Introduction
Ginseng is a popular herbal medicine worldwide.
American ginseng (Panax quinquefolius L.), belonging to
Araliaceae family, is one of the most commonly used botanicals in
the United States. Asian ginseng (Panax ginseng C. A.
Meyer), another well-known species in the same family, has a long
history of use in East Asian countries with favorable health
benefits (1–3). Previous reports suggested that long-term
ginseng consumption is associated with a decreased risk for
multiple human cancers (4,5). It is generally believed that the active
constituents in both ginsengs are a group of triterpene glycosides,
referred to as ginseng saponins or ginsenosides, in which more than
100 have been isolated from botanical sources (6–8). It has
been shown that ginsenoside Rb1 acts as a major protopanaxadiol
(PPD) group saponin (9).
Like many other herbal medicines, ginseng is taken
orally. After ginseng ingestion, these natural ginsenosides are
inevitably exposed to gut microflora in the gastrointestinal tract,
and these compounds are biotransformed into active metabolites by
the enteric microbiome before being absorbed (10,11). These
metabolites are mainly involved in the deglycosylation reaction via
stepwise cleavage of the sugar moieties, ultimately producing their
pharmacological effects (10,12,13).
Based on our previous studies, via enteric
microbiota activities, ginsenoside Rb1 could be converted into
compound K, a major bioactive metabolite absorbed into the systemic
circulation (13,14). Our former research has demonstrated
the significant anti-proliferative and pro-apoptotic effects of
compound K using two selected human colorectal cancer cell lines,
HCT-116 and SW-480 (15). However,
the comparison between ginsenoside Rb1 and compound K on the
inflammatory-linked colorectal cancer has not been reported.
HT-29 is another human colorectal cancer cell line.
These HT-29 cells are not only resistant to chemotherapeutic
agents, but also secrete inflammatory cytokine when induced by
lipopolysaccharide (LPS). Thus, the HT-29 cell line could serve a
good model for inflammatory-linked colon cancer studies. In this
project, in addition to HCT-116, HT-29 cells were used to evaluate
whether compound K possesses anticancer effects on chemo-resistant
cell lines. The related mechanisms of action were explored.
Further, its inflammatory-linked chemopreventive effects were
investigated.
Materials and methods
Reagents and materials
Reference compounds including ginsenoside Rb1 and
compound K were obtained from Indofine Chemical Company
(Somerville, NJ, USA) and ChromaDex Inc. (Irvine, CA, USA),
respectively, whose purities were more than 98% determined by
HPLC-DAD. General anaerobic medium (GAM) was obtained from Kayon
Biological Technology Co. Ltd. (Shanghai, China). Acetonitrile
(ACN) and formic acid of HPLC grade were from Merck KGaA
(Darmstadt, Germany). Deionized water (18 MΩ-cm) was supplied with
a Milli-Q water system (Millipore, Milford, MA, USA). Trypsin,
McCoy's 5A, and phosphate-buffered saline were obtained from
Mediatech, Inc. (Herndon, VA, USA). Penicillin and streptomycin
were obtained from Sigma-Aldrich (St. Louis, MO, USA). An MTS assay
kit, CellTiter 96 Aqueous Solution Cell Proliferation Assay, was
obtained from Promega (Madison, WI, USA). A FITC-Annexin V
apoptosis detection kit was obtained from BD Biosciences
(Rockville, MD, USA). PI/RNase staining buffer was obtained from BD
Biosciences Pharmingen (San Diego, CA, USA). All cell culture
plasticware were obtained from Falcon Labware (Franklin Lakes, NJ,
USA) and Techno Plastic Products (Trasadingen, Switzerland).
Plant materials and extraction
The air-dried roots of American ginseng (P.
quinquefolius L.) were purchased from Roland Ginseng, LLC
(Wausau, WI, USA). The voucher samples were deposited at the Tang
Center for Herbal Medicine Research at the University of Chicago
(Chicago, IL, USA). The powdered American ginseng was extracted
with 70% ethanol twice by the heat-reflux method. The combined
filtrate was condensed under vacuum and then lyophilized to yield
dried American ginseng extract (AGE).
Biotransformation of AGE by human
fecal microflora
The present study protocol was approved by the
Institutional Review Board at the University of Chicago; patients
provided oral consent for sample collection. Fecal samples were
collected from six healthy adult volunteers (non-smokers without
antibiotic consumption more than 6 months before the experiment)
between September 2013 and September 2015. All the fresh fecal
samples were mixed. The 5 g of mixed feces were homogenized with 30
ml of cold physiological saline and filtered through gauze to
obtain the resulting fecal liquid. One microliter of fecal liquid
was mixed with 8 ml of GAM containing 3 mg of AGE. They were
anaerobically incubated at 37°C for 24 h. The reaction mixture was
extracted with water-saturated n-butanol for three times. Then the
combined n-butanol solution was dried under the nitrogen stream and
re-dissolved in methanol. The solution was centrifuged at 13,000 ×
g for 10 min before analysis.
LC-Q-TOF-MS analysis
Chromatographic analysis was performed on an Agilent
1290 LC instrument (Agilent Technologies, GmbH, Waldbronn, Germany)
with a binary pump, an online degasser, an auto plate-sampler, and
a thermostatically controlled column compartment. The separation
was carried out on an Agilent C18 column (4.6×250 mm, 5 µm). A
gradient mobile phase system of water (0.1% formic acid, eluent A)
and ACN (0.1% formic acid, eluent B) was applied as follows: 79% A
and 21% B (0 min), 79% A and 21% B (16 min), 70% A and 30% B (27
min), 55% A and 45% B (34 min), 25% A and 75% B (40 min), 100% B
(46 min). The flow rate was set at 1 ml/min and the injected sample
was 2 µl.
Detection was performed by a 6520 Q-TOF mass
spectrometer (Agilent Technologies, Santa Clara, CA, USA) with a
dual electrospray ionization (ESI) source. The mass parameters for
detection were optimized in the following: Drying gas flow rate, 10
l/min; drying gas temperature, 320°C; nebulizer, 35 psig;
capillary, 3500 V; OCT RFV, 750 V; and fragmentor voltage, 120 V.
The range m/z from 100 to 2,000 were recorded in the negative mode.
Agilent MassHunter Acquisition Software version B.04.00 (Agilent
Technologies) was applied to monitor the operations and data
acquisitions.
Cell culture
The human colorectal cancer cell line HT-29 was
obtained from the American Type Tissue Collection (Rockville, MD,
USA). The cell line was maintained in McCoy's 5A which was
supplemented with 10% FBS, penicillin (100 IU/ml) and streptomycin
(100 µg/ml). The HT-29 cells were incubated in a humidified
atmosphere with 5% CO2 at 37°C and subcultured every two
days.
