Introduction
Lung cancer is the number one malignant tumor
threatening the life of human beings. The survival chance of
patients is strongly diminished even after surgical resection,
neoadjuvant chemotherapy, postoperative routine enhanced
chemotherapy and radiotherapy, or targeted gene therapy (1) and this is mainly due to the still mostly
unknown pathogenesis of lung cancer and the lack of effective
early-stage detection methods. Molecular research suggests the
cancer is caused by the coeffect of external environment and the
genetic changes (2). Epigenetic
modifications, including the methylation of DNA and histone
modification, inactivation of chromosome X and the regulation of
non-coding RNA (miRNAs) are major factors increasing the occurrence
of tumors. miRNAs regulate the transcription and translation of
effector proteins in approximately 30% of the total mRNA pool and
thus interfere in the physiological and pathological behavior of
cells (3). Bioinformatic surveys have
shown that miR-126 is abnormally expressed in many malignant
tumors, such as lung, breast and colorectal cancers and it is also
involved in a variety of biological behavior, including cell
proliferation, differentiation, invasion, metastasis and apoptosis
(4,5).
Clinical studies have also confirmed miR-126 is highly expressed in
non-small cell lung cancer (NSCLC) patients, who invariably suffer
from poor clinical prognoses (6).
Signal transducers and activators of the transcription 3 (STAT3)
transcription factor protein can promote cell proliferation and
inhibit cell apoptosis and the STAT3 signal pathway is also
activated by many extracellular signals, such as non-receptor
tyrosine kinase, cytokines and growth factors. Once STAT3 is
delivered to the nucleus, it binds with the promoters on the target
genes of vascular epithelial growth factor (VEGF) and the relative
receptor (VEGFR), as well as the epithelial growth factor (EGF) and
the relative receptor (EGFR); thereby regulating cell
proliferation, angiogenesis and apoptosis and directly influencing
the occurrence and progression of tumors (7,8). In this
study, hypothesized miR-126 is involved in induction of the STAT3
signal pathway regulating the malignant behavior of NSCLC cells.
Our results identified new potential targets for NSCLC
intervention.
Materials and methods
Experimental materials
A549 cells purchased from Sangon Biotechnology
(Shanghai, China) were cultivated in RPMI-1640 media containing 10%
fetal bovine serum (FBS; Invitrogen-Life Technologies, Carlsbad,
CA, USA) in a CO2 incubator (HyClone, Logan, UT, USA),
and the medium was refreshed every other day. Each culture was
terminated upon 85% confluence of cells by digestion using trypsin.
Cells were harvested and washed using phosphate-buffered saline
(PBS) and cell suspensions were prepared (2×106
cells/ml) and split for subsequent experiments.
Amplification of plasmids
Empty miR-126 overexpression and low-expression
plasmids were purchased from Applied Biosystems (Foster City, CA,
USA) and E. coli DH5α was purchased from Bio-Rad
Laboratories (Hercules, CA, USA). Amplification was carried out for
bacteria containing different plasmids followed by centrifugation
at 10,000 × g for 5 min to precipitate the bacteria. Then, 250 µl
ZL-I/RNAse A mixture was added followed by vibration to fully
suspend the cells into solution. ZL-II (250 µl) was subsequently
added to the cell suspension to obtain a clear cell lysate. Next,
350 µl ZL-III buffer was added to produce a white flocculent
sediment centrifuged at 10,000 × g for 10 min. A Mu-Pu plasmid
micro-separation column (Corning Inc., Corning, NY, USA) was used
to extract the supernatant for centrifugation at 10,000 × g for 5
min and the flow-through was discarded. Then, 500 µl ZL buffer was
added followed by centrifugation at 10,000 × g for 5 min and DNA
was obtained after washing with 720 µl ethanol buffer. The
substrate in the column was dried through centrifugation at 10,000
× g for 5 min. Finally, 100 µl sterile deionized water was added
and the plasmid was eluted with a last centrifugation at 10,000 × g
for 5 min.
G418 experiment
A549 cells were inoculated onto 12-well plates (1
ml/well) and cultivated for 24 h to adhere to the wall. Then the
culture medium was removed and in each well, 1-ml RPMI-1640 culture
medium amounts containing G418 and 10% FBS were added, in which
serial gradient concentrations of G418 were within 100 and 1,000
µg/ml (at 100 µg/ml increments). Cells in a well without G418 were
set as the control group. The plates was transferred into the
incubator (37°C, 5% CO2) for culture. After the 2nd week
of treatment, the screening dosage was that doubling the minimal
and effective lethal dosage and the sustaining dosage was set at
200 µg/ml less than the screening dosage.
