catenin pathway is associated with the
pathogenesis of liver cirosis and could represent a promising novel
target for fibrotic diseases (36–40). The
results from these previous reports are consistent with those of
the present study, demonstrating that ZBP-89 expression was
elevated in fibrosis or cirrhosis tissues of the liver. Therefore,
the direct cause of poor prognosis in patients HCC with high ZBP-89
expression may also be cirrhosis. However, the specific mechanisms
by which ZBP-89 is involved in the formation of liver cirrhosis or
how liver cirrhosis induces the high expression of ZBP-89 remains
unclear and require more in-depth research.
The present study used the METAVIR scoring system
for liver fibrosis scoring. In the peritumoral tissues, The
pathological status of hepatocirrhosis, and the DFS and OS rates
was examined in the current study; although there was a trend of
different survival rates among patients with different severity of
liver cirrhosis, the differences were not significant (Fig. 2E and F).
The present study had several limitations. First,
the clinicopathological data and samples were collected from a
single institution, which the data inadequate for further
stratified analysis. Furthermore, the sample size was not large,
and a prospective multi-center study consisting of a large number
of patients who are uniformly classified and treated is required in
the future. Evaluating only one slide per patient is a further
limitation of the present study. Additionally, intratumoral and
peritumoral expression was not directly compared in the current
study as previous studies (20,22,24) have
already investigated the intratumoral ZBP-89 expression and
concluded that high intratumoral ZBP-89 expression is associated
with improved survival rates.
In conclusion, the results of the present study
indicate that high expression of ZBP-89 in peritumoral HCC tissues
was associated with a shorter DFS time in HCC patients following
curative hepatectomy. Additionally, high ZBP-89 expression in
peritumoral HCC tissue was positively associated with the presence
of liver cirrhosis in HCC patients, indicating that cirrhosis with
high peritumoral ZBP-89 expression may be a contributing factor to
the poor prognosis of patients with HCC. Therefore, peritumoral
ZBP-89 expression may be a good prognostic marker to predict DFS
time in HCC patients following curative hepatectomy and may provide
novel insights into the molecular mechanisms of HCC initiation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472339 and
81402041).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WQS analyzed and interpreted the patient data
regarding the HCC and CC performed the histological examination of
the peritumoral liver slides, both of them were the major
contributors in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to publish
This study was approved by the Joint Chinese
University of Hong Kong-New Territories East Cluster Clinical
Research Ethics Committee. Informed written consent was obtained
from all patients involved in the study.
Consent for publication
Informed written consent was obtained from all
patients involved in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zuo TT, Zheng RS, Zhang SW, Zeng HM and
Chen WQ: Incidence and mortality of liver cancer in China in 2011.
Chin J Cancer. 34:508–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryerson AB, Eheman CR, Altekruse SF, Ward
JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM,
et al: Annual report to the nation on the status of cancer,
1975–2012, featuring the increasing incidence of liver cancer.
Cancer. 122:1312–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Utsunomiya T, Shimada M, Imura S, Morine
Y, Ikemoto T and Mori M: Molecular signatures of noncancerous liver
tissue can predict the risk for late recurrence of hepatocellular
carcinoma. J Gastroenterol. 45:146–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jain RK: Normalizing tumor
microenvironment to treat cancer: Bench to bedside to biomarkers. J
Clin Oncol. 31:2205–2218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llovet JM, Paradis V, Kudo M and
Zucman-Rossi J: Tissue biomarkers as predictors of outcome and
selection of transplant candidates with hepatocellular carcinoma.
Liver Transpl. 17 Suppl 2:S67–S71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu LC, Hsu CH, Hsu C and Cheng AL: Tumor
heterogeneity in hepatocellular carcinoma: Facing the challenges.
Liver Cancer. 5:128–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao Q, Wang ZC, Duan M, Lin YH, Zhou XY,
Worthley DL, Wang XY, Niu G, Xia Y, Deng M, et al: Cell culture
system for analysis of genetic heterogeneity within hepatocellular
carcinomas and response to pharmacologic agents. Gastroenterology.