Cell proliferation analysis
Ginsenoside Rb1 and compound K were dissolved in
DMSO and stored in small aliquots at −20°C prior to use. The HT-29
cells were seeded in 96-well plates at a density of 5,000
cells/well, after incubating for 24 h the cells were treated with
different concentrations of Rb1 and compound K, and the final
concentration of DMSO was 0.5%. Controls were exposed to culture
medium containing 0.5% DMSO without drugs. All experiments were
performed in triplicate and repeated for 3 times. Following the
indicated incubation durations, cell proliferation was detected by
means of the MTS assay according to the manufacturer's
instructions. Briefly, the medium was replaced with 100 µl of fresh
medium and 20 µl of MTS reagent (CellTiter 96 Aqueous Solution) in
each well, and the plate was returned to the incubator for 1–2 h,
then 60 µl aliquot of medium from each well was transferred to an
ELISA 96-well plate and its absorbance at 490 nm was read by an
automated microplate reader (Epoch; Bio-Tek Instruments, Winooski,
VT, USA). Since 0.5% DMSO did not influence the proliferation of
the cell line, results were expressed as percent of control (DMSO
vehicle set at 100%).
Cell cycle and apoptosis analysis
using flow cytometry
Cells were seeded in 24-well tissue culture plates.
On the second day, the medium was replaced with experimental
compounds added. Cells were cultured for 48 h before they were
harvested. These cells were fixed gently with 80% ethanol in a
freezer for 2 h and were then treated with 0.25% Triton X-100 for 5
min on an ice bath. Cells were resuspended in 300 µl of PBS
containing 40 µg/ml propidium iodide (PI) and 0.1 mg/ml RNase. Then
the cells were incubated in dark for 20 min at room temperature,
and cell cycle analysis was performed using a LSRII flow cytometer
(BD Biosciences, Franklin Lakes, NJ) and FlowJo 10.1.0 software
(Tree Star, Ashland, OR, USA). For each measurement, at least
10,000 cells were counted.
The apoptosis assay was performed according to the
following steps. After treatment for 48 h, the culture medium
containing floating cells was collected, and trypsin was added to
detach the adherent cells. Then the firstly collected culture
medium was added back to inactivate the trypsin. After being
pipetted gently, the cells were centrifuged at 2,000 rpm for 5 min.
The supernatant was decanted and cells were stained with
FITC-Annexin V and PI according to the manufacturer's instructions.
Untreated cells served as control. The cells were analyzed
immediately after staining using flow cytometry. For each
measurement, at least 20,000 cells were counted.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
HCT-116 cells were treated with 30 µM of compound K
for 6, 12 or 24 h. Total RNA was isolated using the RNAeasy kit
(Qiagen, Hilden, Germany). First strand cDNA was synthesized using
a SuperScript II First-Strand Synthesis System (Invitrogen,
Carlsbad, CA). RT-qPCR was performed using the following primers
(5′-3′): CDKN1A forward, CCTCATCCCGTGTTCTCCTTT, and reverse,
GTACCACCCAGCGGACAAGT; CDK6 forward, TGGAGACCTTCGAGCACC and reverse,
CACTCCAGGCTCTGGAACTT; CCND1 forward ACGAAGGTCTGCGCGTGTT and
reverse, CCGCTGGCCATGAACTACCT; CCNE1 forward,
ATCAGCACTTTCTTGAGCAACA and reverse, TTGTGCCAAGTAAAAGGTCTCC; TP53
forward, TGGCATTTGCACCTACCTCAC, and reverse, AACTCCCTCTACCTAACCAGC;
BAX, forward, GAGAGGTCTTTTTCCGAGTGG, and reverse,
GCTGGCAAAGTAGAAAAGGGC; BCL2 forward, GGCCTTCTTTGAGTTCGGTG and
reverse, ACAGGGCGATGTTGTCCAC; GAPDH forward, TGCACCACCAACTGCTTAGC
and reverse, GGCATGGACTGTGGTCATGAG. GAPDH was used as an internal
reference gene to normalize the expression of selected genes.
