Introduction
Gastric cancer is a malignancy with a
multi-factorial etiology (1). Owing
to a lack of effective screening methods, the majority of patients
are diagnosed at an advanced disease stage. For these patients,
systemic chemotherapy is the main treatment method (2). Cisplatin is one of the most potent
antitumor agents and displays a good curative effect against a wide
variety of solid tumor types, including gastric cancer (3). Cisplatin exerts a cytotoxic effect by
interacting with DNA to form DNA adducts, which can activate a
number of downstream signal transduction pathways, and eventually
leads to activation of apoptosis (4).
Unfortunately, the majority of gastric cancer patients fail to
respond to chemotherapy owing to the development of drug resistance
(5). Therefore, the development of
methods to overcome drug resistance and improve clinical treatment
efficacy is required.
Recent studies have shown that taxol resistance
protein 1 (TXR1), which was first identified as a taxol
resistance-associated gene in prostate cancer, was differently
expressed in a number of tumor types, including gastric cancer
(6), breast cancer (7) and non-small cell lung cancer (8). It has been reported that high levels of
TXR1 mRNA expression resulted in the development of resistance to
taxol in HeLa cells (9). The
expressions of TXR1 and thrombospondin 1 (TSP1) were significantly
correlated with treatment outcome in lung adenocarcinomas (10). A previous study confirmed that TXR1
regulated the cytotoxicity of taxanes in vitro through
decreasing the expression of TSP1 in gastric cancer (6). TXR1 regulates not only taxol resistance
but also oxaliplatin response; it was reported that overexpression
of TXR1 evoked oxaliplatin resistance by regulating TSP1 (11,12).
However, the association between TXR1 expression and cisplatin
resistance in gastric cancer remains unknown.
In order to clarify the association between TXR1 and
cisplatin response in gastric cancer, the expression of TXR1 in
cisplatin resistance and sensitive gastric cancer tissues was
determined using reverse transcription-quantitative polymerase
chain reaction (RT-qPCR), western blot analysis and
immunohistochemistry. Next, TXR1 expression was altered in gastric
cancer cells by the transfection of lentivirus or small interfering
RNA (siRNA), and then cisplatin response was tested using an MTS
assay. Animal experiments proved the effect of TXR1 in inducing
cisplatin resistance in vivo. Further investigation revealed
that TXR1 regulated cisplatin resistance via apoptosis. Overall,
the results of the present study revealed that TXR1 served a
notable function in the response to cisplatin via apoptosis in
gastric cancer in vitro and in vivo.
Materials and methods
Patients and tissues
Tissue specimens were obtained between January 2009
and December 2016. Tissue samples from patients with primary
gastric cancer were collected from 18 patients with gastric cancer
who received cisplatin-containing chemotherapy and then underwent
surgical resection at Beijing Friendship Hospital, Capital Medical
University (Beijing, China). Patients were excluded from the study
if previous chemotherapy or radiotherapy treatment had been given
or if treatment was not successfully completed. A total of 12 males
and 6 females were included in those patients, of which the age
ranged from 32 to 65 years. The median age of those patients was
55.67±6.19 years. Tissues were obtained following chemotherapy
treatment. The requirement for informed patient consent was waived
by the Ethics Committee of Beijing Friendship Hospital, who
approved the present study.
Patients were divided into two groups according to
the response to chemotherapy. Patients that exhibited complete or
partial response to cisplatin were included in the
cisplatin-sensitive category. Patients with progressive disease or
stable disease following cisplatin treatment were included in the
cisplatin-resistant category. Complete response was defined as
complete eradication of all evaluable disease, confirmed by biopsy,
for at least 4 weeks. Partial response was defined as a decrease of
at least 30% of total size for at least 4 weeks.
Patients with progressive disease included those
that exhibited increase in tumor size and/or worsening of the
shape, or novel intragastric lesions. Patients with stable disease
were defined as those that exhibited changes in tumor size or shape
that were less than a partial response but were not progressive
disease.
Cell culture and treatments
The human gastric cancer SGC-7901 cell line was
purchased from National Infrastructure of Cell Line Resource
(Beijing, China) and stored in of General Surgery Laboratory in the
Beijing Friendship Hospital. A cisplatin-resistant subline was
established and termed the SGC-7901/DDP cell line. SGC-7901 cells
were exposed continuously to 0.01 µg/ml cisplatin at the beginning
and the cisplatin concentration was gradually increased in 2-fold
increments (0.125, 0.25, 0.5, 1, 2, 4 and 8 µg/ml). Finally, the
surviving cells were maintained at 0.1 µg/ml cisplatin. Cell lines
were cultured in DMEM supplemented with 10% fetal bovine serum, 100
U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and maintained in a 5%
CO2 humidified incubator at 37°C. Cisplatin was
purchased from Qilu Pharmaceutical Co., Ltd. (Jinan, China).
Pirarubicin was obtained from Shenzhen Main Luck Pharmaceuticals,
Inc. (Shenzhen, China). Fluorouracil (5-FU) was purchased from
Shanghai Xudong Haipu Pharmaceutical Co., Ltd. (Shanghai, China).
The lentivirus containing TXR1, TXR1-targeting si-TXR1 or negative
control for lentivirus and siRNAs were obtained from Shanghai
GenePharma Co., Ltd. Transfection was performed with 100 nm small
interfering RNA (siRNA) using the Lipofectamine™ RNAiMAX reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Cells were collected after 48 h for
subsequent experimentations.
The sequences of siRNAs were as follows: si-TXR1
duplex sense, 5′-CAGUGAUAGUAGACAAGAATT-3 and anti-sense,
5′-UUCUUGUCUACUAUCACUGTT-3; and si-NC duplex sense,
5′-UUCUCCGAACCUUCAGUTT-3 and anti-sense,
5′-ACGUGACACGUUCCGAGAATT-3′.
RT-qPCR
Total RNA from cell lines and patient tissues was
extracted with TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
in accordance with the manufacturer's protocol. The quality and
quantity of RNA were assessed using a NanoDrop ND-1000
spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). RNA samples were stored at −80°C for subsequent experiments.
