Introduction
Gastric cancer is the second leading cause of
cancer-associated mortality in China (1). Due to a lack of screening protocols, the
majority of patients often present with locally advanced disease or
even metastasis at initial diagnosis. As surgery alone is not
sufficient to guarantee curative treatment in patients with
advanced disease, adjuvant therapies must be considered in order to
increase the possibility of cure in surgically treated patients
with a high risk of recurrence (2,3). While
chemotherapy has been widely used in the clinical treatment of
gastric cancer, its effects on the immune status of patients remain
unknown.
Tumor immune tolerance serves an important role in
contributing to the occurrence and development of gastric cancer
(4). Previous studies have indicated
that the outcome of an immune response towards a tumor is primarily
determined by the type of immune response elicited (5,6).
Regulatory T cells (Tregs) have been demonstrated to
induce tumor immunosuppression and therefore have a prominent role
in the development of cancer (7–9). Previous
studies have demonstrated that the level of Tregs, which
are usually evaluated by immunohistochemical quantification of
Forkhead box protein P3 (FoxP3)-positive T cells, are associated
with a poor outcome (10–13).
The present study explored the effect of folinic
acid (FA)/fluorouracil (5-FU)/oxaliplatin (FOLFOX-6) chemotherapy
on Tregs and the immune function of patients with
gastric cancer prior to and following chemotherapy. It was
indicated that chemotherapy may inhibit the secretion of C-C motif
chemokine ligand 20 (CCL20) to prevent the migration of
Tregs, thereby assisting in enhancing the antitumor
immune response.
Materials and methods
Patients and specimens
The clinical records of 38 patients with gastric
cancer who underwent curative surgical intervention of primary
tumors between June 2011 and September 2014 at the Second
Affiliated Hospital, Zhejiang University School of Medicine
(Hangzhou, China) were collected. Written informed consent was
obtained from the patients and the study was approved by the Ethics
Committee of the Second Affiliated Hospital. Tumor stages were
classified according to the 7th edition of the American Joint
Committee on Cancer Tumor Node Metastasis Classification (14). Patients treated with neoadjuvant
chemotherapy were excluded. Blood was obtained from patients before
and after chemotherapy and healthy control.
Chemotherapy
Venous blood (3 ml) was obtained from patients with
gastric cancer (n=38) and from healthy individuals (n=31), using an
EDTA K2 anticoagulant mining vessel (BD Biosciences, Franklin
Lakes, NJ, USA). Blood was obtained from the gastric cancer
patients 1 day prior to chemotherapy and 1 month following
chemotherapy. Chemotherapy consisted of 65 mg/m2
intravenously (IV) oxaliplatin on day 1, 200 mg/m2 FA IV
as a 2 h infusion, followed by 400 mg/m2 bolus 5-FU IV
and a 22 h infusion of 5,600 mg/m2 5-FU IV on days 1 and
2, every 2 weeks (15). All patients
who were included in the present study completed at least three
cycles of chemotherapy.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were
isolated by Ficoll density-gradient (GE Healthcare, cat. no.
17-1440-03) centrifugation (400 × g for 30 min at 18°C). Antibodies
specific for mouse FITC-conjugated anti-cluster of differentiation
(CD)4 (clone RM4-5, cat. no. 317407), mouse PE-conjugated anti-CD25
(clone BC96, cat. no. 302605), mouse APC-conjugated
anti-interleukin receptor 7 (CD127; clone A019D5, cat. no. 351315),
mouse APC/CY7-conjugated anti-CC-motif chemokine receptor 6 (CCR6;
clone G034E3, cat. no. 353431) and mouse PE/CY7-conjugated
anti-IFN-γ (clone B27, cat. no. 506517) were purchased from
BioLegend, Inc., (San Diego, CA, USA). A total of 5 µl antibody was
added per million cells in 100 µl staining Buffer (BioLegend, Inc.;
cat. no. 420201). IFN-γ was detected by the Intracellular Cytokine
Staining™ kit from BD Pharmingen (BD Biosciences),
according to the manufacturer's protocol, and was quantified by
flow cytometry.
PBMCs were blocked with Fc Receptor Blocking
Solution at room temperature for 5 min (BioLegend, Inc.; cat. no.
422301). After 10 min, red cells were removed by lysis buffer
(BioLegend, Inc.; cat. no. 420301). Then, cells were incubated with
antibody at 4°C for 30 min, washed once with PBS. The cells were
resuspended in 0.5 ml PBS. Flow cytometric analysis was performed
on a BD FACSCanto-II instrument (BD Biosciences). The data was
analyzed by FlowJo V10 (FlowJo LLC, Ashland, OR, USA).