Relative gene expression levels were determined using the
2−ΔΔCq method.
Inflammatory cytokine IL-8 secretion
test
The HT-29 cells were seeded in 96-well plates and
cultured for 48 h. Different concentrations of Rb1 or compound K
plus 100 ng/ml LPS added in the medium were set as the experimental
group, while for control group there were only 100 ng/ml LPS and
medium without any drugs. After incubation for 6 h, the culture
medium was collected, the secreted IL-8 was quantified by
enzyme-linked immunosorbent assay (ELISA; Thermo Fisher Scientific,
MA, USA), and anti-inflammatory activities were calculated.
Statistical analysis
Results are expressed as the mean ± standard error,
and experiments were performed in triplicate with 3 repeats. A
Student's t-test and one-way analysis of variance with Tukey's post
hoc test were performed to evaluate the significance of the
differences between ginseng treatment and model groups. All
statistical analyses were performed using SPSS version 22.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
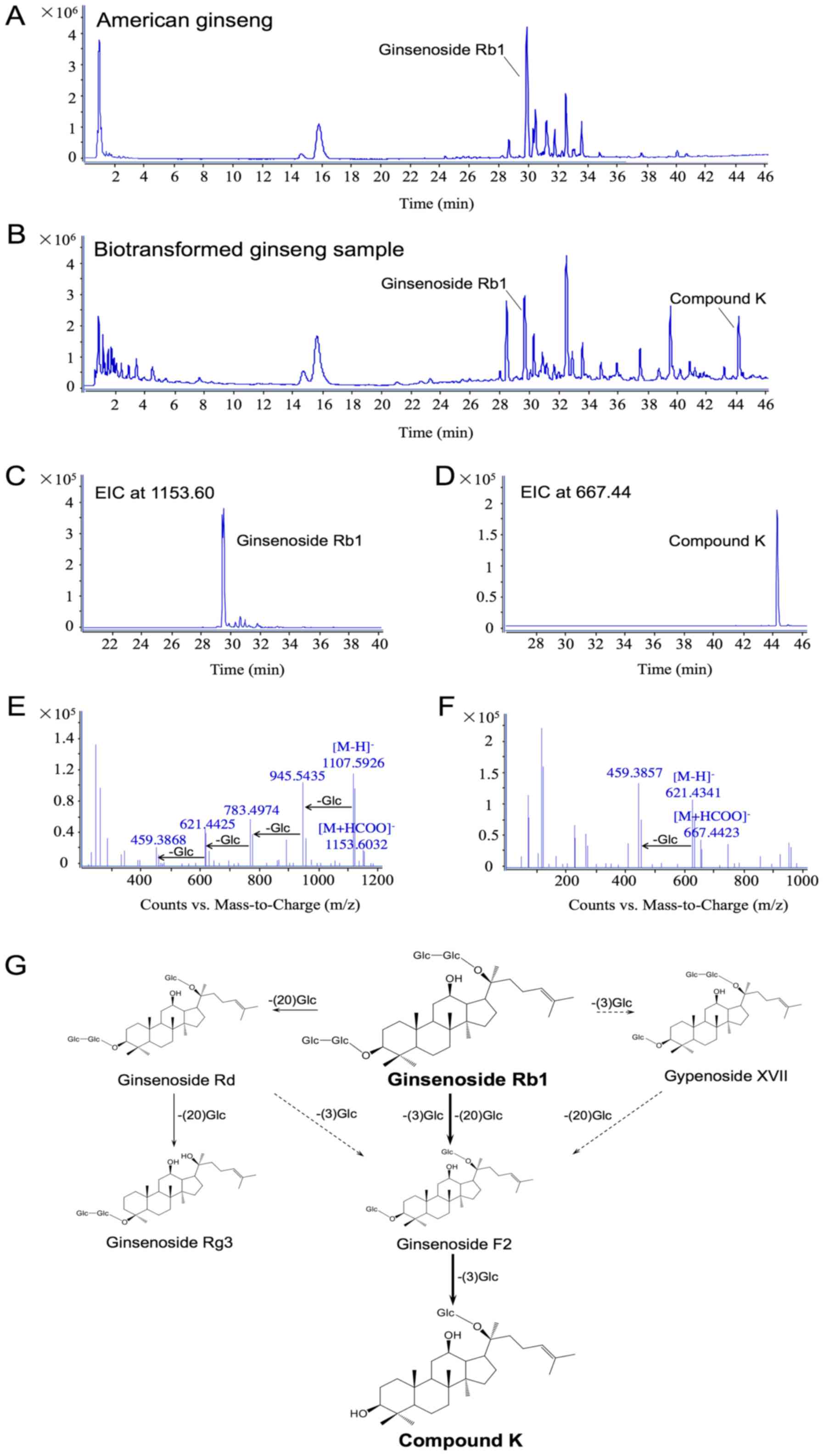
The biotransformation of ginsenoside Rb1 to compound
K by human enteric microflora. As shown in Fig. 1A, the typical total ion chromatography
(TIC) of AGE is displayed in the negative ion mode. Using
LC-Q-TOF-MS analysis, there was a total of 20 major ginseng
saponins detected in the extract, and ginsenoside Rb1 occupied the
highest peak as the primary constituent. When the ginseng extract
was incubated with human gut microflora for 24 h, the peak of
ginsenoside Rb1 was significantly decreased (Fig. 1B) in comparison with the original
un-transformed sample. Compound K was identified as one of its main
metabolites. The extracted ion chromatograms (EICs) of ginsenoside
Rb1 and compound K are further confirmed from the intricate matrix
of the biotransformed ginseng sample with a narrow mass window of
0.01 Da in Fig. 1C and D,
respectively. Fig. 1E and F show the
data of ginsenoside Rb1 and compound K in the negative MS/MS ion
mode. Since formic acid was added in the mobile phase, ginsenoside
Rb1, the typical solvent adduct ion [M+HCOO]− (m/z
1153.6032) and deprotonated molecular ion [M-H]− (m/z
1107.5926), as well as a series of successive losses of 162 Da
corresponding to glucose moiety were observed. Compound K has
similar MS/MS behavior to [M+HCOO]− (m/z 667.4423),
[M-H]− (m/z 621.4341), and [M-H-Glc]− (m/z
459.3857) in the negative ion mode. Combined with the metabolites
detected in this study, the metabolic pathways of Rb1 by human
intestinal microflora are proposed in Fig. 1G.
The major route was initiated when ginsenoside Rb1
eliminated C-3 and C-20 glucoses to form ginsenoside F2, and was
further converted into compound K by a C-3 glucose elimination,
which is easily absorbed into the circulation. Another route was
produced from ginsenoside Rb1 to gypenoside XVII by the glucose
elimination at C-3, and thus to ginsenoside F2 and compound K. In
addition, ginsenoside Rb1 selectively eliminated the C-20 glucose
chain to generate ginsenoside Rd and Rg3. Fig. 1 also indicates that compound K is the
major metabolite of ginsenoside Rb1 by human gut microflora.
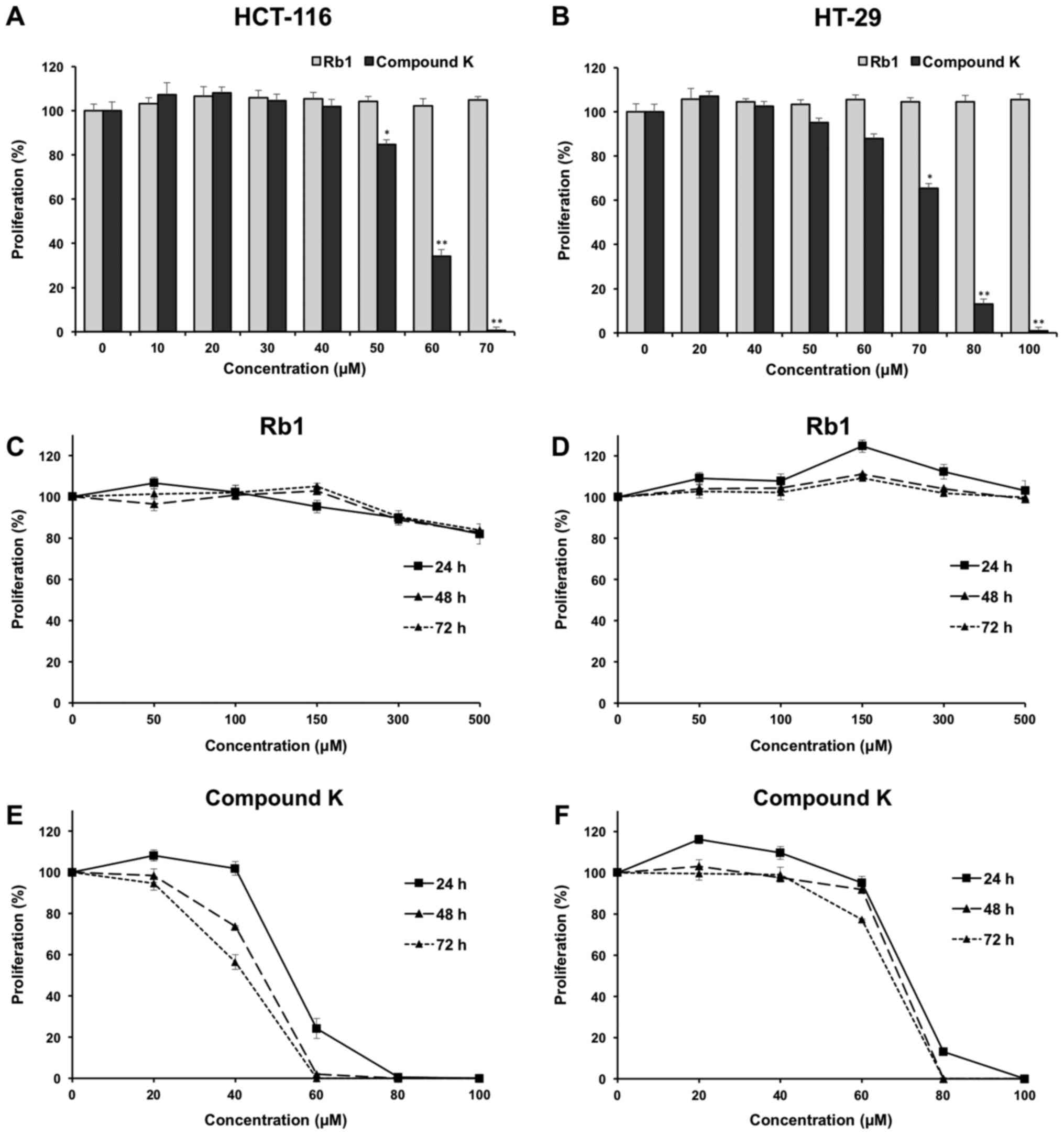
Effects of Rb1 and compound K on colorectal cancer
cell proliferation. The anti-proliferative effects of the
ginsenoside Rb1 and compound K were evaluated using two human
colorectal cancer cell lines, HCT-116 and HT-29. Fig. 2A shows the concentration-related
anti-proliferative effects of compound K on HCT-116 colorectal
cancer cells after a 6 h treatment, and similar results were
observed in HT-29 cells (Fig.