The Reverse Transcription system (cat. no. A3500; Promega
Corporation, Madison, WI, USA) was used to generate cDNA libraries.
qPCR was performed with the SYBRGreen mixture (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The PCR thermocycling conditions were as follows: 5 min
incubation at 94°C, followed by 30 cycles of 94°C for 30 sec, 60°C
for 30 sec and 72°C for 30 sec, with a final incubation for 5 min
at 72°C. Relative expression was calculated using 2−ΔΔCq
method using ABI7500 software (13).
β-actin was used for normalization. All PCR amplifications were
performed in triplicate and the experiment was repeated three
times. Primers were obtained from Sangon Biotech Co., Ltd
(Shanghai, China). The primers used were as follows: TXR1 forward,
AAGGTTGCTGGGAAGTAGAGTC and reverse, ATTGGGCTAAGGAGGAGAGGTA; TSP1
forward, CGTGGTCATCTTGTTCTGTGA and reverse, AGGGTTTCCCGTTCATCTG;
and β-actin forward, GCACCACACCTTCTACAATG and reverse,
TGCTTGCTGATCCACATCTG.
Western blot analysis
Total protein was extracted from cells using a total
protein extraction kit (Nanjing Keygen Biotech Co., Ltd. Nanjing)
and quantified using the BCA method (Tiangen Biotech Co., Ltd.,
Beijing, China) according to the manufacturer's instructions.
Protein samples (8 µg/lane) were separated using 12% SDS-PAGE and
transferred to a polyvinylidene difluoride (PVDF) membrane. PVDF
membranes were blocked with 5% skimmed milk at room temperature for
1 h and incubated with anti-β-actin (cat. no. ab6276; 1:3,000)
anti-TSP1 (cat. no. ab88529; 1:500; both Abcam, Cambridge, MA, USA)
and anti-TXR1 (cat. no. SAB1101786; 1:500; Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany) primary antibodies overnight at 4°C.
Membranes were washed 3 times with TBST and subsequently incubated
with goat anti-rabbit/goat anti-mouse IgG secondary antibodies
(cat. no. ZB2301/ZB2305; 1:3,000; OriGene Technologies, Inc.,
Beijing, China) conjugated to horseradish peroxidase at room
temperature for 1 h, and peroxidase activity was detected by
enhanced chemiluminescence (EMD Millipore, Billerica, MA, USA).
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections (4-µm
thick) were deparaffinized in xylene, rehydrated in a graded
ethanol series (100, 95, 90, 80 and 70%) and treated with 3%
hydrogen peroxide solution at room temperature for 10 min. Epitope
retrieval was performed in boiling water (>95°C) with 0.01 M
citric acid sodium buffer solution (pH 6.0) for 40 min. The
sections were blocked with 5% goat serum at room temperature for 20
min and incubated with TXR1 antibodies (cat. no. HPA046219; 1:100;
Sigma-Aldrich; Merck KGaA) overnight at 4°C. Next, goat anti-rabbit
IgG conjugated to biotin (cat. no. TA130017; 1:200; OriGene
Technologies, Inc., Beijing, China) was added to the slices, and
incubated at 37°C for 30 min. Subsequently, detection was performed
by 3,3′-diaminobenzidine (DAB) chromogenic reaction. Staining
results were visualized using an Olympus CX31-LV320 light
microscope (Olympus Corporation, Tokyo, Japan) at a magnification
of ×200.
MTS assay
Drug response in gastric cancer cells was analyzed
in triplicate using the CellTiter 96 AQueous One Solution Cell
Proliferation assay (MTS assay; Promega Corporation) in accordance
with the manufacturer's protocols. In total, 3,000 cells/well were
seeded in 96-well plates and exposed to various concentrations of
drugs (cisplatin ranging from 0.125 to 8 µg/ml in 2-fold
increments; pirarubicin ranging from 0.25 to 16 µg/ml in 2-fold
increment and 5-FU ranging from 0.5 to 32 µg/ml in 2-fold
increments). After 48 h of treatment, 20 µl MTS was added to the
medium and the cells were incubated in a 5% CO2
incubator at 37°C for 2–4 h. The absorbance was measured at 490 nm
using a plate reader.
Apoptosis analysis
Caspase-3/7 activity was tested using the
Caspase-Glo3/7-assay (Promega Corporation) according with the
manufacturer's protocol. In total, 3,000 cells/well were planted
into 96-well plate and treated with cisplatin at the dosage of
0.25, 0.5, 1, 2 or 4 µg/ml for 48 h, then
Caspase-Glo3/7® reagent was added and luminescence was
recorded by Fluostar Optima plate reader (BMG Labtechnologies GmbH,
Inc., Durham, NC, USA) at 37°C and the enzymatic activity was
calculated.
Xenograft mouse model
A total of 20 male BALB/c nude mice (4–6 weeks old,
18–22 g) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China) and kept in the Animal
Laboratory of Beijing Friendship Hospital for experimentation. Mice
were maintained under a 6/18 h light-dark cycle at 22±1°C. The mice
had ad libitum access to tap water and food. Care was taken
to avoid environmental stress prior to and during the course of the
experiments (including noise, smell or cage crowding). Animal
studies were performed in conformity with applicable laws and
guidelines and were approved by the Laboratory Animal Center at
Beijing Friendship Hospital. 1×106 cells were inoculated
subcutaneously into the armpit of right forelimb of the mice to
generate the xenograft model. When the tumor volume reached 100
mm3, the mice were given the appropriate treatment. In
total, 20 mice were divided into 4 groups: i) The SGC-7901/DDP NC
group, consisting of mice injected with 1×106
SGC-7901/DDP cells and intraperitoneally treated with physiological
saline twice a week for 4 weeks; ii) SGC-7901/DDP cisplatin group,
consisting of mice injected with 1×106 SGC-7901/DDP
cells and intraperitoneally treated with 2.5 mg/kg cisplatin twice
a week for 4 weeks; iii) the SGC-7901/DDP/si-TXR1 group, consisting
of mice injected with 1×106 SGC-7901/DDP cells
transfected with lentivirus to knockdown expression of TXR1 and
injected into mice subcutaneously. Then mice were treated
intraperitoneally with 2.5 mg/kg cisplatin twice a week for 4
weeks; and iv) the SGC-7901/DDP lentivirus group, consisting of
mice injected with 1×106 SGC-7901/DDP cells that were
treated with lentivirus that interfered with TXR1 expression
intratumorally for 4 weeks, 107 transduction units per
mouse, twice a week. Tumor size was measured twice per week. Tumor
volume was calculated as follows:
Volume=length/width2/2.