ELISA
CCL20 was detected by a CCL20 ELISA kit (cat. no.
DM3A00; R&D Systems, Inc., Minneapolis, MN, USA), following the
manufacturer's protocol.
Statistical analysis
The data were analyzed using the Prism 5 software
(GraphPad Software, Inc., San Diego, CA, USA). Continuous variables
are presented as the mean ± standard deviation, and compared using
the unpaired Student's t-test between two groups or one-way
analysis of variance between multiple groups followed by Tukey's
post-hoc test. Dichotomous variables were compared using a
χ2 test or Fisher's exact test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Distribution of T cell subsets in the
peripheral blood of patients with gastric cancer
A total of 38 patients were enrolled in the present
study, including 23 males and 16 females. The age of the patients
ranged from 43 to 78 years, with a median age of 64 years. A total
of 31 patients had grade 0–1 ECOG performance status; the other 7
patients had grade 2 ECOG performance status (16) (Table I).
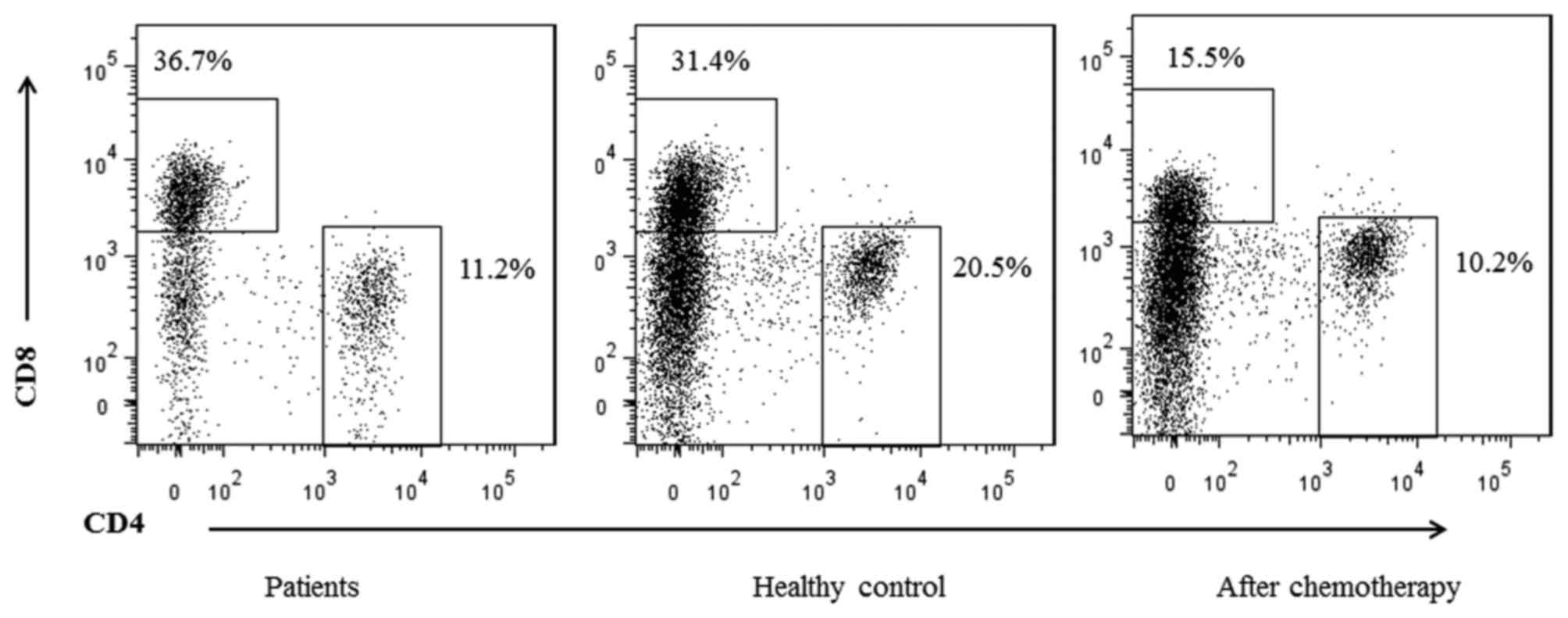
The proportions of CD4+ and CD8+ T cells in
the peripheral blood of patients with gastric cancer and normal
control individuals were compared. The percentage of
CD4+ T cells was significantly decreased compared with
that of the normal controls (22.34±3.37 vs. 11.39±5.91%; P=0.03).