2B).
The effects of compound K on HCT-116 and HT-29 are
illustrated in Fig. 2E and F,
respectively. As shown in the figure, at the concentration of 60
µM, compound K exerted prominent growth suppression in HCT-116
cells. After treatment at 24, 48 and 72 h, compound K inhibited
HCT-116 cell growth by 75.81±3.65, 98.01±0.41 and 100%,
respectively (both P<0.01), indicating a time-related effect
manner. Similar results were observed in HT-29 cells (Fig. 2F). In contrast, Rb1 did not obviously
inhibit cell growth at concentrations of 0–500 µM for HCT-116 and
HT-29 (Fig. 2C and D).
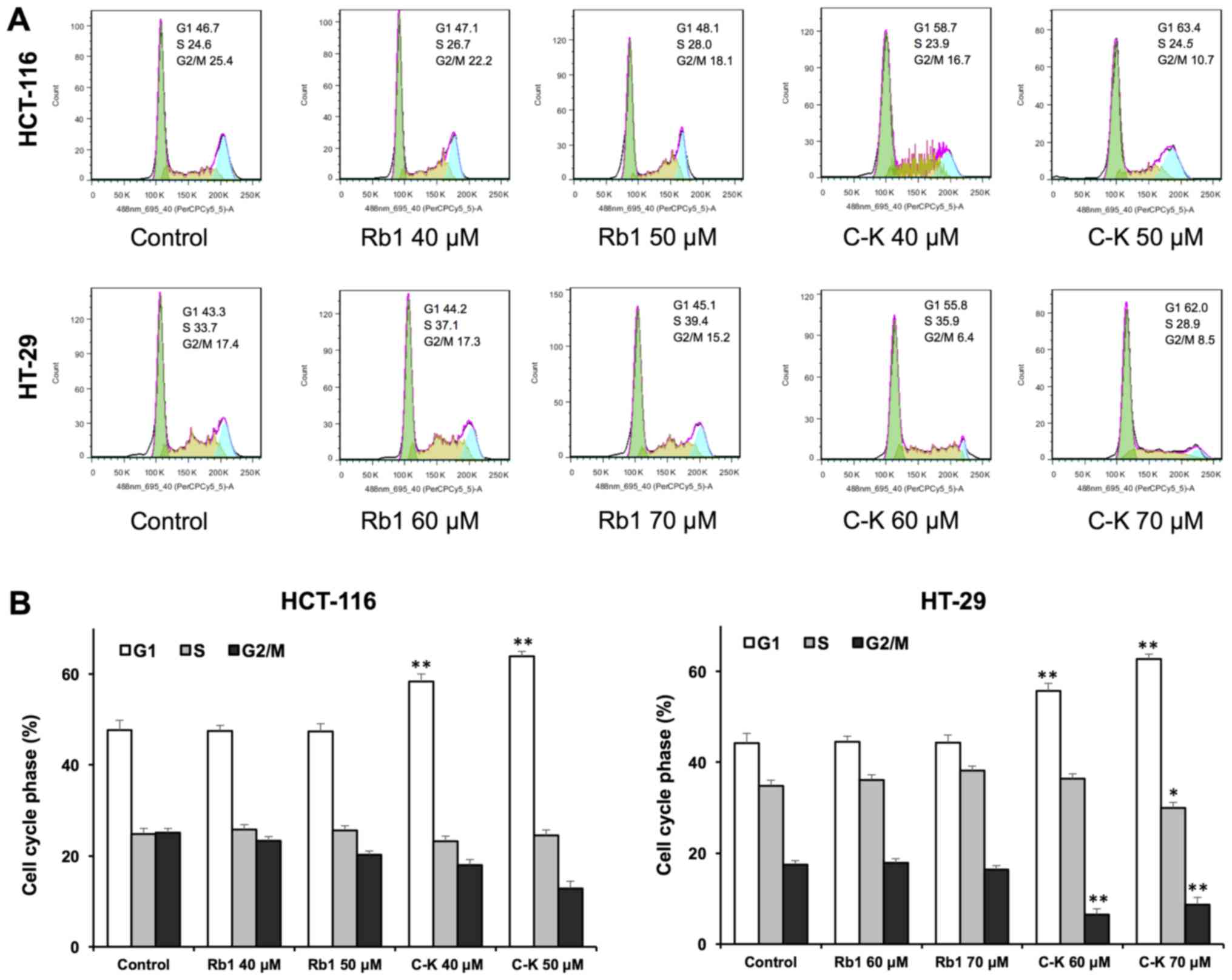
Effects of Rb1 and compound K on cell
cycle
Antiproliferative evaluation suggested that compound
K was active in inhibiting the human colorectal cancer cell growth.
To explore whether this was because of cell cycle arrest at a
specific phase, cell cycle profile was assayed using flow
cytometry. As shown in Fig. 3, the
effects of compound K on the cell cycle profile were observed at
concentrations as low as 40 µM. Treatment of HCT-116 cells with 40
and 50 µM compound K for 48 h increased the percentage of cells in
the G1 phase to 58.33±1.19 and 63.90±0.56%, respectively, compared
to 47.63±1.14% in vehicle-treated cells (all P<0.01). Treatment
of HT-29 cells with 60 and 70 µM compound K for 48 h increased G1
phase cells to 55.67±0.71 and 62.70±0.66% respectively, compared to
44.17±0.81% in vehicle-treated cells (all P<0.01). Thus, in both
cancer cell lines, compound K significantly increased the number of
human colorectal cancer cells in G1 phase.
On the other hand, Rb1 treatment did not influence
the cell cycle at 20 and 40 µM. These results suggested that
compound K, the Rb1 metabolite, could significantly induce G1 phase
cell cycle arrest in both HCT-116 and HT-29 cell lines.