Statistical analysis
The results are presented as the mean ± standard
deviation. All data were analyzed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Significant differences were assessed
using a standard one-way analysis of variance followed by Dunnett's
multiple comparisons test or Student's t-test. The
Kolmogorov-Smirnov test for normality was applied to assess normal
distribution. P<0.05 was considered to indicate a statistically
significant difference.
Results
Association between TXR1 level
expression level and cisplatin response in gastric cancer
tissues
The present study included 18 patients with gastric
cancer who were treated with cisplatin-based chemotherapy at
Beijing Friendship Hospital. Patients were divided into two groups
according to the response to chemotherapy and the expression of
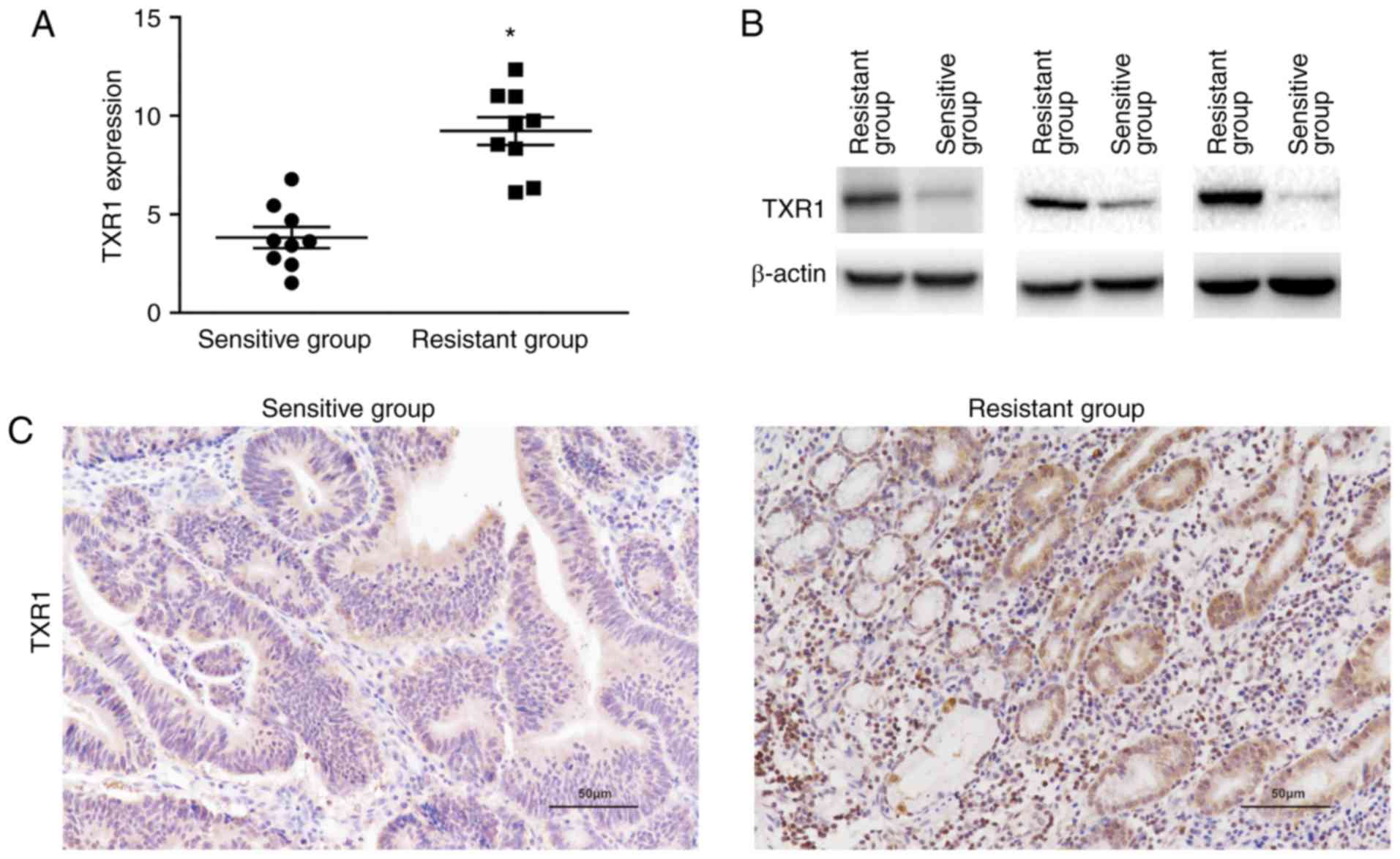
TXR1 was assessed in these two groups. Results revealed that TXR1
expression was significantly elevated in the cisplatin-resistant
group (Fig. 1A), which indicated that
TXR1 might be a regulator of cisplatin resistance. Representative
TXR1 expression in cisplatin-resistant and -sensitive tissues was
assessed using western blot and immunohistochemistry, depicted in
Fig. 1B and C, respectively.
Establishment a cisplatin-resistant
sublines with SGC-7901 cell line
To investigate the association between TXR1
expression and cisplatin-resistance in gastric cancer cell lines, a
cisplatin-resistant gastric cancer cell sublines was established.