However, the percentage of CD8+ T cells was increased in
the patients with gastric cancer compared with the controls
(36.81±5.33 vs. 29.84±2.01%, respectively; P=0.01; Fig. 1; Table
II). In addition, the ratio of CD4+ T cells to
CD8+ T cells was significantly decreased in the patient
group compared with the healthy control group (P=0.005; Table II). These results indicated that the
patients with gastric cancer exhibited impaired cellular immune
function compared with the healthy controls. The proportions of
CD4+ T cells and CD8+ T cells in the PBMCs of
patients with gastric cancer prior to and following chemotherapy
were additionally assessed by flow cytometric analysis (Fig. 1). Prior to chemotherapy, the
CD4+ and CD8+ T cells accounted for
11.39±5.91% and 36.81±5.33%, respectively, in the patient group.
Although the number of T cells decreased following chemotherapy
(the proportions of CD4+ and CD8+ cells were
8.99±7.31% and 16.00±4.51%, respectively), the ratio of
CD4+/CD8+ T cells was increased compared with
the ratio prior to chemotherapy [0.56±0.22 vs. 0.31±0.17,
respectively (P<0.05); Table
III].
 | Table I.Clinical data of the 38 patients with
gastric cancer. |
Table I.
Clinical data of the 38 patients with
gastric cancer.
|
Characteristics | Value |
|---|
| Age, years; median
(range) | 64 (43–78) |
| Sex, n |
|
|
Male | 23 |
|
Female | 16 |
| AJCC stage, n |
|
| 0 | 1 |
| I | 7 |
| II | 11 |
|
III | 19 |
| IV | 0 |
| Performance status,
n |
|
| ECOG
0–1 | 31 |
|
ECOG-2 | 7 |
| Histopathological
type, n |
|
|
Intestinal | 24 |
|
Diffuse | 12 |
|
Unknown | 2 |
 | Table II.T cell subsets in PBMCs from patients
and controls. |
Table II.
T cell subsets in PBMCs from patients
and controls.