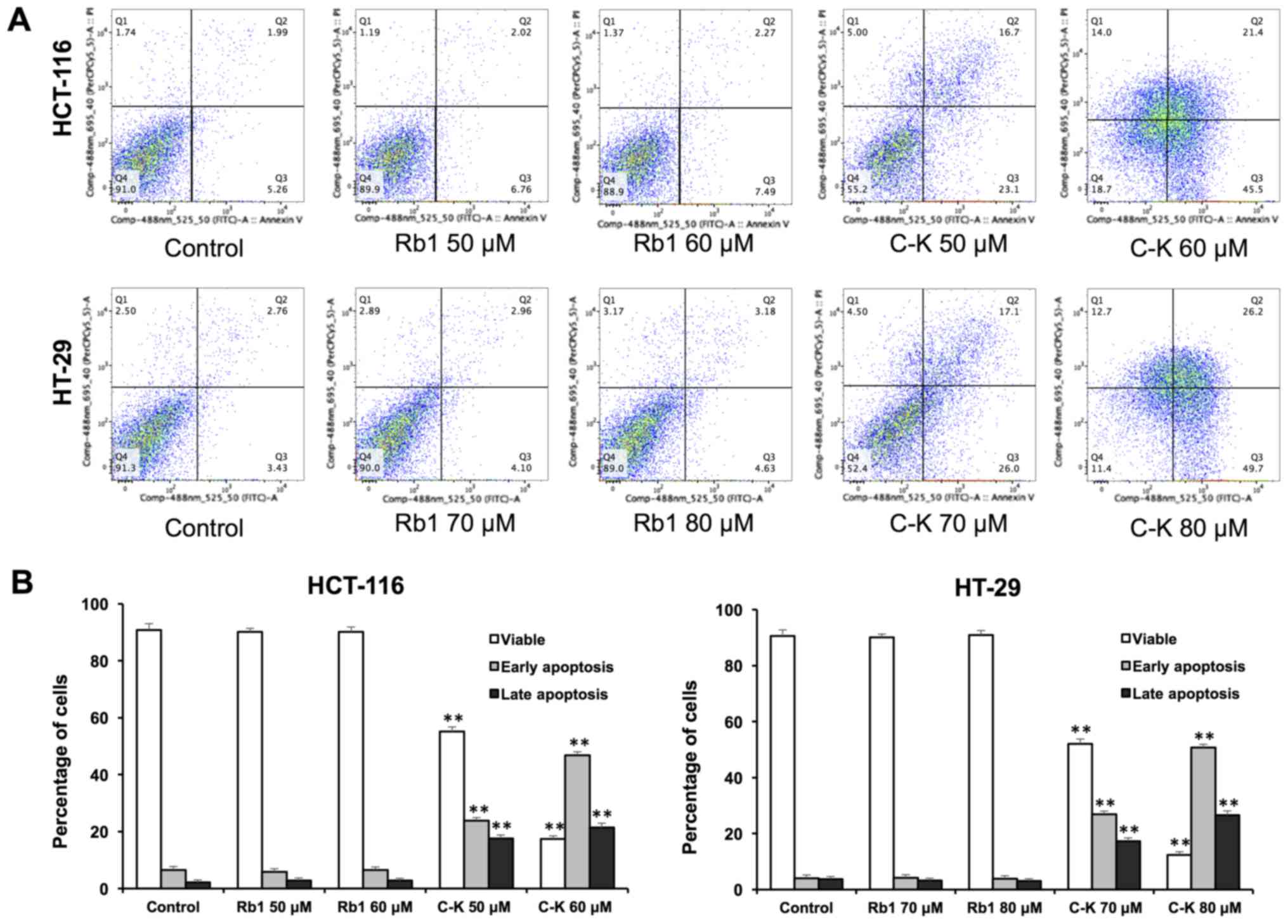
Effects of Rb1 and compound K on cell
apoptosis
The apoptotic effects of Rb1 and compound K were
evaluated by flow cytometry after staining with Annexin V and PI.
Annexin V can be detected in both early and late stages of
apoptosis, whereas PI-stained cells only in late apoptosis or
necrosis. Early apoptotic cells were positive for Annexin V and
negative for PI (lower right quadrant); late apoptotic cells were
stained for both Annexin V and PI (upper right quadrant).
As shown in Fig. 4,
following treatment with 60 µM of compound K for 48 h, the
percentage of early and late apoptotic HCT-116 cells was 46.85±1.37
and 21.38±1.08%, respectively (control, 6.54±1.28 and 2.12±1.17%).
When treated with 80 µM compound K for 48 h, the percentage of
early and late apoptotic HT-29 cells was 50.69±1.20 and
26.52±1.58%, respectively (control: 4.02±1.21 and 3.77±1.90%). In
contrast, Rb1 did not induce apoptosis with the same concentrations
indicating that only compound K significantly induces cell
apoptosis.
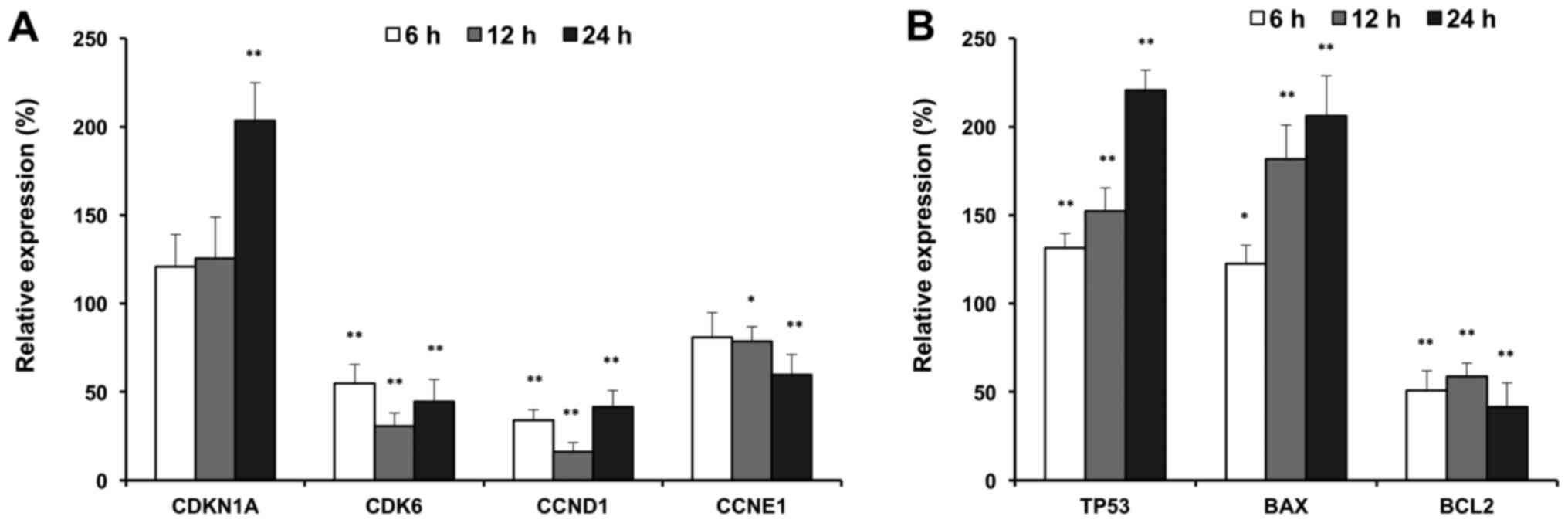
Effects of compound K on cell cycle
and apoptotic-related gene expression
To further explore the antiproliferative mechanisms,
we evaluated the effects of compound K on selected cell cycle and
apoptotic related genes. As shown in Fig.
5, compound K significantly up-regulated expression of p21
(CDKN1A), and downregulated the expressions of CDK6, cyclin D and
cyclin E, which are G1 phase arrest regulators (Fig. 5A). In addition, compound K
up-regulated the expressions of p53 and Bax, which are
pro-apoptotic genes. In contrast, compound K downregulated the
expression of Bcl2, which is an anti-apoptotic gene (Fig. 5B). Cell cycle and apoptotic related
gene expression supported our observations with flow cytometry.