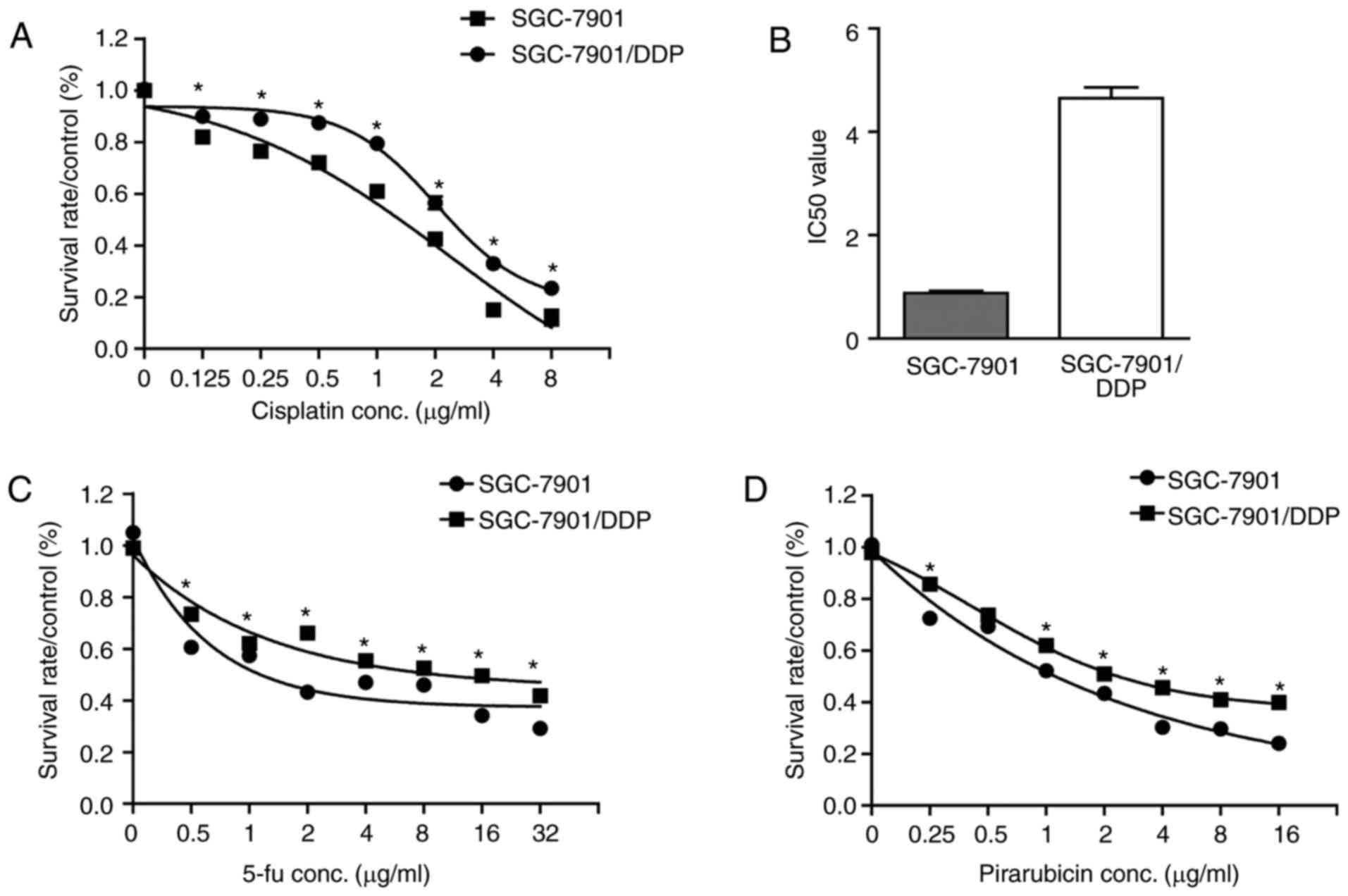
This subline proved ~6-fold resistant to cisplatin (Fig. 2A and B). This level of resistance was
confirmed to be stable over 3 months in continuous culture.
Dose-response data for sensitive cells and resistant
sublines demonstrated that cisplatin-resistant cells were
cross-resistant to 5-FU and pirarubicin (Fig. 2C and D).
Cisplatin sensitivity of gastric
cancer cells is associated with endogenous TXR1 expression
The expression of TXR1 was assessed in
cisplatin-resistant and -sensitive cell lines. Results revealed
that TXR1 was significantly increased in cisplatin-resistant cells
(Fig. 3A), indicating that TXR1 has a
role in regulating cisplatin response.
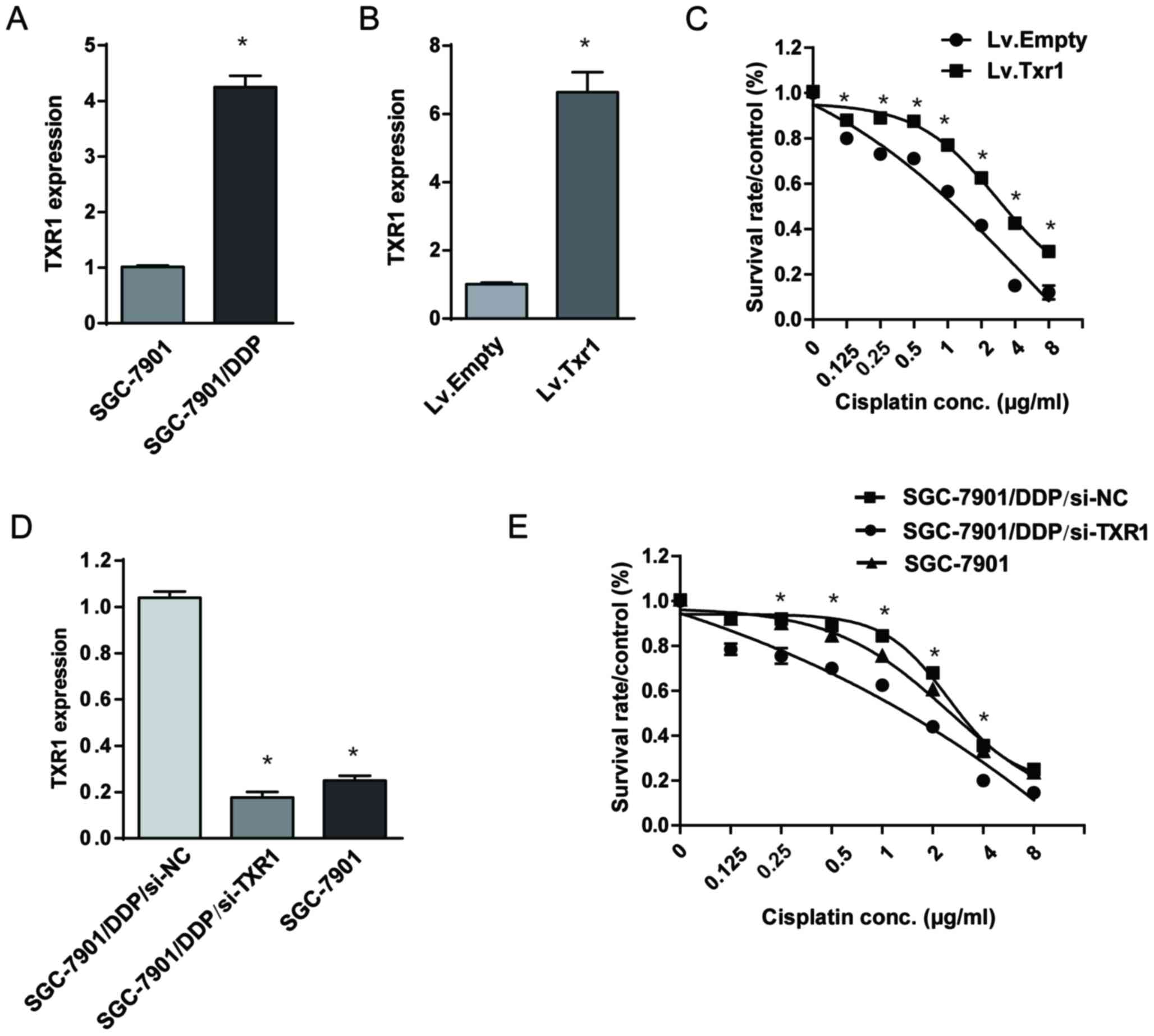 | Figure 3.Cisplatin sensitivity of GC cells is
associated with endogenous TXR1 expression. (A) The expression of
TXR1 in SGC-7901/DDP cell line and its parental cell were tested by
RT-qPCR. β-actin was used for normalization. *P<0.05, compared
with SGC-7901. (B and C) Lv.TXR1 or Lv.NC were transfected into
SGC-7901 cells. (B) TXR1 level was confirmed by RT-qPCR and (C)
cisplatin sensitivity were determined by MTS assays. β-actin was
used for normalization. *P<0.05, compared with Lv.Empty. (D and
E) si-NC or si-TXR1 were transfected into SGC-7901/DDP cells. (D)
TXR1 level was confirmed by RT-qPCR and (E) cisplatin sensitivity
were tested by MTS assay. β-actin was used for normalization.
*P<0.05, compared with SGC-7901/DDP/si-NC. Points, mean values
for three independent experiments; error bars, mean± standard. GC,
gastric cancer; TXR1, taxol resistance protein 1; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Lv.TXR1,