152:232–242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xue R, Li R, Guo H, Guo L, Su Z, Ni X, Qi
L, Zhang T, Li Q, Zhang Z, et al: Variable intra-tumor genomic
heterogeneity of multiple lesions in patients with hepatocellular
carcinoma. Gastroenterology. 150:998–1008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Essien BE, Sundaresan S, Ocadiz-Ruiz R,
Chavis A, Tsao AC, Tessier AJ, Hayes MM, Photenhauer A,
Saqui-Salces M, Kang AJ, et al: Transcription factor ZBP-89 drives
a feedforward loop of β-catenin expression in colorectal cancer.
Cancer Res. 76:6877–6887. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borghaei RC, Gorski G, Seutter S, Chun J,
Khaselov N and Scianni S: Zinc-binding protein-89 (ZBP-89)
cooperates with NF-kB to regulate expression of matrix
metalloproteinases (MMPs) in response to inflammatory cytokines.
Biochem Biophys Res Commun. 471:503–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye CG, Chen GG, Ho RLK, Merchant JL, He ML
and Lai PBS: Epigenetic upregulation of Bak by ZBP-89 inhibits the
growth of hepatocellular carcinoma. Biochim Biophys Acta.
1833:2970–2979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leupold JH, Asangani IA, Mudduluru G and
Allgayer H: Promoter cloning and characterization of the human
programmed cell death protein 4 (pdcd4) gene: Evidence for ZBP-89
and Sp-binding motifs as essential Pdcd4 regulators. Biosci Rep.
32:281–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai L and Merchant JL: Transcription
factor ZBP-89 is required for STAT1 constitutive expression.
Nucleic Acids Res. 31:7264–7270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai L and Merchant JL: Transcription
factor ZBP-89 cooperates with histone acetyltransferase p300 during
butyrate activation of p21waf1 transcription in human cells. J Biol
Chem. 275:30725–30733. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng Y, Wang X, Xu L, Pan H, Zhu S, Liang
Q, Huang B and Lu J: The transcription factor ZBP-89 suppresses p16
expression through a histone modification mechanism to affect cell
senescence. FEBS J. 276:4197–4206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Zhang X, Salmon M and Zehner ZE: The
zinc finger repressor, ZBP-89, recruitshistone deacetylase 1 to
repress vimentin gene expression. Genes Cells. 12:905–918. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Diab IH and Zehner ZE: ZBP-89
represses vimentin gene transcription by interacting with the
transcriptional activator, Sp1. Nucleic Acids Res. 31:2900–2914.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Remington MC, Tarle SA, Simon B and
Merchant JL: ZBP-89, a Kruppel-type zinc finger protein, inhibits
cell proliferation. Biochem Biophys Res Commun. 237:230–234. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Merchant JL, Iyer GR, Taylor BR, Kitchen
JR, Mortensen ER, Wang Z, Flintoft RJ, Michel JB and Bassel-Duby R:
ZBP-89, a Kruppel-like zinc finger protein, inhibits epidermal
growth factor induction of the gastrin promoter. Mol Cell Biol.
16:6644–6653. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang CZ, Cao Y, Yun JP, Chen GG and Lai
PB: Increased expression of ZBP-89 and its prognostic significance
in hepatocellular carcinoma. Histopathology. 60:1114–1124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taniuchi T, Mortensen ER, Ferguson A,
Greenson J and Merchant JL: Overexpression of ZBP-89, a zinc finger
DNA binding protein, in gastric cancer. Biochem Biophys Res Commun.
233:154–160. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan SM, Wu HN, He F, Hu XP, Zhang ZY,
Huang MY, Wu X, Huang CY and Li Y: High expression of zinc-binding
protein-89 predicts decreased survival in esophageal squamous cell
cancer. Ann Thorac Surg. 97:1966–1973. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nilton A, Sayin VI, Zou ZV, Sayin SI,
Bondjers C, Gul N, Agren P, Fogelstrand P, Nilsson O, Bergo MO and
Lindahl P: Targeting Zfp148 activates p53 and reduces tumor
initiation in the gut. Oncotarget. 7:56183–56192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai MY, Luo RZ, Li YH, Dong P, Zhang ZL,
Zhou FJ, Chen JW, Yun JP, Zhang CZ and Cao Y: High-expression of
ZBP-89 correlates with distal metastasis and poor prognosis of
patients in clear cell renal cell carcinoma. Biochem Biophys Res
Commun. 426:636–642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai L, Logsdon C and Merchant JL:
Regulation of epithelial cell growth by ZBP-89: Potential relevance
in pancreatic cancer. Int J Gastrointest Cancer. 31:79–88. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Altman DG, McShane LM, Sauerbrei W and
Taube SE: Reporting recommendations for tumor marker prognostic
studies (REMARK): Explanation and elaboration. PLoS Med.