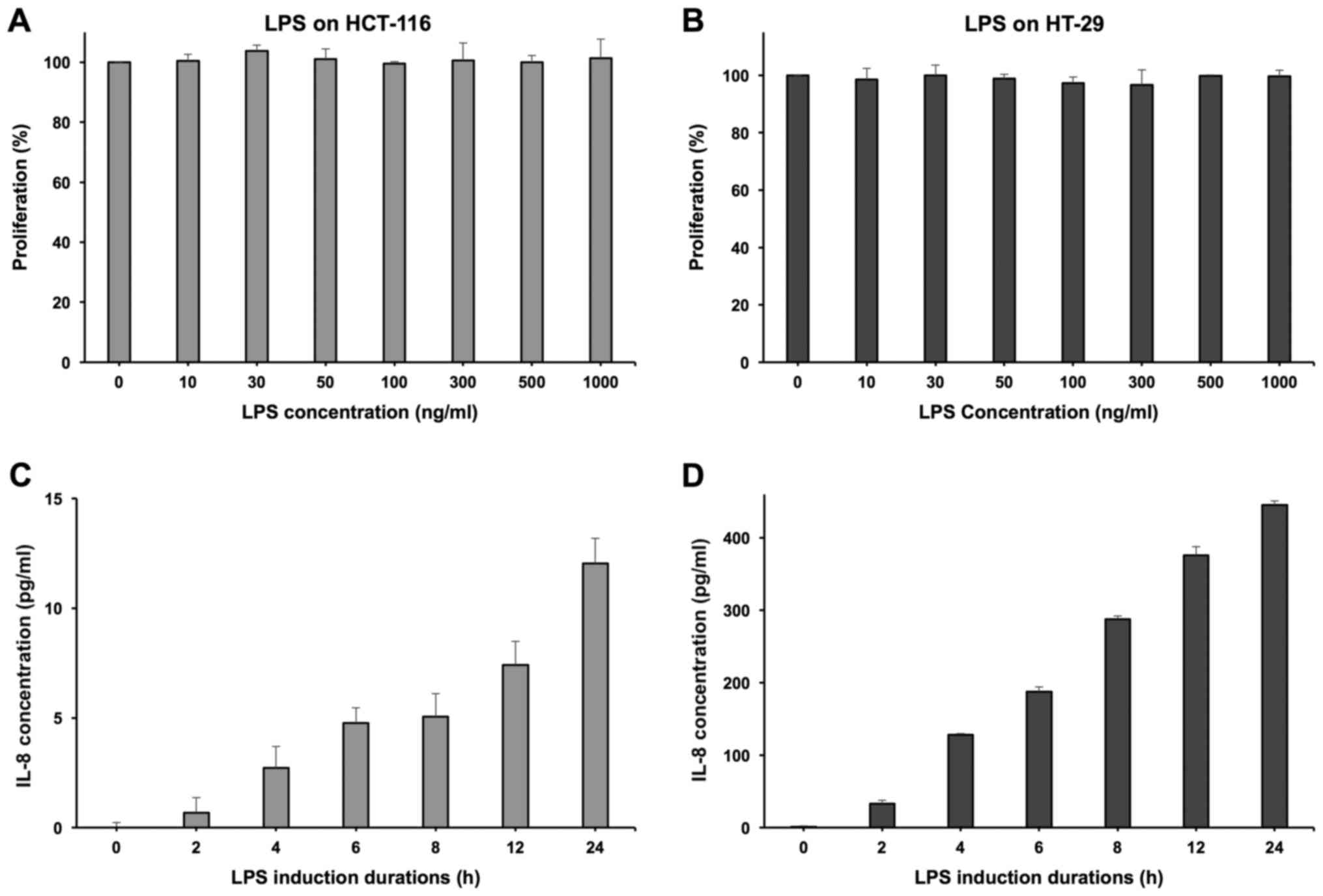
Effects of Rb1 and compound K on
inflammatory cytokine IL-8 secretion of colorectal cancer cell
LPS was used to induce HCT-116 and HT-29 cell lines
to secrete the inflammatory cytokine IL-8. At the concentration of
100 ng/ml, LPS did not show any significant influence on the cell
growth of both cell lines (Fig. 6A and
B). Induced by LPS, HT-29 cells can secrete IL-8 effectively
and demonstrated a positive correlation with the induction time
(Fig. 6D). However, the HCT-116 cell
line showed a relatively limited IL-8 secretion level (Fig. 6C), so the HT-29 cell line was selected
to conduct the further experimentation of anti-inflammatory
evaluation (16).
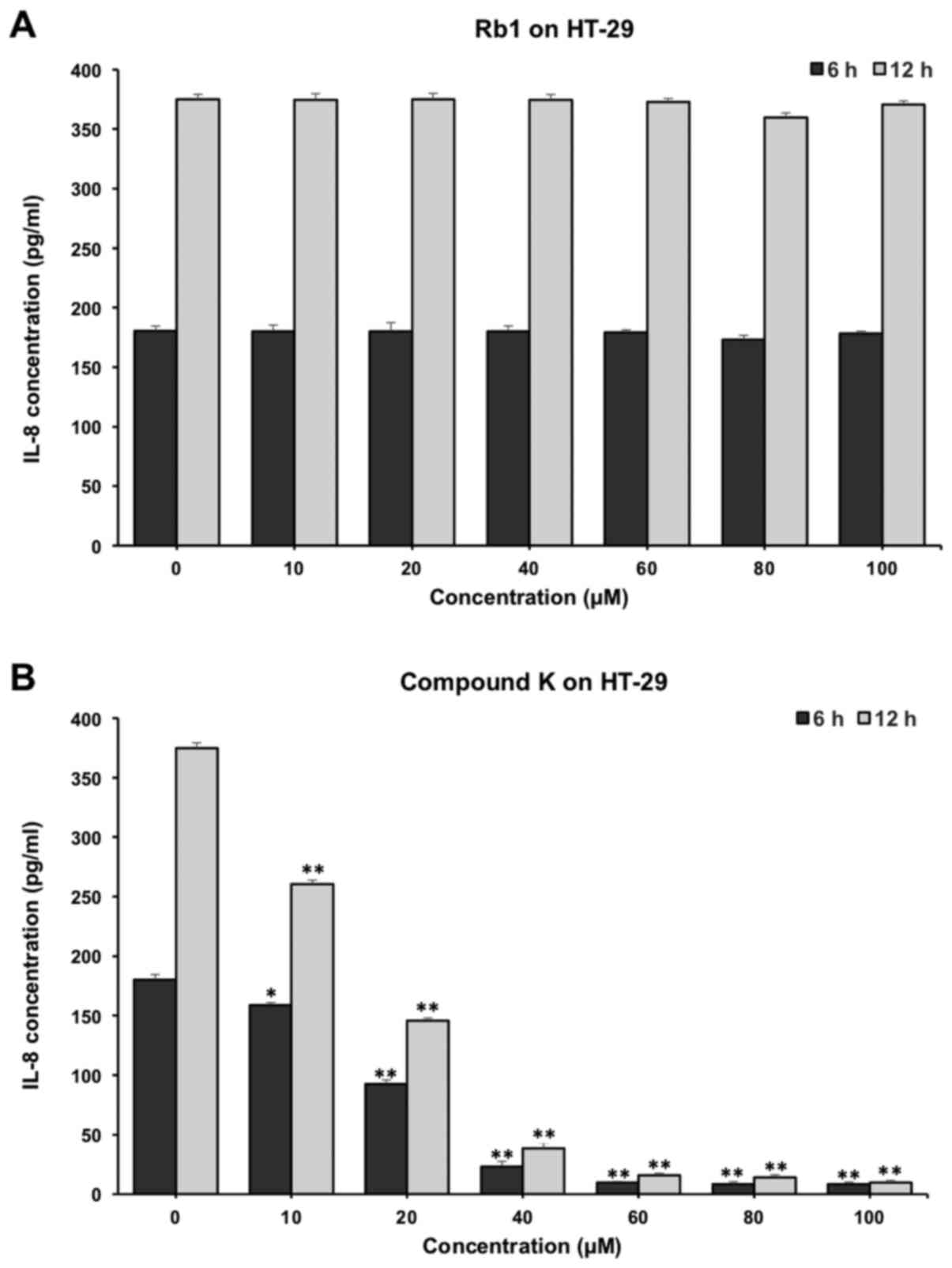
As shown in Fig. 7,
compound K significantly reduced the IL-8 production in HT-29
cells. When incubated with 20 µM compound K of for 6 and 12 h, the
concentrations of IL-8 were reduced from 180.33±4.32 to 92.56±3.46
and 70.05±2.32 pg/ml respectively (P<0.01) (Fig. 7B). In contrast, Rb1 did not show
obvious anti-inflammatory effects on HT-29 cells (Fig. 7A).