Cell transfection
Cell transfections were performed in accordance with
the manufacturer's instructions in the Lipofectamine 2000
(Invitrogen Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) insert and the transfection efficiency was
assayed using reverse transcription polymerase chain reaction
(RT-PCR). The primers to amplify a 256 bp fragment of miR-126 were
synthesized by Beijing Zhongshan Golden Bridge Biotechnology
(Beijing, China): Forward 5′-GCCAGTCAGATGTGGATGAA-3′ and reverse,
5′-CCCAACACTGGCACCAGTAA-3′. The primers for a 225 bp fragment of
reduced glyceraldehyde-phosphate dehydrogenase (GAPDH) to use as
the internal gene reference were: Forward,
5′-CGCGAGAAGATGACCCAGAT-3′ and reverse, 5′-GCACTGTGTTGGCGTACAGG-3′.
An RT-PCR kit (Takara, Otsu, Japan) was used to synthesize cDNA and
the PCR amplification system was set as follows: 5 µl cDNA + 2 µl
10X Ex Buffer + 1.6 µl dNTP mixture (10 mM) + 0.2 µl polymerase Ex
Taq HS + 1 µl upstream primer and 1 µl downstream primer and the
system was diluted to 20 µl by adding water. The amplification
conditions were set up with an initial 94°C for 5 min denaturation
step; then 35 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C
for 30 sec; and a final extension at 72°C for 5 min. Each PCR
product was identified by 2% agarose gel electrophoresis and the
images obtained by ultraviolet spectrometry were analyzed by a gel
imaging system. Digital photographs were used for analysis of gray
values.
Determination of cell cycle status,
proliferation, migration capabilities and apoptosis
susceptibility
After 24 h cultures, methyl thiazolyl tetrazolium
(MTT) from R&D Systems (Minneapolis, MN, USA) was applied to
detect cell proliferation rate using a standard assay protocol.
Cell migration distance was measured performing conventional
scratch assays viewed under a microscope from Olympus (Tokyo,
Japan). The cell cycle status of cells was determined through flow
cytometry, using a FACSCaliber flow cytometer from BD Biosciences
(Franklin Lakes, NJ, USA). The caspase-3 mRNA expression levels in
cells were detected using standard RT-PCR methods (see below) and
the STAT3 protein expression levels were detected using western
blotting assays as detailed below.
Detecting the mRNA expression level of
caspase-3 through RT-PCR
Primer sequences of caspase-3 were synthesized by
Beijing Zhongshan Golden Bridge. The primer sequences are: Forward,
5′-GGTTTCATCCAGGATCGAGCAGG-3′ and reverse,
5′-ACAAAGATGGTCACGGTCTGCC-3′, yielding a 325 bp fragment. GAPDH:
Forward, 5′-CGCGAGAAGATGACCCAGAT-3′ and reverse,
5′-GCACTGTGTTGGCGTACAGG-3′, amplifying a 225 bp fragment. The
reaction system consisted of 2 µl cDNA + 1 µl upstream primer and 1
µl downstream primer + 0.5 µl polymerase Ex Taq HS + 1 µl 10X Ex
Buffer and then was diluted to 25 µl. Reaction conditions were set
as follows: 95°C for 5 min; 30 cycles of 95°C for 30 sec, 72°C for
30 sec and 72°C for 60 sec; with a final extension at 72°C for 5
min.
Detecting the protein expression level
of STAT3 through western blot analysis
Western blotting experiments were carried out
according to the kit manufacturer's instructions. Mouse anti-human
STAT3 and internal reference β-actin monoclonal antibodies were
used as the primary antibodies (dilution, 1:2,000; cat. nos.
sc-71792 and sc-130300) and a goat anti-mouse immunoglobulin G
polyclonal antibody as the secondary antibody (dilution, 1:500;
cat. no. sc-362267). All antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The total protein
extraction kit was purchased from Kangchen Biotech (Shanghai,
China), a bicinchoninic acid (BCA) kit for assay of protein
concentration and an enhanced chemiluminescent (ECL) kit were
purchased from Guangdong Bochuan Biotechnology (Guangdong, China).
The Lab Works 4.5 gel imaging software was purchased from
Invitrogen Life Technologies.