9:e10012162012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moons KG, Altman DG, Reitsma JB and
Collins GS: Transparent Reporting of a Multivariate Prediction
Model for Individual Prognosis or Development Initiative: New
guideline for the reporting of studies developing, validating, or
updating a multivariable clinical prediction model: The TRIPOD
statement. Adv Anat Pathol. 22:303–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pirisi M, Leutner M, Pinato DJ, Avellini
C, Carsana L, Toniutto P, Fabris C and Boldorini R: Reliability and
reproducibility of the edmondson grading of hepatocellular
carcinoma using paired core biopsy and surgical resection
specimens. Arch Pathol Lab Med. 134:1818–1822. 2010.PubMed/NCBI
|
|
29
|
Mohamadnejad M, Tavangar SM, Sotoudeh M,
Kosari F, Khosravi M, Geramizadeh B, Montazeri G, Estakhri A,
Mirnasseri MM, Fazlollahi A, et al: Histopathological study of
chronic hepatitis B: A comparative study of ishak and METAVIR
scoring systems. Int J Organ Transplant Med. 1:171–176.
2010.PubMed/NCBI
|
|
30
|
Chan JK: The wonderful colors of the
hematoxylin-eosin stain in diagnostic surgical pathology. Int J
Surg Pathol. 22:12–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang CZ, Chen GG and Lai PB:
Transcription factor ZBP-89 in cancer growth and apoptosis. Biochim
Biophys Acta. 1806:36–41. 2010.PubMed/NCBI
|
|
32
|
Gao XH, Liu QZ, Chang W, Xu XD, Du Y, Han
Y, Liu Y, Yu ZQ, Zuo ZG, Xing JJ, et al: Expression of ZNF148 in
different developing stages of colorectal cancer and its prognostic
value: A large Chinese study based on tissue microarray. Cancer.
119:2212–2222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bai L and Merchant JL: ZBP-89 promotes
growth arrest through stabilization of p53. Mol Cell Biol.
21:4670–4683. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okada M, Tessier A, Bai L and Merchant JL:
P53 mutants suppress ZBP-89 function. Anticancer Res. 26:2023–2028.
2006.PubMed/NCBI
|
|
35
|
Fang J, Jia J, Makowski M, Xu M, Wang Z,
Zhang T, Hoskins JW, Choi J, Han Y, Zhang M, et al: Functional
characterization of a multi-cancer risk locus on chr5p15.33 reveals
regulation of TERT by ZNF148. Nat Commun. 8:150342017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiang T, Zhang S, Cheng N, Ge S, Wen J,
Xiao J and Wu X: Oxidored-nitro domain-containing protein 1
promotes liver fibrosis by activating the Wnt/β-catenin signaling
pathway in vitro. Mol Med Rep. 16:5050–5054. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miao CG, Yang YY, He X, Huang C, Huang Y,
Zhang L, Lv XW, Jin Y and Li J: Wnt signaling in liver fibrosis:
Progress, challenges and potential directions. Biochimie.
95:2326–2335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng JH, She H, Han YP, Wang J, Xiong S,
Asahina K and Tsukamoto H: Wnt antagonism inhibits hepatic stellate
cell activation and liver fibrosis. Am J Physiol Gastrointest Liver
Physiol. 294:G39–G49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Whittaker S, Marais R and Zhu AX: The role
of signaling pathways in the development and treatment of
hepatocellular carcinoma. Oncogene. 29:4989–5005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo Y, Xiao L, Sun L and Liu F:
Wnt/beta-catenin signaling: A promising new target for fibrosis
diseases. Physiol Res. 61:337–346.. 2012.PubMed/NCBI
|