| Group | Patients
(n=38) | Healthy controls
(n=31) | P-value |
|---|
|
CD4+/PBMC (%) | 11.39±5.91 | 22.34±3.37 | 0.032a |
|
CD8+/PBMC (%) | 36.81±5.33 | 29.84±2.01 | 0.010a |
|
CD4+/CD8+ |
0.31±0.17 |
0.74±0.13 | 0.005a |
 | Table III.T cell subsets in PBMCs between
patients prior to and following chemotherapy. |
Table III.
T cell subsets in PBMCs between
patients prior to and following chemotherapy.
| Group | Prior to
chemotherapy | Following
chemotherapy | P-value |
|---|
|
CD4+/PBMC, % | 11.39±5.91 |
8.99±7.31 | 0.021a |
|
CD8+/PBMC, % | 36.81±5.33 | 16.00±4.51 | 0.001a |
|
CD4+/CD8+ ratio |
0.31±0.17 |
0.56±0.22 | 0.001a |
Effect of chemotherapy on T cell
subsets
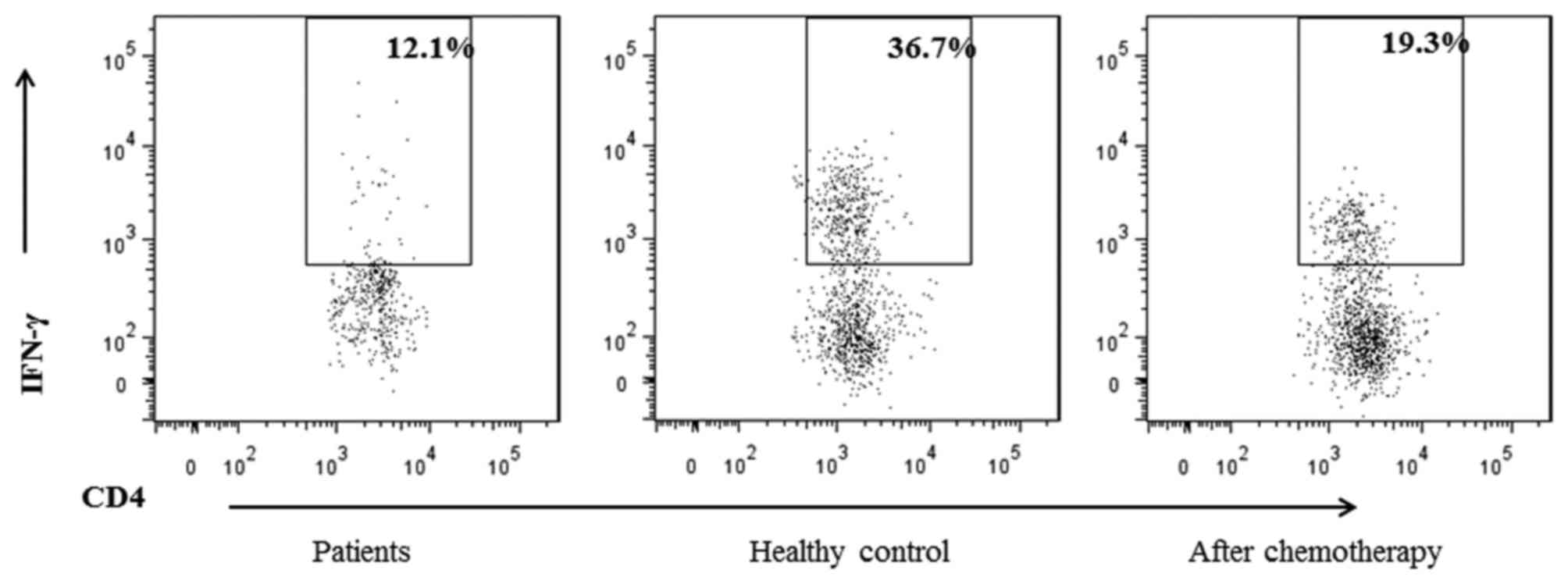
To evaluate the effect of chemotherapy on the
function of the T cells, the production of interferon (IFN)-γ by
CD4+ T cells in patients was investigated. It was
identified that the percentage of IFN-γ+ T cells from
the CD4+ T cells was decreased in the patient cohort
compared with healthy controls (Table
IV), but was increased following chemotherapy compared with
prior to chemotherapy (Fig. 2;
Table V).
 | Table IV.Distribution of CD4+ T
cell subsets in peripheral blood mononuclear cells between patients
and controls. |
Table IV.
Distribution of CD4+ T
cell subsets in peripheral blood mononuclear cells between patients
and controls.
| Group | Patients
(n=38) | Healthy controls
(n=31) | P-value |
|---|
|
IFN-γ+/CD4+ (%) | 13.15±3.99 | 37.7±4.41 | 0.033a |
|
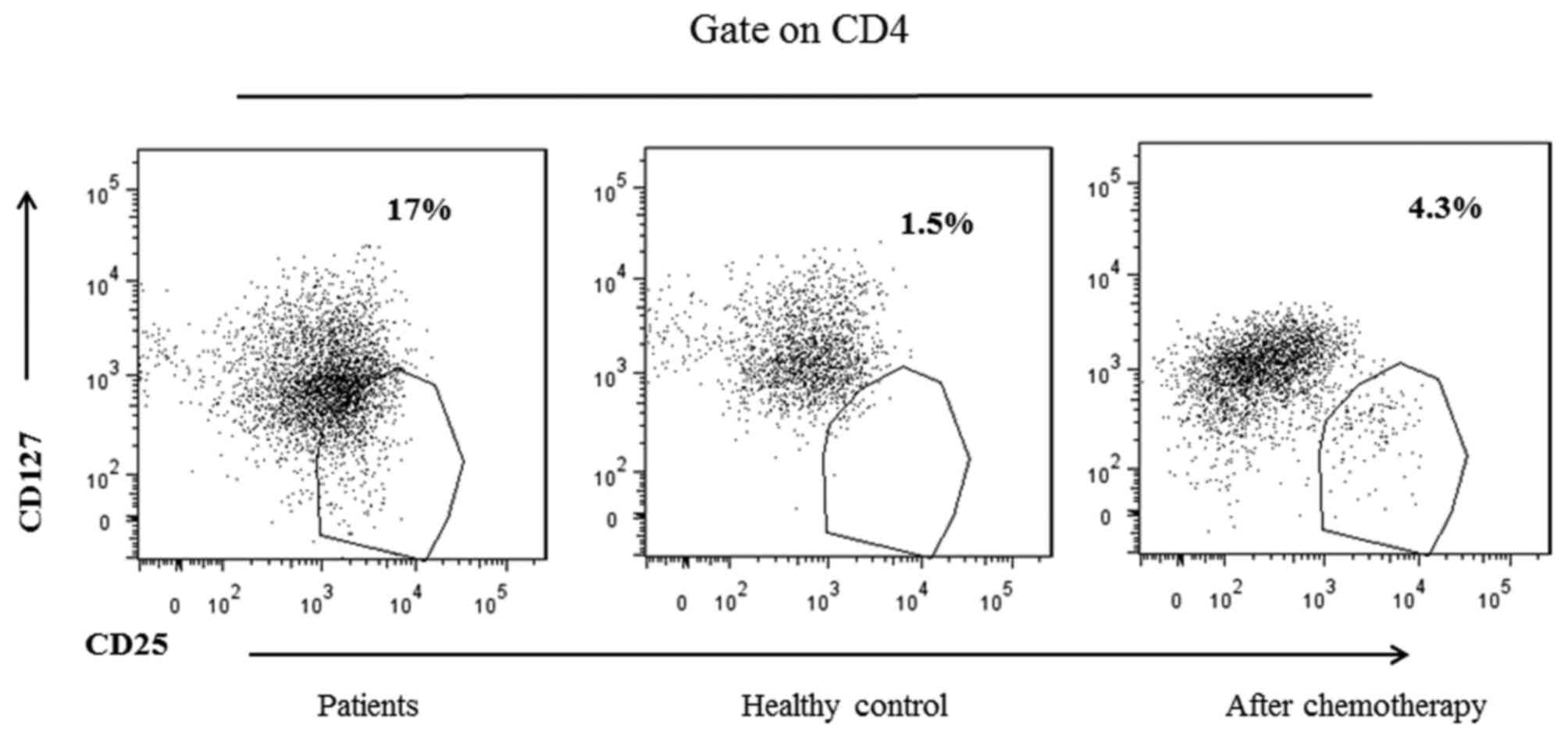
Treg/CD4+ (%) | 18.33±2.51 |
1.5±0.31 | 0.002a |
 | Table V.Distribution of CD4+ T
cell subsets in peripheral blood mononuclear cells between patients
prior to and following chemotherapy. |
Table V.