lentivirus bearing TXR1; NC, negative control; si-NC, NC short
interfering RNA. |
To investigate the role of TXR1 in cisplatin
resistance, TXR1 lentivirus (or a control equivalent) was
transfected into SGC-7901 cells, which significantly increased the
TXR1 expression level (Fig. 3B).
Next, the SGC-7901 cells transfected with control or TXR1
lentivirus were treated with cisplatin at various concentrations.
The results revealed that TXR1 overexpression significantly
increased the resistance of cisplatin (Fig. 3C). Next, SGC-7901/DDP cells were
transfected with siRNA to knock-down TXR1 (Fig. 3D). The resistance to cisplatin was
significantly decreased in SGC-7901/DDP/si-TXR1 cells compared with
SGC-7901/DDP/si-NC cells (P<0.05; Fig.
3E). Similarly, the resistance of cisplatin was decreased in
SGC-7901 cells compared with SGC-7901/DDP/si-NC cells, where the
TXR1 level was lower compared with SGC-7901 cells (Fig. 3E). In summary, the results of the
present study indicated a notable role of TXR1 in cisplatin
response in gastric cancer.
TXR1 enhance the cisplatin resistance
in gastric cancer in vivo
To investigate the effect of TXR1 in cisplatin
resistance in vivo, a xenograft model was established in
BALB/c nude male mice.
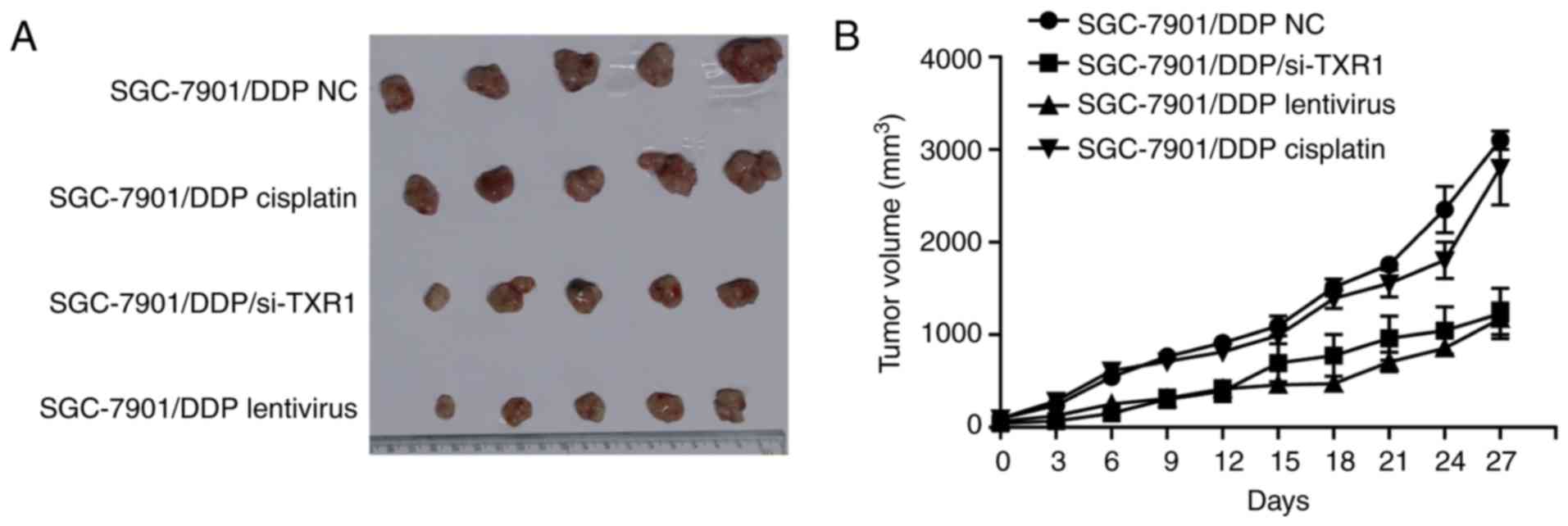
As shown in Fig. 4A and
B, the difference in tumor volumes in the SGC-7901/DDP NC and
SGC-7901/DDP cisplatin groups was not significant, owing to the
cisplatin resistance of SGC-7901/DDP cells. Compared with the
SGC-7901/DDP cisplatin group, the tumor volume in the
SGC-7901/DDP-siTXR1 group was significantly smaller, indicating
that TXR1 has a notable role in cisplatin response in vivo.