Statistical analysis
SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analyses. The measurement data are presented
as mean ± standard deviation (SD). One-way analysis of variance was
adopted for intergroup comparisons and least significant difference
test (LSD-t) was performed for paired comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
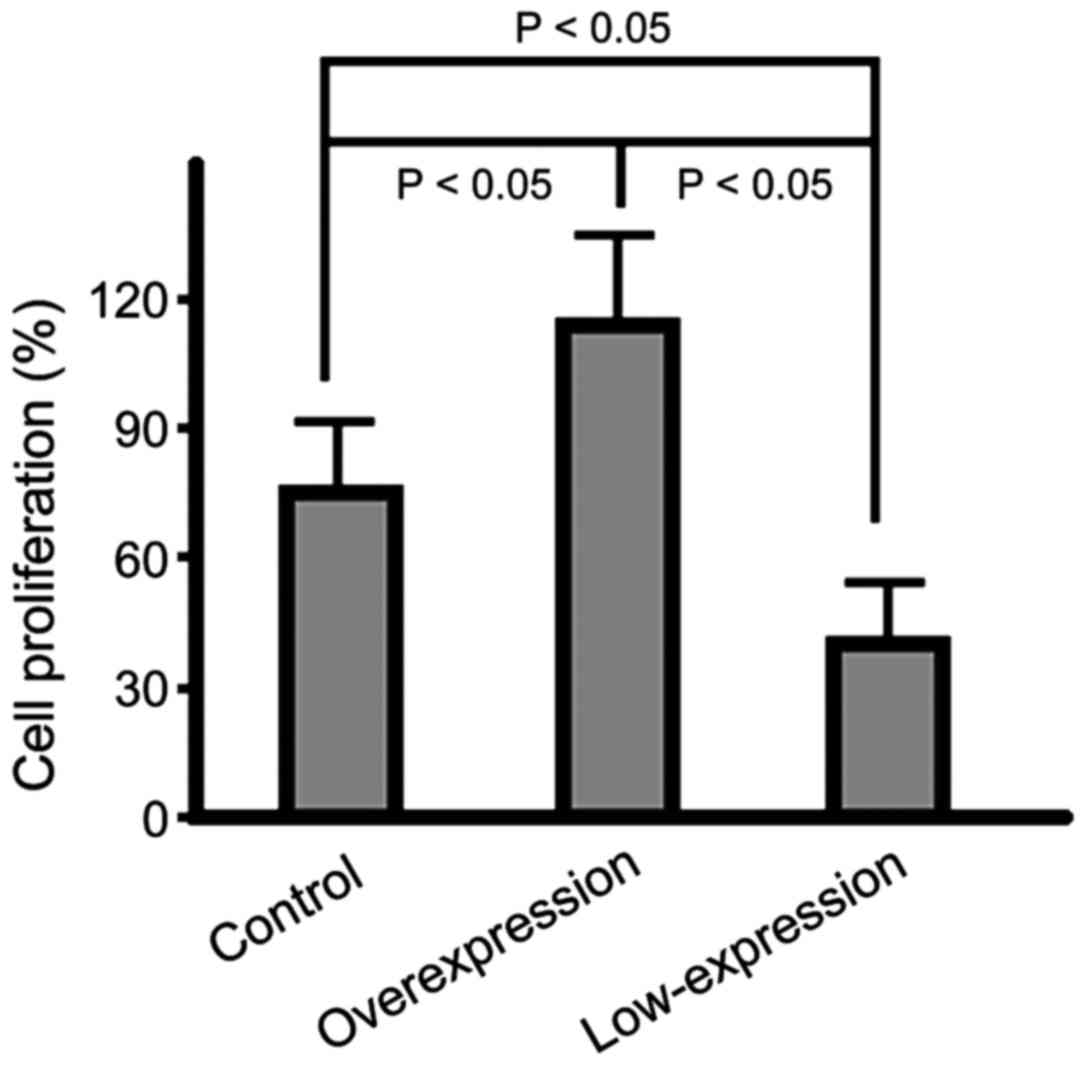
Cell proliferation rates in the three
groups
In the overexpression group, the cell proliferation
rate was significantly higher than that in the control group
(p<0.05) and the rate in the low-expression group was the lowest
among the three groups (p<0.05); the difference had statistical
significance (Fig. 1).