Distribution of CD4+ T
cell subsets in peripheral blood mononuclear cells between patients
prior to and following chemotherapy.
| Group | Prior to
chemotherapy | Following
chemotherapy | P-value |
|---|
|
IFN-γ+/CD4+ (%) | 13.15±3.99 | 20.1±5.79 | 0.001a |
|
Treg/CD4+ (%) | 18.33±2.51 |
5.5±4.11 | 0.001a |
Furthermore, whether the chemotherapy affected
immunosuppressive Tregs cells was examined. Liu et
al (17) suggested that CD127
expression is inversely correlated with FoxP3 in Tregs
cells; thus, CD127 was examined instead of FoxP3 in the present
study (Fig. 3). It was identified
that the proportion of Tregs was significantly increased
in the patients vs. healthy controls (Table IV), but decreased following
chemotherapy compared with prior to chemotherapy (Table V). These results suggest that
chemotherapy may promote antitumor immunity and partially restore
cellular immune function in patients with gastric cancer.
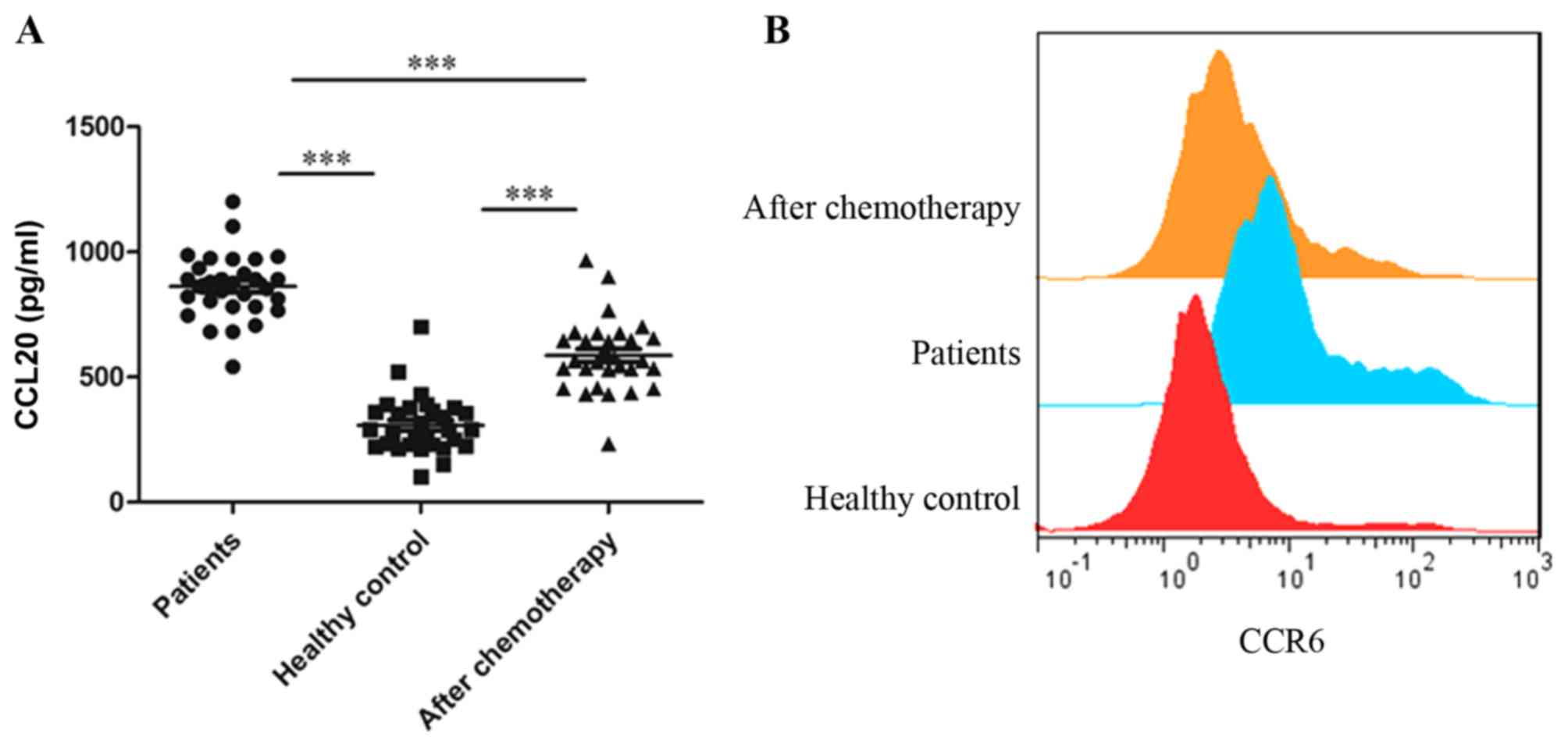
CCL20 is required for the recruitment
of CCR6+ Tregs in patients with gastric
cancer
It has been established that the CCL20/CCR6 signal
mediates the migration of Tregs to the tumor
microenvironment in human liver cancer (11,18). To
identify whether this occurred in gastric cancer, the concentration
of CCL20 in 30 patients and 30 healthy controls from the original
cohort was detected. The results indicated that the level of CCL20
was significantly increased prior to chemotherapy in patients
compared with the healthy controls (Fig.
4A). After chemotherapy, the expression of CCL20 significantly
decreased. CCR6 has been established as the sole receptor for the
chemokine CCL20 (11). As expected,
it was identified that the expression of CCR6 on Tregs
was significantly decreased following chemotherapy (P<0.01;
Fig. 4B). These data suggested that
CCL20 was required for the recruitment of CCR6+
Tregs in patients with gastric cancer.
Discussion
Due to the low chemotherapeutic sensitivity of
gastric cancer, surgery and chemotherapy is the primary course of
treatment, and gastric cancer has a 5-year overall survival rate of
30–60% in surgically curable cases (19,20). The
survival benefits of chemotherapy are well-recognized, but the
effect of chemotherapy on immune cells remains unknown. Tumors are
believed to be controlled by the immune system through a process