Similarly, lentivirus was injected into tumor to silence TXR1
expression, generating similar data to the SGC-7901/DDP/si-TXR1
group, which further confirmed these results.
TXR1 enhance the cisplatin resistance
through inducing apoptosis
Owing to the inverse association observed between
TXR1 levels and cisplatin sensitivity, additional mechanistic
experiments were warranted. As apoptosis is the predominant
mechanism of cisplatin-induced toxicity, an apoptosis assay was
performed using a caspase-glo3/7 assay. The results of this assay
revealed that TXR1-knockdown significantly increased the
cisplatin-induced activity of caspase-3/7, whereas
TXR1-overexpression reduced the activity of caspase-3/7 in SGC-7901
cells (Fig. 5A and B). These data
indicated that TXR1 expression enhances the cytotoxic effect of
cisplatin by sensitizing the gastric cancer cells to
cisplatin-induced apoptosis.
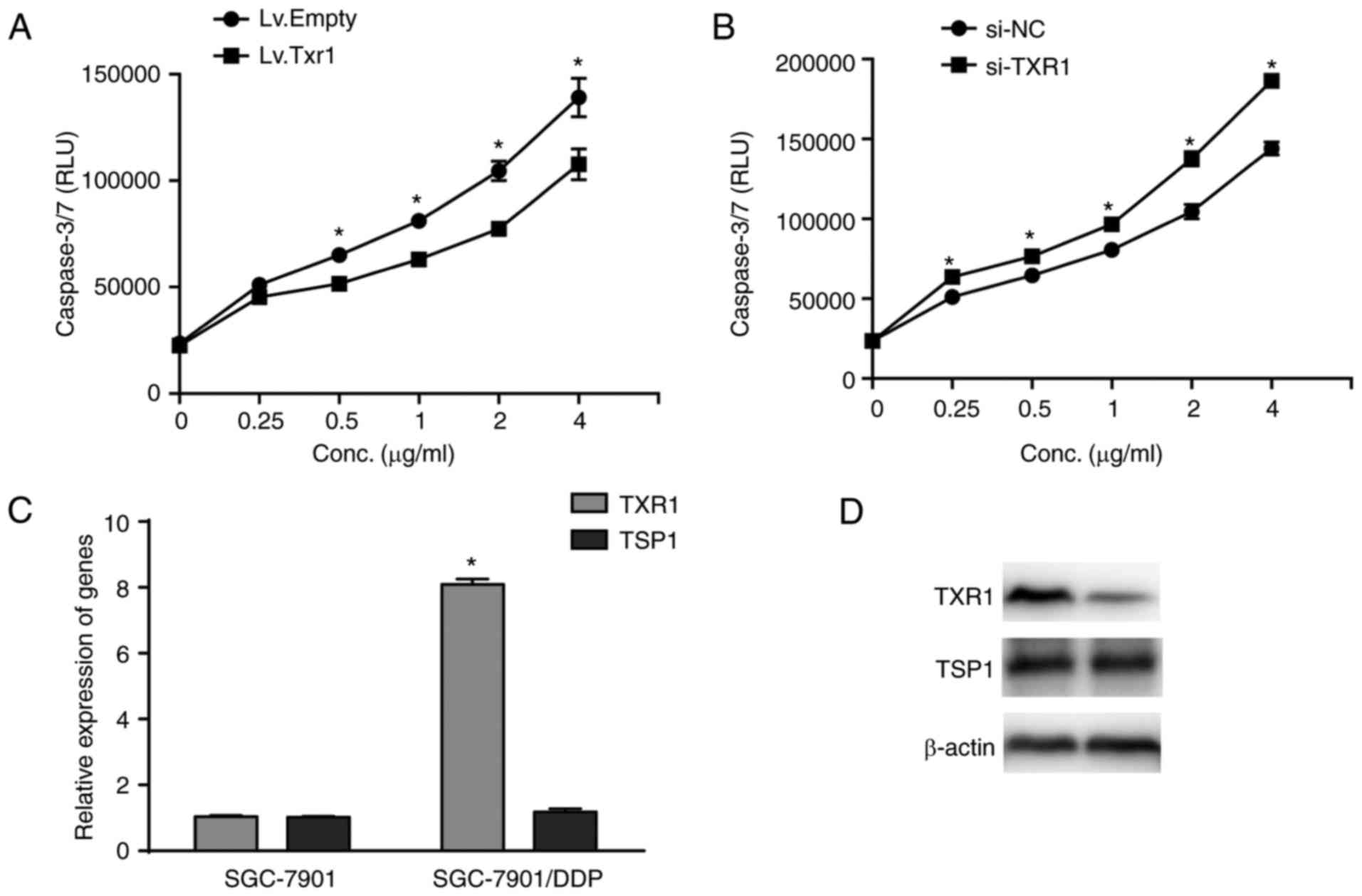 | Figure 5.TXR1 enhances cisplatin resistance by
inhibiting apoptosis. (A) Lv.TXR1 was transfected into SGC-7901
cells to overexpress TXR1, then the caspase3/7 activity was
assessed using the Caspase-Glo3/7-assay. Each point represents the
mean RLU ± SEM of triplicate samples from two independent
experiments. *P<0.05, vs. Lv.Empty. (B) SGC-7901 cells were
transfected with si-TXR1 to reduce the expression of TXR1, then
caspase-3/7 activity was assessed using the Caspase-Glo3/7-assay.