Discussion
Colorectal cancer, one of the fourth leading causes
of cancer-related death worldwide, is known to be a common
malignant disease with a high mortality rate and gradually
increased incidence (17–19). Colorectal cancer is a multifactorial
disease involving genetic, environmental and lifestyle risk
factors. In the development of colorectal cancer, chronic
inflammation has been identified as a key predisposing factor
(20,21). The inflammatory state of the colon can
strongly influence the process of colorectal cancer, and subtle
inflammation in otherwise healthy colonic tissues may cause the
conversion of a healthy colon into a dysplastic colon, as well
(22).
Due to the disadvantageous effects of chemotherapy,
which may worsen the patient's quality of life prominently, it is
necessary and meaningful to identify non-toxic chemicals extracted
from herbal medicines for the treatment of colorectal cancer. In
this study, HT-29 chemo-resistant cancer cells were used for the
evaluation of the effects of compound K.
Ginseng is one of the herbs that proved to be
beneficial for the treatment. According to many studies, ginseng is
active in maintaining health and effective in the prevention and
treatment of cancers by different mechanisms (23–25).
Ginseng is not only effective in reducing chemotherapy side effects
and improving the quality of patient's life, it also plays a
crucial role in inflammation reduction and thereby reduces the risk
of cancer (26,27). Ginsenoside Rb1 is a major component of
American ginseng and Asian ginseng. The products of ginseng are
almost always taken orally just like many other herbal medicines.
After being ingested into the gut, ginsenoside Rb1 is degraded by
intestinal microbiota and then stepwise converted into compound K;
this is a main metabolic pathway of Rb1 (28,29). As an
important metabolite of Rb1, compound K could be absorbed into the
systemic circulation and exert remarkable biofunctions (30,31).
In this study, using LC-Q-TOF-MS, we examined the
biotransformation of Rb1 by human gut microbiota, and compound K
was identified as a main metabolite of Rb1. Compared with its
parent compound Rb1, compound K shows a distinct anti-tumor effect
in both HCT-116 and HT-29 cell lines. Compound K can also
significantly arrest HCT-116 and HT-29 cells in the G1 phase, and
induce cell apoptosis. In contrast, Rb1 did not exert any obvious
influence on the cancer cell proliferation, cell cycle and
apoptosis. RT-qPCR data supported our flow cytometry results, that
compound K regulated the expressions of G1 phase cell cycle and
apoptotic related genes.
We compared the anti-inflammatory effects of Rb1 and
compound K using LPS to induce HCT-116 and HT-29 human colorectal
cancer lines to secrete inflammatory cytokine IL-8. The IL-8
production indicates that the HT-29 cell line is a suitable in
vitro model to evaluate the anti-inflammatory responses
(16). Compound K, the Rb1
metabolite, was observed to have significant anti-inflammatory
effects, even at low concentrations, while Rb1 did not show any
anti-inflammatory effects. Of note, the concentrations of compound
K to be observed with anti-tumor effects are relatively higher than
that for anti-inflammatory ones, which may imply that the compound
K at low doses can play an anti-inflammatory role in the early
stage of colorectal cancer with inflammatory conditions, and an
anti-tumor role for high doses.
In conclusion, compound K is an important metabolite
of Rb1 and possesses even more significant bio-functions compared
with Rb1. Compound K can significantly induce G1 phase cell cycle
arrest and apoptosis in both HCT-116 and HT-29 cell lines. Compound
K can benefit the treatment of cancer-associated inflammation and
exert anticancer effects in a concentration related manner, using
HT-29 cells. Future in vivo observations are needed to
evaluate the clinical utility of compound K in colorectal cancer
chemoprevention.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by grants
from China Postdoctoral Science Foundation (grant no. 2017M610830),
National Natural Science Foundation of China (grant no. 81603378),
Natural Science Foundation of Jiangsu Province (grant no.
BK20160545) and The National Institutes of Health/National Center
for Complementary and Integrative Health (grant nos. AT004418 and
AT005362).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CY conceived the study and led the design of this
research, and QW and XN made substantial contributions to the
conception and design. HY, JW, CW and JZ made contributions to the
acquisition of data. WH, CS and LL participated in the analysis and
interpretation of data. HY and JW drafted the manuscript, CS
revised it critically for important intellectual content, and CW
gave final approval of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board at the University of Chicago; patients provided oral
consent for sample collection.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Qi LW, Wang CZ and Yuan CS: Isolation and
analysis of ginseng: Advances and challenges. Nat Prod Rep.
28:467–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim DH: Chemical Diversity of Panax
ginseng, Panax quinquifolium, and Panax notoginseng. J
Ginseng Res. 36:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park J, Bui PTC, Song H, Kim SK, Rhee DK,
Kim EY, Rhyu MR, Lee MS and Lee YJ: Ginseng on nuclear hormone
receptors. Am J Chin Med. 45:1147–1156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yennurajalingam S, Tannir NM, Williams JL,
Lu Z, Hess KR, Frisbee-Hume S, House HL, Lim ZD, Lim KH, Lopez G,
et al: A double-blind, randomized, placebo-controlled trial of
Panax ginseng for cancer-related fatigue in patients with
advanced cancer. J Natl Compr Canc Netw. 15:1111–1120. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wan JY, Huang WH, Zheng W, Park CW, Kim
SH, Seo DB, Shin KS, Zeng JX, Yao H, Sava-Segal C, et al: Multiple
effects of ginseng berry polysaccharides: Plasma cholesterol level
reduction and enteric neoplasm prevention. Am J Chin Med.