termed immunosurveillance, which includes an equilibrium phase and
eventual immune escape. The results from a previous study using a
mouse model indicated that chemotherapy may selectively inhibit
Tregs, while sparing effector T cells (21). Of the compounds included in the
chemotherapy regimens of the present study, oxaliplatin and 5-FU
are hypothesized to induce immunogenic cell death and partially
deplete or transiently inactivate inhibitory immune cells (22). Therefore, we hypothesized that
chemotherapy may result in immunomodulation in patients,
suppressing the inhibitory immune cell function.
Cellular immunity serves a key role in the antitumor
immune response (23). T cells are
critical in the immune regulation and surveillance involved in
cellular immunity, and are divided into two major subsets:
CD4+ and CD8+. The ratio of
CD4+/CD8+ cells significantly affects the
host immune function; a decreased CD4+/CD8+
ratio impairs the immune systems of patients and promotes the
development of tumors (24). A
previous study indicated that chemotherapy may selectively decrease
naïve CD4+ T cells, but preserved the activated
CD4+ or CD8+ and memory T cells (25). However, this particular study compared
the fraction of CD4+ cells among the total mononuclear
cells in the bone marrow of patients with that of healthy controls
(13.7±5.0 vs. 22.9±6.6, respectively), which may not take into
account the complex cell composition of the bone marrow.
Furthermore, from the same data set, an increase of the ratio of
CD4+/CD8+ (1.63 vs. 1.55) was identified in
the patients received chemotherapy, compared with the healthy
control (25). We hypothesize that
the percentage of CD4 in peripheral blood may better reflect the
immune status of patients, as there are numerous cell types
existing in BM, including progenitors and naïve T cells. Consistent
with this previous study, to the best of our knowledge, the present
study demonstrated for the first time the effects of systemic
treatment on the peripheral immune cell populations of patients
with gastric cancer. Although total counts were reduced following
chemotherapy, the proportion of CD4+/CD8+
cells increased compared with before chemotherapy, suggesting that
chemotherapy may enhance the cellular immune function in patients
with gastric cancer.