Points on graph represent the mean RLU ± SEM of triplicate samples
from two independent experiments. *P<0.05 vs. si-NC. (C and D)
the level of TXR1 and TSP1 in SGC-7901/DDP cell line and its
parental cell were detected by (C) reverse
transcription-quantitative polymerase chain reaction and (D)
western blot. β-actin was used for normalization. *P<0.05,
compared with SGC-7901. Points, mean values for three independent
experiments. Error bars, ± SEM. TXR1, taxol resistance protein 1;
Lv.TXR1, lentivirus bearing TXR1; RLU, relative luminescence units;
SD, standard deviation; si-TXR1, small interfering RNA targeting
TXR1. |
The TXR1/TSP1 regulatory pathway has been recently
described to be involved in drug resistance in cancer (6,14).
Therefore, the expression of TSP1 in SGC-7901 and SGC-7901/DDP
cells was examined at the transcriptional and translational level
using RT-qPCR and western blot analysis. Results revealed that
there were no significant differences TSP1 at either the mRNA or
protein level in SGC-7901/DDP cells (Fig.
5C and D). These results indicated that TSP1 may not be
involved in the cisplatin response regulated by TXR1.
Discussion
Due to a lack of effective screening methods, a
majority of patients with gastric cancer are diagnosed at an
advanced disease stage when the tumor is inoperable; and for these
patients, systemic chemotherapy is the main treatment option
(15,16). Cisplatin is the basis of multiple
treatment regimens; it exerts its cytotoxicity activity mainly in
combination with other chemotherapy drugs by inducing apoptosis in
cancer cells (4). However, with
multiple drug treatments, cancer cells frequently acquire
resistance to the cytotoxic effects of cisplatin (17). The problems caused by cisplatin
resistance seem to be more severe than they were in the past
(18).
The mechanism of drug resistance contains a series
of pathological changes induced by expression diversification of
large-scale genes in numerous cell lines (19–21).
Recent studies showed that TXR1 was a taxol-resistance-associated
agent, which also regulates oxaliplatin response in gastric cancer
(4,6).
TXR1 was previously shown to be closely associated with oxaliplatin
response in vitro. However, to the best of our knowledge,
the association between TXR1 and cisplatin resistance in
vitro and in vivo was unknown. It was inferred that TXR1
may have a role in cisplatin resistance. The differential
expression of TXR1 between cisplatin-resistant and
cisplatin-sensitive gastric cancer tissues provided clues for this
hypothesis. Subsequently, the SGC-7901/DDP cell line was
established. Compared with its parental SGC-7901 cell line, TXR1
expression was elevated in the SGC-7901/DDP cell line.
Overexpression of TXR1 led to the development of cisplatin
resistance in SGC-7901 cell lines, whereas downregulation of TXR1
reversed the resistance induced by TXR1 in the SGC-7901/DDP cell
line. All results indicated that TXR1 may protect gastric cancer
cells from cisplatin cytotoxicity in the gastric cancer SGC-7901
cell line. The results of the in vivo experiment further
confirmed that TXR1 was likely associated with cisplatin
resistance. In the present study, TXR1 exerted effects as an
inducer of cisplatin resistance and could be considered to be
cancer-promoting gene. This result is consistent with previous
research, in which TXR1 expression caused multiple drug resistance
(8,22). Downregulation of the apoptotic signal
is a common characteristic of multi-drug resistance (23). Although cisplatin is a potent inducer
of apoptosis, resistance is considered to be developed when tumor
cells fail to undergo apoptosis at clinical drug concentrations
(24,25). Thus, the present study assessed the
apoptosis rate induced by changes to TXR1 expression. Caspase-3/7
have been reported to be activated by chemotherapy-induced tumor
cell death and considered to be an early biomarker for evaluating
apoptosis-inducing antitumor effects (26). The activity of Caspase-3/7 was
detected to evaluate the rate of apoptosis induced by cisplatin.
Results revealed that the upregulation of TXR1 caused an increase
in the apoptotic rate, whereas downregulation of TXR1 caused a
decrease. Thus, we hypothesize that TXR1 induces cisplatin
resistance by regulating apoptosis.
It has been reported that the TXR1 protein impedes
taxane-induced apoptosis via downregulation of thrombospondin 1
(TSP1) (14,27). TSP1 has been suggested to serve a
notable role in preventing angiogenesis and inducing apoptosis in
malignant cells (28). Thus, the
present study also analyzed TSP1 gene expression. Unexpectedly,
changes in the expression of TSP1 were not observed in
cisplatin-resistant cells. Therefore, TSP1 was not involved in
cisplatin response in gastric cancer. The specific molecular
mechanism of cisplatin resistance induced by TXR1 requires further
investigation.
In conclusion, to the best of our knowledge, the
present study reveals, to the best of our knowledge for the first
time, that TXR1-knockdown enhanced chemosensitivity to cisplatin in
gastric cancer cells by inducing apoptosis. The identification of
TXR1 as an inducer of chemoresistance in patients undergoing
cisplatin treatment provides a theoretical basis for novel
approaches to overcoming chemotherapy resistance.
Acknowledgements
Not applicable.