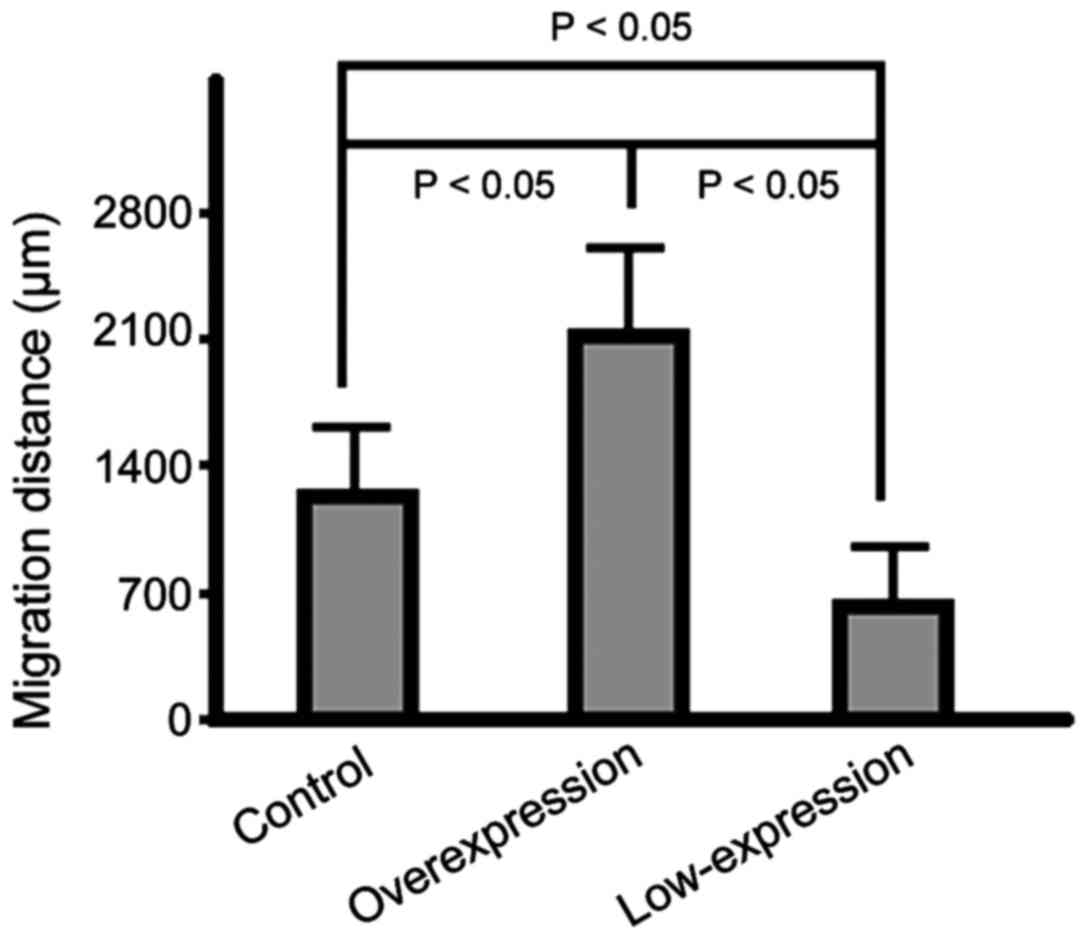
Distance of cell migration in scratch
assays in the three groups
The migration capabilities of the cells in the
overexpression group were significantly increased compared to those
of cells in the control group. The cells that migrated the shortest
distance were those in the low-expression group (p<0.05)
(Fig. 2).
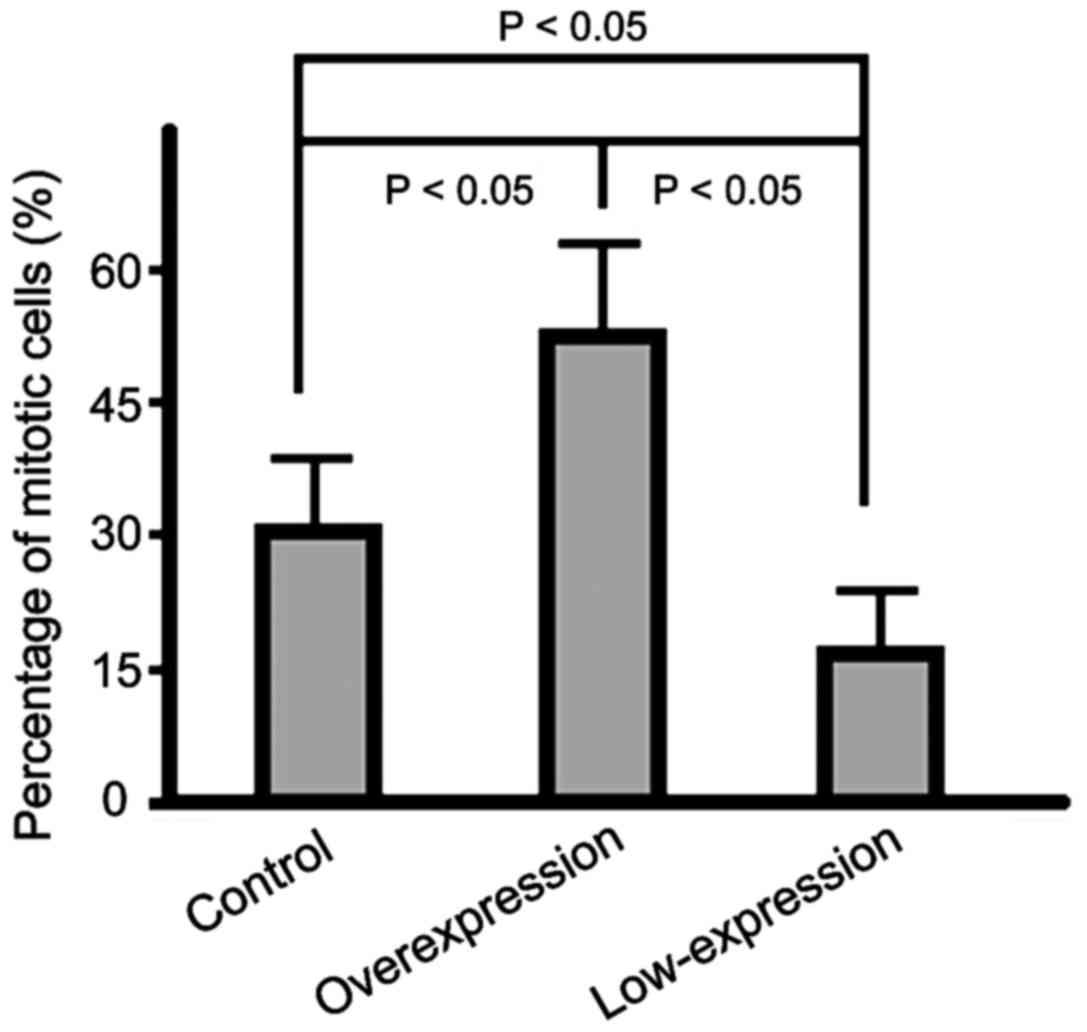
Cell cycle status of cells in the
three groups