Tregs serve a critical role in the
maintenance of self-tolerance and suppression of autoimmune disease
(26,27). Tregs are also essential for
the immunopathogenesis of cancer, in which they may prevent the
anti-tumor immune response in a non-antigen-specific manner
(28). There is emerging evidence
suggesting that higher frequencies of Tregs in tumors
microenvironment are associated with a poor outcome (29,30).
However, the role of Tregs remains controversial in
gastric cancer, particularly in the peripheral blood: Previous
studies have demonstrated that CD4+CD25+
(Tregs) cells comprise 5–10% of peripheral
CD4+ T cells in healthy controls (11); however, this is inaccurate for the
Tregs definition. The most specific cell marker of
Tregs identified is the nuclear transcription factor
FoxP3 (31). As Liu et al
(17) demonstrated, CD127 expression
was inversely correlated with FoxP3 expression. In the present
study, the combined expression of CD4, CD25 and CD127 was used to
calculate the population of Treg cells out of the total
CD4+ population; this proportion was 1.5±0.31% in
healthy controls, but was significantly increased in patients with
gastric cancer (18.33±2.51%). Furthermore, the function of
CD4+ T cells was also investigated in patients with
gastric cancer. Increased IFN-γ production following chemotherapy
was identified, which is predominantly mediated by CD4+
T cells subsequent to the development of antigen-specific immunity.
Taken together, the data from the present study indicated that
chemotherapy may effectively suppress the quantity and function of
Tregs.
The high fraction of Tregs in the
peripheral blood raises the question how these cells migrate to the
tumor. It has been revealed that CCL20 is important in recruiting
circulating Tregs into tumor tissue in the liver cancer
(11,18). In the present study, an increase in
the level of CCL20 was also observed in the patient group compared
with healthy controls. Furthermore, as the only known receptor for
CCL20 (32), the level of CCR6 was
significantly higher in the Tregs of patients.
Therefore, the elevation of Tregs is due to, at least in
part, their selective migration in response to CCL20.
In conclusion, the present study suggested that
chemotherapy may promote cellular immune function and inhibit
immunosuppression in patients with gastric cancer. These results
provide additional understanding of the mechanism of FOLFOX-6
chemotherapy on gastric cancer, and provide important insights into
the immune status of patients with gastric cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Scientific Research Foundation of the Health Department of Zhejiang
Province (grant no., 2011KYA069) and the Natural Science Foundation
of Zhejiang Province, China (grant nos., LY15H150004, LY17H160035
and LQ15H160010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HR and LW conceived the idea. LW and DZ performed
the experiments. HR analyzed the data. HR, LW and DZ wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from the patients and
the study was approved by the Ethics Committee of the Second
Affiliated Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim NK, Park YS, Heo DS, Suh C, Kim SY,
Park KC, Kang YK, Shin DB, Kim HT, Kim HJ, et al: A phase III
randomized study of 5-fluorouracil and cisplatin versus
5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil
alone in the treatment of advanced gastric cancer. Cancer.
71:3813–3818. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wöhrer SS, Raderer M and Hejna M:
Palliative chemotherapy for advanced gastric cancer. Ann Oncol.
15:1585–1595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi HS, Ha SY, Kim HM, Ahn SM, Kang MS,
Kim KM, Choi MG, Lee JH, Sohn TS, Bae JM, et al: The prognostic
effects of tumor infiltrating regulatory T cells and myeloid
derived suppressor cells assessed by multicolor flow cytometry in
gastric cancer patients. Oncotarget. 7:7940–7951. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gajewski TF, Schreiber H and Fu YX: Innate
and adaptive immune cells in the tumor microenvironment. Nat
Immunol. 14:1014–1022. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goodman AM, Kato S, Bazhenova L, Patel SP,
Frampton GM, Miller V, Stephens PJ, Daniels GA and Kurzrock R:
Tumor mutational burden as an independent predictor of response to
immunotherapy in diverse cancers. Mol Cancer Ther. 16:2598–2608.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Curiel TJ: Tregs and rethinking cancer
immunotherapy. J Clin Invest. 117:1167–1174. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DeNardo DG and Coussens LM: Inflammation
and breast cancer. Balancing immune response: Crosstalk between
adaptive and innate immune cells during breast cancer progression.