Funding
The present study was supported by the grants from
National Natural Science Foundation of China (no. 81172317 to
Zhigang Bai).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ and ZB participated in the design of the study
and performed the majority of the analyses. SD drafted the
manuscript. SD and JY conceived and coordinated the study. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was provided by the Ethics
Committee of Beijing Friendship Hospital (reference no.
BJFH-EC/2014-051), and the requirement for informed patient consent
was waived by the Ethics Committee of Beijing Friendship
Hospital.
Consent for publication
The present study was granted an exemption by the
Ethics Committee of Beijing Friendship Hospital as the patients
cannot be traced.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kato M and Asaka M: Recent knowledge of
the relationship between Helicobacter pylori and gastric cancer and
recent progress of gastroendoscopic diagnosis and treatment for
gastric cancer. Jpn J Clin Oncol. 40:828–837. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikeda F and Kiyohasa Y: The epidemiology
of gastric cancer: The Hisayama Study. Fukuoka Igaku Zasshi.
106:195–201. 2015.(In Japanese). PubMed/NCBI
|
|
3
|
De Milito A and Fais S: Tumor acidity,
chemoresistance and proton pump inhibitors. Future Oncol.
1:779–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers (Basel). 3:1351–1371.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bai ZG, Qu X, Han W, Ma XM, Zhao XM and
Zhang ZT: Expression of taxol resistance gene 1 correlates with
gastric cancer patient clinical outcome and induces taxol
resistance. Mol Med Rep. 3:1071–1078. 2010.PubMed/NCBI
|
|
7
|
Zhang H, Qu X, Ma X, Wang T, Yang Y, Ge Z,
Zhang Z, Bai Z, Gao Y, Yuan Z and Wang Z: TXR1 and TSP1 expression
varies by the molecular subtypes of breast cancer patients who
received previous docetaxel-based first-line chemotherapy. Exp Biol
Med (Maywood). 241:1919–1923. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papadaki C, Tsaroucha E, Kaklamanis L,
Lagoudaki E, Trypaki M, Tryfonidis K, Mavroudis D, Stathopoulos E,
Georgoulias V and Souglakos J: Correlation of BRCA1, TXR1 and TSP1
mRNA expression with treatment outcome to docetaxel-based
first-line chemotherapy in patients with advanced/metastatic
non-small-cell lung cancer. Br J Cancer. 104:316–323. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bi W, Wang Y, Sun G, Zhang X, Wei Y, Li L
and Wang X: Paclitaxel-resistant HeLa cells have up-regulated
levels of reactive oxygen species and increased expression of taxol
resistance gene 1. Pak J Pharm Sci. 27:871–878. 2014.PubMed/NCBI
|
|
10
|
Papadaki C, Mavroudis D, Trypaki M,
Koutsopoulos A, Stathopoulos E, Hatzidaki D, Tsakalaki E,
Georgoulias V and Souglakos J: Tumoral expression of TXR1 and TSP1
predicts overall survival of patients with lung adenocarcinoma
treated with first-line docetaxel-gemcitabine regimen. Clin Cancer
Res. 15:3827–3833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bi J, Bai Z, Ma X, Song J, Guo Y, Zhao J,
Yi X, Han S and Zhang Z: Txr1: An important factor in oxaliplatin
resistance in gastric cancer. Med Oncol. 31:8072014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu L, Bai Z, Ma X, Wang T, Yang Y and
Zhang Z: Effects of taxol resistance gene 1 expression on the
chemosensitivity of SGC-7901 cells to oxaliplatin. Exp Ther Med.
11:846–852. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Amerongen R and Berns A: TXR1-mediated
thrombospondin repression: A novel mechanism of resistance to
taxanes? Genes Dev. 20:1975–1981. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Songun I, Keizer HJ, Hermans J,
Klementschitsch P, de Vries JE, Wils JA, van der Bijl J, van
Krieken JH and van de Velde CJ: Chemotherapy for operable gastric
cancer: Results of the Dutch randomised FAMTX trial. The Dutch
Gastric Cancer Group (DGCG). Eur J Cancer. 35:558–562. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: A
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuruo T, Naito M, Tomida A, Fujita N,
Mashima T, Sakamoto H and Haga N: Molecular targeting therapy of
cancer: Drug resistance, apoptosis and survival signal. Cancer Sci.
94:15–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Housman G, Byler S, Heerboth S, Lapinska
K, Longacre M, Snyder N and Sarkar S: Drug resistance in cancer: An
overview. Cancers (Basel). 6:1769–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rueff J and Rodrigues AS: Cancer drug
resistance: A brief overview from a genetic viewpoint. Methods Mol
Biol. 1395:1–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colloca G, Venturino A and Checcaglini F:
Second-line chemotherapy in metastatic docetaxel-resistant prostate
cancer: A review. Med Oncol. 29:776–785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Neill CF, Ormerod MG, Robertson D,
Titley JC, Cumber-Walsweer Y and Kelland LR: Apoptotic and
non-apoptotic cell death induced by cis and trans analogues of a
novel ammine(cyclohexylamine)dihydroxodichloroplatinum(IV) complex.
Br J Cancer. 74:1037–1045. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Henkels KM and Turchi JJ: Induction of
apoptosis in cisplatin-sensitive and -resistant human ovarian
cancer cell lines. Cancer Res. 57:4488–4492. 1997.PubMed/NCBI
|
|
26
|
Ye D, Shuhendler AJ, Pandit P, Brewer KD,
Tee SS, Cui L, Tikhomirov G, Rutt B and Rao J: Caspase-responsive
smart gadolinium-based contrast agent for magnetic resonance
imaging of drug-induced apoptosis. Chem Sci. 4:3845–3852. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lih CJ, Wei W and Cohen SN: Txr1: A
transcriptional regulator of thrombospondin-1 that modulates
cellular sensitivity to taxanes. Genes Dev. 20:2082–2095. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sid B, Sartelet H, Bellon G, El Btaouri H,
Rath G, Delorme N, Haye B and Martiny L: Thrombospondin 1: A
multifunctional protein implicated in the regulation of tumor
growth. Crit Rev Oncol Hematol. 49:245–258. 2004. View Article : Google Scholar : PubMed/NCBI
|