The percentage of cells in mitotic phase in the
overexpression group was significantly higher than that in the
control group and the percentage in the low-expression group was
the lowest (p<0.05) (Fig. 3).
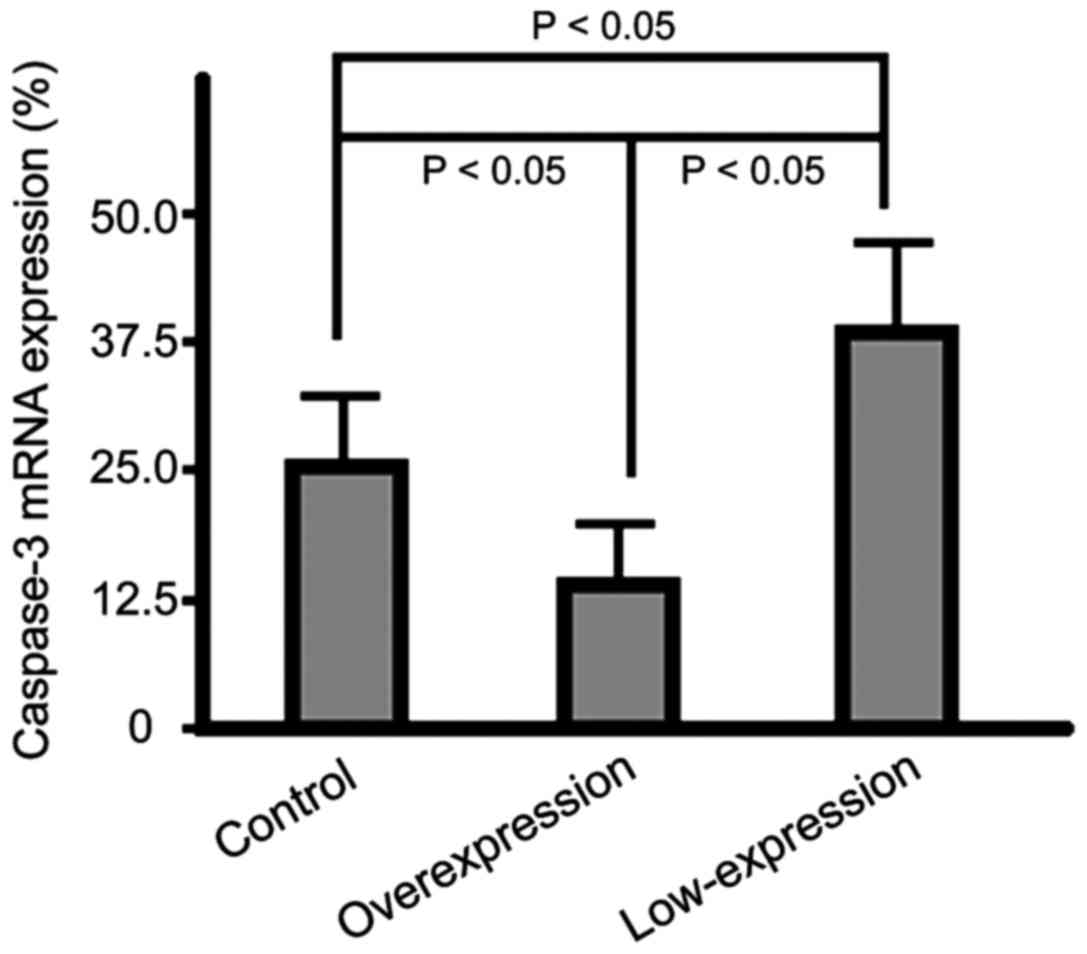
mRNA expression level of caspase-3 in
cells of the three groups
The mRNA expression levels of caspase-3 of cells in
the overexpression group were significantly lower than those in the