45:1293–1307. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi LW, Wang CZ and Yuan CS: Ginsenosides
from American ginseng: Chemical and pharmacological diversity.
Phytochemistry. 72:689–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan CS, Wang CZ, Wicks SM and Qi LW:
Chemical and pharmacological studies of saponins with a focus on
American ginseng. J Ginseng Res. 34:160–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SJ, Ha N, Kim Y and Kim MG: Changes in
the ginsenoside content during fermentation using an appliance for
the preparation of red ginseng. Am J Chin Med. 44:1595–1606. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du XH, Zhao YL, Yang DF, Liu Y, Fan K,
Liang ZS and Han RL: A correlation model of UPLC fingerprints and
anticoagulant activity for quality assessment of Panax
notoginseng by hierarchical clustering analysis and multiple
linear regression analysis. Anal Met. 7:2985–2992. 2015. View Article : Google Scholar
|
|
10
|
Wan JY, Liu P, Wang HY, Qi LW, Wang CZ, Li
P and Yuan CS: Biotransformation and metabolic profile of American
ginseng saponins with human intestinal microflora by liquid
chromatography quadrupole time-of-flight mass spectrometry. J
Chromatogr A. 1286:83–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao J, Chen H, Kang D, Shao Y, Shen B, Li
X, Yin X, Zhu Z, Li H, Rao T, et al: Qualitatively and
quantitatively investigating the regulation of intestinal
microbiota on the metabolism of Panax notoginseng saponins.
J Ethnopharmacol. 194:324–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Z, Yang J, Cheng C, Huang Y, Du F, Wang
F, Niu W, Xu F, Jiang R, Gao X and Li C: Combinatorial metabolism
notably affects human systemic exposure to ginsenosides from orally
administered extract of Panax notoginseng roots (Sanqi).
Drug Metab Dispos. 41:1457–1469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wan JY, Wang CZ, Liu Z, Zhang QH, Musch
MW, Bissonnette M, Chang EB, Li P, Qi LW and Yuan CS: Determination
of American ginseng saponins and their metabolites in human plasma,
urine and feces samples by liquid chromatography coupled with
quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt
Technol Biomed Life Sci. 1015–1016:62–73. 2016. View Article : Google Scholar
|
|
14
|
Wang CZ, Kim KE, Du GJ, Qi LW, Wen XD, Li
P, Bauer BA, Bissonnette MB, Musch MW, Chang EB and Yuan CS:
Ultra-performance liquid chromatography and time-of-flight mass
spectrometry analysis of ginsenoside metabolites in human plasma.
Am J Chin Med. 39:1161–1171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang CZ, Du GJ, Zhang Z, Wen XD, Calway T,
Zhen Z, Musch MW, Bissonnette M, Chang EB and Yuan CS: Ginsenoside
compound K, not Rb1, possesses potential chemopreventive activities
in human colorectal cancer. Int J Oncol. 40:1970–1976.
2012.PubMed/NCBI
|
|
16
|
Grimoud J, Durand H, de Souza S, Monsan P,
Ouarné F, Theodorou V and Rogues C: In vitro screening of
probiotics and synbiotics according to anti-inflammatory and
anti-proliferative effects. Int J Food Microbiol. 144:42–50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Troeung L, Sodhi-Berry N, Martini A,
Malacova E, Ee H, O'Leary P, Lansdorp-Vogelaar I and Preen DB:
Increasing incidence of colorectal cancer in adolescents and young
adults aged 15–39 years in Western Australia 1982–2007: Examination
of colonoscopy history. Front Public Health. 5:1792017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Campos FGCM, Figueiredo MN, Monteiro M,
Nahas SC and Cecconello I: Incidence of colorectal cancer in young
patients. Rev Col Bras Cir. 44:208–215. 2017.(In English,
Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ullman TA and Itzkowitz SH: Prognosis of
colorectal cancer in inflammatory bowel disease: Data from a state
registry. Dig Dis Sci. 62:1850–1851. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ehrlich AC, Patel S, Meillier A, Rothstein
RD and Friedenberg FK: Chemoprevention of colorectal cancer in
inflammatory bowel disease. Expert Rev Anticancer Ther. 17:247–255.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brennan CA and Garrett WS: Gut microbiota,
inflammation, and colorectal cancer. Annu Rev Microbiol.
70:395–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang CZ, Huang WH, Zhang CF, Wan JY, Wang
Y, Yu C, Williams S, He TC, Du W, Musch MW, et al: Role of
intestinal microbiome in American ginseng-mediated colon cancer
protection in high fat diet-fed AOM/DSS mice. Clin Transl Oncol.
20:302–312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai D, Zhang CF, Williams S, Yuan CS and
Wang CZ: Ginseng on cancer: Potential role in modulating
inflammation-mediated angiogenesis. Am J Chin Med. 45:13–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HS, Kim MK, Lee M, Kwon BS, Suh DH and
Song YS: Effect of red ginseng on genotoxicity and health-related
quality of life after adjuvant chemotherapy in patients with
epithelial ovarian cancer: A randomized, double blind,
placebo-controlled trial. Nutrients. 9:pii: E772. 2017. View Article : Google Scholar
|
|
26
|
Yu T, Rhee MH, Lee J, Kim SH, Yang Y, Kim
HG, Kim Y, Kim C, Kwak YS, Kim JH and Cho JY: Ginsenoside Rc from
Korean red ginseng (Panax ginseng C.A. Meyer) attenuates
inflammatory symptoms of gastritis, hepatitis and arthritis. Am J
Chin Med. 44:595–615. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Poudyal D, Le PM, Davis T, Hofseth AB,
Chumanevich A, Chumanevich AA, Wargovich MJ, Nagarkatti M,
Nagarkatti PS, Windust A and Hofseth LJ: A hexane fraction of
American ginseng suppresses mouse colitis and associated colon
cancer: Anti-inflammatory and proapoptotic mechanisms. Cancer Prev
Res (Phila). 5:685–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tawab MA, Bahr U, Karas M, Wurglics M and
Schubert-Zsilavecz M: Degradation of ginsenosides in humans after
oral administration. Drug Metab Dispos. 31:1065–1071. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim KA, Jung IH, Park SH, Ahn YT, Huh CS
and Kim DH: Comparative analysis of the gut microbiota in people
with different levels of ginsenoside Rb1 degradation to compound K.
PLoS One. 8:e624092013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiao J, Chen D, Lin XX, Peng SF, Xiao MF,
Huang WH, Wang YC, Peng JB, Zhang W, Ouyang DS and Chen Y:
Screening of drug metabolizing enzymes for the ginsenoside compound
K in vitro: An efficient anti-cancer substance originating from
Panax ginseng. PLoS One. 11:e01471832016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Xu Y, Zhu Y and Li X: Anti-cancer
effects of ginsenoside compound K on pediatric acute myeloid
leukemia cells. Cancer Cell Int. 13:242013. View Article : Google Scholar : PubMed/NCBI
|