Breast Cancer Res. 9:2122007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carreras J, Lopez-Guillermo A, Fox BC,
Colomo L, Martinez A, Roncador G, Montserrat E, Campo E and Banham
AH: High numbers of tumor-infiltrating FOXP3-positive regulatory T
cells are associated with improved overall survival in follicular
lymphoma. Blood. 108:2957–2964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH,
Taki-Eldin A and Zheng SS: Selective recruitment of regulatory T
cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor
progression and predicts poor prognosis. PLoS One. 6:e246712011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng L, Zhang H, Luan Y, Zhang J, Xing Q,
Dong S, Wu X, Liu M and Wang S: Accumulation of foxp3+ T regulatory
cells in draining lymph nodes correlates with disease progression
and immune suppression in colorectal cancer patients. Clin Cancer
Res. 16:4105–4112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Droeser R, Zlobec I, Kilic E, Güth U,
Heberer M, Spagnoli G, Oertli D and Tapia C: Differential pattern
and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor
infiltrating lymphocytes in ductal and lobular breast cancers. BMC
Cancer. 12:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keam B, Im SA, Han SW, Ham HS, Kim MA, Oh
DY, Lee SH, Kim JH, Kim DW, Kim TY, et al: Modified FOLFOX-6
chemotherapy in advanced gastric cancer: Results of phase II study
and comprehensive analysis of polymorphisms as a predictive and
prognostic marker. BMC Cancer. 8:1482008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee
MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth
Fazekas B, et al: CD127 expression inversely correlates with FoxP3
and suppressive function of human CD4+ T reg cells. J Exp Med.
203:1701–1711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia
XF, Sun X, Li GG, Hu QD, Fu QH and Liang TB: Hypoxia-induced
epithelial-to-mesenchymal transition in hepatocellular carcinoma
induces an immunosuppressive tumor microenvironment to promote
metastasis. Cancer Res. 76:818–830. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shurin GV, Tourkova IL, Kaneno R and
Shurin MR: Chemotherapeutic agents in noncytotoxic concentrations
increase antigen presentation by dendritic cells via an
IL-12-dependent mechanism. J Immunol. 183:137–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vincent J, Mignot G, Chalmin F, Ladoire S,
Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C and
Ghiringhelli F: 5-Fluorouracil selectively kills tumor-associated
myeloid-derived suppressor cells resulting in enhanced T
cell-dependent antitumor immunity. Cancer Res. 70:3052–3061. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao J, Xiao J, Zhou Y, Liu Z and Wang C:
Effect of transcatheter arterial chemoembolization on cellular
immune function and regulatory T cells in patients with
hepatocellular carcinoma. Mol Med Rep. 12:6065–6071. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shah W, Yan X, Jing L, Zhou Y, Chen H and
Wang Y: A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes
and a high percentage of CD4(+)FOXP3(+) regulatory T cells are
significantly associated with clinical outcome in squamous cell
carcinoma of the cervix. Cell Mol Immunol. 8:59–66. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Solomayer EF, Feuerer M, Bai L, Umansky V,
Beckhove P, Meyberg GC, Bastert G, Schirrmacher V and Diel IJ:
Influence of adjuvant hormone therapy and chemotherapy on the
immune system analysed in the bone marrow of patients with breast
cancer. Clin Cancer Res. 9:174–180. 2003.PubMed/NCBI
|
|
26
|
Kono K, Kawaida H, Takahashi A, Sugai H,
Mimura K, Miyagawa N, Omata H and Fujii H: CD4(+)CD25high
regulatory T cells increase with tumor stage in patients with
gastric and esophageal cancers. Cancer Immunol Immunother.
55:1064–1071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ke X, Wang J, Li L, Chen IH, Wang H and
Yang XF: Roles of CD4+CD25(high) FOXP3+ Tregs in lymphomas and
tumors are complex. Front Biosci. 13:3986–4001. 2008.PubMed/NCBI
|
|
28
|
Ladoire S, Martin F and Ghiringhelli F:
Prognostic role of FOXP3+ regulatory T cells infiltrating human
carcinomas: The paradox of colorectal cancer. Cancer Immunol
Immunother. 60:909–918. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haas M, Dimmler A, Hohenberger W,
Grabenbauer GG, Niedobitek G and Distel LV: Stromal regulatory
T-cells are associated with a favourable prognosis in gastric
cancer of the cardia. BMC Gastroenterol. 9:652009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gooden MJ, de Bock GH, Leffers N, Daemen T
and Nijman HW: The prognostic influence of tumour-infiltrating
lymphocytes in cancer: A systematic review with meta-analysis. Br J
Cancer. 105:93–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mougiakakos D, Choudhury A, Lladser A,
Kiessling R and Johansson CC: Regulatory T cells in cancer. Adv
Cancer Res. 107:57–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghadjar P, Rubie C, Aebersold DM and
Keilholz U: The chemokine CCL20 and its receptor CCR6 in human
malignancy with focus on colorectal cancer. Int J Cancer.
125:741–745. 2009. View Article : Google Scholar : PubMed/NCBI
|