control group cells and the highest expression level was found in
the cells of the low-expression group (p<0.05) (Fig. 4).
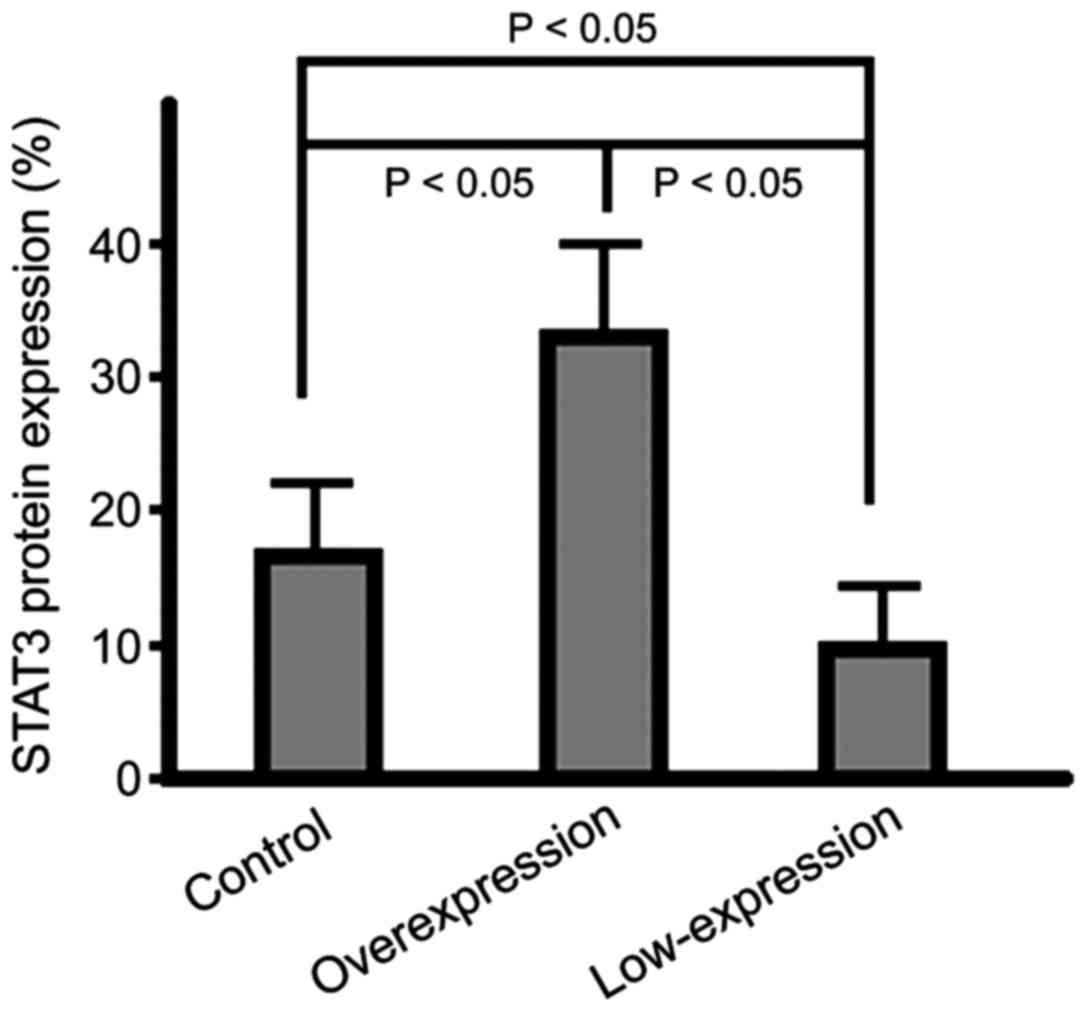
Protein expression level of STAT3 in
cells
The STAT3 protein expression in cells of the
overexpression group were significantly lower than those of cells
in the control group and the highest expression level was
identified in the low-expression group cells (p<0.05) (Fig. 5).
Discussion
The miRNAs are high evolutionarily conserved
non-coding single-chain nucleotide chains with lengths of 18 to 24
nucleotides, that can bind to proteins to form RNA-induced
silencing complexes (RISCs) that degrade mRNAs or silence their
expression through direct mRNA binding interactions (9). Each miRNA can regulate multiple target
genes and potentially all of the genetic pathways are regulated by
miRNAs. The miR17/20 regulate the interleukin-8, the miR17-92
regulate the p21 and miR181 regulates the B-cell lymphoma-2 (Bcl-2)
pathways, thereby exerting a major regulatory role in the
occurrence and progression of malignant tumors (10,11).
It has been reported that the expression of miR-126
varies during different stages of development in mouse and human
pulmonary tissues and that it is mainly expressed in the
endothelial cells of lung tissues and the epithelial cells of
bronchi (12).
Through this study, we found that compared with the
control group, the cell proliferation rate in cells in miR-126
overexpression group was significantly elevated, the distance of
cell migration was extended, the ratio of cells in mitotic phase
was increased, the mRNA expression level of caspase-3 was
remarkably reduced and the protein expression level of STAT3 was
elevated. Furthermore, the results in the low-expression group were
contrary to those in the overexpression group and the differences
had statistical significance. These results suggest that miR-126,
is a cancer-promoting gene, which can mediate the activation of the
STAT3 signaling pathway and thus regulate the malignant behavior of
NSCLC. Nevertheless, a different study suggested that miR-126 may
have cancer suppressing effects (13)
and the fact that miR-126 can suppress the expression of target
genes SPRED1 and PIK3R2 (negatively regulating the Erk and PIK3R2
signal pathways), that it can inhibit expression of chemotactic
factor CXCL12 and block the apoptosis of vascular endothelial cells
indicates that miR-126 could regulate embryonic development and
tissue growth and sustain the vascular homeostasis. The apparent
contradiction with our results may be associated with the variance
between the in vivo and in vitro environment of tumor
cells and the characteristics of expression in different types of
tumor cells. For example, another study confirmed that in
peripheral blood of patients with acute myelogenous leukemia, the
level of miR-126 expression is elevated due to the decrease in
methylation in its promoter region, leading to inhibition of cell
apoptosis (14), a consequence more
in agreement with our own findings.
It has been shown that STAT3 expression in lung
cancer tissues is significantly higher than that in normal
para-carcinoma tissues and that the expression levels significantly
correlate with the differentiation, lymphatic metastasis and TNM
staging of NSCLC tumors (15). Even
more, the STAT3 signaling pathway is closely associated with the
persistent activation of EGFR (16).
Importantly, Tang et al (17)
showed that the expression of STAT3 in EGFR-mutant lung cancer
cells is significantly higher than that in the wild-type cells and
the sensitivity to the target therapy of gefitinib is also
improved. EGFR, is a member of the erbB family (a receptor of
tyrosine protein kinase, TPK), involved in the occurrence of tumors
through activation of multiple downstream target genes by enabling
the acidification site of tyrosine at the C terminal domain of the
STAT3 protein (18,19).
In conclusion, abnormal expression of miR-126 is
closely associated with the malignant behavior of NSCLC cells and
the STAT3 signaling pathway is probably the main target leading to
that effect. Thus, futher studies should confirm our results that
suggest intervening in the activity or expression of miR-126 and
STAT3 may be an effective treatment against NSCLC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shandong Provincial
Natural Science Foundation, China (ZR2016HM80).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ contributed significantly to writing the
manuscript and cell culture. JW analyzed and interpreted RT-PCR. JC
performed and analyzed western blotting. XY performed G418
experiment and helped the conception of the study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fang W and Ruan W: Advances in surgical
treatment of early stage non-small cell lung cancer. Asian Pac J
Surg Oncol. 2:1–10. 2017.
|
|
2
|
Lee PN, Forey BA and Coombs KJ: Systematic
review with meta-analysis of the epidemiological evidence in the
1900s relating smoking to lung cancer. BMC Cancer. 12:3852012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leidinger P, Galata V, Backes C, Stähler
C, Rheinheimer S, Huwer H, Meese E and Keller A: Longitudinal study
on circulating miRNAs in patients after lung cancer resection.
Oncotarget. 6:16674–16685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Del Vescovo V, Grasso M, Barbareschi M and
Denti MA: MicroRNAs as lung cancer biomarkers. World J Clin Oncol.
5:604–620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tai HC, Chang AC, Yu HJ, Huang CY, Tsai
YC, Lai YW, Sun HL, Tang CH and Wang SW: Osteoblast-derived
WNT-induced secreted protein 1 increases VCAM-1 expression and
enhances prostate cancer metastasis by down-regulating miR-126.
Oncotarget. 5:7589–7598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu W, Zhou K, Zha Y, Chen D, He J, Ma H,
Liu X, Le H and Zhang Y: Diagnostic value of serum miR-182,
miR-183, miR-210, and miR-126 levels in patients with early-stage
non-small cell lung cancer. PLoS One. 11:e01530462016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun CC, Li SJ, Zhang F, Zhang YD, Zuo ZY,
Xi YY, Wang L and Li DJ: The novel miR-9600 suppresses tumor
progression and promotes paclitaxel sensitivity in non-small-cell
lung cancer through altering STAT3 expression. Mol Ther Nucleic
Acids. 5:e3872016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu F, Dai C, Fu Y, Loo JF, Xia D, Gao SP,
Ma Z and Chen Z: Physalin A exerts anti-tumor activity in non-small
cell lung cancer cell lines by suppressing JAK/STAT3 signaling.
Oncotarget. 7:9462–9476. 2016.PubMed/NCBI
|
|
9
|
Hou J, Meng F, Chan LW, Cho WC and Wong
SC: Circulating plasma MicroRNAs as diagnostic markers for NSCLC.
Front Genet. 7:1932016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu Z, Willmarth NE, Zhou J, Katiyar S,
Wang M, Liu Y, McCue PA, Quong AA, Lisanti MP and Pestell RG:
microRNA 17/20 inhibits cellular invasion and tumor metastasis in
breast cancer by heterotypic signaling. Proc Natl Acad Sci USA.
107:8231–8236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong J, Zheng S, Zhang L, Wang Y and Meng
J: Induction of K562 cell apoptosis by As4S4 via down-regulating
miR181. Med Sci Monit. 23:144–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Lan H, Huang X, Liu B and Tong Y:
MicroRNA-126 inhibits tumor cell growth and its expression level
correlates with poor survival in non-small cell lung cancer
patients. PLoS One. 7:e429782012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Lu J, Sun M, Mi S, Zhang H, Luo RT,
Chen P, Wang Y, Yan M, Qian Z, et al: Distinct microRNA expression
profiles in acute myeloid leukemia with common translocations. Proc
Natl Acad Sci USA. 105:15535–15540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin Z, Zhang Y, Li Y, Lv T, Liu J and Wang
X: Prognostic significance of STAT3 expression and its correlation
with chemoresistance of non-small cell lung cancer cells. Acta
Histochem. 114:151–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Shi Y, Yuan Q, Liu X, Yan B, Chen L,
Tao Y and Cao Y: Epstein-Barr virus encoded LMP1 regulates cyclin
D1 promoter activity by nuclear EGFR and STAT3 in CNE1 cells. J Exp
Clin Cancer Res. 32:902013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang J, Guo F, Du Y, Liu X, Qin Q, Liu X,
Yin T, Jiang L and Wang Y: Continuous exposure of non-small cell
lung cancer cells with wild-type EGFR to an inhibitor of EGFR
tyrosine kinase induces chemoresistance by activating STAT3. Int J
Oncol. 46:2083–2095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaganathan S, Yue P, Paladino DC,
Bogdanovic J, Huo Q and Turkson J: A functional nuclear epidermal
growth factor receptor, SRC and Stat3 heteromeric complex in
pancreatic cancer cells. PLoS One. 6:e196052011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lo HW, Cao X, Zhu H and Ali-Osman F:
Cyclooxygenase-2 is a novel transcriptional target of the nuclear
EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol Cancer Res.
8:232–245. 2010. View Article : Google Scholar : PubMed/NCBI
|